Abstract
Objective
The aims of the following experiments were to characterize anti-diabetic in vitro and in vivo activity of the polyphenol-rich aqueous extract of Rutgers Scarlet Lettuce.
Materials / Methods
Rutgers Scarlet Lettuce (RSL) extract (RSLE) and isolated compounds were evaluated for inhibitory effects on glucose production as well as tumor necrosis factor alpha (TNFα)-dependent inhibition of insulin activity in H4IIE rat hepatoma cells. Additionally, high fat diet-induced obese mice were treated with RSLE (100 or 300 mg/kg), Metformin (250 mg/kg) or vehicle (water) for 28 days by oral administration and insulin and oral glucose tolerance tests were conducted. Tissues were harvested at the end of the study and evaluated for biochemical and physiological improvements in metabolic syndrome conditions.
Results
A polyphenol-rich RSLE, containing chlorogenic acid, cyanidin malonyl-glucoside and quercetin malonyl-glucoside, was produced by simple boiling water extraction at pH 2. In vitro, RSLE and chlorogenic acid demonstrated dose-dependent inhibition of glucose production. In vivo, RSLE treatment improved glucose metabolism measured by oral glucose tolerance tests, but not insulin tolerance tests. RSLE treated groups had a lower ratio of liver weight to body weight as well as decreased total liver lipids compared to control group after 28 days of treatment. No significant differences in plasma glucose, insulin, cholesterol, and triglycerides were observed with RSLE treated groups compared to vehicle control.
Conclusion
RSLE demonstrated anti-diabetic effects in vitro and in vivo and may improve metabolic syndrome conditions of fatty liver and glucose metabolism.
Keywords: Red lettuce, anthocyanins, flavonols, chlorogenic acid, metabolic syndrome
Introduction
Modification of diet and exercise are the primary means by which most individuals can significantly counter the health problems of obesity and associated conditions of metabolic syndrome, including type 2 diabetes, dyslipidemia, and cardiovascular disease. Diets rich in fruits and vegetables help reduce the risk of developing chronic diseases according to epidemiological studies [1, 2]. Development of functionally-enhanced foods containing beneficial phytochemicals and offering health and wellness benefits beyond basic nutrition, may provide additional advantages in combating the metabolic syndrome. Prominent functional foods include blueberries, green tea, cocoa, and cinnamon; although clinical research is not definitive on their preventative and treatment properties [3, 4]. Recently, it was reported that polyphenols, but not sugars, were efficiently concentrated and stabilized from blueberry and grape juices to produce a phytochemically-enhanced food ingredient which demonstrated anti-diabetic effects in animal models [5-7]. Polyphenol rich fruits and vegetables also showed anti-diabetic effects is several clinical studies [8, 9].
Development and production of plants with enhanced nutritional and functional value is on the rise due to burgeoning consumer interest [10]. Red lettuce varieties with high polyphenolic content (close to 10% dry weight), named Rutgers Scarlet Lettuce (RSL), were developed through non-transgenic tissue culture approaches and targeted screens for purple anthocyanin coloration in leaf tissue, (unpublished data, Cheng et al. 2013). RSL accumulated high contents of hydroxycinnamates and quercetin glycosides. Hydroxycinnamates are abundant in coffee, especially green coffee beans, and have been reported to have anti-diabetic effects [11]. Chlorogenic acid is one of the most abundant hydroxycinnamates and coffee extracts decreased plasma glucose compared to control when administered with carbohydrate in humans [12]. Quercetin is another ubiquitous phytochemical reported to have anti-inflammatory activity, improve insulin sensitivity, and reduce blood pressure and other conditions relevant to metabolic syndrome [13-15]. The high content of hydroxycinnamic acids, cyanidin-glycosides and quercetin glycosides suggest that RSL may also have beneficial effects against metabolic syndrome and type 2 diabetes.
In this study, we produced an aqueous polyphenol-rich extract from RSL, termed RSLE, and characterized its in vitro and in vivo anti-diabetic activity including inhibition of glucose production and attenuation of tumor necrosis factor alpha (TNFα)-induced insulin resistance in H4IIE hepatoma cells. Additionally, high fat diet-induced obese mice were treated with RSLE for 28 days by oral administration and oral glucose and insulin tolerance tests were conducted. Tissues were harvested at the end of the study and evaluated for biochemical and physiological improvements in metabolic syndrome conditions. Our results suggest that RSL improves glucose metabolism and attenuates lipid accumulation in the liver.
Materials and Methods
Phytochemical Analysis
RSL plants were maintained in growth chambers under following conditions: 19 °C day, 16 °C night, 16 h light/8 h dark photoperiod, 65 % relative humidity, and 225 µE m-2s-1 light intensity provided by cool white fluorescent lamps. Two varieties of RSL were harvested for analyses: NFR-S-4 and NBR-S-16. Voucher specimens of NFR-S-4 (accession # 139699) and NBR-S-16 (accession # 139700) were deposited in the Chrysler Herbarium at Rutgers University. NFR-S-4 was used to produce the RSLE use in this study.
Outer leaves were harvest from 2-3 month old plants, fresh weights were recorded and leaves were frozen at -80 °C prior to lyophilizatio n. Dry weights were recorded and leaves were ground to a fine powder with a mortar and pestle. Samples were extracted with a methanolic solvent to efficiently extract polyphenols as described by [16] with minor modifications. Briefly, 0.5 g of dried material was extracted with 15 mL of solvent (CH4O/H2O/C2H4O2; 85:14.5:0.5) three times. Samples were pooled and filtered through 0.45 μm PTFE filters (VWR) prior to analyses.
