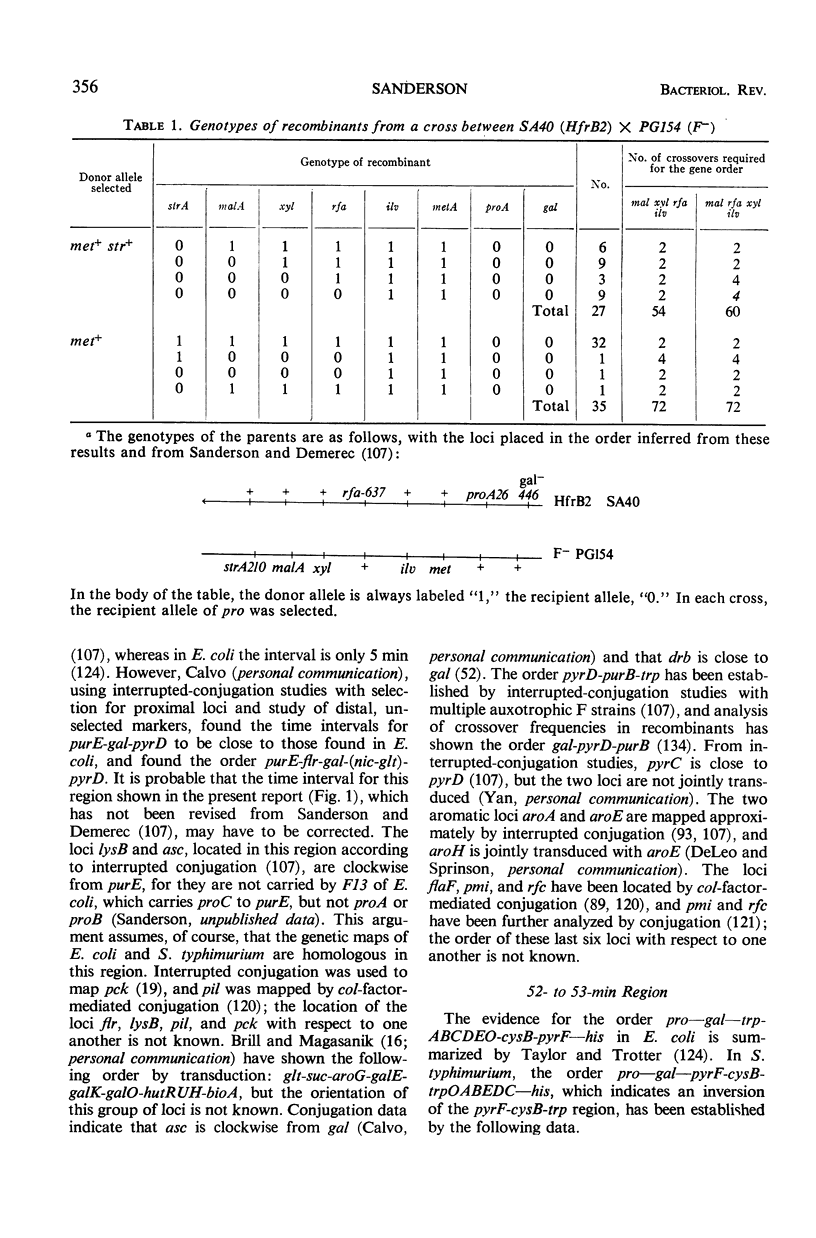
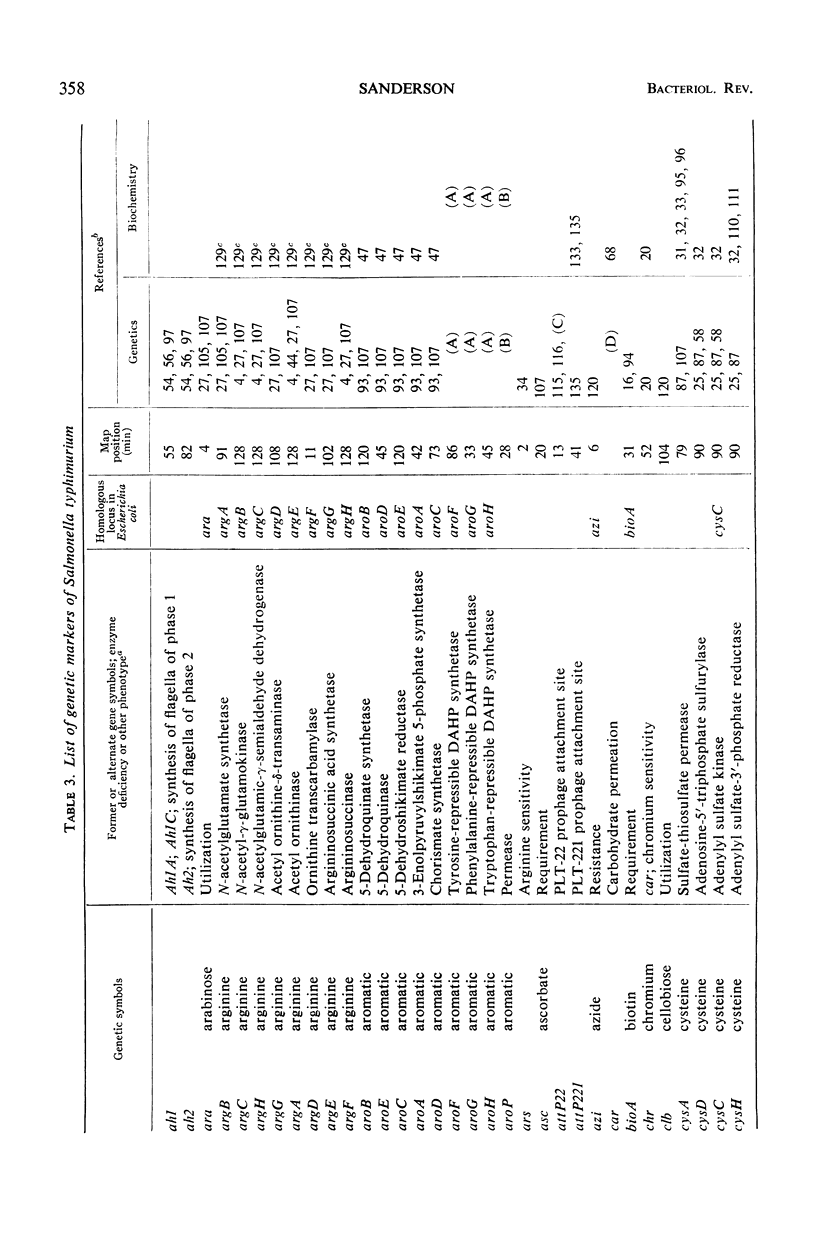
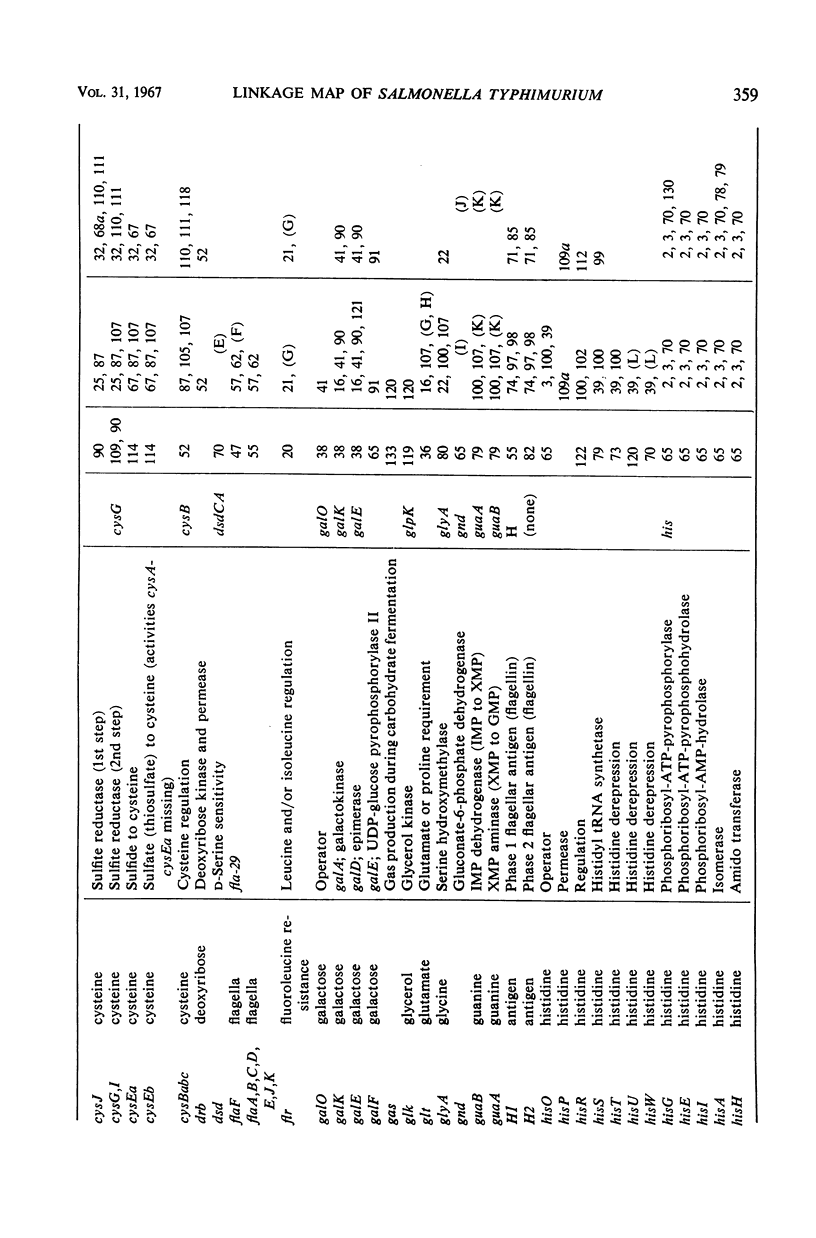
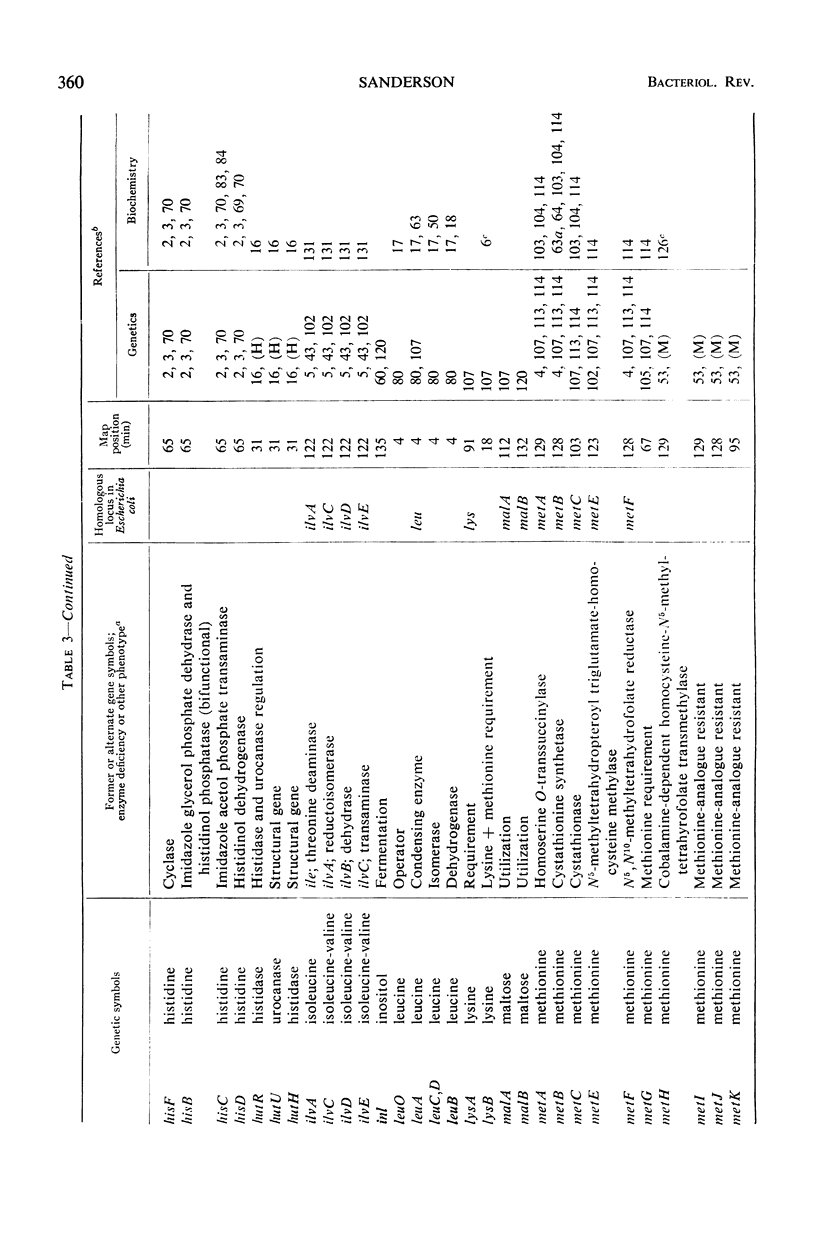
Full text
PDF


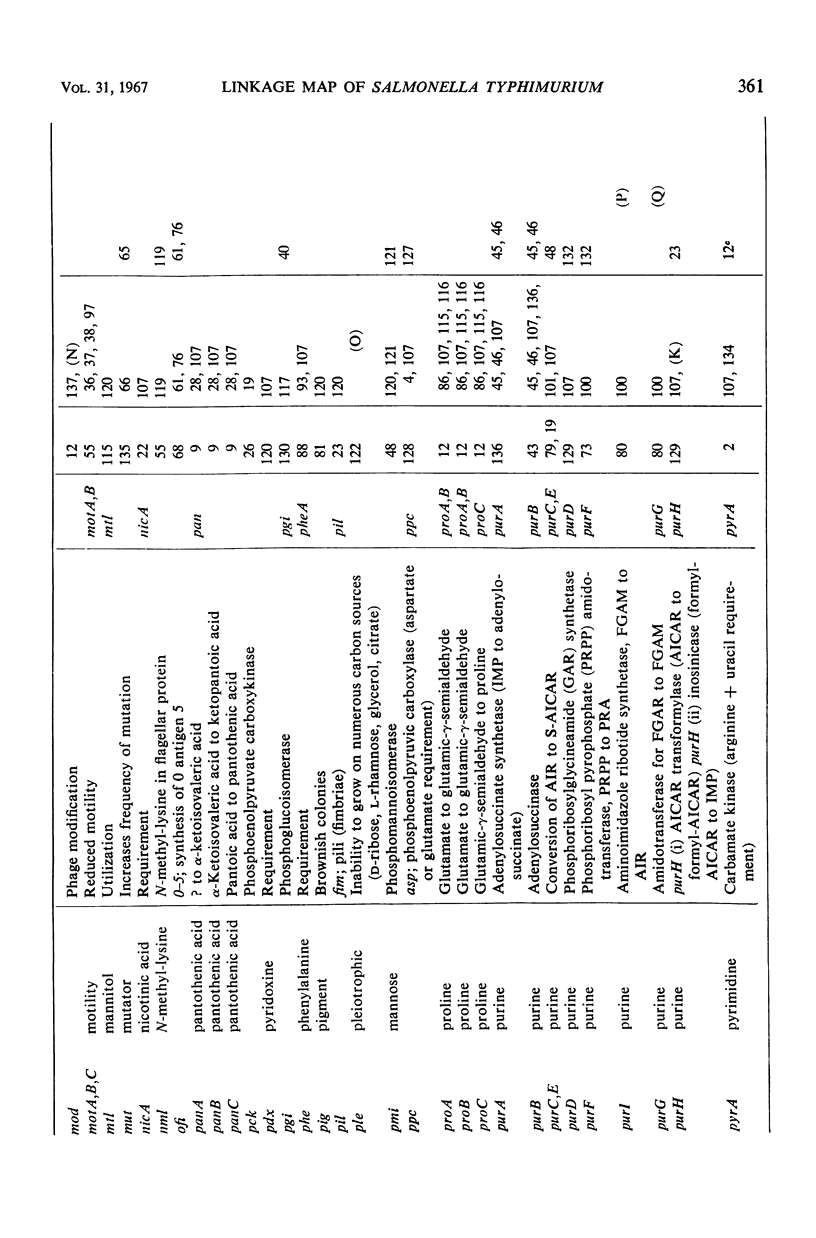
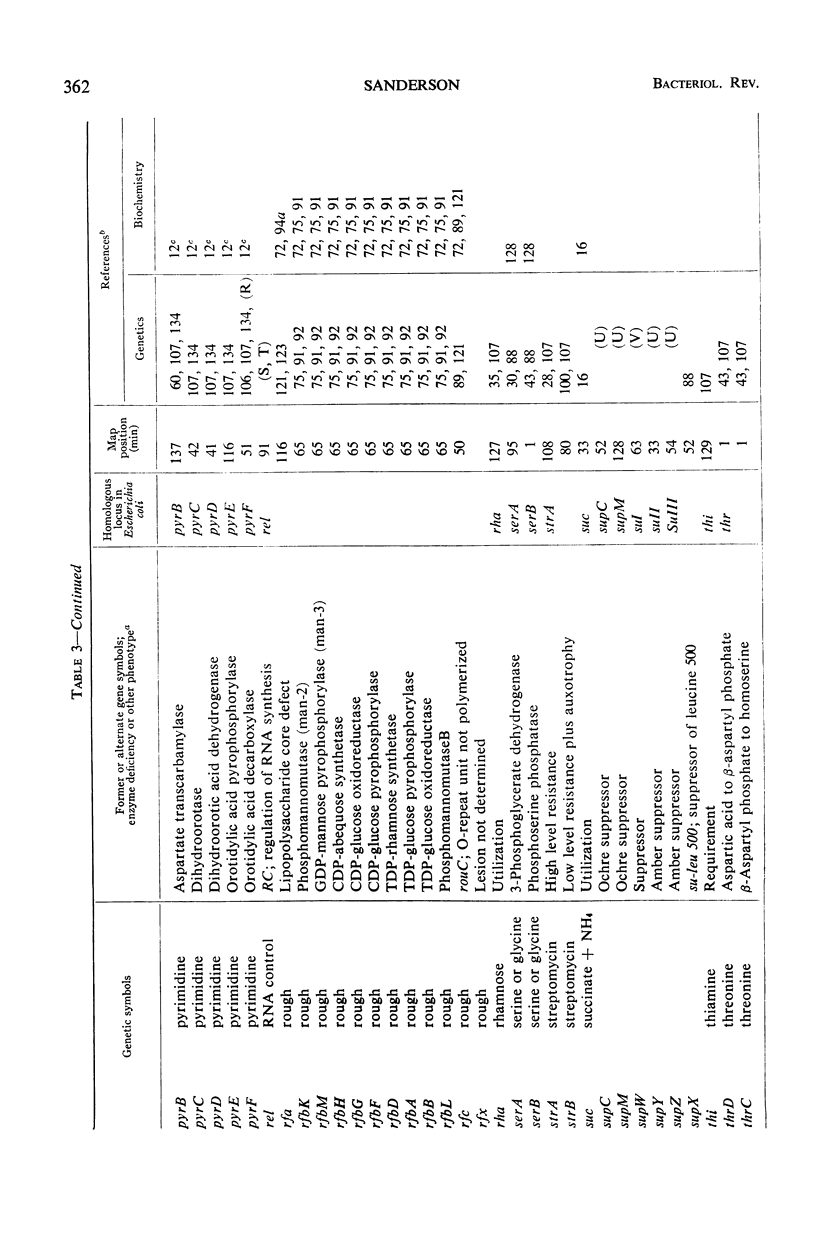
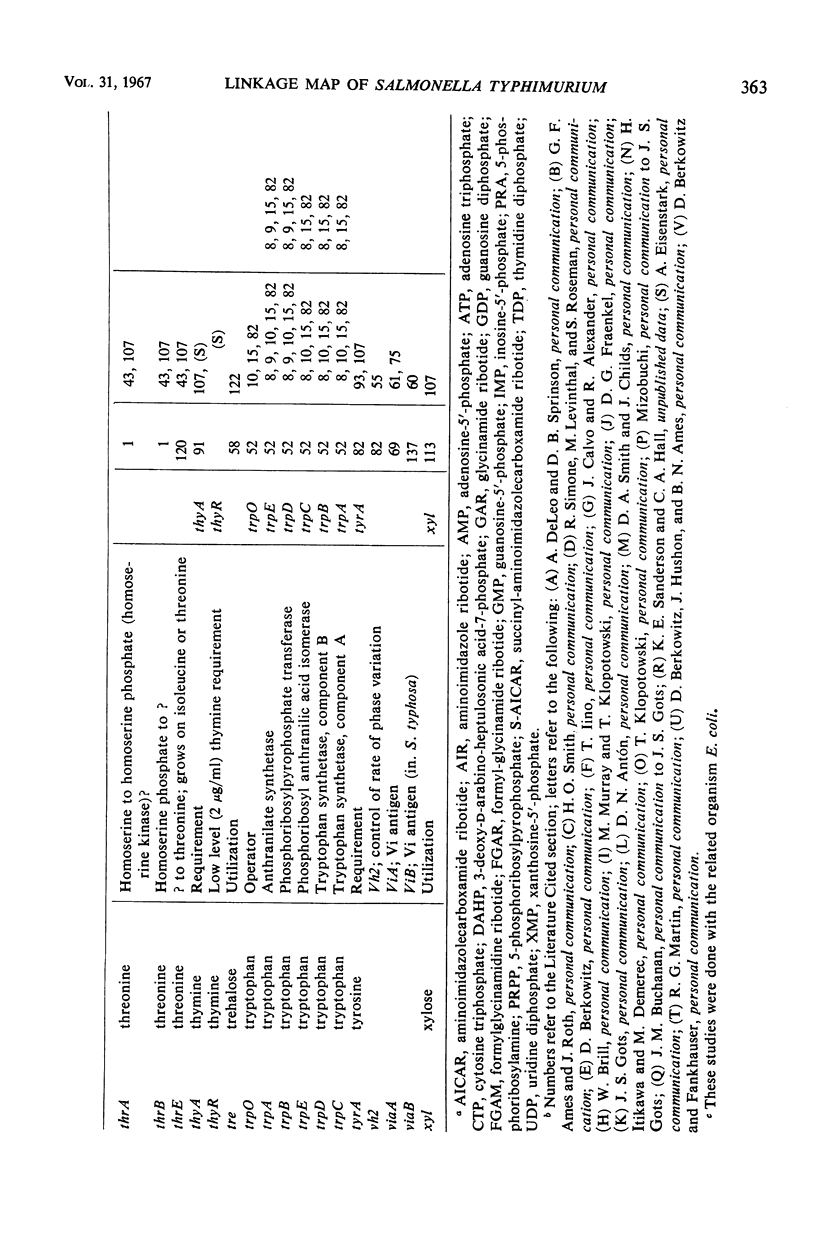
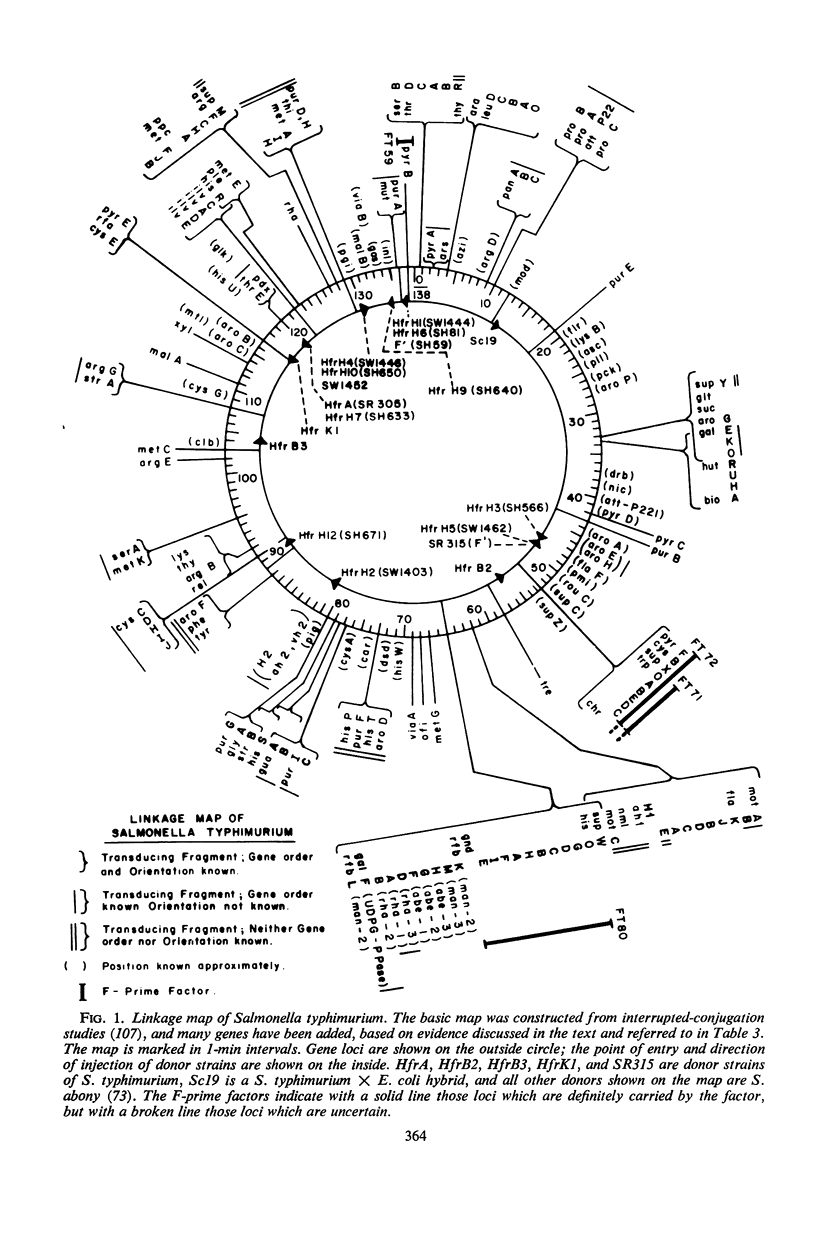







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG F. B., WAGNER R. P. ISOLEUCINE-VALINE REQUIRING MUTANTS OF SALMONELLA TYPHIMURIUM. Genetics. 1964 Nov;50:957–965. doi: 10.1093/genetics/50.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B. N., Garry B. COORDINATE REPRESSION OF THE SYNTHESIS OF FOUR HISTIDINE BIOSYNTHETIC ENZYMES BY HISTIDINE. Proc Natl Acad Sci U S A. 1959 Oct;45(10):1453–1461. doi: 10.1073/pnas.45.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong F. B. Orientation and order of loci of the met-arg region in the Salmonella typhimurium linkage map. Genetics. 1967 Jul;56(3):463–466. doi: 10.1093/genetics/56.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACK K. J., WESTAWAY E. G. Studies on a mutant strain of Escherichia coli which requires both methionine and lysine for growth. J Gen Microbiol. 1962 Jan;27:41–50. doi: 10.1099/00221287-27-1-41. [DOI] [PubMed] [Google Scholar]
- BALBINDER E. The fine structure of the loci tryC and tryD of Salmonella typhimurium. I. Determination of the order of mutational sites by three-point transduction tests. Genetics. 1962 Apr;47:469–482. doi: 10.1093/genetics/47.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKMANN I., SUBBAIAH T. V., STOCKER B. A. ROUGH MUTANTS OF SALMONELLA TYPHIMURIUM. II. SEROLOGICAL AND CHEMICAL INVESTIGATIONS. Nature. 1964 Mar 28;201:1299–1301. doi: 10.1038/2011299a0. [DOI] [PubMed] [Google Scholar]
- BECKWITH J. R., PARDEE A. B., AUSTRIAN R., JACOB F. Coordination of the synthesis of the enzymes in the pyrimidine pathway of E. coli. J Mol Biol. 1962 Dec;5:618–634. doi: 10.1016/s0022-2836(62)80090-4. [DOI] [PubMed] [Google Scholar]
- BURNS R. O., UMBARGER H. E., GROSS S. R. THE BIOSYNTHESIS OF LEUCINE. III. THE CONVERSION OF ALPHA-HYDROXY-BETA-CARBOXYISOCAPROATE TO ALPHA-KETOISOCAPROATE. Biochemistry. 1963 Sep-Oct;2:1053–1058. doi: 10.1021/bi00905a024. [DOI] [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. A multifunctional enzyme complex in the tryptophan pathway of Salmonella typhimurium: comparison of polarity and pseudopolarity mutations. Cold Spring Harb Symp Quant Biol. 1966;31:203–214. doi: 10.1101/sqb.1966.031.01.028. [DOI] [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. Evidence for two sites for initiation of gene expression in the tryptophan operon of Salmonella typhimurium. J Mol Biol. 1967 Jun 28;26(3):423–436. doi: 10.1016/0022-2836(67)90313-0. [DOI] [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. The functional organization of the tryptophan gene cluster in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1966 Jul;56(1):111–118. doi: 10.1073/pnas.56.