Abstract
Many current pharmaceutical therapies for systolic heart failure target intracellular [Ca2+] ([Ca2+]i) metabolism, or cardiac troponin C (cTnC) on thin filaments, and can have significant side-effects, including arrhythmias or adverse effects on diastolic function. In this study, we tested the feasibility of directly increasing the Ca2+ binding properties of cTnC to enhance contraction independent of [Ca2+]i in intact cardiomyocytes from healthy and myocardial infarcted (MI) hearts. Specifically, cardiac thin filament activation was enhanced through adenovirus-mediated over-expression of a cardiac troponin C (cTnC) variant designed to have increased Ca2+ binding affinity conferred by single amino acid substitution (L48Q). In skinned cardiac trabeculae and myofibrils we and others have shown that substitution of L48Q cTnC for native cTnC increases Ca2+ sensitivity of force and the maximal rate of force development. Here we introduced L48Q cTnC into myofilaments of intact cardiomyocytes via adeno-viral transduction to deliver cDNA for the mutant or wild type (WT) cTnC protein. Using video-microscopy to monitor cell contraction, relaxation, and intracellular Ca2+ transients (Fura-2), we report that incorporation of L48Q cTnC significantly increased contractility of cardiomyocytes from healthy and MI hearts without adversely affecting Ca2+ transient properties or relaxation. The improvements in contractility from L48Q cTnC expression are likely the result of enhanced contractile efficiency, as intracellular Ca2+ transient amplitudes were not affected. Expression and incorporation of L48Q cTnC into myofilaments was confirmed by Western blot analysis of myofibrils from transduced cardiomyocytes, which indicated replacement of 18±2% of native cTnC with L48Q cTnC. These experiments demonstrate the feasibility of directly targeting cardiac thin filament proteins to enhance cardiomyocyte contractility that is impaired following MI.
Keywords: cardiomyocyte contractility, Ca2+ sensitivity, cardiomyocytes, adenovirus, gene therapy, myocardial infarction
1. INTRODUCTION
Heart failure is characterized by decreased systolic function and has a variety of etiologies, with one of the most common being myocardial infarction (MI). In addition to the formation of a fibrotic scar that follows an MI, normal processes in surviving cardiomyocytes in non-infarct regions can be impaired, such as intracellular Ca2+ handling [1–3], β-adrenergic responsiveness [4], and contractile apparatus function [5,6] [7,8]. While there is still debate about the extent and mechanisms of changes in myofilament properties that occur post-MI, changes in Ca2+ sensitivity of thin filament activation are thought to underlie, at least in part, the reduced contractility of failing myocardium [5,9–13].
Numerous strategies have been developed to compensate for the deleterious changes that occur in cardiomyocytes following MI. Most approaches have focused on increasing intracellular Ca2+ as a means to overcome decreased cardiomyocyte contractility, but these have the potential to cause arrhythmias or eventually impair diastolic function [14]. Ca2+ sensitizing pharmaceutical agents that target the myofilaments, such as calmidazolium, bepridil, and levosimenden, have also been developed and tested. These agents improve the contractility of failing myocardium by increasing Ca2+ binding to the N-terminus of cardiac troponin C (cTnC) to enhance contractile activation. Unfortunately, they are not completely specific to cTnC and can have off-target effects on other EF-hand calcium binding proteins and other proteins involved in excitation-contraction coupling [15,16]. One pharmacological treatment currently under development is omecamtiv mecabril, which is a small molecule capable of directly activating myosin and thus modulating cardiac contractility [17,18]. This treatment does not manipulate intracellular Ca2+ in order to increase contractility; instead, it interacts with myosin and enhances the transition to the strongly actin-bound state. The direct interaction of the small molecule with myosin to enhance contraction and systolic function does not appear to interfere the kinetics of contraction or relaxation [19]. Ongoing clinical trials with omecamtiv mecabril have also yielded promising results that suggest that a targeted approach to improving systolic function without perturbing intracellular Ca2+ or contraction/relaxation kinetics may be a viable therapeutic approach [17,20].
Another approach to enhance cardiomyocyte contractility, while minimizing concomitant off-target effects, is genetic manipulation of thin filament proteins that regulate Ca2+ dependent activation of cardiac myofilaments. cTnC is an attractive myofilament protein target because it binds Ca2+ to initiate myofilament contraction, and manipulation of its Ca2+ binding properties can result in enhanced cardiac contractility without requiring increased intracellular [Ca2+]. Previous work by us [21] and others [22] has demonstrated that a single amino acid substitution of leucine at position 48 with glutamine (L48Q) in cTnC increases the Ca2+ binding affinity of the troponin complex (cTn). We have also demonstrated that when cTn containing L48Q cTnC (L48Q cTnC-cTn) was exchanged into demembranated cardiac muscle or cardiac myofibrils, the Ca2+ sensitivity of force and the rate of force development was increased without impairing maximal force generation or relaxation [23,24]. We recently reported that exchange of L48Q cTnC-cTn into demembranated cardiac muscle increased thin filament activation at submaximal Ca2+ concentrations independent of strong crossbridge binding. This effectively increased contraction at submaximal intracellular Ca2+ levels that occur during cardiomyocyte twitch contraction [24]. We have also demonstrated that engraftment of neonatal cardiomyocytes into the infarct zone of adult rat hearts enhances the myofilament Ca2+ sensitivity of cardiac tissue in the non-infarcted regions and is a major contributor to the improvement in systolic function [9]. Taken together, these studies suggest that enhancing myofilament Ca2+ sensitivity, via incorporation of L48Q cTnC into myofilaments, could improve contraction of cardiomyocytes from both healthy and MI hearts.
In this study, we show that adenovirus-mediated in vitro expression of L48Q cTnC significantly improved the contraction of cardiomyocytes from healthy hearts without altering intracellular Ca2+ transients. It also improved the depressed contraction of cardiomyocytes from hearts following MI, where Ca2+ transients were depressed. These increases in contraction occurred without prolonging relaxation. Additionally, the increased contractility occurred with only an ~20% myofilament incorporation of L48Q cTnC, as indicated by Western blot analysis. Our results suggest that acute expression and myofilament incorporation of L48Q cTnC in intact cardiomyocytes from either healthy or failing hearts increases cardiac contractile properties. Future in vivo studies will be required to determine the effects of L48Q cTnC incorporation into myofilament on whole heart function.
2. METHODS
2.1 Animal and cardiomyocyte preparation
These studies were approved by the University of Washington (UW) Animal Care Committee and conducted in accordance with federal guidelines. Animals were cared for in accordance with US NIH Policy on Humane Care and Use of Laboratory Animals in the Department of Comparative Medicine at UW.
Adult rat cardiomyocytes were isolated from 6–8 week old female Fischer 344 rat hearts using aortic retrograde perfusion for enzymatic (collagenase/protease) dispersion of cells as previously described [25,26]. For cardiomyocytes isolated from infarcted hearts, adult female Fischer 344 rats 6–8 weeks old were infarcted via permanent ligation of the left descending coronary artery using a surgical suture, and access to the heart was obtained via thoracotomy [9]. Sham operated (healthy control) animals received only the thoracotomy procedure. Once cardiac dysfunction was documented in MI rats by at least a > 25% reduction in left ventricular fractional shortening, which was measured by echocardiography at 4–6 weeks post-infarction, cardiomyocytes were isolated as previously described [9,25,26]. Cardiomyocytes from healthy and infarcted hearts were subsequently transduced with adenoviral vectors and cultured for 48–60 hours, as described below in Section 2.3, to allow sufficient transgene expression.
2.2 Echocardiography
Echocardiography was performed as described by Laflamme et al. [27]. Rats were lightly sedated with isoflurane and monitored by continuous electrocardiography (ECG) via three limb leads. Echocardiographic measurements were taken using a GE Vivid7 echocardiography system with an 11 MHz convex transducer of parasternal long-axis and short-axis images at the mid-papillary muscle level to ensure evaluation of the infarcted region of the hearts. M-mode measurements on the short-axis view were taken to obtain left ventricular end-diastolic (LVEDD) and systolic dimensions (LVESD). Fractional shortening (FS) was calculated as (LVEDD − LVESD) / LVEDD×100%. Measurements were made on at least three cardiac cycles by two blinded echocardiographers and averaged for each data value.
2.3 Plasmid design and virus production
The AdEasy™ system was used as originally described [28,29] to generate recombinant adenoviral vectors to express histidine-tagged (C-terminal 6-His) WT cTnC and L48Q cTnC, and Flag tagged (C-terminal Flag tag) WT cTnC-Flag and L48Q cTnC-Flag from the cytomegalovirus (CMV) promoter. For both sets of viruses, there was a second expression cassette within each virus that also contained green fluorescent protein (GFP) as a reporter protein to identify transduced cells via fluorescence microscopy. HEK 293 cell lines were used to produce high titer adenoviral preparations of 108–109 [28,29]. Cardiomyocytes were transduced at ~200–250 infectious units per cell (MOI) for 48–60 hours. We achieved nearly 100% transfection efficiency and gene transfer as grossly indicated by green fluorescence with microscopy. Cell survival over this period was assessed as described previously [26] and was consistent with previous studies using cardiomyocytes [9,26,30,31]. There were no differences in cell viability between transduced and non-transduced cardiomyocytes, and cellular characteristics are presented in Table 1. The viruses containing histidine-tagged (C-terminal 6-His) WT cTnC and L48Q cTnC were used for all of the contractility measurements, but the His tag was not well-suited for determination of myofilament incorporation of tagged cTnC via Western blot. A second set of viruses containing WT cTnC-Flag and L48Q cTnC-Flag were made using a commercially available and more effective tag system to determine myofilament incorporation of cTnC-Flag via Western blot. There were no significant differences in the effects on healthy cardiomyocyte contraction or Ca2+ transient properties from transduction with either His tag or Flag tag AV systems (Supplemental Tables 6–9).
