Abstract
The eighteen incompatible medicaments is an important theory in traditional Chinese medicine. The theory suggests that drugs in the eighteen incompatible medicaments can be toxic when used together. Veratrum nigrum L. and Radix paeoniae alba belong to the eighteen incompatible medicaments and have been prohibited for thousands of years. This study offers preliminary insight into the mechanism and chemical constituents responsible for the incompatibility and toxicity of these two agents. Specifically, we performed toxicology studies to identify and quantify the constituent substances of the two agents. Experiments revealed that acute toxicity increases when the dose of V. nigrum L. is higher than, or equal to, RPA. UPLC-TOF-MS analysis showed that, although the volumes of V. nigrum L. were the same, the content of some veratrum alkaloids changed significantly and had a trend toward a highly positive correlation (|r| ≥ 0.8) with toxicity. This suggests that the increased toxicity of the V. nigrum L. and RPA combination was due mainly to increased content of the special veratrum alkaloids. The cytotoxicity of veratridine in SH-SY5Y cells was decreased with increasing paeoniflorin concentrations. This study provides insight into the mechanism behind the incompatibility theory of TCM.
1. Introduction
Traditional Chinese medicine (TCM) is an important part of Chinese culture and makes significant contributions to the prosperity and health of the Chinese population. TCM has become more popular worldwide because of its efficacy and curative effects. However, it is important to ensure that these treatments are safe. Confirming the compatibility of TCM is currently the major method used to ensure its safety and efficacy [1]. TCM has formed a unique incompatibility theory over thousands of years, and the typical principles of prescriptions include the eighteen incompatible medicaments. This means that these specific agents can be toxic when used in combination [2]. However, some studies have used combinations of drugs in the eighteen incompatible medicaments to treat incurable diseases [2]. Therefore, it is important to determine whether these agents are incompatible when used in combination, and the reasons behind any incompatibility.
In this study, we used Veratrum nigrum L. (V. nigrum L.) and Radix paeoniae alba (RPA), which are two agents belonging to the eighteen incompatible medicaments whose concurrent use has been prohibited for thousands of years. Although there are no cases describing the compatibility of V. nigrum L. and RPA in modern Chinese medicine, fewer than 20 prescriptions using a combination of V. nigrum L. and RPA have been described to treat incurable diseases such as tumors, hemorrhoids, carbuncle, and breast carbuncle [3]. Therefore, it is necessary to determine whether the use of V. nigrum L. and RPA in combination should be prohibited, as well as the reasons for any incompatibility [4].
V. nigrum L. is the dried roots and rhizomes of Veratrum nigrum L. It has been used for medicinal purposes for thousands of years in China and was used in Europe during the Middle Ages [5] despite its well-known poisonous characteristics [4]. It is used to treat hypertension, stroke, excessive phlegm, and epilepsy. However, aqueous extracts are toxic and irritate the digestive tract mucosa, nucleus nervi vagi, and central nervous system [6, 7].
RPA, the dried root of Paeonia lactiflora Pall without bark, has been used as a medicinal herb in traditional Chinese medicine for centuries and exerts a wide range of pharmacological activities. In ancient pharmacology, RPA was used to calm liver wind, relieve pain, nourish blood, regulate menstrual functions, and suppress sweating [8]. In modern pharmacology, RPA decoctions could be used to treat rheumatoid arthritis, systemic lupus erythematosus, hepatitis, dysmenorrhea, muscle cramping and spasms, and long-standing fever [4, 9, 10].
In this study, UPLC/TOF MS, multivariate statistical analysis, and typical metabolomics approach were used to identify the key chemical markers that are responsible for the increased toxicity and the combination of V. nigrum L. and RPA. In addition, cellular and mouse acute toxicity assays were used to identify the toxic effects of this combination. The compatibility of V. nigrum L. and RPA has not yet been validated by modern science; therefore, it is important to provide preliminary insight into the mechanism of their reported incompatibility, as well as to identify and quantify their toxic chemical constituents.
2. Materials and Methods
2.1. Chemicals and Materials
RPA from Panan County (Zhejiang, China; lot 100714) and V. nigrum L. from Huajia County (Changchun, China; lot 100701) were purchased from Anhui BBCA Tongling Chinese herbal medicine company. Formic acid (CNW Technologies GmbH) and acetonitrile (Fisher Scientific, Fair Lawn, NJ, USA) were both of chromatographic purity. Deionized water was prepared using a Millipore water purification system. Veratridine (D00114760) was purchased from Merck (Merck KGaA, Germany), and paeoniflorin was purchased from Sigma-Aldrich. The MTS cell proliferation assay kit was purchased from Promega. Roswell Park Memorial Institute 1640 (RPMI-1640) and fetal bovine serum (FBS) were purchased from Gibco BRL (Invitrogen, USA).
2.2. Preparation of Decoctions
Based on a previous study, the LD50 of aqueous extracts of V. nigrum L. and RPA after intragastric administration were 2.566 g/kg and 160 g/kg, respectively [11]. In this study, the dose of V. nigrum L. was fixed at 2.566 g/kg, and the RPA dose varied from 0.2566 to 25.66 g/kg.
The doses of V. nigrum L. and RPA in each of 12 groups are shown in Table 1. Each group was extracted using deionized water (700 mL) for 1 h during microboiling under reflux. The extracts were filtered through three layers of gauze, and the drug extraction was then repeated. The filtrates were combined and were then concentrated to 100 mL at 60−70°C under reduced pressure. Samples were then shaken, calibrated, and stored at 4°C.
Table 1.
Doses of V. nigrum L. and RPA in the 12 decoction groups.
| Group | V. nigrum L. dose (g) | RPA dose (g) | Water (mL) | Final volume (mL) | Drug concentration ratio (V. nigrum L.: RPA) |
|---|---|---|---|---|---|
| A | 6.415 | 0.6415 | 700 | 100 | 10 : 1 |
| B | 6.415 | 0.802 | 700 | 100 | 8 : 1 |
| C | 6.415 | 1.069 | 700 | 100 | 6 : 1 |
| D | 6.415 | 1.604 | 700 | 100 | 4 : 1 |
| E | 6.415 | 3.208 | 700 | 100 | 2 : 1 |
| F | 6.415 | 6.415 | 700 | 100 | 1 : 1 |
| G | 6.415 | 12.83 | 700 | 100 | 1 : 2 |
| H | 6.415 | 25.66 | 700 | 100 | 1 : 4 |
| I | 6.415 | 38.49 | 700 | 100 | 1 : 6 |
| J | 6.415 | 51.32 | 700 | 100 | 1 : 8 |
| K | 6.415 | 64.15 | 700 | 100 | 1 : 10 |
| L | 6.415 | 0 | 700 | 100 | — |
All decoctions were centrifuged at 13,000 rpm for 10 min using a Heraeus Labofuge 400R refrigerated centrifuge (Thermo Scientific, USA). The supernatants were then filtered through a 0.22 μM aqueous microporous membrane and were stored at 4°C for UPLC-TOF-MS analysis. Groups were prepared in triplicate [12, 13].