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith J. R., Signer E. R. Transposition of the lac region of Escherichia coli. I. Inversion of the lac operon and transduction of lac by phi80. J Mol Biol. 1966 Aug;19(2):254–265. doi: 10.1016/s0022-2836(66)80003-7. [DOI] [PubMed] [Google Scholar]
- Berg C. M., Curtiss R., 3rd Transposition derivatives of an Hfr strain of Escherichia coli K-12. Genetics. 1967 Jul;56(3):503–525. doi: 10.1093/genetics/56.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume A. J., Balbinder E. The tryptophan operon of Salmonella typhimurium. Fine structure analysis by deletion mapping and abortive transduction. Genetics. 1966 Mar;53(3):577–592. doi: 10.1093/genetics/53.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns R. O., Calvo J., Margolin P., Umbarger H. E. Expression of the leucine operon. J Bacteriol. 1966 Apr;91(4):1570–1576. doi: 10.1128/jb.91.4.1570-1576.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo R. A., Calvo J. M. Lack of end-product inhibition and repression of leucine synthesis in a strain of Salmonella typhimurium. Science. 1967 May 26;156(3778):1107–1109. doi: 10.1126/science.156.3778.1107. [DOI] [PubMed] [Google Scholar]
- Corwin L. M., Fanning G. R., Feldman F., Margolin P. Mutation leading to increased sensitivity to chromium in Salmonella typhimurium. J Bacteriol. 1966 Apr;91(4):1509–1515. doi: 10.1128/jb.91.4.1509-1515.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMEREC M., GILLESPIE D. H., MIZOBUCHI K. GENETIC STRUCTURE OF THE CYST REGION OF THE SALMONELLA GENOME. Genetics. 1963 Aug;48:997–1009. doi: 10.1093/genetics/48.8.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMEREC M., OHTA N. GENETIC ANALYSES OF SALMONELLA TYPHIMURIUM X ESCHERICHIA COLI HYBRIDS. Proc Natl Acad Sci U S A. 1964 Aug;52:317–323. doi: 10.1073/pnas.52.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DREYFUSS J. CHARACTERIZATION OF A SULFATE- AND THIOSULFATE-TRANSPORTING SYSTEM IN SALMONELLA TYPHIMURIUM. J Biol Chem. 1964 Jul;239:2292–2297. [PubMed] [Google Scholar]