Table 1.
Cell characteristics
| n | N | SL (µm) | Cell length (µm) | |
|---|---|---|---|---|
| Healthy | ||||
| Non-transduced | 54 | 5 | 1.83 ± 0.03 | 89.2 ± 2.0 |
| WT cTnC | 43 | 5 | 1.82 ± 0.02 | 91.4 ± 1.9 |
| L48Q cTnC | 60 | 5 | 1.84 ± 0.02 | 93.2 ± 2.4 |
| MI | ||||
| Sham | 27 | 3 | 1.77 ± 0.02 | 91.8 ± 2.9 |
| MI | 48 | 8 | 1.79 ± 0.02 | 95.2 ± 2.3 |
| MI+WT cTnC | 41 | 6 | 1.78 ± 0.02 | 95.9 ± 3.5 |
| MI+L48Q cTnC | 51 | 7 | 1.79 ± 0.02 | 91.7 ± 2.5 |
n = # of cells, N = # of animals; No statistical significance between any groups.
2.4 Cardiomyocyte contractile assessments and statistical analysis
Cell shortening and re-lengthening of arbitrarily selected stimulated cardiomyocytes was monitored and recorded using IonOptix SarcLen system video microscopy with a 40× objective (Olympus UWD 40) and 25× intermediate lenses (IonOptix, Milton, MA, USA) [6,26]. Contractile assessments were performed at room temperature (22–24°C) and at 37°C in fresh modified Tyrodes buffer using 0.5 Hz, 1 Hz and 2 Hz stimulation frequencies [26]. Only cells that followed field stimulation 1:1 and with resting sarcomere lengths above 1.65 µm were measured. Average cardiomyocyte length and sarcomere length is reported, and there was no difference in either measurement between transduced and non-transduced cells (Table 1). Ca2+ transients induced by electrical stimulation were measured in Fura-2 loaded adult cardiomyocytes using IonOptix equipment as described [6,26]. Briefly, Ca2+ transients were recorded by measuring Fura-2 fluorescence passed through a 510 nm emission filter to a photomultiplier tube using the interpolated pseudo-ratiometric method with 380 nm excitation during, and 360 nm excitation at the onset and end of 20 second recording events. Fura-2 fluorescence was measured using an IonOptix spectrophotometer (Stepper Switch) attached to a fluorescence microscope. Emitted Fura-2 fluorescence was collected by the 40× objective, passed through a 510nm filter and detected by a photomultiplier tube. Contraction, relaxation, and Ca2+ transients were analyzed later using proprietary software (IonOptix) to determine the magnitudes and rates of these properties. Experiments were performed by 3 different experimentalists, with at least 2 rotating on a given day, and all data was analyzed in duplicate by 3 different analysts. No differences were found in the measured results between experimentalists. Statistical differences were determined by ANOVA, with Student-Newman-Keuls and/or Dunnett as a post-hoc tests (SigmaPlot 12.5). Differences at p-value < 0.05 were considered statistically significant. Data is displayed as mean ± S.E.M.
2.5 SDS-PAGE and Western blot analysis
WT-Flag and L48Q cTnC-Flag incorporation into myofibrils in cardiomyocytes was determined by SDS-PAGE and Western blot analysis. Cardiomyocytes were plated at 6–9×104 cells per well in 6-well tissue culture dishes, and were cultured under the same conditions that were used for the contractility analysis [26]: MOI of ~200–250 infectious units/cell and cultured for 48–60 hours. To determine the amount of Flag tagged cTnC that was incorporated into the myofilaments, cardiomyoctyes were harvested and demembranated to remove the sarcolemma and cytosolic milieu and leave only the myofibrils. The demembranated cardiomyocytes were then re-suspended in lysis buffer [26] and stored at −20°C until they were used. In preparation for SDS-PAGE and Western blotting, total protein content from the myofibril preparations was measured using the Bradford colormetric protein assay where the protein content is determined by measuring the absorbance of 595nm light. Once total protein content for each sample was determined, myofibril preparations were re-suspended in Laemmli sample. Determination of total protein content before SDS-PAGE allowed loading of equal amounts of total protein (20 µg). SDS-PAGE separated proteins were transferred to a nitrocellulose membrane and probed with rabbit polyclonal anti-TnC (Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit polyclonal anti-Flag (Sigma-Aldrich, St. Louis, MO) after blocking with 5% milk (w/v in Tris-buffered saline containing 0.1% v/v Tween-20). Antibody incubations were carried out in 1% milk (w/v in Tris-buffered saline containing 0.1% v/v Tween-20). The same nitrocellulose membrane was probed with anti-TnC or anti-Flag, and was then stripped and re-blocked before probing with a second antibody, anti-TnC or anti-Flag, which ever was not used first. The order of probing did not affect either antibody signal. The same membrane was probed twice to confirm that the larger molecular weight band in the anti-TnC blot was indeed cTnC-Flag. Protein bands were quantified using ImageJ gel analysis toolkit (NIH), and the expression of each cTnC-Flag was calculated relative to the amount of native cTnC present in the anti-TnC Western blot. This analysis generated a relative amount (%) of cTnC-Flag that was incorporated into the myofilaments of AV-L48Q cTnC or AV-WT cTnC transduced cardiomyocytes. Similarly, analysis of L48Q cTnC-cTn incorporation into the myofilaments of demembranated trabeculae amount was conducted via Western blotting using rabbit polyclonal antibodies against cTnT (sc-8121 1:500, Santa Cruz Biotechnology, Santa Cruz, CA). L48Q cTnC-cTn complexes contained cmyc-cTnT to allow identification of exchanged cTn from native cTn. cTnT protein bands were quantified using ImageJ gel analysis toolkit (NIH), and incorporation of cmyc-cTnT was calculated relative to the amount of non-tagged cTnT for both native and recombinant proteins.
2.6 Whole cardiac troponin (cTn) exchange and force-pCa measurements
Expression, purification and complexation of recombinant cTn subunits was as previously described in detail [20]. The L48Q cTnC was generated by site directed mutagenesis was performed using the QuikChange II Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) to generate the L48Q cTnC mutation. cTn complexes containing L48Q cTnC also contained cmyc-tag labeled cTnT. Proteins were stored at −80°C before use.
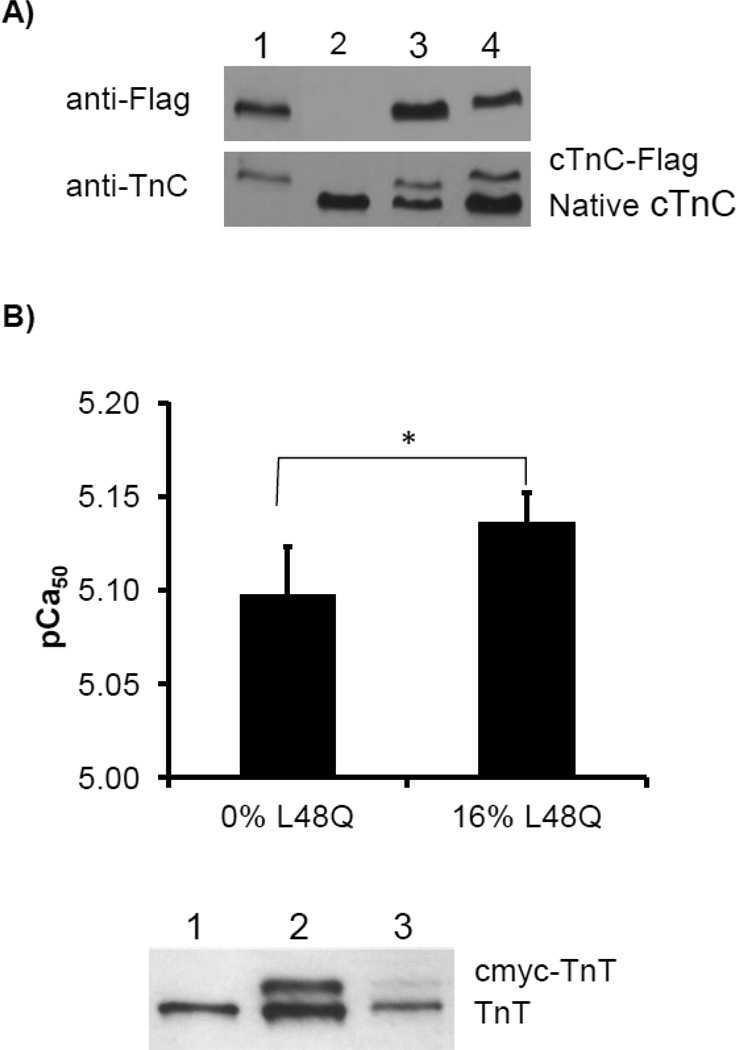
The force-pCa relationship in demembranated right ventricular trabeculae from rats (male Sprague-Dawley, 200–250g) was determined after passive overnight exchange of native cTn with recombinant cTn complexes containing either 100% WT cTnC or a mix of 75% WT and 25% L48Q-cTnC, cmycTnT, cTnI as described [24]. Force-pCa data were fit by the Hill equation as previously described [20]. The reported pCa50 values represent the means of the values from the individual fits, ± standard error of the means (SEM). Means are compared with Student’s T-test with significance at the 95% confidence level (p<0.05). Statistical analysis was performed using Excel (Microsoft, Redmond, WA), SigmaPlot (Systat, Richmond, CA), and Fityk (Wojdyr, 2010). Following mechanical measurements, trabeculae were processed for Western blot analysis to determine the amount of WT vs. L48Q cTnC containing cTn in myofibrils [20]. The presence of cmyc-tagged cTnT in the complexes containing L48Q-cTnC allowed subsequent analysis of actual amount of L48Q-cTnC incorporation via Western blotting (Fig. 2).