2.3. Animals and Experimental Design
Seven-week-old Kunming (KM) mice (18−22 g) were obtained from the Experimental Animal Center of the Academy of Military Medical Sciences with the certificate of conformity SCXK-(Army) 2007-004. Animal subjects were housed at the SPF animal center of the Academy of Military Medical Sciences (Beijing, China) according to the regulations of the animal care committee. Mice were housed at a constant temperature of 25 ± 1°C with 50 ± 20% humidity, with a 12 h light/dark cycle and 10–15 air changes per hour. Mice were allowed free access to water and food during the experimental period.
Mice were acclimatized to the facilities and environment for 3 days before the experiments. Two hundred and forty mice were randomized into 12 groups (A–L), with 10 mice per group. Food was removed from all animals 12 h before the experiments. Mice received decoctions at 0.4 mL/10 g via gavage and were observed for 14 days.
2.4. UPLC-MS
2.4.1. Liquid Chromatography
UPLC was performed on a Waters Acquity UPLC system (Waters, Milford, MA, USA) equipped with a binary solvent delivery system, an autosampler, and a photodiode-array detection (PDA) system. Chromatography was performed using a Waters ACQUITY BEHC18 column (100 mm × 2.1 mm, 1.7 μm) [12] The mobile phase consisted of (A) water containing 0.1% formic acid and (B) acetonitrile containing 0.1% formic acid. The eluting conditions were as follows: isocratic 2% B (0-1 min), linear gradients of 2−5% B (1-2 min), 5–20% B (2–5 min), 20–30% B (5–7 min), 30–33% B (7–10 min), 33–36% B (10–13 min), 36–40% B (13–17 min), 40–100% B (17-18 min), 100–2% B (18-19 min), and 2% B (19-20 min). The flow rate was 0.5 mL/min. The sample chamber was maintained at 4°C, with the column at 45°C. The injection volume was 5 μL [12, 14, 15].
2.4.2. Mass Spectrometry
Mass spectrometry was performed using a Waters SYNAPT mass spectrometer equipped with an electrospray ionization (ESI) source. Samples were injected twice: once in positive ESI mode and once in negative ESI mode. The data acquisition range was 100–1500 Da. The lock spray reference scan frequency was 20 s, with a reference cone voltage of 30 V. The MS source temperature was set at 100°C, and the desolvation temperature was 450°C with a gas flow of 900 L/h. The lock mass compound was leucine enkephalin (200 pg/μL), with an m/z of 556.2771 in the positive ion mode and 554.2615 in negative ion mode. The capillary voltages were set to 2.9 kV for ESI+ and 3 kV for ESI−. The cone voltage was 40 kV, and the collision energies were 6 V (trap) and 4 V (transfer), with 2.00 mL/min trap gas flow [16].
2.5. SH-SY5Y Cell Cultures and Cell Proliferation Assay
SH-SY5Y cells (ATCC, Manassas, USA) were cultured in RPMI-1640 supplemented with 10% FBS. Cells were maintained at 37°C in an incubator with a saturated humidity atmosphere of 95% air and 5% CO2. They were cultured in a 96-well plate and treated with different drug combinations. An MTS cell proliferation assay was performed, and the OD at 490 nm was read using a VICTOR X plate reader (Perkin Elmer, USA).
2.6. Data Analysis
The UPLC-TOF-MS data of all samples were analyzed using MassLynx4.1 software (Waters, Manchester, UK), and principal component analysis (PCA) was used for data analysis. Pearson correlation coefficients were used to identify relationships between the study parameters and mortality. The chemical markers in each group were identified using the V. nigrum L. and RPA chemical databases [12]. For data analyses, we compared the correlation between chemical composition and the acute toxicity of V. nigrum L. and RPA between the 12 groups. Experimental values are expressed as means ± standard deviations (SD). Statistical analyses were performed using two-tailed Student's t-tests. A value of P < 0.01 was considered statistically significant.
3. Results and Discussion
3.1. Mouse Acute Toxicity
Mice in the 12 groups were administered the decoctions by intragastric administration. Death occurred 5−10 min after administration. Toxicity was manifested predominantly as trembling, convulsions, and spasms. The major organs showed no obvious lesions at necropsy by the naked eye [6, 17, 18]. Mouse mortality is shown in Table 2.
Table 2.
Mice mortality after the intragastric administration of different proportion decoctions of V. nigrum L. and RPA.
| Group | V. nigrum L. dose (g) | RPA dose (g) | Crude drug concentration ratio | Female mortality | Male mortality | Total mortality |
|---|---|---|---|---|---|---|
| A | 6.415 | 0.6415 | 10 : 1 | 100% | 90% | 95% |
| B | 6.415 | 0.802 | 8 : 1 | 100% | 100% | 100% |
| C | 6.415 | 1.069 | 6 : 1 | 100% | 60% | 80% |
| D | 6.415 | 1.604 | 4 : 1 | 100% | 60% | 80% |
| E | 6.415 | 3.208 | 2 : 1 | 90% | 70% | 80% |
| F | 6.415 | 6.415 | 1 : 1 | 90% | 100% | 95% |
| G | 6.415 | 12.83 | 1 : 2 | 50% | 70% | 60% |
| H | 6.415 | 25.66 | 1 : 4 | 60% | 60% | 60% |
| I | 6.415 | 38.49 | 1 : 6 | 0 | 20% | 10% |
| J | 6.415 | 51.32 | 1 : 8 | 0 | 0 | 0 |
| K | 6.415 | 64.15 | 1 : 10 | 0 | 0 | 0 |
| L | 6.415 | 0 | 0 | 70% | 70% | 70% |
Group L (with a V. nigrum L. dose of the LD50 2.566 g/kg) had a mortality of 70%. Groups A−F were treated with the same dose of V. nigrum L., but the groups fed a lower ratio of RPA had a higher mortality than group L. Groups G−K had a higher proportion of RPA and lower mortality than group L, suggesting that toxicity was minimized. Groups B and F were the most toxic with mortalities of 100% and 95%, respectively. Overall analyses showed that mortality increased when the dose of V. nigrum L. was less than the dose of RPA. Conversely, toxicity decreased when the proportion of RPA increased. The trends in mortality are shown in Figure 1.
Figure 1.
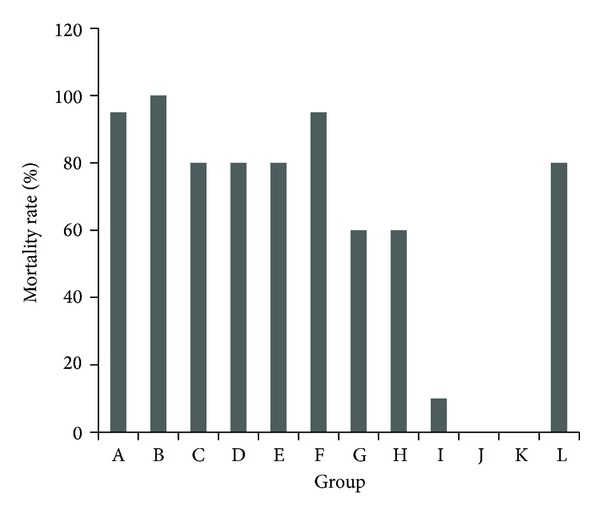
Total mortality of mice after the intragastric administration of decoctions containing different proportions of V. nigrum L. and RPA. The groups represent the combinations shown in Table 1.
Female mouse mortality decreased with increasing proportions of RPA, whereas males mortality exhibited two peaks at groups B and F, which both had 100% (Figure 2).