- Dalal F. R., Gots R. E., Gots J. S. Mechanism of adenine inhibition in adenine-sensitive mutants of Salmonella typhimurium. J Bacteriol. 1966 Feb;91(2):507–513. doi: 10.1128/jb.91.2.507-513.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss J., Pardee A. B. Regulation of sulfate transport in Salmonella typhimurium. J Bacteriol. 1966 Jun;91(6):2275–2280. doi: 10.1128/jb.91.6.2275-2280.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E., BARON L. S. Mutation to L-rhamnose resistance and transduction to L-rhamnose utilization in Salmonella typhosa. J Bacteriol. 1959 Nov;78:675–686. doi: 10.1128/jb.78.5.675-686.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto M. Genetic Studies of Paralyzed Mutants in Salmonella. II. Mapping of Three mot Loci by Linkage Analysis. Genetics. 1966 Nov;54(5):1069–1076. doi: 10.1093/genetics/54.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto M. Genetic studies of paralyzed mutant in Salmonella. I. Genetic fine structure of the mot loci in Salmonella typhimurium. Genetics. 1966 Sep;54(3):715–726. doi: 10.1093/genetics/54.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL D., OSBORN M. J., HORECKER B. L., SMITH S. M. Metabolism and cell wall structure of a mutant of Salmonella typhimurium deficient in phosphoglucose isomerase. Biochem Biophys Res Commun. 1963 Jun 20;11:423–428. doi: 10.1016/0006-291x(63)90086-x. [DOI] [PubMed] [Google Scholar]
- FUKASAWA T., NIKAIDO H. Galactose mutants of Salmonella typhimurium. Genetics. 1961 Oct;46:1295–1303. doi: 10.1093/genetics/46.10.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLLUB E. G., GOTS J. S. Purine metabolism in bacteria. VI. Accumulations by mutants lacking adenylosuccinase. J Bacteriol. 1959 Sep;78:320–325. doi: 10.1128/jb.78.3.320-325.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS S. R., BURNS R. O., UMBARGER H. E. THE BIOSYNTHESIS OF LEUCINE. II. THE ENZYMIC ISOMERIZATION OF BETA-CARBOXY-BETA-HYDROXYISOCAPROATE AND ALPHA-HYDROXY-BETA-CARBOXYISOCAPROATE. Biochemistry. 1963 Sep-Oct;2:1046–1052. doi: 10.1021/bi00905a023. [DOI] [PubMed] [Google Scholar]
- Gemski P., Jr, Stocker B. A. Transduction by bacteriophage P22 in nonsmooth mutants of Salmonella typhimurium. J Bacteriol. 1967 May;93(5):1588–1597. doi: 10.1128/jb.93.5.1588-1597.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanville E V, Demerec M. Threonine, Isoleucine, and Isoleucine-Valine Mutants of Salmonella Typhimurium. Genetics. 1960 Oct;45(10):1359–1374. doi: 10.1093/genetics/45.10.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatzer L., Labrie D. A., Armstrong F. B. Transduction of Salmonella typhimurium-Salmonella montevideo hybrids. Genetics. 1966 Aug;54(2):423–432. doi: 10.1093/genetics/54.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gots J. S., Gollub E. G. SEQUENTIAL BLOCKADE IN ADENINE BIOSYNTHESIS BY GENETIC LOSS OF AN APPARENT BIFUNCTIONAL DEACYLASE. Proc Natl Acad Sci U S A. 1957 Sep 15;43(9):826–834. doi: 10.1073/pnas.43.9.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman P. E., Rusgis C., Stahl R. C. Orientation of the histidine operon in the Salmonella typhimurium linkage map. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1332–1335. doi: 10.1073/pnas.53.6.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino T., Enomoto M. Genetical studies of non-flagellate mutants of Salmonella. J Gen Microbiol. 1966 Jun;43(3):315–327. doi: 10.1099/00221287-43-3-315. [DOI] [PubMed] [Google Scholar]