Figure 2.
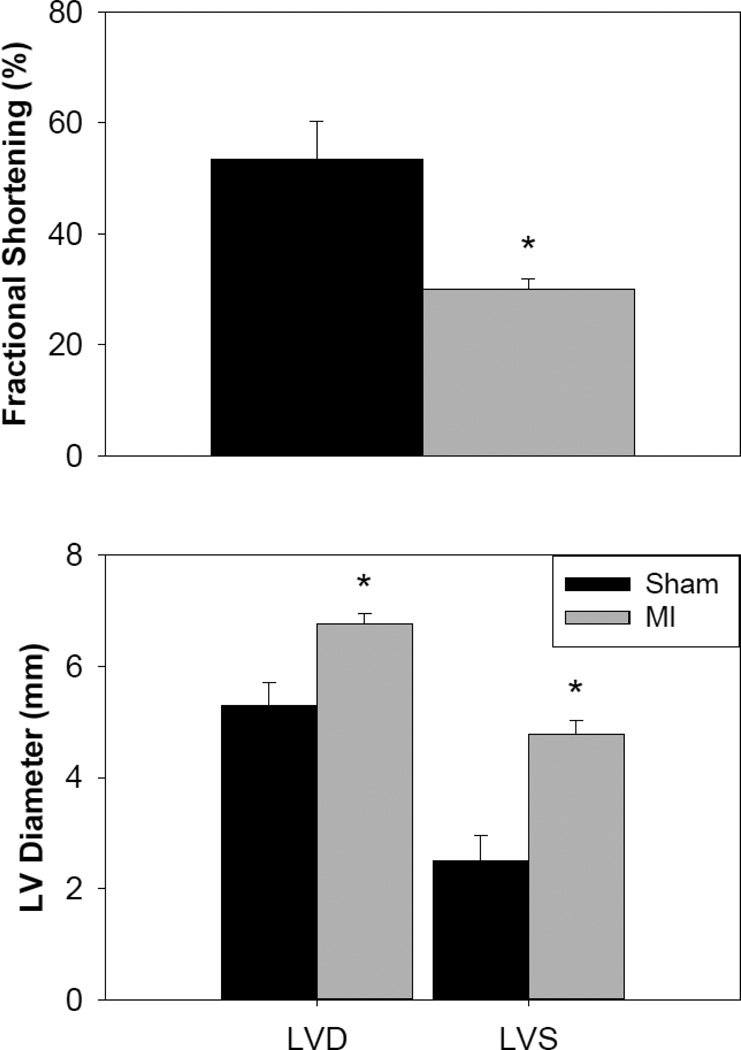
Assessment of cardiac function after myocardial infarction by echocardiography. Factional shortening of infarcted hearts (A, n= 6) was significantly reduced compared to sham operated (healthy control) hearts (sham, n = 3). Both diastolic and systolic LV diameters significantly increased after MI. Values are means ± S.E.M.; * = p<0.05 as compared to sham operated (healthy control).
3. RESULTS
3.1 Cardiomyocyte transduction and cellular characteristics
Cardiomyocytes from healthy and MI hearts were enzymatically isolated and then transduced with adenovirus (AV) constructs containing genes for L48Q cTnC + GFP (AV-L48Q cTnC) or WT cTnC + GFP (AV-WT cTnC). After 48–60 hours of AV-cTnC expression, there were no differences in cell viability between transduced and non-transduced cardiomyocytes from healthy and MI hearts, which was consistent with previous studies using cardiomyocytes [9,26,30,31]. There were also no differences in cell length or sarcomere length between groups (Table 1), which suggests that over-expression of L48Q or WT cTnC did not alter the resting state of cardiomyocytes or induce Ca2+ independent activation.
3.2 Contraction and Ca2+ transient properties of isolated healthy cardiomyocytes
The effects of L48Q cTnC over-expression on the contractile properties of cardiomyocytes from healthy hearts are summarized in Table 2. Cardiomyocytes from healthy hearts transduced with AV-L48Q cTnC had significantly increased magnitude (% cell shortening) and rate of cell shortening with no significant effects on relaxation times (RT50, RT90) when compared to non-transduced cells (Table 2). Additionally, transduction of healthy cardiomyocytes with AV-WT cTnC had no effects on the magnitude of cell shortening or relaxation times (RT50, RT90), but did slightly slow the rate of cell shortening at 0.5 Hz as compared to non-transduced cells (Table 2) However, the reduction in rate of cell shortening was not observed at higher stimulation frequencies (data not shown). These results suggest that the over-expression of the L48Q cTnC variant, and not simply over-expression of cTnC, markedly increased contractility of healthy cardiomyocytes.
Table 2.
Contraction and Ca2+ transient values for cardiomyocytes from healthy hearts at 0.5 Hz stimulation.
| Cell Shortening (%) |
Cell Shortening Rate (µm/s) |
RT50 (ms) | RT90 (ms) | Baseline Ca2+ (Fura-2 ratio units |
Peak Ca2+ (Fura-2 ratio units) |
DT50 (ms) | DT90 (ms) | |
|---|---|---|---|---|---|---|---|---|
| Non-transduced | 6.6 ± 0.4 | 77.7 ± 4.4 | 120 ± 12 | 303 ± 29 | 1.11 ± 0.02 | 1.25 ± 0.03 | 191 ± 19 | 556 ± 54 |
| WT cTnC (Control) | 5.4 ± 0.4 | 59.4 ± 4.2* | 135 ± 15 | 369 ± 42 | 1.16 ± 0.03 | 1.26 ± 0.03 | 230 ± 20 | 682 ± 60 |
| L48Q cTnC | 9.0 ± 0.5* | 115.1 ± 7.2* | 138 ± 10 | 367 ± 25 | 1.12 ± 0.02 | 1.25 ± 0.03 | 225 ± 20 | 636 ± 38 |
= p<0.05 as compared to non-transduced; ANOVA analysis and post-hoc tests with Dunnett’s test for both transduced conditions.
To determine if the increases in contractile properties resulted from changes myofilament properties per se or were influenced by changes in intracellular Ca2+ behavior during stimulation, we simultaneously examined the Ca2+ transient properties of cardiomyocytes from healthy hearts. These data are also summarized in Table 2. Ca2+ transient peak or Ca2+ transient decay times, measured as the 50% (DT50) and 90% (DT90) time to return to baseline, did not differ between cardiomyocytes transduced with AV-L48Q cTnC or AV-WT cTnC (Table 2). These data suggest that increases in the contractile properties of healthy cardiomyocytes transduced with AV-L48Q cTnC occurred via enhanced myofilament Ca2+ sensitivity and not by significantly altering the Ca2+ transient properties.
3.3 Cardiomyocyte and cardiac trabeculae myofilament protein analysis
To determine the extent of WT or L48Q cTnC incorporation into the myofilaments that was responsible for the changes in healthy cardiomyocyte contractility, cardiomyocytes were transduced with AV constructs to express WT or L48Q cTnC-Flag + GFP. Myofilament incorporation of WT or L48Q cTnC-Flag was demonstrated by Western blot ratiometric analysis of purified myofibrils from transduced cardiomyocytes probed with anti-cTnC (Santa Cruz Biotechnology, Santa Cruz, CA) for total cTnC content and anti-Flag (Sigma-Aldrich, St. Louis, MO) for transduced protein content (Fig. 1A). Figure 1A shows a representative example of the anti-TnC and anti-Flag Western blots. Western blot bands from transduced cardiomyocyte myofibrils were quantified by densitometry to compare total cTnC content vs. cTnC-Flag (transduced protein) content and approximate the extent of myofilament incorporation. Three batches of cardiomyocytes were run in duplicate and averaged results indicate equivalent replacement of native cTnC with either WT cTnC-Flag (22±5%) or L48Q cTnC-Flag (18±2%).
Figure 1.
Analysis of myofilament incorporation of L48Q cTnC in transduced, cultured cardiomyocytes and demembranated trabeculae. A) Representative Western blots against Flag and TnC show that myofibrils from transduced, cultured cardiomyocytes contain 22±5% WT cTnC-Flag or 18±2% L48Q cTnC-Flag of the total myofilament cTnC content. Lane 1: WT cTnC-Flag from HEK 293 cells (positive control), lane 2: non-transduced cardiomyocytes (negative control), lane 3: WT cTnC-Flag transduced cardiomyocytes, lane 4: L48Q cTnC-Flag transduced cardiomyocytes. B) In demembranated trabeculae, after passive cTn exchange in solution containing 25%/75% L48Q/WT cTnC-cTn, Ca2+ sensitivity of force was significantly increased compared to WT cTnC-cTn exchanged trabeculae (pCa 5.14±0.02 vs. 5.10±0.03, n = 4 and 6, respectively, p<0.05). Inclusion of cmyc-cTnT in L48Q cTnC-containing cTn complexes allowed for assessment of incorporation via Western blotting since cmyc-TnT travels slower on SDS-PAGE. Lane 1: recombinant cTnT, lane 2: mix of cmyc-cTnT and cTnT, lane 3: actual exchanged trabecula showing 16/84% cmyc-cTnT/cTnT incorporation. Average incorporation measured in three sets of preparation equaled 16±4%.
To further demonstrate that an ~20% replacement of endogenous cTnC with L48Q cTnC can significantly enhance cardiomyoycte contraction, we also examined how a similar level of L48Q cTnC incorporation into myofilaments affected the Ca2+ sensitivty of demembranted cardiac trabeculae. By using whole cTn exchange solutions with various ratio amounts of L48Q cTnC-cTn and WT cTnC-cTn, we found that a ratio of as little as 25% L48Q cTnC-cTn significantly increased the Ca2+ sensitivity of force (pCa50; Fig. 1B). Subsequent Western blot analysis of these trabeculae showed that the actual amount of incorporated L48Q-cTnC was 16±2% (Fig. 1B). These results suggest that as little as ~15–20% replacement of endogenous cTnC with L48Q cTnC is sufficient to increase the myofilament Ca2+ sensitivity of intact cardiomyocytes to increase contractility.