Figure 2.
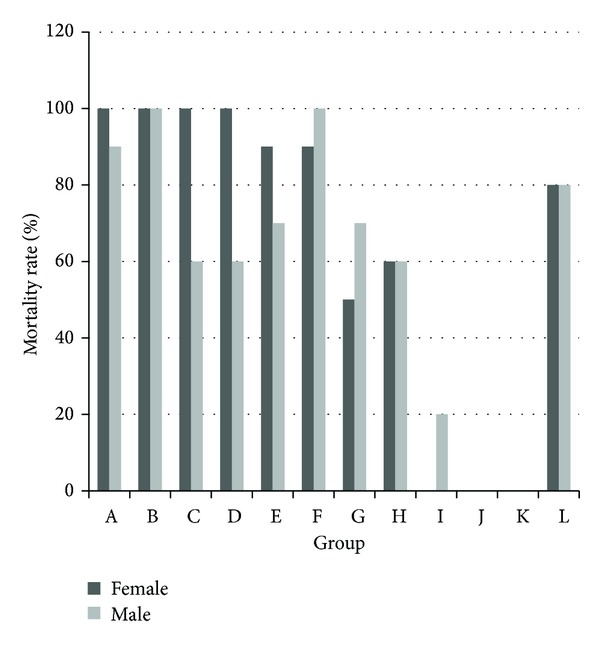
Mortality of male and female mice after the intragastric administration of decoctions containing different proportions of V. nigrum L. and RPA. The groups represent the combinations shown in Table 1.
3.2. Identification and Quantification of the Chemical Components of V. nigrum L. and RPA
To explain the cause of acute toxicity of the combined use of V. nigrum L. and RPA, we compared the chemical composition of the 12 groups using a supervised orthogonal partial least squared discriminant analysis (OPLS-DA). After Pareto scaling with mean centering, the data from both the positive and negative ion modes were displayed as scores plots (Figure 3). The scores plots clearly revealed that the samples were clustered into 12 groups; replicates of same group were comparable, but the 12 groups could be distinguished easily. This suggests that the changes in chemical compositions of the 12 groups were consistent, and the experiment was reproducible.
Figure 3.
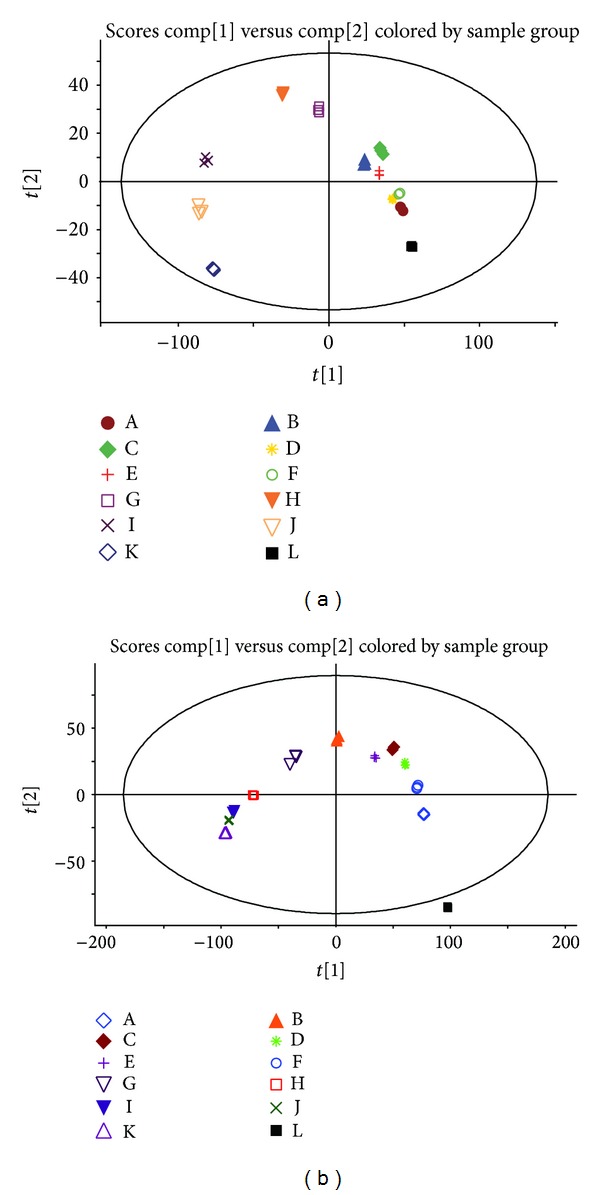
BBThe OPLS-DA score plot of 36 samples obtained using Pareto scaling with mean centering. (a) Positive ion mode. (b) Negative ion mode. The same score plots represent the same group. Both the positive and negative ion modes showed that the samples were clearly clustered into 12 groups. Replicates of the 12 groups could be distinguished easily.
UPLC-TOF-MS is a rapid, specific, and sensitive method used to identify and quantify individual components. Representative BPI chromatograms of the 36 samples in the positive and negative mode ESI are shown in Figure 4.
Figure 4.
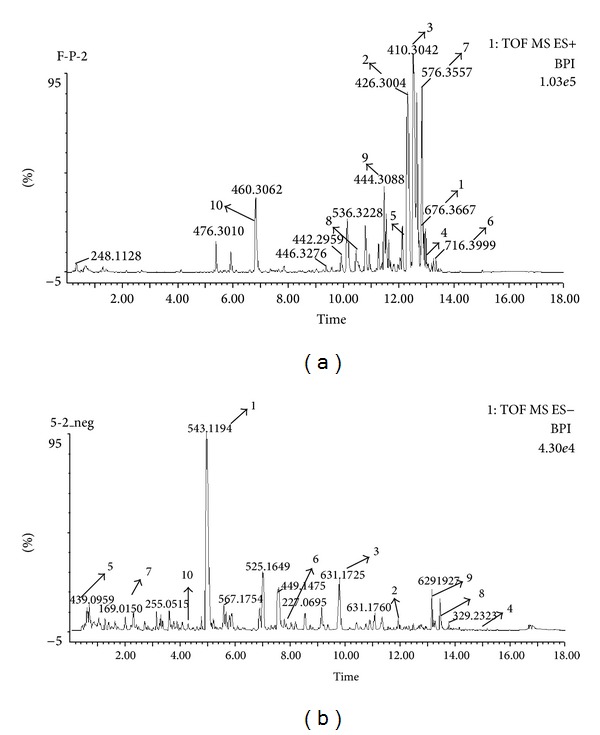
Representative BPI chromatograms. (a) Positive ion mode. (b) Negative ion mode. Some of identified constituents (1–10) are labeled in the BPI chromatogram, and the numbers correspond to Tables 3 and 4.
The contents of some special alkaloids change significantly in different proportion decoctions. To determine which constituents contributed to the differences in toxicity observed with the 12 decoctions, statistical analyses were performed using Pearson's correlation, and the correlation coefficient (r) described the degree of linear correlation between two variables: total mouse mortality and the chemical composition of the 12 decoctions. The r values were between −1 and +1; r > 0 indicates that two variables were positively correlated, whereas r < 0 indicates a negative correlation. The larger the absolute value, the higher the correlation; therefore we defined r = 0.90–1.00 as extremely related and r = 0.80–0.89 as highly related. We extracted the relevant chemical component data when r ≥ 0.8 and identified the individual components using the RPA and V. nigrum L. chemical composition databases.