To produce an aqueous extract, RSLE, fresh leaves of RSL were blended with 100 °C water (adjusted to pH 2 with H2SO4) 1:5 (w/v) in a Vitamix Professional 500 Blender (Cleveland, OH) for 30 s. The lettuce mixture was centrifuged for 5 min at 4000 rpm to pellet solids. The supernatant was vacuum filtered through Whatman® #1 paper and the volume was reduced by rotary evaporation. Samples were lyophilized and stored at -20 °C. For stability tests, extracts were stored in amber glass screw cap vials under accelerated storage conditions (37 °C). Subsamples of extracts were analyzed for total polyphenolic and anthocyanidin content every 7 days for 28 days.
Phytochemical Isolation and Structure Elucidation
Compounds were separated by centrifugal partition chromatography (CPC) on an Armen Spot CPC 250 Light (Saint-Avé, France) instrument using a 210 mL column. A two phase solvent system of CHCl3/C3H8O/H2O (2:4:4) 0.5 % acetic acid was used in ascending mode with the aqueous upper layer as the mobile phase (5 mL/min, elute 300 mL upper phase, extrude 300 mL lower phase). Fractions were collected every 2 min on a CHF122SC fraction collector (Advantec, Dublin, CA) and pooled according to UV monitoring at 254 nm into 11 fractions (A-K).
Reversed phase-high performance liquid chromatography (RP-HPLC) was carried out on a Water System (Waters 616 four channel pump with semi-preparative pump heads operated on a Waters 600 Controller; Waters 490E Programmable Multiwavelength Detector set to monitor at 254, 360 and 520nm; Waters 717 Plus Autosampler) using a Phenomenex semi-preparative Synergi Hydro column (250 × 20 mm, 4 μm) run with a flow rate of 10 mL/min. CPC fraction C was subjected to isocratic RP-HPLC elution with CH3OH/H2O/C2H4O2 (50:45:5) to give cyanidin 3-O-glucoside (Rt 6.2 min) and cyanidin 3-O-(6-malonyl-glucoside) (Rt 7.4 min). FCPC fraction E was subjected to RP-HPLC under the same conditions as above to afford 5-O-caffeoylquinic acid (chlorogenic acid) (Rt 7.3 min) and quercetin glucuronide (Rt 13.5 min). FCPC fractions F and I were combined and subjected to isocratic RP-HPLC with CH3OH/H2O/C2H4O2 (25:70:5) to give quercetin 3-glucoside (Rt 9.4 min) and quercetin 3-(6-malonylglucoside) (Rt 10.6 min). Compound identity was confirmed by comparison of HPLC-MS and NMR data with published values [17-20]. 1H NMR spectra were recorded in DMSO-d6 on a 500 Varian VNMRS 500 MHz. UPLC-MS was performed as previously described in [21] with the addition a positive ionization mode in the Varian 1200L triple quadrupole mass detector (Varian Inc., Palo Alto, CA). The electrospray ionization interface was operated in negative or positive ionization mode and voltage was adjusted to -4.5 kV for the negative mode and 5 kV for the positive mode. The drying gas temperature was 280 °C and the sheath gas was compressed air for the negative ionization mode and nitrogen for the positive ionization mode.
HPLC-UV Quantification
Phytochemicals were quantified by RP-HPLC-UV using standards of chlorogenic acid (Sigma), dicaffeoyl quinic acid (ChromaDex, Irvine, CA), cyanidin 3-glucoside (Polyphenols, Norway) and quercetin 3-glucoside (provided by Professor Karwe at Rutgers University) to generate regression lines for quantification. The column was a Synergi Hydro-RP 80Å column (250 × 4.6 mm, 4 μm). The flow rate was 1 mL/min and 10 μL of each sample or standard was injected. UV detection was set at 254 nm (quercetin glycosides), 320 nm (hydroxycinnamic acids), and 517 nm (anthocyanins). Peak areas were analyzed using and expressed as indicated compound equivalents ± standard error (SE). Compounds were separated using 0.1% trifluoroacetic acid in water (solvent A) and acetonitrile (solvent B). The solvent gradient used was 16% B, 84% A initially; 20% B, 80% A at 15 min and 16% B, 84% A at 32 min to completion. Each run was 60 min and followed by a 15 min equilibration period with 16% B, 84% A.
Analyses of total polyphenols, anthocyanins and oxygen radical absorption capacity
Total polyphenol content was measured by a modified Folin-Ciocalteu method [22] as previously described [21] and expressed as mean gallic acid equivalents of at least three independent experiments. Total monomeric anthocyanin content was determined according to the AOAC pH differential method and recorded as cyanidin 3-glucoside equivalents [23]. Oxygen radical absorption capacity was measured as described [24, 25] and results were expressed as μmol Trolox® equivalents.
In vitro glucose production assay
H4IIE rat hepatoma cells (CRL-1548, American Type Culture Collection, Manassas, VA) were assayed as previously described [21]. Cell viability was measured by the 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT; TCI, Portland, OR) assay [26].