3.4 In vivo cardiac functional assessment
We next sought to determine whether L48Q cTnC could improve the contractility of cardiomyocytes isolated from infarcted hearts (MI). Echocardiography was used to determine the in vivo cardiac function of sham operated (healthy control) and MI hearts 4–6 weeks post-surgery (permanent ligature of the left descending coronary artery), and the measurements are summarized in Figure 2. There were no significant differences in average body weight or heart rate between control (180±3 g; 363±9 beats/min) and MI rats (181±2 g; 333±10 beats/min), but left ventricular fractional shortening (FS) was significantly reduced in MI rat hearts (30.0±1.9%) compared to control (53.5±6.7%) (Fig. 2A). These results are in agreement with previous studies [7,9,32–35] by us and others. The decrease in cardiac function was characterized by both end-diastolic and end-systolic dilation in MI hearts (Fig. 2B). Left-ventricular diameter at end-diastole (LVDD) was significantly larger in MI hearts (6.8±0.2 mm) compared to sham operated control hearts (5.3±0.4 mm). At end-systole, left ventricular diameter (LVSD) of MI hearts (4.8±0.3 mm) was almost twice as large as control hearts (2.5±0.5 mm) (p < 0.05), indicating greatly reduced systolic function (Fig. 2B). There were no significant differences in septal wall or left-ventricular posterior wall thickness between sham and MI rats (data not shown). These results demonstrate that the MI hearts had developed significant systolic dysfunction at the time of cardiomyocyte isolation for in vitro measures of contraction, relaxation, and intracellular Ca2+ transients.
3.5 Contraction and Ca2+ transient properties of cardiomyocytes from MI hearts
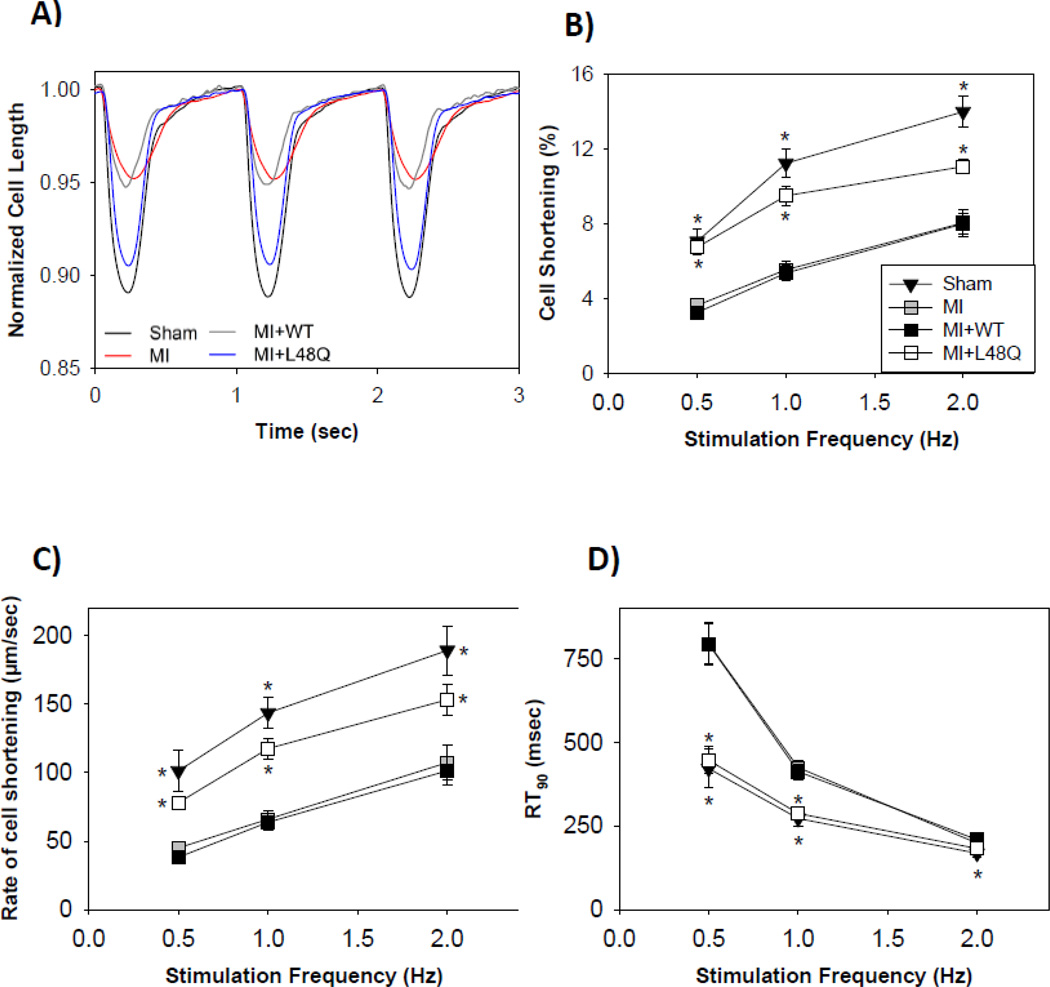
The effects of L48Q cTnC over-expression on the contractile properties of cardiomyocytes from MI hearts measured at 0.5, 1 & 2 Hz stimulation frequency are summarized in Figure 3, Table 3, and Supplemental Tables 1 and 2. After 48–72 hours in culture, the ability of adult cardiomyocytes to respond to higher frequencies found in vivo (4–6 Hz) is diminished, likely due to partial disruption of the T-tubule system over time in culture [36]. Example contractile traces for cardiomyocytes from healthy control and MI hearts at 1 Hz that were non-transduced, or transduced with AV-WT cTnC or AV-L48Q cTnC are shown in Figure 3A. Cardiomyocytes from MI hearts had significantly reduced magnitude and rate of cell shortening at all stimulation frequencies compared to cardiomyocytes from sham operated (healthy control) hearts (Fig. 3B, C; Table 3). Cardiomyocytes from MI hearts relaxed much slower, as indicated by significantly longer relaxation times (RT50, RT90) (Fig. 3D; Table 3). The depression of contractile and relaxation properties of cardiomyocytes from MI hearts agreed with previously published results [7,9,32–35].
Figure 3.
Contractile properties of transduced cardiomyocytes from MI hearts stimulated at 0.5, 1, & 2 Hz. (A) Representative cell length traces at 1 Hz of healthy sham control (black), MI non-transduced (red), MI+WT cTnC (grey), and MI+L48Q cTnC (blue) cardiomyocytes. (B, C) The magnitude and rate of cell shortening was decreased at all frequencies for MI (grey squares) and MI+WT cTnC (black squares) cardiomyocytes compared to healthy sham control (black triangles) at all frequencies. MI+L48Q cTnC (white squares) improved magnitude and rate of cell shortening. (D) Relaxation times were prolonged at all frequencies for MI (grey squares) and MI+WT cTnC (black squares) cardiomyocytes compared to healthy sham control (black), and MI+L48Q cTnC (white squares) slightly improved relaxation times at 0.5 and 1Hz frequencies. Only the healthy sham control cells relaxed significantly faster compared to MI cells at 2 Hz. Values are means ± S.E.M while some error bars are concealed within data points; * = p<0.05 as compared to MI for sham (black triangles) or MI+L48Q cTnC (white squares). Double asterisks indicate significant difference compared to MI where sham and MI+L48Q cTnC data symbols overlap.
Table 3.
Contraction and Ca2+ transient values for cardiomyocytes from MI hearts at 0.5 Hz stimulation frequency
| Fractional Shortening (%) |
Cell shortening rate (µm/sec) |
RT50 (ms) | RT90 (ms) | Baseline Ca2+ (Fura-2 ratio units) |
Peak Ca2+ (Fura-2 ratio units) |
DT50 (ms) | DT90 (ms) | |
|---|---|---|---|---|---|---|---|---|
| Sham | 7.0 ± 0.7* | 101 ± 15* | 146 ± 22* | 421 ± 58* | 1.21 ± 0.02* | 1.55 ± 0.04* | 244 ± 12* | 676 ± 38* |
| MI | 3.6 ± 0.3 | 45 ± 4 | 265 ± 27 | 793 ± 59 | 1.12 ± 0.02 | 1.44 ± 0.03 | 366 ± 19 | 1209 ± 53 |
| MI+WT cTnC | 3.2 ± 0.3 | 38 ± 4 | 262 ± 26 | 792 ± 62 | 1.09 ± 0.01 | 1.35 ± 0.02 | 390 ± 15 | 1304 ± 32 |
| MI+L48Q cTnC | 6.7 ± 0.4* | 78 ± 5* | 150 ± 13* | 446 ± 40* | 1.15 ± 0.02 | 1.43 ± 0.02 | 269 ± 11* | 972 ± 46* |
= p<0.05 as compared to MI; ANOVA analysis and post-hoc tests with Dunnett’s test for all MI conditions; independent t-test for MI vs. Sham.
Transduction with AV-WT cTnC (MI+WT cTnC) did not have any effects on the contractile properties of cardiomyocytes from MI hearts at any stimulation frequency. As was observed in non-transduced cardiomyocytes from MI hearts, MI+WT cTnC cardiomyocytes had significantly reduced magnitude and rate of cell shortening (Fig. 3B, C; Table 3 and Supplemental Tables 1 and 2), and prolonged relaxation times (RT50, RT90) compared to cardiomyocytes from sham operated (healthy control) hearts (Fig. 3D).