According to Pearson's correlation coefficient, 131 compositions had an extremely positive relationship (r ≥ 0.9) with toxicity. However, only 14 of these could be identified and all came from V. nigrum L. In addition, 35 components were highly positively correlated (r ≥ 0.8) to acute toxicity, and these constituents contributed most of the toxicity identified in the 12 decoctions. Therefore, we hypothesized that these components might play important roles in the acute toxicity of this drug combination. We identified that these compounds were all veratrum alkaloids that were detected in each sample in various ionized forms. The chemical components with r ≥ 0.8 are shown in Table 3 [7, 19–24].
Table 3.
The chemical components that were highly positively correlated with toxicity (r ≥ 0.8).
| Number | r | tR (min) | Assigned identity | Molecular formula | Mean measured mass (Da) | Theoretical exact mass (Da) | Mass accuracy (ppm) |
|---|---|---|---|---|---|---|---|
| 1 | 0.9779 | 12.98 | 3-Veratroylgermine | C36H53NO11 | 676.3667 | 675.8061 | 0.1805 |
| 2 | 0.9661 | 12.39 | Jervine | C27H39NO3 | 426.3004 | 425.6035 | 2.3624 |
| 3 | 0.9589 | 12.61 | Veretramine | C27H39NO2 | 410.3042 | 409.6041 | 2.9751 |
| 4 | 0.9581 | 13.43 | Germanitrine | C39H59NO11 | 718.4169 | 717.8859 | 2.5015 |
| 5 | 0.9402 | 12.20 | Germidine | C34H53NO10 | 636.3412 | 635.7853 | 0.5755 |
| 6 | 0.9393 | 13.40 | Germerine | C37H59NO11 | 716.3999 | 693.8645 | 0.3408 |
| 7 | 0.9390 | 12.91 | 3-Angeloylzygadenine | C32H49NO8 | 576.3557 | 575.7334 | 1.2708 |
| 8 | 0.9311 | 10.35 | 11-Deoxojervine | C27H41NO2 | 412.3189 | 411.6199 | 6.4392 |
| 9 | 0.9240 | 1.57 | 1β,3α-Dihydroxy-5β-jervanin-12-en-11-one | C27H41NO4 | 444.3088 | 443.6187 | 2.4040 |
| 10 | 0.9237 | 6.96 | Jervinone | C27H37NO3 | 424.2860 | 423.5876 | 2.0140 |
| 11 | 0.9182 | 11.25 | Neogermbudine | C37H59NO12 | 732.3971 | 709.8639 | 4.9169 |
| 12 | 0.9143 | 4.25 | Germine | C27H43NO8 | 510.3059 | 509.6322 | 1.5549 |
| 13 | 0.9131 | 4.18 | Neogermine, Veramanine | C27H43NO5 | 462.3213 | 461.6340 | 1.4522 |
| 14 | 0.9034 | 11.58 | Veraline B | C27H45NO3 | 432.3465 | 431.6511 | 2.9646 |
| 15 | 0.8978 | 11.16 | Verdine | C27H41NO5 | 460.3056 | 459.6181 | 1.5249 |
| 16 | 0.8903 | 11.63 | Stenophylline A | C36H51NO11 | 674.3474 | 673.7902 | 9.8655 |
| 17 | 0.8870 | 10.35 | Germitrine | C39H61NO12 | 736.4278 | 735.9011 | 0.8288 |
| 18 | 0.8805 | 9.14 | Polydatin | C20H22O8 | 429.0937 | 390.3839 | 3.4849 |
| 19 | 0.8738 | 10.81 | Cervdine | C32H49NO9 | 592.3498 | 591.7328 | 2.0608 |
| 20 | 0.8675 | 12.34 | 3-Veratrum acyl protoveratrine | C36H51NO12 | 690.3482 | 689.7896 | 1.0609 |
| 21 | 0.8671 | 12.89 | Zygadenitic acid δ-lactone-16-angelate | C32H47NO8 | 574.3408 | 573.7175 | 4.8884 |
| 22 | 0.8652 | 12.32 | Maackinine | C39H59NO11 | 718.4171 | 717.8859 | 0.6797 |
| 23 | 0.8641 | 12.38 | Rubijervine; rubivirine; etioline; epirubijervine; Isorubijervine | C27H43NO2 | 414.3357 | 413.6358 | 3.6827 |
| 24 | 0.8639 | 10.49 | Solanidine | C27H43NO3 | 430.3308 | 429.6352 | 3.0494 |
| 25 | 0.8492 | 10.57 | Pseudojervine | C32H49NO8 | 588.3525 | 587.7441 | 1.9710 |
| 26 | 0.8444 | 11.81 | Neojerminalanine | C39H61NO13 | 752.4244 | 751.9005 | 3.0014 |
| 27 | 0.8379 | 8.43 | Zygacine | C29H45NO8 | 536.3223 | 535.6695 | 0.0000 |
| 28 | 0.8358 | 13.20 | Neoverataline B | C27H42N2O9 | 556.3260 | 538.6304 | 4.079 |
| 29 | 0.8201 | 12.80 | 3-Veratroylzygadenine | C36H51NO10 | 696.3187 | 657.7908 | 5.3469 |
| 30 | 0.8176 | 5.83 | Zygadenine | C27H43NO7 | 494.3110 | 493.6328 | 1.5434 |
| 31 | 0.8176 | 0.54 | γ-Aminobutyric acid | C4H9NO2 | 246.0978 | 103.1198 | 1.6121 |
| 32 | 0.8160 | 12.73 | Veratrosine | C33H49NO7 | 616.3528 | 571.7447 | 6.8329 |
| 33 | 0.8128 | 13.22 | Stenophylline A | C37H55NO10 | 674.3901 | 673.8333 | 0.5430 |
| 34 | 0.8119 | 13.34 | 7-Acetyl-15-methylbutyryl-3- veratroylgermine | C43H61NO13 | 838.3791 | 799.9433 | 1.3104 |
| 35 | 0.8017 | 12.13 | Vanilloylzygadenine | C35H49NO10 | 644.3444 | 643.7643 | 1.5156 |
3-Veratroylgermine, jervine, veratramine, germanitrine, germidine, and germerine were the main chemical components that were associated with acute toxicity, with Pearson's correlation coefficients of 0.9779, 0.9661, 0.9589, 0.9581, 0.9402, and 0.9393, respectively. These compounds are all veratrum alkaloids, and the changes in their content are shown in Figure 5. The other 29 components that were highly positively correlated (r ≥ 0.8) with acute toxicity are shown in Figure 6.
Figure 5.
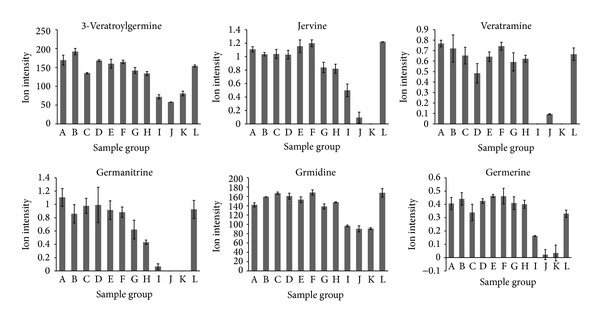
Ion intensity trend plots for 3-veratroylgermine, jervine, veratramine, germanitrine, germidine, and germerine, which were highly positively correlated (r ≥ 0.9393) with toxicity.