Animal care and experimental design
All protocols and standard operating procedures with animals were reviewed according to guidelines approved by the Internal Animal Care and Use Committee at Rutgers University. Five week old male C57Bl/6J mice were purchased from Jackson Labs (Bar Harbor, ME) and cared for as previously describe [21]. A diet-induced obese (DIO) mouse model was induced by providing a very high fat diet (VHFD) containing 60% kcal fat (D12492, Research Diets, New Brunswick, NJ) for 13 weeks [27]. Weekly food intake per cage and individual body weight measurements were determined throughout the study.
After 13 weeks on VHFD, DIO mice were given a preliminary oral glucose tolerance test (OGTT) and divided to balanced response groups. Mice were given daily oral administrations of RSLE (100 or 300 mg/kg) or water control (n = 10 per treatment group) for 28 days. A Metformin group (250 mg/kg) was included as positive control (n = 5). On days 7 and 21 of treatment, mice were given insulin tolerance tests (ITTs). On days 14 and 25 of treatment, mice were given OGTTs. ITTs were administered by fasting mice for 4-6 h prior to insulin i.p. injection (0.75 U/kg) and measuring blood glucose every 20 or 30 min from the tail vein using a glucose using an AlphaTrak glucometer (Abbott Labs Inc., Abbott Park, IL). For OGTT, mice were fasted for 4 h (day 14) or overnight (day 25) prior to an oral glucose challenge (2 g/kg). Blood glucose was recorded every 30 min. Insulin response and glucose metabolism were evaluated at each time point and by calculating the area under the curve (AUC).
After 28 days of gavage, mice were euthanized by CO2 asphyxiation and cardiac puncture. Blood was collected in tubes containing EDTA. Plasma biochemical analyses were performed at Pennington Biomedical Research Center (Baton Rouge, LA). Plasma insulin was determined using an ELISA kit (Crystal Chem, Downers Grove, IL). Plasma glucose, cholesterol, and triglycerides were run on a Beckman DxC 600 Pro (Beckman Coulter, Inc., Brea, CA). Tissues were harvested, weights were recorded for liver, and tissues were snap frozen in liquid nitrogen and stored at -80 °C.
Hepatic lipid extraction
Lipid content of liver samples was determined by Folch's method [28]. Briefly, liver samples (~300 mg) were extracted 20:1 (v/w) with CHCl2/ CH3OH (2:1), solvent was removed by rotary evaporation and lyophilization and dry weights were recorded. Samples were redissolved in 7 mL isopropanol, and triglyceride and cholesterol content were determined colorimetrically according to manufacturer's instructions (Sigma).
Statistical analysis
Data were summarized as mean ± SE. Statistical significance of differential effects of RSLE, Metformin and vehicle control was analyzed in terms of a general linear model analysis of variance. Longitudinal data from oral glucose tolerance tests and insulin tolerance tests were analyzed using a mixed effects model for repeated measures across 120 minutes. Multiple pairwise comparisons were performed using two-directional alternative hypotheses with Dunnett's or Tukey's methods for a global significance level set at α = 0.05. All statistical procedures were performed with SAS 9.3 software (Cary, IN).
Results
Phytochemistry
A boiling water extraction was used to produce RSLE, a rich red colored extract with high content of polyphenols (143.8 ± 3.2 mg/g gallic acid equivalents) and anthocyanins (43.0 ± 3.3 mg/g cyanidin 3-glucoside equivalents). Phytochemical structures of chlorogenic acid (5-O-caffeoylquinic acid), cyanidin 3-O-glucoside, cyanidin 3-O-(6-malonyl-glucoside), quercetin-3-glucoside, quercetin 3-(6-malonylglucoside), quercetin glucuronide were confirmed by comparison of 1H NMR and HPLC-MS data according to previously reported spectra and molecular weights. RSLE yield was 4.4 ± 0.1 % of fresh weight lettuce. Antioxidant capacity of RSLE was 6.2 ± 0.28 mmol Trolox equivalents per g extract. Phytochemical constituents of RSL varieties NFR-S-4 and NBR-S-16 and RSLE are listed in Table 1. Cyanidin 3-glucoside and quercetin glucuronide were detected, but were not within the limits of quantification.
Table 1.
Phytochemical content of Rutgers Scarlet Lettuce (RSL) and RSL extract (RSLE)
| Chlorogenic acid | Cyanidin malonyl-glucoside (C3G eq.) | Cyanidin-3-glucoside | Quercetin malonyl-glucoside (Q3G eq.) | Quercetin-3-glucoside | |
|---|---|---|---|---|---|
| mg/g DW extract | |||||
| RSLE | 54.8 ± 5.7 | 44.9 ± 3.1 | below LOQ | 67.8 ± 5.6 | 10.0 ± 2.5 |
| mg/g DW leaves | |||||
| NFR-S-4 | 24.5 ± 6.1 | 20.4 ± 7.1 | 5.5 ± 2.9 | 19.8 ± 4.9 | 18.6 ± 3.7 |
| NBR-S-16 | 32.6 ± 3.4 | 17.4 ± 2.1 | 3.8 ± 2.1 | 21.1 ± 1.9 | 14.5 ± 7.5 |
| mg/g FW leaves | |||||
| NFR-S-4 | 1.39 ± 0.17 | 1.63 ± 0.57 | 0.81 ± 0.23 | 1.58 ± 0.39 | 1.07 ± 0.60 |
| NBR-S-16 | 2.61 ± 0.27 | 1.39 ± 0.17 | 0.31 ± 0.17 | 1.68 ± 0.15 | 1.16 ± 0.60 |
RSL plant material for varieties NFR-S-4 and NBR-S-16. Compounds were quantified by HPLC using UV peak areas of compound standards. Cyanidin malonyl-glucoside content was quantified as cyanidin 3-glucoside equivalents (C3G eq.). Quercetin malonyl-glucoside content was quantified quercetin 3-glucoside equivalents (Q3G eq.). Results are presented as the means ± SE.