In contrast, transduction with AV-L48Q cTnC (MI+L48Q cTnC) significantly increased the magnitude and rate of cell shortening of cardiomyocytes from MI hearts at all stimulation frequencies (Fig. 3B, C; Table 3 and Supplemental Tables 1 and 2). At 0.5 and 1 Hz, transduction with AV-L48Q cTnC slightly improved relaxation times (RT50, RT90) (Fig. 3D, Table 3 and Supplemental Tables 1 and 2). However, the improvement in relaxation times did not occur at 2 Hz. These results suggest that the over-expression of the L48Q cTnC variant, and not simply over-expression of cTnC, markedly improved the contractile properties of cardiomyocytes from MI hearts at varying stimulation frequencies.
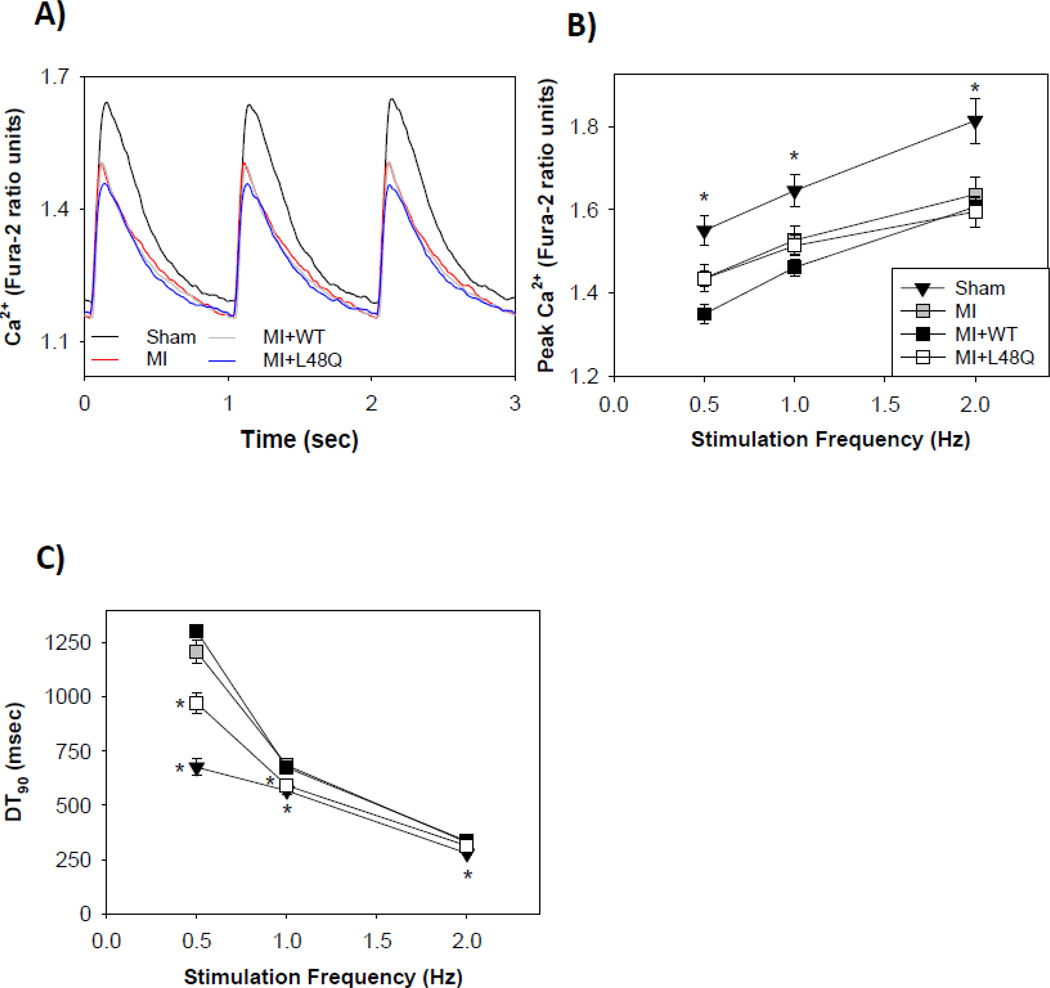
As with cardiomyocytes from healthy (non-operated) hearts (Table 2), we also simultaneously examined the Ca2+ transient properties of cardiomyocytes from MI and sham operated (healthy control) hearts to determine if the improvements in contractile properties resulted from changes myofilament properties per se or were due in part to changes in Ca2+ transient behavior. These data are summarized in Figure 4, Table 3, and Supplemental Tables 1 and 2. Example intracellular Ca2+ transient traces at 1 Hz are shown in Figure 4A. Cardiomyocytes from MI hearts had a significant decrease in the intracellular Ca2+ transient peak compared with cardiomyocytes from sham operated (healthy control) hearts at all stimulation frequencies (Fig. 4E; Table 3 and Supplemental Tables 1 and 2). The cells from MI hearts also had a significantly slower Ca2+ transient decay times (DT50, DT90) at all frequencies (Fig. 4C). The reduction in intracellular Ca2+ transient peak and prolongation of transient decay times for cardiomyocytes from MI hearts agrees with previously published results from failing hearts [2,3,7,9,32–35], and suggests reduced contractility and slower relaxation may result (at least partially) from altered cardiomyocyte Ca2+ handling.
Figure 4.
Intracellular Ca2+ transient properties of transduced cardiomyocytes from MI hearts stimulated at 0.5, 1, & 2 Hz. (A) Representative Ca2+ transients of healthy sham control (black), MI non-transduced (red), MI+WT cTnC (grey), and MI+L48Q cTnC (blue) cardiomyocytes. (B) Peak intracellular Ca2+ release (Fura-2 fluorescence) was depressed in all MI conditions at all frequencies. (C) Intracellular Ca2+ decay times were slowed in MI (grey squares) and MI+WT cTnC (black squares) at all frequencies compared to healthy sham control (black triangles), and MI+L48Q cTnC did not adversely affect Ca2+ decay times at any frequency. Only the healthy sham control cells had significantly faster Ca2+ decay times compared to MI cells at 2 Hz. Values are means ± S.E.M while some error bars are concealed within data points; * = p<0.05 as compared to MI for sham (black triangles) or MI+L48Q cTnC (white squares). Double asterisks indicate significant difference compared to MI where sham and MI+L48Q cTnC data symbols overlap.
Transduction of cardiomyocytes from MI hearts with either AV-L48Q cTnC or AV-WT cTnC did not significantly increase the intracellular Ca2+ transient magnitude or decay times (Fig. 4B, C; Table 3 and Supplemental Tables 1 and 2) for most measurements. However, cardiomyocytes with AV-L48Q cTnC had slightly improved Ca2+ transient decay times (DT90, DT90) at 0.5 and 1 Hz, but not 2 Hz stimulation frequency (Fig. 4C). These results suggest that over-expression of cTnC has little or no effect on the Ca2+ handling properties of MI cardiomyocytes, and that any cytosolic cTnC is not acting as a significant Ca2+ buffer. Additionally, the data suggest that the improvements in contractile properties of MI cells expressing L48Q cTnC primarily resulted from enhancement of myofilament Ca2+ binding properties, not changes in intracellular Ca2+ handling.
Whether or not this is the case, L48Q cTnC was able to significantly increase the contractile performance of these cardiomyocytes, independent of Ca2+ transient behavior. Additionally, while most experiments were performed at room temperature (24°C) to compare with the predominant number of reports for cultured cardiomyocytes in the literature [26,37–41], we did a subset of measurements was made at 37°C to determine if the effects of L48Q cTnC persist at physiological temperature. These data are presented in the Supplement (Supplemental Tables 3–5) and show that the enhancement of contraction with L48Q cTnC is of similar magnitude at 37°C for cardiomyocytes from both normal and infarcted hearts.