Figure 6.
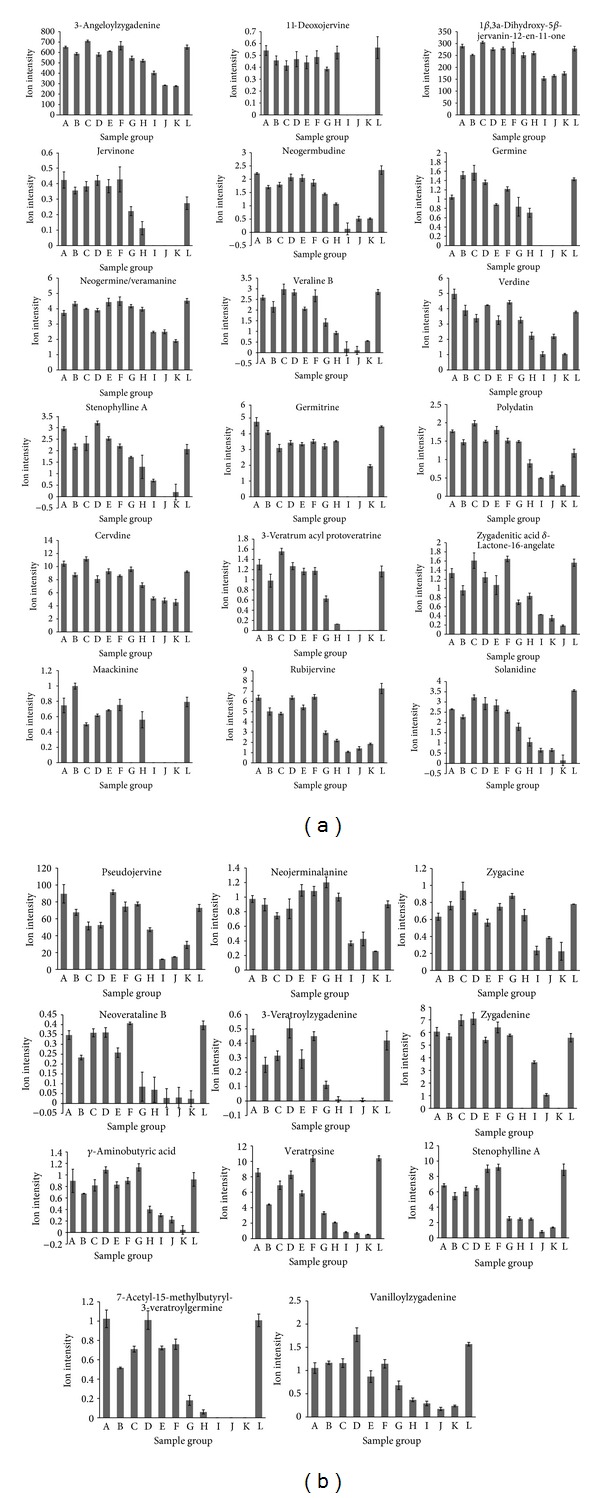
Ion intensity trend plots for the remaining 29 chemical components that were highly positively correlated (r ≥ 0.8) with toxicity.
Interestingly, Pearson's correlation coefficient identified 24 RPA components that were extremely negatively correlated (r ≤ −0.9) with toxicity. The chemical components with r ≤ −0.9 are shown in Table 4 [25–33].
Table 4.
The chemical components that were highly negatively correlated with toxicity (r ≤ −0.9).
| Number | r | tR (min) | Assigned identity | Molecular formula | Mean measured mass (Da) | Theoretical exact mass (Da) | Mass accuracy (ppm) |
|---|---|---|---|---|---|---|---|
| 1 | −0.9811 | 4.95 | Paeoniflorinsulfonate | C23H28O13S | 543.1194 | 544.5256 | 3.3714 |
| 2 | −0.9799 | 12.20 | Benzoyloxy paeoniflorin | C30H32O13 | 599.1785 | 600.1843 | 0.3046 |
| 3 | −0.9793 | 9.77 | Galloyl paeoniflorin | C30H32O15 | 631.1725 | 632.1741 | 0.3856 |
| 4 | −0.9737 | 14.79 | Paeonilacto-ne B | C17H18O6 | 317.1750 | 318.1103 | 3.9209 |
| 5 | −0.9736 | 0.56 | Paeonilacto-ne C | C10H12O4 | 195.0496 | 196.0736 | 5.3192 |
| 6 | −0.9734 | 8.09 | Tetragalloyglucose | C34H28O22 | 787.1008 | 788.5729 | 5.6607 |
| 7 | −0.9728 | 2.30 | Vanillic acid | C7H6O5 | 169.0161 | 170.1195 | 3.7471 |
| 8 | −0.9716 | 13.91 | Desbenzoyl paeoniflorin | C16H24O10 | 375.1868 | 376.1369 | 4.4505 |
| 9 | −0.9708 | 13.52 | Benzoyl paeoniflorin | C30H32O12 | 583.1848 | 584.5679 | 0.6258 |
| 10 | −0.9684 | 4.15 | 6-O-β-D-Glucopyranosyllactinolide | C16H26O9 | 361.1537 | 362.3722 | 9.3435 |
| 11 | −0.9670 | 10.37 | Paeoniflorin; albiflorin R1; albiflorin; mudanpioside I | C23H28O11 | 519.1261 | 480.4618 | 1.5284 |
| 12 | −0.9655 | 11.67 | Gallic acid | C9H10O2 | 151.0750 | 150.1745 | 5.9590 |
| 13 | −0.9640 | 4.93 | Paeonol | C9H10O3 | 165.0545 | 166.1739 | 4.0676 |
| 14 | −0.9625 | 11.67 | Lactiflorin | C23H26O10 | 480.1872 | 462.4465 | 0.4449 |
| 15 | −0.9609 | 6.67 | Isomaltopaeoniflorin | C29H38O16 | 643.2221 | 642.6024 | 2.6569 |
| 16 | −0.9589 | 8.52 | Albiflorin | C23H27O11 | 957.3118 | 479.4539 | 9.3723 |
| 17 | −0.9582 | 6.79 | 1′-O-Benzoylsucrose | C19H26O12 | 464.1757 | 446.4025 | 2.3668 |
| 18 | −0.9567 | 10.57 | Pentagalloylglucose | C41H32O26 | 939.1166 | 940.6772 | 6.6292 |
| 19 | −0.9562 | 8.02 | Oxypaeoniflorin | C23H28O12 | 514.1934 | 496.4612 | 1.8992 |
| 20 | −0.9544 | 11.58 | Ethyl gallate | C9H10O5 | 221.0433 | 198.1727 | 3.2445 |
| 21 | −0.9478 | 10.11 | Eugeniin | C41H30O26 | 937.1010 | 938.6613 | 6.7086 |
| 22 | −0.9395 | 5.85 | Paeonilactinone | C9H14O2 | 153.0904 | 154.2063 | 7.5750 |
| 23 | −0.9352 | 1.13 | Lactinolide | C10H16O4 | 201.1111 | 200.2316 | 7.8907 |
| 24 | −0.9173 | 13.73 | Palbinone | C22H30O4 | 357.2047 | 358.4712 | 5.2115 |
The concentrations of these components increased with increasing amounts of RPA. Paeoniflorin sulfonate, benzoyloxy paeoniflorin, galloyl paeoniflorin, paeonilacto-neB, paeonilacto-neC, and tetragalloyglucose were the major RPA components that were identified, with Pearson's correlation coefficients of −0.9811, −0.9799, −0.9793, −0.9737, −0.9736, and −0.9734, respectively. The changes in content of the 24 components that were highly negatively correlated (r ≤ −0.9) with acute toxicity are shown in Figure 7.