- Iino T. A Stabilizer of Antigenic Phases in Salmonella Abortus-Equi. Genetics. 1961 Nov;46(11):1465–1469. doi: 10.1093/genetics/46.11.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itikawa H., Demerec M. Ditto deletions in the cysC region of the Salmonella chromosome. Genetics. 1967 Jan;55(1):63–68. doi: 10.1093/genetics/55.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON E. M., KRAUSKOPF B., BARON L. S. GENETIC MAPPING OF VI AND SOMATIC ANTIGENIC DETERMINANTS IN SALMONELLA. J Bacteriol. 1965 Aug;90:302–308. doi: 10.1128/jb.90.2.302-308.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. M., Krauskopf B., Baron L. S. Genetic analysis of the ViA-his chromosomal region in Salmonella. J Bacteriol. 1966 Nov;92(5):1457–1463. doi: 10.1128/jb.92.5.1457-1463.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joys T. M., Stocker B. A. Complementation of non-flagellate Salmonella mutants. J Gen Microbiol. 1965 Oct;41(1):47–55. doi: 10.1099/00221287-41-1-47. [DOI] [PubMed] [Google Scholar]
- KIRCHNER C. E. The effects of the mutator gene on molecular changes and mutation of Salmonella typhimurium. J Mol Biol. 1960 Dec;2:331–338. doi: 10.1016/s0022-2836(60)80044-7. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Flavin M. Cystathionine gamma-synthetase of Salmonella. Catalytic properties of a new enzyme in bacterial methionine biosynthesis. J Biol Chem. 1966 Oct 10;241(19):4463–4471. [PubMed] [Google Scholar]
- Kirchner C. E., Rudden M. J. Location of a mutator gene in Salmonella typhimurium by cotransduction. J Bacteriol. 1966 Nov;92(5):1453–1456. doi: 10.1128/jb.92.5.1453-1456.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M., Tomkins G. M. The enzymic synthesis of L-cysteine in Escherichia coli and Salmonella typhimurium. J Biol Chem. 1966 Nov 10;241(21):4955–4965. [PubMed] [Google Scholar]
- Kundig W., Kundig F. D., Anderson B., Roseman S. Restoration of active transport of glycosides in Escherichia coli by a component of a phosphotransferase system. J Biol Chem. 1966 Jul 10;241(13):3243–3246. [PubMed] [Google Scholar]
- LEINWEBER F. J., MONTY K. J. THE METABOLISM OF THIOSULFATE IN SALMONELLA TYPHIMURIUM. J Biol Chem. 1963 Nov;238:3775–3780. [PubMed] [Google Scholar]