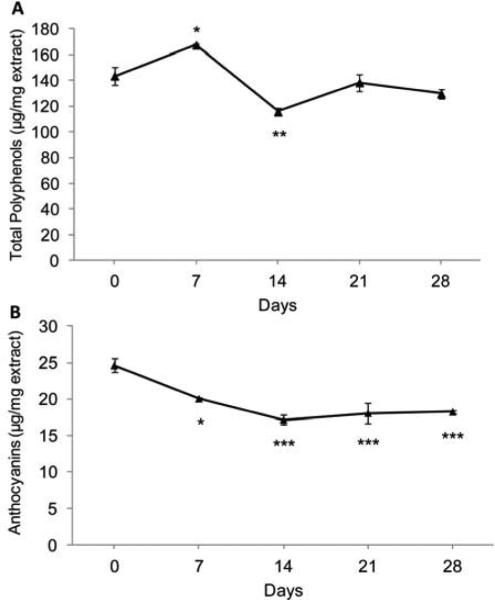
Stability of total polyphenols and anthocyanins in RSLE over time were determined over 4 weeks at 37 °C (Fig. 1). After 4 weeks of storag e, a 10 ± 2 % reduction in total polyphenols was observed, although the difference was not statistically significant. Anthocyanin content was significantly reduced by 26 ± 1% after 4 weeks at 37 °C. Flavonoids, particularly anthocyanins, are known to be heat sensitive [29].
Figure 1.
Stability of (A) total polyphenols and (B) anthocyanins in Rutgers Scarlet Lettuce aqueous extract (RSLE) over one month in accelerated conditions (37 °C). Significant differences compared to day 0 by Dunnett's test (*p < 0.05, **p < 0.01, ***p < 0.001).
Inhibition of glucose production in vitro
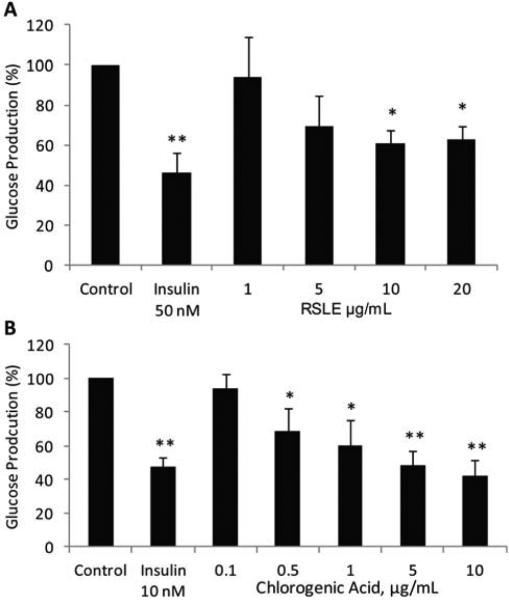
RSLE and compounds isolated from RSLE (chlorogenic acid, cyanidin malonyl-glucoside, quercetin malonyl-glucoside, and quercetin glucuronide) were evaluated for inhibition of glucose production in H4IIE rat hepatoma cells. RSLE demonstrated dose-dependent inhibition of glucose production with statistically significant reduction observed at the RSLE concentrations of 10 and 20 μg/mL (Fig. 2a). Chlorogenic acid, a major constituent in RSLE (55 mg/g extract), also demonstrated a dose-dependent response (Fig. 2b). Cyanidin malonyl-glucoside, quercetin malonyl-glucoside, quercetin glucuronide did not demonstrate significant inhibitory effects on glucose production at concentrations up to 10 μM. Minor components of RSLE, including cyanidin 3-glucoside and quercetin 3-glucoside dose-dependently inhibited glucose production up to 48 % and 56 % at 10 μM, respectively. Results suggest that chlorogenic acid is at least partially responsible for the glucose lowering effect in this assay.
Figure 2.
Inhibition of glucose production by RSLE and chlorogenic acid. H4IIE cells treated with (A) RSLE or (B) chlorogenic acid demonstrated dose-dependent reduction of glucose production. Data are presented as means ± SE of 3-4 independent experiments. Significant differences compared to control were determined by Dunnett's test (* p < 0.05, ** p < 0.01).
Reduction of TNFα-attenuation of insulin signaling
H4IIE hepatoma cells were co-treated with insulin and/or TNFα to demonstrate attenuation of insulin inhibition of glucose production (Fig. 3). However, concurrent treatment with RSLE (20 μg/mL), insulin and TNFα did not significantly reduce glucose response compared to insulin with TNFα treatment. RSLE may have some ability to overcome TNFα attenuation of the inhibition of glucose production by insulin. A more sensitive and robust assay is needed to validate this effect. The trend in reduction in the mean percentage glucose level may be the additive effect of chlorogenic acid lowering glucose production through inhibition of glucose-6-phosphatase translocase [30]. There were no significant differences in cell viability with any of the treatments reported.
Figure 3.