Figure 7.
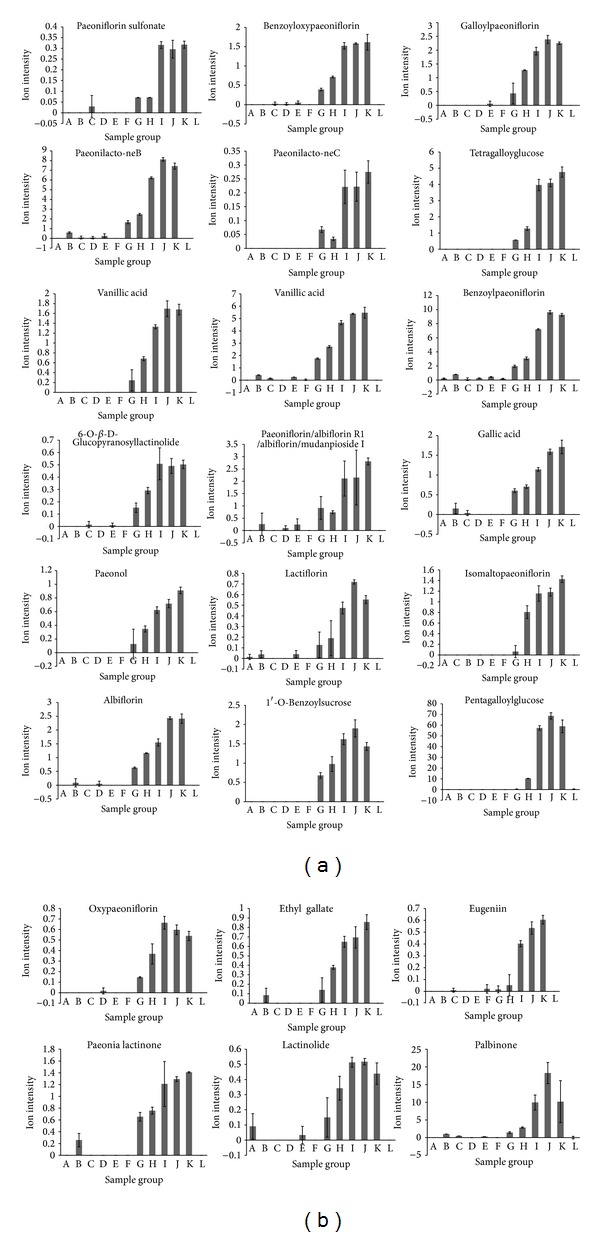
Ion intensity trend plots for the chemical components that were highly negatively correlated with toxicity (r ≥ 0.9).
3.3. Cell Proliferation Assay
To further validate the toxicity of the combination of V. nigrum L. and RPA on neurons, we used veratridine and paeoniflorin, the major pharmacologically active components of V. nigrum L. and peony for in vitro experiments. The effect of these two chemical components on the viability of SH-SY5Y cells was tested using MTS assays. As shown in Figures 8(a) and 8(b), veratridine had an LD50 of 450, whereas paeoniflorin exerted no significant toxicity at doses of 0–2000 μM. Therefore, the dose of veratridine was fixed at 400 μM, and the RPA dose was varied from 50 to 2000 μM. SH-SY5Y cell cytotoxicity was increased when the concentration of paeoniflorin was decreased in the combination (Figure 8(c)). Therefore, paeoniflorin could offset the cytotoxicity caused by veratridine in SH-SY5Y cells [25].
Figure 8.
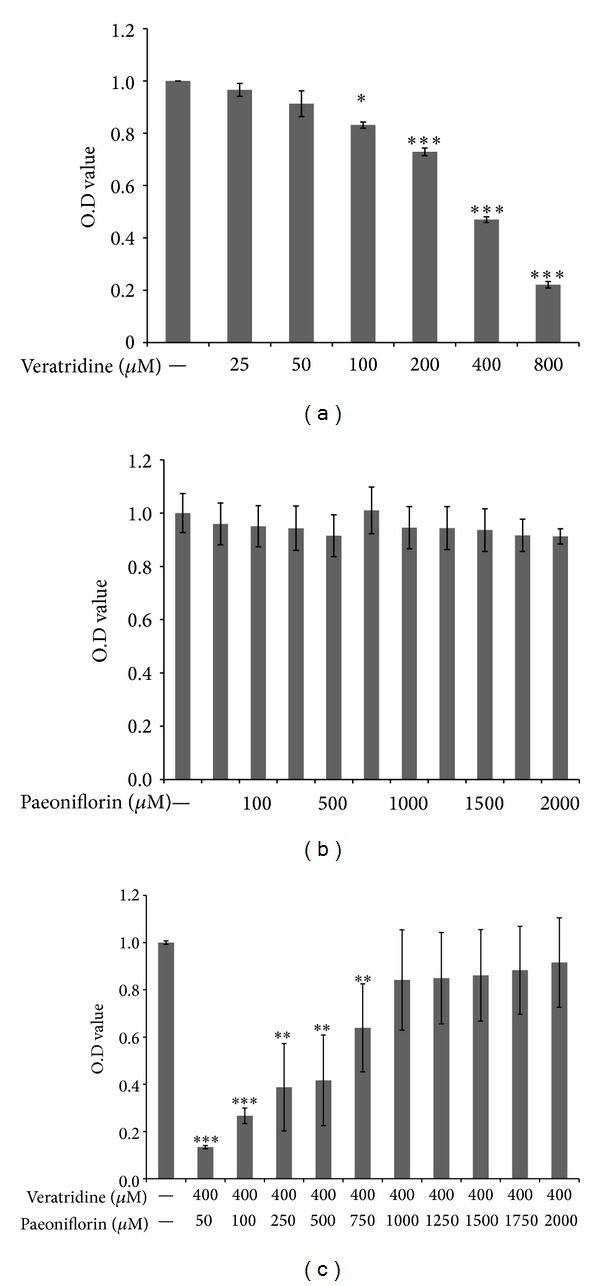
SH-SY5Y cell viability: SH-SY5Y cells were cultured with (a) veratridine (25–800 μM), (b) paeoniflorin (50−2000 μM), and (c) 400 μM veratridine in combination with 50−2000 μM paeoniflorin for 24 h. Cell viability was then assessed using MTS assays. Data are presented as mean ± S.D. Means of three independent experiments, Asterisks indicate a statistically significant difference compared with untreated cells. *P < 0.05; **P < 0.01; ***P < 0.001 versus controL.