- LOPER J. C., ADAMS E. PURIFICATION AND PROPERTIES OF HISTIDINOL DEHYDROGENASE FROM SALMONELLA TYPHIMURIUM. J Biol Chem. 1965 Feb;240:788–795. [PubMed] [Google Scholar]
- LOPER J. C., GRABNAR M., STAHL R. C., HARTMAN Z., HARTMAN P. E. GENES AND PROTEINS INVOLVED IN HISTIDINE BIOSYNTHESIS IN SALMONELLA. Brookhaven Symp Biol. 1964 Dec;17:15–52. [PubMed] [Google Scholar]
- LOWY J., MCDONOUGH M. W. STRUCTURE OF FILAMENTS PRODUCED BY RE-AGGREGATION OF SALMONELLA FLAGELLIN. Nature. 1964 Oct 10;204:125–127. doi: 10.1038/204125a0. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAKELA P. H. HFR males in Salmonella abony. Genetics. 1963 Mar;48:423–429. doi: 10.1093/genetics/48.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIN P. BIPOLARITY OF INFORMATION TRANSFER FROM THE SALMONELLA TYPHIMURIUM CHROMOSOME. Science. 1965 Mar 19;147(3664):1456–1458. doi: 10.1126/science.147.3664.1456. [DOI] [PubMed] [Google Scholar]
- MARGOLIN P. Genetic fine structure of the leucine operon in Salmonella. Genetics. 1963 Mar;48:441–457. doi: 10.1093/genetics/48.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDONOUGH M. W. AMINO ACID COMPOSITION OF ANTIGENICALLY DISTINCT SALMONELLA FLAGELLAR PROTEINS. J Mol Biol. 1965 Jun;12:342–355. doi: 10.1016/s0022-2836(65)80258-3. [DOI] [PubMed] [Google Scholar]
- Margolies M. N., Goldberger R. F. Isolation of the fourth (isomerase) of histidine biosynthesis from Salmonella typhimurium. J Biol Chem. 1966 Jul 25;241(14):3262–3269. [PubMed] [Google Scholar]
- Margolies M. N., Goldberger R. F. Physical and chemical characterization of the isomerase of histidine biosynthesis in Salmonella typhimurium. J Biol Chem. 1967 Jan 25;242(2):256–264. [PubMed] [Google Scholar]
- Margolin P., Bauerle R. H. Determinants for regulation and initiation of expression of tryptophan genes. Cold Spring Harb Symp Quant Biol. 1966;31:311–320. doi: 10.1101/sqb.1966.031.01.041. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Goldberger R. F. Imidazolylacetolphosphate:L-glutamate aminotransferase. Purification and physical properties. J Biol Chem. 1967 Mar 25;242(6):1168–1174. [PubMed] [Google Scholar]
- Martin R. G., Voll M. J., Appella E. Imidazolylacetolphosphate:L-glutamate aminotransferase. Composition and substructure. J Biol Chem. 1967 Mar 25;242(6):1175–1181. [PubMed] [Google Scholar]
- Miyake T, Demerec M. Proline Mutants of Salmonella Typhimurium. Genetics. 1960 Jun;45(6):755–762. doi: 10.1093/genetics/45.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizobuchi K, Demerec M, Gillespie D H. Cysteine Mutants of Salmonella Typhimurium. Genetics. 1962 Nov;47(11):1617–1627. doi: 10.1093/genetics/47.11.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai F. H., Margolin P. ANALYSIS OF UNLINKED SUPPRESSORS OF AN O degrees MUTATION IN SALMONELLA. Proc Natl Acad Sci U S A. 1963 Jul;50(1):140–148. doi: 10.1073/pnas.50.