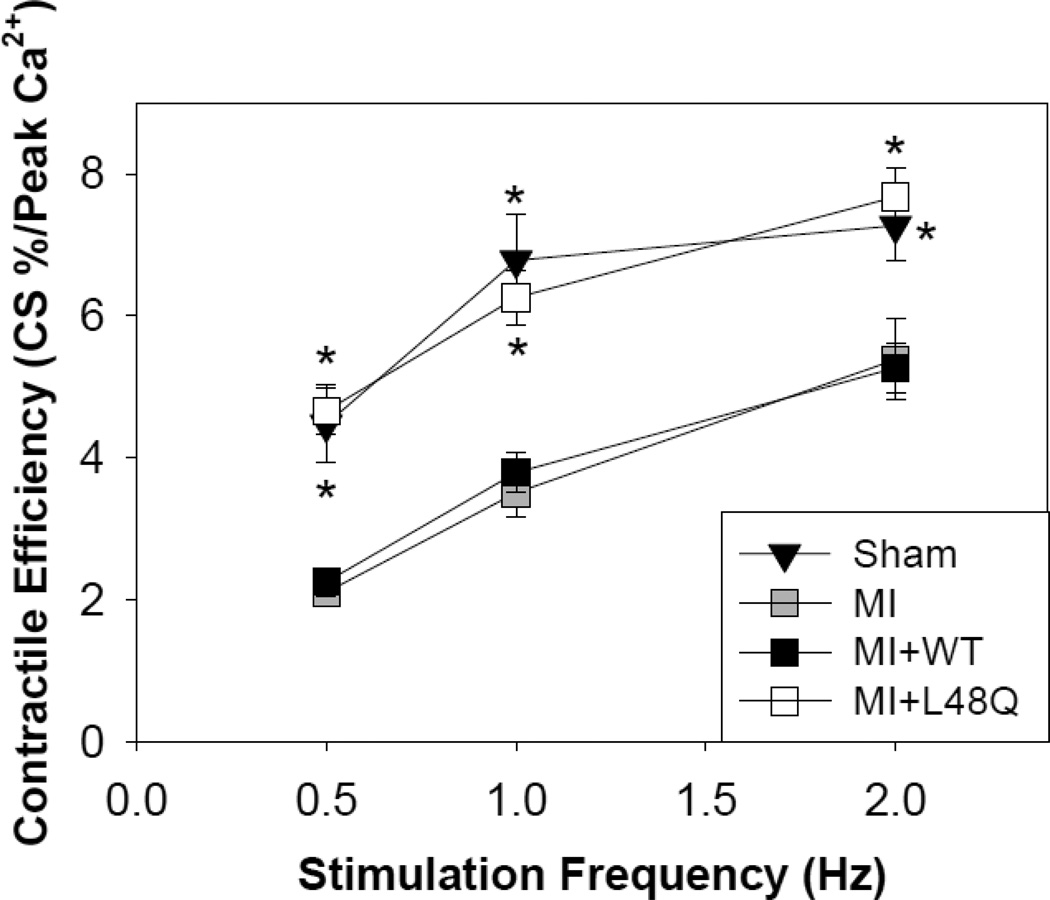
3.6 Cardiomyocyte contractile efficiency
A measure of myofilament responsiveness to Ca2+ (Ca2+ sensitivity of contraction) can be obtained by re-plotting the data in Figures 3 and 4 as the amount of cell shortening (contraction) per unit of peak intracellular Ca2+. The summary of this analysis is illustrated as contractile responsiveness in Figure 5. Cardiomyocytes from MI hearts exhibit a significant decrease in contractile efficiency at all stimulation frequencies compared to cardiomyocytes from sham operated (healthy control) hearts. This reduced efficiency was not affected when cardiomyocytes were transduced with AV-WT cTnC. However, cardiomyocytes from MI hearts transduced with AV-L48Q cTnC exhibited a significant improvement at all frequencies, such that contractile efficiency was almost completely restored to that of cardiomyocytes from sham operated (healthy control) hearts. The significant improvement in the contractile efficiency with AV-L48Q cTnC adds additional support to the idea that there is an enhancement of myofilament responsiveness to activating Ca2+ when L48Q cTnC is being expressed and incorporated into the myofilaments.
Figure 5.
Contractile efficiency assessed as cell shortening divided by peak intracellular Ca2+ release (Fura-2 ratio units). MI+L48Q cTnC transduced cardiomyocytes (white squares) are significantly more responsive to Ca2+ at all stimulation frequencies, which is similar to the Ca2+ responsiveness of healthy control cells (sham operated hearts) (black triangles). MI+WT cTnC transduced cardiomyocytes (black squares) respond to Ca2+ similarly to non-transduced MI cardiomyocytes (grey squares). Values are means ± S.E.M.; * = p<0.05 as compared to MI for sham (black triangles) or MI+L48Q cTnC (white squares). Double asterisks indicate significant difference compared to MI where sham and MI+L48Q cTnC data symbols overlap.
4. DISCUSSION
The main objective of this study was to determine if expression and incorporation of L48Q cTnC into myofilaments could increase the contractile properties of intact cardiomyocytes and improve contractile function following MI. This hypothesis was based on previous results, where L48Q cTnC enhanced the Ca2+ binding properties of cTn and thin filaments in solution, and the Ca2+ sensitivity of myofilament contraction [23,24]. In this study we found that an ~20% myofilament incorporation of L48Q cTnC increased contractility of cardiomyocytes from both healthy hearts and hearts with systolic dysfunction (post-MI). These findings are supported by our previous studies in skeletal muscle where we reported that replacement of ~12.5–25% native fast skeletal TnC (sTnC) with an sTnC variant that increases Ca2+ sensitivity (M80Q sTnCF27W) is sufficient to see a gain of function of demembranated rabbit psoas muscle fibers [42]. Thus, while it may not be the case for all troponin C mutations, it appears that this is the case for these two mutations.
Importantly, the increases in contractile properties of cardiomyocytes with L48Q cTnC occurred without slowing relaxation times or Ca2+ transient decay kinetics. These results suggest that over-expression of L48Q cTnC and incorporation into the myofilaments of intact cardiomyocytes (or even remaining in the cytosol) increases the Ca2+ sensitivity of myofibrils such that significantly greater contraction can occur without an increase in intracellular Ca2+ or compromising relaxation.
Recent work by our group [21,23,24] suggests that the mechanism of by which L48Q cTnC increases contraction of intact cardiomyocytes is by augmented Ca2+ binding to cTn that results in increased thin filament activation at any given [Ca2+]. Our solution studies, and work by others [22], have demonstrated that the L48Q point mutation significantly increases the Ca2+ affinity of cTnC and increases the affinity of cTnC for cTnI in both the presence and absence of Ca2+. Both magnetic resonance spectroscopy (NMR) and molecular dynamic (MD) simulation data indicate that the N-terminal lobe of L48Q cTnC has a more open structure and exposure of the cTnC hydrophobic patch is stabilized following Ca2+ binding [21]. This should increase cTnC-cTnI interaction, allowing enhanced movement of cTm and access to myosin binding sites on actin for a given submaximal [Ca2+]. It may also reduce the reliance on crossbridge-dependent stabilizing the cTnC-cTnI state [43]. This effect should be more pronounced at submaximal [Ca2+], where the slower dissociation rate of L48Q cTnC [22,23] results in more Ca2+ bound to cTn in thin filaments at any given time. These are the physiological conditions present in cardiomyocytes in this study and that occur in the myocardium at the whole organ level. Also, we did not examine myosin content and composition in the cardiomyocytes from MI hearts, but it is possible that changes in myosin isoform could have contributed somewhat to the change of contractile properties of these cells.
An important aspect of L48Q cTnC is that, while it more effectively activated the thin filament and improved contraction, cardiomyocyte relaxation was mainly unaffected in both cardiomyocytes from healthy and MI hearts. Prolonging systole and slowing diastole could have severe in vivo functional consequences by reducing diastolic filling and, subsequently, cardiac output. Experiments by de Tombe et al. [44] in myofibrils demonstrated that increased myofilament Ca2+ sensitivity via exchange with ssTnI or exposure to bepridil does not prolong relaxation, while re-lengthening of cardiomyocytes from transgenic mice expressing ssTnT was significantly slowed, possibly due to slower kinetics of Ca2+ decay [45]. We previously reported that the initial slow phase of relaxation of cardiac myofibrils was increased slightly following ~100% exchange of endogenous cTnC with L48Q cTnC (compared to ~20% L48Q cTnC exchange in the current study), suggesting the rate of thin filament de-activation may be somewhat affected. However, the rate of this slow phase (thought to be dependent of cross-bridge detachment rate) was not affected, and neither was the much larger rapid phase rate of relaxation [23], and thus the total relaxation time was similar to myofibrils containing WT or native cTnC. An additional consideration is that these previous myofibril contraction and relaxation measurements were made during maximal Ca2+ activation at 15°C, and in the presence of a phosphate mop. In the intact in vitro cardiomyocyte, as in the heart, the sarcoplasmic reticulum releases a submaximal amount of Ca2+, which would limit the level of thin filament activation and decrease the time required for Ca2+ re-sequestration after it dissociates from cTn, thus increasing the relaxation rate. An additional factor is that the isometric myofibril experiments utilized ~100% exchange of endogenous cTnC with L48Q cTnC and maximal [Ca2+] (pCa 4.0), while the intact cardiomyocytes in this study contained only ~20% L48Q cTnC, which would reduce the buffering capacity of cTn complexes of thin filaments to maintain thin filament activation. Finally, relaxation properties are different under unloaded (no strain) conditions, such as isolated intact cells in vitro, and the effects of L48Q cTnC on relaxation may differ under loaded conditions (strain) such as those encountered in vivo. While it is possible that greater incorporation of L48Q cTnC into myofibrils of intact cardiomyocytes might begin to slow relaxation, that question is beyond the scope of our current studies.
A consideration of studies where thin filament proteins are over-expressed in muscle cells is if there is an effect on Ca2+ transient behavior, possibly from thin filament proteins in the cytosolic fraction (not yet incorporated into filaments). It has been shown that when cTnI is over-expressed in mouse cardiomyocytes, the non-complexed proteins undergo degradation [46], suggesting that levels of free (cytosolic) cTn subunits are regulated. Interestingly, Lim et al., demonstrated that cardiomyocytes over-expressing mutant cTnC (E59D, D75Y), which is associated with idiopathic dilated cardiomyopathy, markedly decreased contractility while having no effect on intracellular Ca2+ homeostasis [47], and it is another example that it is possible to manipulate thin filament Ca2+ binding affinity without affecting Ca2+ transient behavior. While transgenic expression of ssTnI in myocytes was shown to prolong relaxation and Ca2+ transient decay times [45], work with this transgenic ssTnI mouse model and the myofilament Ca2+ sensitizer, EMD-57033, in a non-transgenic mouse model showed that specifically increasing myofilament Ca2+ sensitivity improved cardiac and myofilament function after ischemiareperfusion injury. This study also showed an improvement in diastolic pressure after ischemia-reperfusion injury [48]. These results provide further support of our hypothesis that targeted increases in myofilament Ca2+ sensitivity through specific manipulation of thin filament Ca2+ binding properties can have a significant impact on cardiac contractility without perturbing intracellular Ca2+ handling or relaxation.