4. Discussion
The eighteen incompatible medicaments (Shi Ba Fan) is a well-known traditional Chinese medicine (TCM) theory. It basically listed eighteen pairs of TCM herbal medicine that are not to be used in combination as they may cause fatal consequences. This principal has been used as guidance for general TCM practices for hundreds of years with little chemical comprehension. In this work, for the first time, we used the UPLC-TOF MS coupled with multivariate statistical analysis in an effort to gain scientific understanding for the causes of the incompatibility.
We previously reported that the LD50 of aqueous extracts of V. nigrum L. and RPA were 2.566 g/kg and 160 g/kg, respectively, after intragastric administration [11]. V. nigrum L. extract was severely toxic, whereas RPA had minimal toxicity. Although the amount of V. nigrum L. in each group was unchanged, the mortality changed significantly with altered ratios of V. nigrum L. to RPA. When the doses of V. nigrum L. were equal to or higher than RPA, particularly ratios of 8 : 1 and 1 : 1, mouse mortality increased significantly. Therefore, components of V. nigrum L. were the major causative factors of toxicity. In addition, RPA modulated the toxicity of V. nigrum L. Therefore, clinical applications should be monitored carefully.
Mice died 5−10 min after drug administration. Toxicity manifested predominantly as trembling, convulsions, and spasms, but the major organs showed no obvious lesions at necropsy by the naked eye. Based on the toxic reactions and the time of death in the current study and according to the guidelines for Chinese natural medicine acute toxicity testing, we speculate that the toxicity was caused predominantly by suppression of the central nervous system and that V. nigrum L. was the major cause of death. V. nigrum L. water extracts exert toxic effects on the digestive tract mucosa, nucleus nervi vagi, and central nervous system [6, 17, 18]. Pharmacological studies of veratrum alkaloids have been performed previously and reported that these alkaloids stimulate the central nervous system and inhibit the brain. This results in spasms, convulsions, coma, drowsiness, and other symptoms of consciousness in animals, which is consistent with the symptoms of the mice in the current study [6, 17].
Veratrum alkaloid is the toxic component of V. nigrum L. We previously investigated the differently expressed genes in SH-SY5Y cells treated with veratridine using cDNA microarrays [34]. Data revealed that veratridine treatment altered the expression of MMP, caused Ca2+ concentration overload, increased reactive oxygen species production, enhanced LDH release, damaged the cell membrane, reduced SH-SY5Y cell viability, and induced apoptosis. Under oxidizing conditions, the MAPK signaling pathway is activated, which increases apoptosis. This study illustrates the neurotoxicity of the combination of V. nigrum L. and reveals the molecular mechanism behind the neurotoxic effects of V. nigrum L. (data not shown).
The amount of V. nigrum L. in each group was the same; the dose of RPA is in ascending order, and the chemical components of RPA are also increased, but the mortality of each group changed significantly with the increase of RPA; so we conclude that higher amount of RPA can have protection effect, which can offset the toxicity of combination of V. nigrum L. and RPA. And pharmacological studies have demonstrated that RPA exerts a wide range of pharmacological activities, including analgesic, sedative, anticonvulsant, and antispasmodic effects on the central nervous system. RPA could also inhibit writhing reactions and antagonize pentylenetetrazol-induced convulsions. The most important active ingredient in RPA is paeoniflorin, which has significant neuroprotective effects [26–34].
In this study, mouse acute toxicity experiments revealed that the mortality of mice changed significantly when the dose of V. nigrum L. was higher than or equal to that of RPA, particularly at ratios of V. nigrum L.: RPA of 8 : 1 and 1 : 1. UPLC-Q-TOF/MS with automated data analysis used to identify and quantify the specific chemical components. According to Pearson's correlation coefficient, most of the chemical components that were positively correlated with toxicity were from V. nigrum L. We conclude that the major veratrum alkaloids of V. nigrum L. caused acute toxicity and death in mice. When the doses of V. nigrum L. were higher than or equal to RPA, the main chemical components of V. nigrum L. and the toxicity of the decoctions were increased. This suggests that V. nigrum L. and RPA have opposing roles. Additional experiments revealed that the cytotoxicity of veratridine in SH-SY5Y cells was decreased with increasing concentrations of paeoniflorin. Therefore, the clinical use of these agents should be considered carefully. This study also provides information to help improve the incompatibility theory of TCM and introduces novel ideas for further studies on the development and application of TCM.
When preparing V. nigrum L. and RPA decoctions, a lower amount of RPA contributes to the dissolution of veratrum alkaloids. Conversely, higher amounts of RPA can exert neuroprotective effects, which can offset the toxicity caused by the combination of V. nigrum L. and RPA. Under these conditions, dissolution is the most important factor for V. nigrum L. toxicity. The results of cell-based experiments also demonstrated that paeoniflorin could offset the neurotoxic effects of veratridine when treated in combination, which excluded the factor of dissolution in preparation.
Acknowledgments
The authors are grateful for financial support from National Nature Science Foundation of China (Grant no. 81073149), National Basic Research Program of China (Grant nos. 2011CB505304 and 2012CB518402), and Natural Science Foundation of Beijing (Grant no. 7112110).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Guo H, Li W, Wang XY, Fan GW. Advance of research on toxic attenuation by compatibility of traditional Chinese medicine prescriptions. Zhongguo Zhongyao Zazhi. 2012;37(1):120–123. [PubMed] [Google Scholar]
- 2.Zhang Y, Hua H, Fan X, Wang C, Duan J. Exploration on eighteen incompatible medicaments of chest pain prescriptions based on association rules mining. Zhongguo Zhongyao Zazhi. 2011;36(24):3544–3547. [PubMed] [Google Scholar]
- 3.Xu C-H, Wang P, Wang Y, et al. Pharmacokinetic comparisons of two different combinations of Shaoyao-Gancao Decoction in rats: competing mechanisms between paeoniflorin and glycyrrhetinic acid. Journal of Ethnopharmacology. 2013;149(2):443–452. doi: 10.1016/j.jep.2013.06.049. [DOI] [PubMed] [Google Scholar]
- 4.Tian DH. Dictionary of Practical Traditional Chinese Medicine II. Beijing, China: People’s Medical; 2002. [Google Scholar]