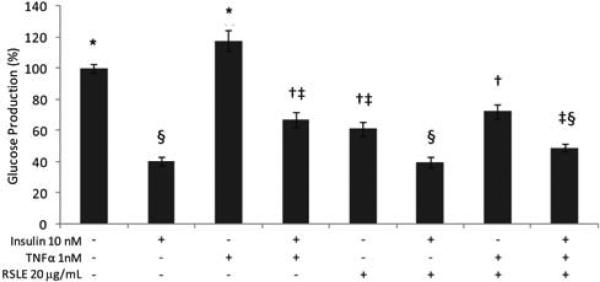
Amelioration of TNFα-attenuated insulin inhibition of glucose production in H4IIE cells. H4IIE cells treated were with insulin, TNFα, and RSLE. Three independent experiments were consolidated and data are presented as means ± SE. Significant differences were determined by Tukey's multiple comparisons test (means with the same symbol are not significantly different, p < 0.05).
Improvement of glucose metabolism and liver lipid accumulation in DIO mice
Daily oral administrations of RSLE improved parameters of metabolic syndrome in DIO mice including glucose metabolism measured by OGTTs and hepatic lipid accumulation. There were no significant differences in daily feed intake or weight change between treatments compared to control groups.
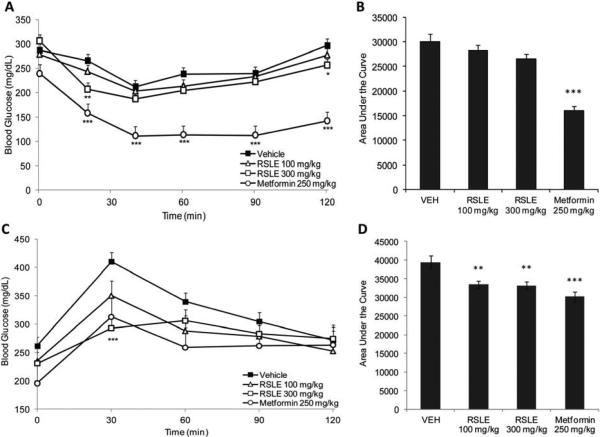
RSLE improved insulin sensitivity acutely after 21 days of treatment showing decreased glucose 20 min post insulin injection (Fig. 4a). However, AUC analysis did not indicate significant improvements in insulin sensitivity with RSLE treatment compared to vehicle control although a decreasing trend was observed (Fig 4b). Metformin (positive control) showed significant improvement in insulin sensitivity compared to vehicle control after 7 days and 21 days of treatment. There were no significant differences with 7 days of RSLE treatment.
Figure 4.
Effects of RSLE on insulin sensitivity and glucose metabolism. DIO mice were treated with vehicle (water), RSLE, or Metformin for 28 days. After 21 days of treatment mice were given an insulin tolerance test (A & B). After 25 days of treatment, mice were given an oral glucose tolerance test (C & D). Data are presented as (A & C) mean blood glucose ± SE and (B & D) area under the curve ± SE (n = 10, except Metformin, n = 5; *p < 0.05; **p < 0.01, ***p < 0.001).
In OGTT, RSLE (100 and 300 mg/kg), as well as Metformin, significantly decreased AUC compared to vehicle control group after 25 days of treatment (Fig. 4d), indicating improvements in glucose metabolism. Blood glucose was significantly lower 30 min after glucose challenge with RSLE 300 mg/kg treatment (Fig 4c). OGTT after 14 days of treatment was not included as blood glucose metabolism was not improved even in the Metformin positive control group.
After 28 days of RSLE treatment, no improvement in plasma glucose, insulin, cholesterol and triglycerides content was observed compared to vehicle control. Significant differences in plasma insulin levels were only observed in the Metformin group compared to vehicle control (data not shown).
The percent liver weight to body weight of mice from all three treatment groups were lower than control, however, only RSLE 300 mg/kg was differentially significantly (Fig. 5a). Total liver lipid content of mice treated with RSLE 300 mg/kg or Metformin was significantly lower than control (Fig. 5b). There were no significant differences in cholesterol or triglyceride concentration in liver lipids compared to vehicle control.
Figure 5.
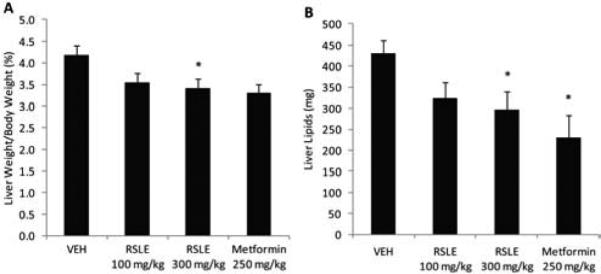
Reduction of hepatic lipid accumulation. DIO mice were treated with vehicle (water), RSLE, or Metformin for 28 days. Livers were harvested and lipids were extracted by Folch's method. Data are the means ± SE (n = 10, except Metformin, n = 4; *p < 0.05).
Discussion
RSL was developed as a novel functional food that contains high levels of bioactive polyphenols. Three major phytochemicals accumulate in RSL: chlorogenic acid, cyanidin malonyl-glucoside, and quercetin malonyl-glucoside; representatives of three important polyphenolic classes: hydroxycinnamic acids, anthocyanins, and flavonols, respectively. While these compound classes commonly accumulate in fruits and vegetables, their particularly high levels in RSL may provide added health benefits as these compounds are associated with anti-diabetic and/or anti-inflammatory activities. This is particularly true because high polyphenol fruits usually contain high levels of sugars with high glycemic index (e.g., glucose) making them more caloric and less useful for the dietary management of diabetes and obesity.