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H. Genetic determination of the O antigens of Salmonella groups B (4,5,12) and C1(6,7). J Bacteriol. 1966 Mar;91(3):1115–1125. doi: 10.1128/jb.91.3.1115-1125.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H., Ziegler L. An F' episome of Salmonella abony. Acta Pathol Microbiol Scand. 1966;67(4):547–554. doi: 10.1111/apm.1966.67.4.547. [DOI] [PubMed] [Google Scholar]
- NAIDE Y., NIKAIDO H., MAEKELAE P. H., WILKINSON R. G., STOCKER B. A. SEMIROUGH STRAINS OF SALMONELLA. Proc Natl Acad Sci U S A. 1965 Jan;53:147–153. doi: 10.1073/pnas.53.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIKAIDO H., FUKASAWA T. The effect of mutation in a structural gene on the inducibility of the enzymes controlled by other genes of the same operon. Biochem Biophys Res Commun. 1961 Apr 7;4:338–342. doi: 10.1016/0006-291x(61)90214-5. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Levinthal M., Nikaido K., Nakane K. Extended deletions in the histidine-rough-B region of the Salmonella chromosome. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1825–1832. doi: 10.1073/pnas.57.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Nikaido K., Mäkelä P. H. Genetic determination of enzymes synthesizing O-specific sugars of Salmonella lipopolysaccharides. J Bacteriol. 1966 Mar;91(3):1126–1135. doi: 10.1128/jb.91.3.1126-1135.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Demerec M., Eisenstark A. Genetic analysis of aromatic mutants of Salmonella typhimurium. Genetics. 1967 Jun;56(2):341–351. doi: 10.1093/genetics/56.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J. Biosynthesis and structure of the core region of the lipopolysaccharide in Salmonella typhimurium. Ann N Y Acad Sci. 1966 Jun 30;133(2):375–383. doi: 10.1111/j.1749-6632.1966.tb52377.x. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Prestidge L. S., Whipple M. B., Dreyfuss J. A binding site for sulfate and its relation to sulfate transport into Salmonella typhimurium. J Biol Chem. 1966 Sep 10;241(17):3962–3969. [PubMed] [Google Scholar]
- Pardee A. B. Purification and properties of a sulfate-binding protein from Salmonella typhimurium. J Biol Chem. 1966 Dec 25;241(24):5886–5892. [PubMed] [Google Scholar]
- Pearce U., Stocker B. A. Variation in composition of chromosome fragments transduced by phage P22. Virology. 1965 Nov;27(3):290–296. doi: 10.1016/0042-6822(65)90108-x. [DOI] [PubMed] [Google Scholar]
- ROWBURY R. J. SYNTHESIS OF CYSTATHIONINE AND ITS CONTROL IN SALMONELLA TYPHIMURIUM. Nature. 1964 Aug 29;203:977–978. doi: 10.1038/203977a0. [DOI] [PubMed] [Google Scholar]