MI and cardiac diseases of the sarcomere, such as dilated cardiomyopathy (DCM), commonly lead to heart failure [49–51]. Within these causes of heart failure, some patterns have emerged that demonstrate potential similarities in the changes of contractile properties and disease state. For example, most DCM mutations in myofilament proteins result in decreased Ca2+ sensitivity of force [52–56]. Additionally, perturbations in myofilament properties, including decreased Ca2+ sensitivity, are thought to underlie (at least in part) the decreased contractility of failing myocardium post-MI [5,9–13]. If myofilament Ca2+ sensitivity in failing myocardium from DCM or MI is decreased, one logical therapeutic approach would be to return the Ca2+ sensitivity to normal levels to limit adaptive changes that occur with reduced systolic performance. However, little is known about whether direct manipulation of myofilament Ca2+ sensitivity might be beneficial for systolic dysfunction. Some work has shown that increasing myofilament Ca2+ sensitivity exhibits promise as a means to improve contractility in models of systolic dysfunction. One study demonstrated the use of a Ca2+ sensitizing agent, pimobendan, in a DCM mouse model significantly improved cardiac performance and morphology [57]. In another study, we demonstrated that enhanced myofilament Ca2+ sensitivity in the non-infarct region of failing hearts following neonatal rat cardiomyocyte engraftment was a major contributor to the improvement in whole heart function following MI [9]. These results suggest that modulation of thin filament properties to enhance myofilament Ca2+ sensitivity may be effective in improving cardiac muscle contractility, and possibly whole heart function, in hearts with systolic dysfunction.
5. CONCLUSIONS
This study demonstrated that expression of L48Q cTnC and incorporation into cardiac myofibrils enhanced Ca2+ sensitivity of the myofilaments and markedly improved contractility in cardiomyocytes from healthy and MI hearts without adverse effects on relaxation or Ca2+ transient behavior. The use of engineered proteins, such as L48Q cTnC, may serve as an alternative approach to other strategies of increasing in myofilament Ca2+ sensitivity through protein [58] or pharmacological [59,60] means, which may have off-target effects. In this in vitro set of studies, we demonstrated that replacement of a minority (~20%) of native cTnC by L48Q cTnC was effective in improving the contractile properties of cardiomyocytes from healthy and MI hearts. Future in vivo work, using approaches more compatible to animal models, will be required to determine the effects of L48Q cTnC expression on whole heart function, and whether this can improve systolic function in damaged or diseased hearts without impairing diastolic function.
Supplementary Material
Highlights.
We over-expressed a Ca2+ sensitizing cTnC variant, L48Q, in intact rat cardiomyocytes
Over-expression resulted in ~15–20% myofilament incorporation of L48Q cTnC
L48Q cTnC increased the contractility of cardiomyocytes from healthy & infarcted hearts
L48Q cTnC did not significantly affect relaxation or Ca2+ transient properties
Acknowledgments
We thank Drs. Hans Reinecke, Jennifer Davis, and N. Scott Blair for their assistance in adenoviral design, production, and troubleshooting. We also thank Kate Buckley for her help with cardiomyocyte isolations and assessment of cardiomyocyte contractility.
Sources of Funding
Funding support for this work was provided by from NSF DGE-0718124 (ERF), AHA FA41247 (FSK and MR), NIH HL65497 (MR), R21 HL091368 (MR and CEM), R01 HL084642 (CEM) and P01 HL004374 (CEM).
Non-standard Abbreviations and Acronyms
- MI
myocardial infarction
- cTnC
cardiac troponin C
- GFP
green fluorescent protein
- RT50, RT90
time to 50% and 90% relaxation
- DT50, DT90
time to 50% and 90% Ca2+ decay
- WT
wild-type
- L48Q
single amino acid substitution of leucine with glutamine at position 48 in cTnC
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The author’s hold a provisional patent application (UW ref. 45511.01US1) on L48Q cTnC. A full patent application has been filed.
References
- 1.Benitah J-P, Alvarez JL, Gómez AM. L-type Ca(2+) current in ventricular cardiomyocytes. J Mol Cell Cardiol. 2010;48:26–36. doi: 10.1016/j.yjmcc.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 2.Hobai IA, O’Rourke B. Decreased Sarcoplasmic Reticulum Calcium Content Is Responsible for Defective Excitation-Contraction Coupling in Canine Heart Failure. Circulation. 2001;103:1577–1584. doi: 10.1161/01.cir.103.11.1577. [DOI] [PubMed] [Google Scholar]
- 3.Piacentino V, Weber CR, Chen X, Weisser-Thomas J, Margulies KB, Bers DM, et al. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003;92:651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- 4.Sabbah HN. Biologic rationale for the use of beta-blockers in the treatment of heart failure. Heart Fail Rev. 2004;9:91–97. doi: 10.1023/B:HREV.0000046363.59374.23. [DOI] [PubMed] [Google Scholar]
- 5.LeWinter MM. Functional consequences of sarcomeric protein abnormalities in failing myocardium. Hear Fail Rev. 2005;10:249–257. doi: 10.1007/s10741-005-5254-4. [DOI] [PubMed] [Google Scholar]
- 6.Palmer BM. Thick filament proteins and performance in human heart failure. Heart Fail Rev. 2005;10:187–197. doi: 10.1007/s10741-005-5249-1. [DOI] [PubMed] [Google Scholar]
- 7.Holt E, Tønnessen T, Lunde PK, Semb SO, Wasserstrom Ja, Sejersted OM, et al. Mechanisms of cardiomyocyte dysfunction in heart failure following myocardial infarction in rats. J Mol Cell Cardiol. 1998;30:1581–1593. doi: 10.1006/jmcc.1998.0724. [DOI] [PubMed] [Google Scholar]
- 8.Li P, Park C, Micheletti R, Li B, Cheng W, Sonnenblick EH, et al. Myocyte performance during evolution of myocardial infarction in rats: effects of propionyl-L-carnitine. Am J Physiol. 1995;268:H1702–H1713. doi: 10.1152/ajpheart.1995.268.4.H1702. [DOI] [PubMed] [Google Scholar]
- 9.Moreno-Gonzalez A, Korte FS, Dai J, Chen K, Ho B, Reinecke H, et al. Cell therapy enhances function of remote non-infarcted myocardium. J Mol Cell Cardiol. 2009;47:603–613. doi: 10.1016/j.yjmcc.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belin RJ, Sumandea MP, Kobayashi T, Walker LA, Rundell VL, Urboniene D, et al. Left ventricular myofilament dysfunction in rat experimental hypertrophy and congestive heart failure. Am J Physiol Heart Circ Physiol. 2006;291:H2344–H2353. doi: 10.1152/ajpheart.00541.2006. [DOI] [PubMed] [Google Scholar]
- 11.Belin RJ, Sumandea MP, Allen EJ, Schoenfelt K, Wang H, Solaro RJ, et al. Augmented protein kinase C-alpha-induced myofilament protein phosphorylation contributes to myofilament dysfunction in experimental congestive heart failure. Circ Res. 2007;101:195–204. doi: 10.1161/CIRCRESAHA.107.148288. [DOI] [PubMed] [Google Scholar]
- 12.Hamdani N, Kooij V, van Dijk S, Merkus D, Paulus WJ, Remedios CD, et al. Sarcomeric dysfunction in heart failure. Cardiovasc Res. 2007;77:649–658. doi: 10.1093/cvr/cvm079. [DOI] [PubMed] [Google Scholar]
- 13.Wolff MR, Whitesell LF, Moss RL. Calcium Sensitivity of Isometric Tension Is Increased in Canine Experimental Heart Failure. Circ Res. 1995;76:781–789. doi: 10.1161/01.res.76.5.781. [DOI] [PubMed] [Google Scholar]
- 14.Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest. 2005;115:2305–2315. doi: 10.1172/JCI26381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kass Da, Solaro RJ. Mechanisms and use of calcium-sensitizing agents in the failing heart. Circulation. 2006;113:305–315. doi: 10.1161/CIRCULATIONAHA.105.542407. [DOI] [PubMed] [Google Scholar]
- 16.Wyskovsky W, Hauptner R, Suko J. Drug-induced calcium release from heavy sarcoplasmic reticulum of skeletal muscle. Biochim Biophys Acta. 1988;938:89–96. doi: 10.1016/0005-2736(88)90125-3. [DOI] [PubMed] [Google Scholar]
- 17.Cleland JGF, Teerlink JR, Senior R, Nifontov EM, Mc Murray JJV, Lang CC, et al. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet. 2011;378:676–683. doi: 10.1016/S0140-6736(11)61126-4. [DOI] [PubMed] [Google Scholar]
- 18.Shen Y-T, Malik FI, Zhao X, Depre C, Dhar SK, Abarzúa P, et al. Improvement of cardiac function by a cardiac Myosin activator in conscious dogs with systolic heart failure. Circ Heart Fail. 2010;3:522–527. doi: 10.1161/CIRCHEARTFAILURE.109.930321. [DOI] [PubMed] [Google Scholar]
- 19.Malik FI, Hartman JJ, Elias Ka, Morgan BP, Rodriguez H, Brejc K, et al. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science. 2011;331:1439–1443. doi: 10.1126/science.1200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garg V, Frishman WH. A new approach to inotropic therapy in the treatment of heart failure: cardiac myosin activators in treatment of HF. Cardiol Rev. 21:155–159. doi: 10.1097/CRD.0b013e318275889c. n.d. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Robertson IM, Li MX, McCully ME, Crane ML, Luo Z, et al. Structural and functional consequences of the cardiac troponin C L48Q Ca(2+)-sensitizing mutation. Biochemistry. 2012;51:4473–4487. doi: 10.1021/bi3003007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tikunova SB, Davis JP. Designing calcium-sensitizing mutations in the regulatory domain of cardiac troponin C. J Biol Chem. 2004;279:35341–35352. doi: 10.1074/jbc.M405413200. [DOI] [PubMed] [Google Scholar]