Regular consumption of polyphenol-rich RSL and RSLE may contribute to the prevention and treatment of metabolic syndrome. The development of obesity includes changes in lipid accumulation (oxidation and storage), glucose production, insulin response and inflammatory status in the liver due to obesity [31]. Nonalcoholic fatty liver disease or hepatic steatosis is the manifestation of metabolic syndrome in the liver. RSLE showed evidence of countering some of these conditions such as improving glucose metabolism and attenuating liver lipid accumulation in vivo and inhibition of glucose production in vitro. In other studies, dietary supplementation of green or red lettuce also improved tissue oxidation, lipid metabolism, plasma cholesterol, as well as antioxidant status compared to control diets [32, 33]. In addition to polyphenol content, vitamin, carotenoid, and fiber content of lettuce may also contribute to health benefits [34].
Our in vitro results suggest that inhibition of hepatic glucose production by RSLE is likely due to very high chlorogenic acid content. Chlorogenic acid is ubiquitous in the plant kingdom and good sources include apples, pear, apricots and berries. The main dietary source of chlorogenic acid is coffee, providing 20 - 675 mg of chlorogenic acid per 200 mL cup depending on the coffee bean, roast and brew [35]. There is a growing body of research on the benefits of coffee [36, 37], coffee polyphenols [11, 38], as well as chlorogenic acid [39, 40] in rodent models of metabolic syndrome. Chlorogenic acid is an inhibitor of hepatic glucose-6-phosphate translocase, and has also been shown to lower fasting blood glucose in db/db mice, as well as stimulate glucose transport in skeletal muscle, and improve glucose and lipid metabolism through AMPK activation [30, 41]. Mice given an oral pretreatment with chlorogenic acid (3.5 mg/kg body weight) demonstrated a lower rise in blood glucose compared to control following an oral glucose challenge [40]. Likewise, RSLE also showed a lower rise and lower level of blood glucose compared to vehicle control when given an OGTT. Chlorogenic acid containing-foods, such as RSL, may help to attenuate the development of the metabolic syndrome. Future studies may include incorporation of RSL or RSLE through dietary supplementation to investigate long term benefits for combating the metabolic syndrome.
Effects of long term dietary supplementation with chlorogenic acid have been reported with mixed results. Mice given a high-fat diet supplemented with chlorogenic acid (0.2 g/kg diet) for 8 weeks resulted in lower body weight, plasma leptin and insulin levels, as well as improved lipid metabolism, compared to high fat diet control [39]. However, in another study, dietary supplementation with chlorogenic acid (1 g/kg diet) for 12 weeks seemed to increase glucose intolerance as well as increase insulin resistance and lipid accumulation compared with high fat diet control [42]. While the study by Mubarak et al. [42] used a higher concentration of chlorogenic acid supplementation than Cho et al. [39], accounting for the reported feed intakes, mice consumed daily chlorogenic acid doses of approximately 0.15 mg/kg body weight [42] or 1.3 mg/kg body weight [39], respectively. In comparison, RSLE oral administration delivered a greater single daily chlorogenic acid dose of 5.5 mg/kg body weight (100 mg RSLE/kg) or 16.5 mg/kg (300 mg RSLE/kg). The different effects in DIO mice may be due to the dose of chlorogenic acid delivered as well as the use of different mouse strains. Additionally, the presence of flavonoids in RSLE, including cyanidin- and quercetin-glycosides may have also contributed to in vivo anti-diabetic effects. For instance, cyanidin 3-glucoside prevented obesity-associated insulin resistance in HFD-fed and db/db mice [43] and ameliorated hyperglycemia and insulin sensitivity in DIO mice [44]. Additionally, quercetin has been shown to improve inflammatory status in obese rats and improve metabolic syndrome conditions [15] as well as reduce systolic blood pressure and plasma oxidized LDL in overweight subjects [14]. The complex interactions of these phytochemicals are interesting avenues for further exploration given their associated health benefits and prominence in fruits and vegetables.
As lettuce is widely and regularly consumed around the world, benefits from RSL consumption could have significant impact. Though further pre-clinical and clinical research is needed, improvements observed in glucose metabolism and lipid accumulation from RSLE treatment suggest that RSLE may be a valuable health-promoting food ingredient.
Acknowledgements
The authors thank Jennifer C. Rood, Julia Dreifus and Fiona Lee for technical assistance.
Funding
This project was supported by P50AT002776-01 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS), which funds the Botanical Research Center and Botanical Research Center Pilot Program Sub award 5P50AT002776-08 S12-50318. DMC and CW were supported by NIH training grant T32: 5T32AT004094-04. PRS was supported by SENESCYT-2011 Ecuadorian fellowship.
Abbreviations
- RSL
Rutgers Scarlet Lettuce
- RSLE
Rutgers Scarlet Lettuce extract
- OGTT
oral glucose tolerance test
- ITT
insulin tolerance test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: DMC, NP and IR hold a patent on Rutgers Scarlet Lettuce (RSL). IR has equity in Nutrasorb, LLC.
Author contributions
DMC wrote the manuscript and designed the experiments. NP produced and maintained plant materials. PK conducted animal experiments. AP conducted LC-MS analysis and provided phytochemical expertise. CW performed compound isolation and NMR analyses. PRS, CW, PK and DMC conducted animal tissue analyses. WDJ provided statistical assistance. IR provided guidance and oversight. All authors reviewed and approved the manuscript.