- ROWBURY R. J. THE ACCUMULATION OF O-SUCCINYLHOMOSERINE BY ESCHERICHIA COLI AND SALMONELLA TYPHIMURIUM. J Gen Microbiol. 1964 Nov;37:171–180. doi: 10.1099/00221287-37-2-171. [DOI] [PubMed] [Google Scholar]
- Roth J. R., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium II. Histidine regulatory mutants having altered histidyl-tRNA synthetase. J Mol Biol. 1966 Dec 28;22(2):325–333. doi: 10.1016/0022-2836(66)90135-5. [DOI] [PubMed] [Google Scholar]
- Roth J. R., Antón D. N., Hartman P. E. Histidine regulatory mutants in Salmonella typhimurium. I. Isolation and general properties. J Mol Biol. 1966 Dec 28;22(2):305–323. doi: 10.1016/0022-2836(66)90134-3. [DOI] [PubMed] [Google Scholar]
- Roth J. R., Hartman P. E. Heterogeneity in P22 transducing particles. Virology. 1965 Nov;27(3):297–307. doi: 10.1016/0042-6822(65)90109-1. [DOI] [PubMed] [Google Scholar]
- Roth J. R., Sanderson K. E. Orientation of the isoleucine-valine genes in the Salmonella typhimurium linkage map. Genetics. 1966 May;53(5):971–976. doi: 10.1093/genetics/53.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDERSON K. E., DEMEREC M. THE LINKAGE MAP OF SALMONELLA TYPHIMURIUM. Genetics. 1965 Jun;51:897–913. doi: 10.1093/genetics/51.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAFLER S., MINTZER L., SCHAFLER C. Acquisition of lactose fermenting properties by salmonellae. II. Role of the medium. J Bacteriol. 1960 Feb;79:203–212. doi: 10.1128/jb.79.2.203-212.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ N. M. SUPPRESSION OF A LAC O-O MUTATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Oct;88:996–1001. doi: 10.1128/jb.88.4.996-1001.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUBBAIAH T. V., STOCKER B. A. ROUGH MUTANTS OF SALMONELLA TYPHIMURIUM. I. GENETICS. Nature. 1964 Mar 28;201:1298–1299. doi: 10.1038/2011298a0. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E. Information transfer in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1335–1340. doi: 10.1073/pnas.53.6.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifrin S., Ames B. N., FerroLuzzi-Ames G. Effect of the alpha-hydrazino analogue of histidine on histidine transport and arginine biosynthesis. J Biol Chem. 1966 Jul 25;241(14):3424–3429. [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Kinetic properties of the TPNH-specific sulfite and hydroxylamine reductase of Salmonella typhimurium. Biochem Biophys Res Commun. 1964 Oct 14;17(3):201–205. doi: 10.1016/0006-291x(64)90383-3. [DOI] [PubMed] [Google Scholar]
- Silbert D. F., Fink G. R., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium 3. A class of regulatory mutants deficient in tRNA for histidine. J Mol Biol. 1966 Dec 28;22(2):335–347. doi: 10.1016/0022-2836(66)90136-7. [DOI] [PubMed] [Google Scholar]