- 23.Kreutziger KL, Piroddi N, McMichael JT, Tesi C, Poggesi C, Regnier M. Calcium binding kinetics of troponin C strongly modulate cooperative activation and tension kinetics in cardiac muscle. J Mol Cell Cardiol. 2011;50:165–174. doi: 10.1016/j.yjmcc.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korte FS, Feest ER, Razumova MV, Tu A-Y, Regnier M. Enhanced Ca2+ binding of cardiac troponin reduces sarcomere length dependence of contractile activation independently of strong crossbridges. Am J Physiol Heart Circ Physiol. 2012;303:H863–H870. doi: 10.1152/ajpheart.00395.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santana LF, Kranias EG, Lederer WJ. Calcium sparks and excitation-contraction coupling in phospholamban-deficient mouse ventricular myocytes. J Physiol. 1997;503(Pt 1):21–29. doi: 10.1111/j.1469-7793.1997.021bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korte FS, Dai J, Buckley K, Feest ER, Adamek N, Geeves MA, et al. Upregulation of cardiomyocyte ribonucleotide reductase increases intracellular 2 deoxy-ATP, contractility, and relaxation. J Mol Cell Cardiol. 2011;51:894–901. doi: 10.1016/j.yjmcc.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laflamme Ma, Chen KY, Naumova AV, Muskheli V, Fugate Ja, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 28.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo J, Deng Z-L, Luo X, Tang N, Song W-X, Chen J, et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc. 2007;2:1236–1247. doi: 10.1038/nprot.2007.135. [DOI] [PubMed] [Google Scholar]
- 30.Badrian B, Bogoyevitch MA. Gene expression profiling reveals complex changes following MEK-EE expression in cardiac myocytes. Int J Biochem Cell Biol. 2007;39:349–365. doi: 10.1016/j.biocel.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Badrian B, Bogoyevitch MA. Changes in the transcriptional profile of cardiac myocytes following green fluorescent protein expression. DNA Cell Biol. 2007;26:727–736. doi: 10.1089/dna.2007.0604. [DOI] [PubMed] [Google Scholar]
- 32.Li RK, Jia ZQ, Weisel RD, Mickle DA, Zhang J, Mohabeer MK, et al. Cardiomyocyte transplantation improves heart function. Ann Thorac Surg. 1996;62:654–660. doi: 10.1016/s0003-4975(96)00389-x. discussion 660–1. [DOI] [PubMed] [Google Scholar]
- 33.Müller-Ehmsen J, Whittaker P, Kloner RA, Dow JS, Sakoda T, Long TI, et al. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–116. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 34.Scorsin M, Hagege AA, Marotte F, Mirochnik N, Copin H, Barnoux M, et al. Does transplantation of cardiomyocytes improve function of infarcted myocardium? Circulation. 1997;96:II, 188–193. [PubMed] [Google Scholar]
- 35.Huwer H, Winning J, Vollmar B, Welter C, Löhbach C, Menger MD, et al. Long-term cell survival and hemodynamic improvements after neonatal cardiomyocyte and satellite cell transplantation into healed myocardial cryoinfarcted lesions in rats. Cell Transplant. 2003;12:757–767. doi: 10.3727/000000003108747361. [DOI] [PubMed] [Google Scholar]
- 36.Sacconi L, Ferrantini C, Lotti J, Coppini R, Yan P, Loew LM, et al. Action potential propagation in transverse-axial tubular system is impaired in heart failure. Proc Natl Acad Sci U S A. 2012;109:5815–5819. doi: 10.1073/pnas.1120188109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cazorla O, Lucas A, Poirier F, Lacampagne A, Lezoualc’h F. The cAMP binding protein Epac regulates cardiac myofilament function. Proc Natl Acad Sci U S A. 2009;106:14144–14149. doi: 10.1073/pnas.0812536106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheehan KA, Arteaga GM, Hinken AC, Dias FA, Ribeiro C, Wieczorek DF, et al. Functional effects of a tropomyosin mutation linked to FHC contribute to maladaptation during acidosis. J Mol Cell Cardiol. 2011;50:442–450. doi: 10.1016/j.yjmcc.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawashima H, Satoh H, Saotome M, Urushida T, Katoh H, Hayashi H. Protein phosphatase inhibitor-1 augments a protein kinase A-dependent increase in the Ca2+ loading of the sarcoplasmic reticulum without changing its Ca2+ release. Circ J. 2009;73:1133–1140. doi: 10.1253/circj.cj-08-0871. [DOI] [PubMed] [Google Scholar]
- 40.Shah AM, Prendergast BD, Grocott-Mason R, Lewis MJ, Paulus WJ. The influence of endothelium-derived nitric oxide on myocardial contractile function. Int J Cardiol. 1995;50:225–231. doi: 10.1016/0167-5273(95)02381-6. [DOI] [PubMed] [Google Scholar]
- 41.Vogelpohl I, Vetter R, Heger J, Ebermann L, Euler G, Schultheiss H-P, et al. Transgenic overexpression of heart-specific adenine nucleotide translocase 1 positively affects contractile function in cardiomyocytes. Cell Physiol Biochem. 2011;27:121–128. doi: 10.1159/000325214. [DOI] [PubMed] [Google Scholar]
- 42.Feest ER, Tu A, Luo C, Nowakowski SGRM. Effect of varying mutant troponin C content on contractile properties of striated muscle. Biophys J. 2009;96:228a. [Google Scholar]
- 43.Dong WJ, Jayasundar JJ, An J, Xing J, Cheung HC. Effects of PKA phosphorylation of cardiac troponin I and strong crossbridge on conformational transitions of the N-domain of cardiac troponin C in regulated thin filaments. Biochemistry. 2007;46:9752–9761. doi: 10.1021/bi700574n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Tombe PP, Belus A, Piroddi N, Scellini B, Walker JS, Martin AF, et al. Myofilament calcium sensitivity does not affect cross-bridge activation-relaxation kinetics. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1129–R1136. doi: 10.1152/ajpregu.00630.2006. [DOI] [PubMed] [Google Scholar]
- 45.Fentzke RC, Buck SH, Patel JR, Lin H, Wolska BM, Stojanovic MO, et al. Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin I in the heart. J Physiol. 1999;517(Pt 1):143–157. doi: 10.1111/j.1469-7793.1999.0143z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng H-Z, Hossain MM, Huang X-P, Jin J-P. Myofilament incorporation determines the stoichiometry of troponin I in transgenic expression and the rescue of a null mutation. Arch Biochem Biophys. 2009;487:36–41. doi: 10.1016/j.abb.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim CC, Yang H, Yang M, Wang C-K, Shi J, Berg Ea, et al. A novel mutant cardiac troponin C disrupts molecular motions critical for calcium binding affinity and cardiomyocyte contractility. Biophys J. 2008;94:3577–3589. doi: 10.1529/biophysj.107.112896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arteaga GM, Warren CM, Milutinovic S, Martin AF, Solaro RJ. Specific enhancement of sarcomeric response to Ca2+ protects murine myocardium against ischemia-reperfusion dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H2183–H2192. doi: 10.1152/ajpheart.00520.2005. [DOI] [PubMed] [Google Scholar]
- 49.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functio. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 50.Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270–276. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 51.Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308–1320. doi: 10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- 52.Morimoto S, Lu Q-W, Harada K, Takahashi-Yanaga F, Minakami R, Ohta M, et al. Ca(2+)-desensitizing effect of a deletion mutation Delta K210 in cardiac troponin T that causes familial dilated cardiomyopathy. Proc Natl Acad Sci U S A. 2002;99:913–918. doi: 10.1073/pnas.022628899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu Q-W, Morimoto S, Harada K, Du C-K, Takahashi-Yanaga F, Miwa Y, et al. Cardiac troponin T mutation R141W found in dilated cardiomyopathy stabilizes the troponin T-tropomyosin interaction and causes a Ca2+ desensitization. J Mol Cell Cardiol. 2003;35:1421–1427. doi: 10.1016/j.yjmcc.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Mirza M, Marston S, Willott R, Ashley C, Mogensen J, McKenna W, et al. Dilated cardiomyopathy mutations in three thin filament regulatory proteins result in a common functional phenotype. J Biol Chem. 2005;280:28498–28506. doi: 10.1074/jbc.M412281200. [DOI] [PubMed] [Google Scholar]
- 55.Du C-K, Morimoto S, Nishii K, Minakami R, Ohta M, Tadano N, et al. Knock-in mouse model of dilated cardiomyopathy caused by troponin mutation. Circ Res. 2007;101:185–194. doi: 10.1161/CIRCRESAHA.106.146670. [DOI] [PubMed] [Google Scholar]
- 56.Rajan S, Ahmed RPH, Jagatheesan G, Petrashevskaya N, Boivin GP, Urboniene D, et al. Dilated cardiomyopathy mutant tropomyosin mice develop cardiac dysfunction with significantly decreased fractional shortening and myofilament calcium sensitivity. Circ Res. 2007;101:205–214. doi: 10.1161/CIRCRESAHA.107.148379. [DOI] [PubMed] [Google Scholar]
- 57.Du CK, Morimoto S, Nishii K, Minakami R, Ohta M, Tadano N, et al. Knock-in mouse model of dilated cardiomyopathy caused by troponin mutation. Circ Res. 2007;101:185–194. doi: 10.1161/CIRCRESAHA.106.146670. [DOI] [PubMed] [Google Scholar]
- 58.Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, et al. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest. 2008;118:3893–3903. doi: 10.1172/JCI36642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Solaro RJ, Gambassi G, Warshaw DM, Keller MR, Spurgeon HA, Beier N, et al. Stereoselective actions of thiadiazinones on canine cardiac myocytes and myofilaments. Circ Res. 1993;73:981–990. doi: 10.1161/01.res.73.6.981. [DOI] [PubMed] [Google Scholar]
- 60.White J, Lee JA, Shah N, Orchard CH. Differential effects of the optical isomers of EMD 53998 on contraction and cytoplasmic Ca2+ in isolated ferret cardiac muscle. Circ Res. 1993;73:61–70. doi: 10.1161/01.res.73.1.61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.