References
- 1.Bazzano LA, He J, Ogden LG, et al. Fruit and vegetable intake and risk of cardiovascular disease in US adults: the first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am J Clin Nutr. 2002;76(1):93–9. doi: 10.1093/ajcn/76.1.93. [DOI] [PubMed] [Google Scholar]
- 2.Hung HC, Joshipura KJ, Jiang R, et al. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer I. 2004;96(21):1577–84. doi: 10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- 3.van Dam RM, Naidoo N, Landberg R. Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases: Review of recent findings. Curr Opin Lipidol. 2013;24(1):25–33. doi: 10.1097/MOL.0b013e32835bcdff. [DOI] [PubMed] [Google Scholar]
- 4.Qin B, Panickar KS, Anderson RA. Cinnamon: Potential role in the prevention of insulin resistance, metabolic syndrome, and type 2 diabetes. J Diabetes Sci Technol. 2010;4(3):685–693. doi: 10.1177/193229681000400324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grace MH, Ribnicky DM, Kuhn P, et al. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine. 2009;16(5):406–15. doi: 10.1016/j.phymed.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roopchand DE, Kuhn P, Poulev A, et al. Biochemical analysis and in vivo hypoglycemic activity of a grape polyphenol-soybean flour complex. J Agr Food Chem. 2012;60(36):8860–5. doi: 10.1021/jf300232h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roopchand DE, Kuhn P, Rojo LE, et al. Blueberry polyphenol-enriched soybean flour reduces hyperglycemia, body weight gain and serum cholesterol in mice. Pharmacol Res. 2013;68(1):59–67. doi: 10.1016/j.phrs.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muraki I, Imamura F, Manson JE, et al. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ. 2013;347:f5001. doi: 10.1136/bmj.f5001. doi: 10.1136/bmj.f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stull AJ, Cash KC, Johnson WD, et al. Bioactives in Blueberries Improve Insulin Sensitivity in Obese, Insulin-Resistant Men and Women. J Nutr. 2010;140(10):1764–8. doi: 10.3945/jn.110.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bumgarner NR, Scheerens JC, Kleinhenz MD. Nutritional yield: a proposed index for fresh food improvement illustrated with leafy vegetable data. Plant Food Hum Nutr. 2012;67(3):215–22. doi: 10.1007/s11130-012-0306-0. [DOI] [PubMed] [Google Scholar]
- 11.Murase T, Yokoi Y, Misawa K, et al. Coffee polyphenols modulate whole-body substrate oxidation and suppress postprandial hyperglycaemia, hyperinsulinaemia and hyperlipidaemia. Br J Nutr. 2012;107(12):1757–65. doi: 10.1017/S0007114511005083. [DOI] [PubMed] [Google Scholar]
- 12.Iwai K, Narita Y, Fukunaga T, et al. Study on the postprandial glucose responses to a chlorogenic acid-rich extract of decaffeinated green coffee beans in rats and healthy human subjects. Food Sci Technol Res. 2012;18(6):849–60. [Google Scholar]
- 13.Chuang CC, Martinez K, Xie G, et al. Quercetin is equally or more effective than resveratrol in attenuating tumor necrosis factor-alpha-mediated inflammation and insulin resistance in primary human adipocytes. Am J Clin Nutr. 2010;92(6):1511–21. doi: 10.3945/ajcn.2010.29807. [DOI] [PubMed] [Google Scholar]
- 14.Egert S, Bosy-Westphal A, Seiberl J, et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br J Nutr. 2009;102(7):1065–74. doi: 10.1017/S0007114509359127. [DOI] [PubMed] [Google Scholar]
- 15.Rivera L, Moron R, Sanchez M, et al. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity. 2008;16(9):2081–7. doi: 10.1038/oby.2008.315. [DOI] [PubMed] [Google Scholar]
- 16.Wu X, Gu L, Prior RL, et al. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J Agric Food Chem. 2004;52:7846–7856. doi: 10.1021/jf0486850. [DOI] [PubMed] [Google Scholar]
- 17.Aoki H, Kuze N, Kato Y, et al. Anthocyanins isolated from purple corn (Zea mays L.). Foods and Food Ingredients J Japan. 2002;199:41–45. [Google Scholar]
- 18.Ferreres F, Gil M, Castaner M, et al. Phenolic metabolites in red pigmented lettuce (Lactuca sativa). Changes with minimal processing and cold storage. J Agric Food Chem. 1997;45(11):4249–54. [Google Scholar]
- 19.Morishita H, Iwahashi H, Osaka N, et al. Chromatographic separation and identification of naturally occurring chlorogenic acids by 1H nuclear magnetic resonance spectroscopy and mass spectrometry. J Chromatogr A. 1984;315:253–360. doi: 10.1016/s0021-9673(01)90742-3. [DOI] [PubMed] [Google Scholar]
- 20.Wald B, Wray V, Galensa R, et al. Malonated flavonol glycosides and 3, 5-dicaffeoylquinic acid from pears. Phytochemistry. 1989;28(2):663–664. [Google Scholar]
- 21.Cheng DM, Kuhn P, Poulev A, et al. In vivo and in vitro antidiabetic effects of aqueous cinnamon extract and cinnamon polyphenol-enhanced food matrix. Food Chem. 2012;135(4):2994–3002. doi: 10.1016/j.foodchem.2012.06.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In: Packer L, editor. Methods in Enzymology. Academic Press; New York: 1999. pp. 152–178. [Google Scholar]
- 23.AOAC Official Method 2005.02. Total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines. J AOAC Int. 2005;88:1269–1270. [PubMed] [Google Scholar]
- 24.Held P. Performing oxygen radical absorbance capacity (ORAC) assays with Synergy™ HT Multi-Mode Microplate Reader - ORAC antioxidant tests. 2005 http://www.biotek.com/resources/articles/performing-orac-assays html.