- Smith D. A., Childs J. D. Methionine genes and enzymes of Salmonella typhimurium. Heredity (Edinb) 1966 May;21(2):265–286. doi: 10.1038/hdy.1966.22. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Levine M. Gene order in prophage P22. Virology. 1965 Oct;27(2):229–231. doi: 10.1016/0042-6822(65)90166-2. [DOI] [PubMed] [Google Scholar]
- Stocker B. A., Wilkinson R. G., Mäkelä P. H. Genetic aspects of biosynthesis and structure of Salmonella somatic polysaccharide. Ann N Y Acad Sci. 1966 Jun 30;133(2):334–348. doi: 10.1111/j.1749-6632.1966.tb52375.x. [DOI] [PubMed] [Google Scholar]
- TAYLOR A. L., THOMAN M. S. THE GENETIC MAP OF ESCHERICHIA COLI K-12. Genetics. 1964 Oct;50:659–677. doi: 10.1093/genetics/50.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THEODORE T. S., ENGLESBERG E. MUTANT OF SALMONELLA TYPHIMURIUM DEFICIENT IN THE CARBON DIOXIDE-FIXING ENZYME PHOSPHOENOLPYRUVIC CARBOXYLASE. J Bacteriol. 1964 Oct;88:946–955. doi: 10.1128/jb.88.4.946-955.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. T., Weissbach H. N5-methyltetrahydrofolate-homocysteine transmethylase. Partial purification and properties. J Biol Chem. 1967 Apr 10;242(7):1502–1508. [PubMed] [Google Scholar]
- Voll M. J., Appella E., Martin R. G. Purification and composition studies of phosphoribosyladenosine triphosphate:pyrophosphate phosphoribosyltransferase, the first enzyme of histidine biosynthesis. J Biol Chem. 1967 Apr 25;242(8):1760–1767. [PubMed] [Google Scholar]
- Wagner R P, Bergquist A. Nature of the Genetic Blocks in the Isoleucine-Valine Mutants of Salmonella. Genetics. 1960 Oct;45(10):1375–1386. doi: 10.1093/genetics/45.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO N., ANDERSON T. F. Genomic masking and recombination between serologically unrelated phages P22 and P221. Virology. 1961 Aug;14:430–439. doi: 10.1016/0042-6822(61)90334-8. [DOI] [PubMed] [Google Scholar]
- Yan Y., Demerec M. Genetic analysis of pyrimidine mutants of Salmonella typhimurium. Genetics. 1965 Sep;52(3):643–651. doi: 10.1093/genetics/52.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B. G., Hartman P. E. Sites of P22 and P221 prophage integration in Salmonella typhimurium. Virology. 1966 Feb;28(2):265–270. doi: 10.1016/0042-6822(66)90150-4. [DOI] [PubMed] [Google Scholar]
- ZINDER N. D. Hybrids of Escherichia and Salmonella. Science. 1960 Mar 18;131(3403):813–815. doi: 10.1126/science.131.3403.813. [DOI] [PubMed] [Google Scholar]
- ZINDER N. D. Sexuality and mating in salmonella. Science. 1960 Mar 25;131(3404):924–926. doi: 10.1126/science.131.3404.924. [DOI] [PubMed] [Google Scholar]