- 5.Gaillard Y, Pepin G. LC-EI-MS determination of veratridine and cevadine in two fatal cases of Veratrum album poisoning. Journal of Analytical Toxicology. 2001;25(6):481–485. doi: 10.1093/jat/25.6.481. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Gao G-Y, Li S-Y, Zhao W-J, Guo Y-T, Liang Z-J. Effects of Veratrum nigrum alkaloids on central catecholaminergic neurons of renal hypertensive rats. Acta Pharmacologica Sinica. 2000;21(1):23–28. [PubMed] [Google Scholar]
- 7.Wang L, Li W, Liu Y. Hypotensive effect and toxicology of total alkaloids and veratramine from roots and rhizomes of Veratrum nigrum L. in spontaneously hypertensive rats. Pharmazie. 2008;63(8):606–610. [PubMed] [Google Scholar]
- 8.Jiang YP, Liu YG, Chen HC. Effect of aqueous extract of Radix Paeoniae Rubra against Carbon Tetrachloride induced liver fibrosis in rat. Herald of Medicine. 2004;23:527–529. [Google Scholar]
- 9.He D-Y, Dai S-M. Anti-inflammatory and immunomodulatory effects of Paeonia lactiflora Pall., a traditional Chinese herbal medicine. Frontiers in Pharmacology. 2011 doi: 10.3389/fphar.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng C, Liu M, Shi X, et al. Pharmacokinetic properties of paeoniflorin, albiflorin and oxypaeoniflorin after oral gavage of extracts of Radix Paeoniae Rubra and Radix Paeoniae Alba in rats. Journal of Ethnopharmacology. 2010;130(2):407–413. doi: 10.1016/j.jep.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 11.Zhang XX, Wang YG, Ma ZC, Gao Y, Xiao CR. Uniform designed research on the compatibility toxicity of Veratridine and peony. Zhong Hua Zhong Yi Yao Za Zhi. 2013;28(10):2901–2904. [Google Scholar]
- 12.Liang QD, Gao Y, Ma J, et al. Chemical comparison of dried rehmannia root and prepared rehmannia root by UPLC-TOF MS and HPLC-ELSD with multivariate statistical analysis. Acta Pharmaceutica Sinica B. 2013;3(1):55–64. [Google Scholar]
- 13.Wang Y, He S, Cheng X, Lu Y, Zou Y, Zhang Q. UPLC-Q-TOF-MS/MS fingerprinting of traditional Chinese formula SiJunZiTang. Journal of Pharmaceutical and Biomedical Analysis. 2013;80:24–33. doi: 10.1016/j.jpba.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Kang L-P, Yu K, Zhao Y, et al. Characterization of steroidal glycosides from the extract of Paris Polyphylla var. Yunnanensis by UPLC/Q-TOF MSE. Journal of Pharmaceutical and Biomedical Analysis. 2012;62:235–249. doi: 10.1016/j.jpba.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann A, Butcher P, Maden K, Widmer M. Ultra-performance liquid chromatography coupled to time of flight mass spectrometry (UPLC-TOF): a novel tool for multiresidue screening of veterinary drugs in urine. Analytica Chimica Acta. 2007;586(1-2):13–21. doi: 10.1016/j.aca.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Zhou S, Ma ZC, Liang QD, et al. UPLC/Q-TOF-MS based chemical profiling approach to evaluate chemical composition of augmentation toxicity in combination of Radix aconiti and Pinellia praeparata . Acta Chimica Sinica. 2012;70(3):284–290. [Google Scholar]
- 17.Lin N, Gao X. Experimental observation on the absorption of Veratrum nigrum L. in the upper digestive tract. Zhongguo Zhong Yao Za Zhi. 1992;17(1):43–45. [PubMed] [Google Scholar]
- 18.Wang Z-Z, Zhao W-J, Zhang X-S, et al. Protection of Veratrum nigrum L. var. ussuriense Nakai alkaloids against ischema-reperfusion injury of the rat liver. World Journal of Gastroenterology. 2007;13(4):564–571. doi: 10.3748/wjg.v13.i4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christov V, Mikhova B, Ivanova A, et al. Steroidal alkaloids of Veratrum lobelianum Bernh. and Veratrum nigrum L. Zeitschrift fur Naturforschung; C Journal of Biosciences. 2010;65(3-4):195–200. doi: 10.1515/znc-2010-3-405. [DOI] [PubMed] [Google Scholar]
- 20.Christov V, Mikhova B, Selenge D. (-)-Veranigrine, a new steroidal alkaloid from Veratrum nigrum L. Fitoterapia. 2009;80(1):25–27. doi: 10.1016/j.fitote.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Cong Y, Wang J-H, Wang R, Zeng Y-M, Liu C-D, Li X. A study on the chemical constituents of Veratrum nigrum L. processed by rice vinegar. Journal of Asian Natural Products Research. 2008;10(7-8):619–624. doi: 10.1080/10286020802133266. [DOI] [PubMed] [Google Scholar]
- 22.Li H-L, Tang J, Liu R-H, Zhang C, Zhang W-D. Two new flavanone glycosides from Veratrum nigrum L. Natural Product Research. 2009;23(2):122–126. doi: 10.1080/14786410801887664. [DOI] [PubMed] [Google Scholar]
- 23.Cong Y, Zhou Y-B, Chen J, Zeng Y-M, Wang J-H. Alkaloid profiling of crude and processed Veratrum nigrum L. through simultaneous determination of ten steroidal alkaloids by HPLC-ELSD. Journal of Pharmaceutical and Biomedical Analysis. 2008;48(3):573–578. doi: 10.1016/j.jpba.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Wang L-S, Zhao D-Q, Tao H-M, Liu Y-H. Determination of vetatramine in Veratrum nigrum by HPLC-ELSD. Zhongguo Zhongyao Zazhi. 2008;33(7):791–792. [PubMed] [Google Scholar]
- 25.Wang Q, Guo H-Z, Huo C-H, et al. Chemical constituents in root of Paeonia lactiflora. Chinese Traditional and Herbal Drugs. 2007;38(7):972–976. [Google Scholar]
- 26.Tan J-J, Zhao Q-C, Yang L, Shang Z-P, Du Z-Q, Yan M. Chemical constituents in roots of Paeonia lactiflora. Chinese Traditional and Herbal Drugs. 2010;41(8):1245–1248. [Google Scholar]
- 27.Zhang Q, Wang ZZ, Xiao W, et al. UPLC characteristic chromatographic profile of Paeoniae Radix Alba. Zhongguo Zhongyao Zazhi. 2011;36(6):712–714. [PubMed] [Google Scholar]
- 28.Gao XR, Tian Y. Active principles of Paeonia lactiflora Pall. Chinese Journal of New Drugs. 2006;15:416–418. [Google Scholar]
- 29.Luo N-C, Ding W, Wu J, et al. UPLC-Q-TOF/MS coupled with multivariate statistical analysis as a powerful technique for rapidly exploring potential chemical markers to differentiate between radix paeoniae alba and radix paeoniae rubra. Natural Product Communications. 2013;8(4):487–491. [PubMed] [Google Scholar]
- 30.Zhao WK, Guo Y, Yasuhiro T, Tohru K. Isolation and structure determination of echinuline from Veratrum nigrum L. var. ussuriense Nakai. Zhongguo Zhong Yao Za Zhi. 1991;16(7):425–426. [PubMed] [Google Scholar]
- 31.Zhao W, Guo Y, Yasuhirb T, Kikuchi T. Isolation and structure determination of aurantiamide acetate from Veratrum nigrum L.var.ussuriense Nakai. Zhongguo Zhongyao Zazhi. 1998;23(1):p. 41. [PubMed] [Google Scholar]
- 32.Yang L, Xia XH, Zhu Q, Tan XP. Study on the rule of influence of two purification methods on the chemical compositions in aqueous solution of paeoniae radix alba. Zhong Yao Cai. 2012;36:118–121. [PubMed] [Google Scholar]
- 33.Zang XY, Li X. Development of the study on chemical constituentsof Paeonia lact if lora Pall. Journal of Shenyang Pharmaceutical University. 2002;19:70–73. [Google Scholar]
- 34.Wang Y-L, Wang Y-G, Liang Q-D, et al. Using cDNA microarray to screen differently expressed genes in SH-SY5Y treated by veratridine. Chinese Pharmacological Bulletin. 2013;29(5):643–647. [Google Scholar]