- 25.Prior RL, Hoang H, Gu LW, et al. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL)) of plasma and other biological and food samples. J Agric Food Chem. 2003;51:3273–3279. doi: 10.1021/jf0262256. [DOI] [PubMed] [Google Scholar]
- 26.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 27.Surwit RS, Feinglos MN, Rodin J, et al. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metab Clin Exp. 1995;44(5):645–651. doi: 10.1016/0026-0495(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 28.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 29.Ioannou I, Hafsa I, Hamdi S, et al. Review of the effects of food processing and formulation on flavonol and anthocyanin behaviour. J Food Eng. 2012;111(2):208–17. [Google Scholar]
- 30.Hemmerle H, Burger H, Below P, et al. Chlorogenic acid and synthetic chlorogenic acid derivatives: Novel inhibitors of hepatic glucose-6-phosphate translocase. J Med Chem. 1997;40(2):137–45. doi: 10.1021/jm9607360. [DOI] [PubMed] [Google Scholar]
- 31.Lumeng CN. Innate immune activation in obesity. Mol Aspects Med. 2013;34(1):12–29. doi: 10.1016/j.mam.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicolle C, Cardinault N, Gueux E, et al. Health effect of vegetable-based diet: lettuce consumption improves cholesterol metabolism and antioxidant status in the rat. Clin Nutr. 2004;23(4):605–14. doi: 10.1016/j.clnu.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Lee JH, Felipe P, Yang YH, et al. Effects of dietary supplementation with red-pigmented leafy lettuce (Lactuca sativa) on lipid profiles and antioxidant status in C57BL/6J mice fed a high-fat high-cholesterol diet. Brit J Nutr. 2009;101(8):1246–54. doi: 10.1017/S0007114508073650. [DOI] [PubMed] [Google Scholar]
- 34.Nicolle C, Carnat A, Fraisse D, et al. Characterisation and variation of antioxidant micronutrients in lettuce (Lactuca sativa folium). J Sci Food Agric. 2004;84(15):2061–9. [Google Scholar]
- 35.Clifford MN. Chlorogenic acids and other cinnamates - Nature, occurrence and dietary burden. J Sci Food Agr. 1999;79:362–72. [Google Scholar]
- 36.Fukushima Y, Kasuga M, Nakao K, et al. Effects of Coffee on Inflammatory Cytokine Gene Expression in Mice Fed High-Fat Diets. J Agr Food Chem. 2009;57(23):11100–5. doi: 10.1021/jf901278u. [DOI] [PubMed] [Google Scholar]
- 37.Vitaglione P, Morisco F, Mazzone G, et al. Coffee reduces liver damage in a rat model of steatohepatitis: The underlying mechanisms and the role of polyphenols and melanoidins. Hepatology. 2010;52(5):1652–61. doi: 10.1002/hep.23902. [DOI] [PubMed] [Google Scholar]
- 38.Murase T, Misawa K, Minegishi Y, et al. Coffee polyphenols suppress diet-induced body fat accumulation by downregulating SREBP-1c and related molecules in C57BL/6J mice. AM J Physiol-Endoc M. 2011;300(1):E122–33. doi: 10.1152/ajpendo.00441.2010. [DOI] [PubMed] [Google Scholar]
- 39.Cho A, Jeon S, Kim M, et al. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem Toxicol. 2010;48(3):937–43. doi: 10.1016/j.fct.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Bassoli BK, Cassolla P, Borba-Murad GR, et al. Chlorogenic acid reduces the plasma glucose peak in the oral glucose tolerance test: effects on hepatic glucose release and glycaemia. Cell Biochem Funct. 2008;26(3):320–8. doi: 10.1002/cbf.1444. [DOI] [PubMed] [Google Scholar]
- 41.Ong KW, Hsu A, Tan BKH. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by AMPK activation. Biochem Pharmacol. 2013;85(9):1341–51. doi: 10.1016/j.bcp.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Mubarak A, Hodgson JM, Considine MJ, et al. Supplementation of a High-Fat Diet with Chlorogenic Acid Is Associated with Insulin Resistance and Hepatic Lipid Accumulation in Mice. J Agric Food Chem. 2013;61(18):4371–8. doi: 10.1021/jf400920x. [DOI] [PubMed] [Google Scholar]
- 43.Guo H, Xia M, Zou T, et al. Cyanidin 3-glucoside attenuates obesity-associated insulin resistance and hepatic steatosis in high-fat diet-fed and db/db mice via the transcription factor FoxO1. J Nutr Biochem. 2012;23(4):349–60. doi: 10.1016/j.jnutbio.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 44.Sasaki R, Nishimura N, Hoshino H, et al. Cyanidin 3-glucoside ameliorates hyperglycemia and, insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem Pharmacol. 2007;74(11):1619–27. doi: 10.1016/j.bcp.2007.08.008. [DOI] [PubMed] [Google Scholar]