Abstract
The Ron receptor tyrosine kinase plays a regulatory role in the inflammatory response to acute lung injury induced by intranasal administration of bacterial lipopolysaccharide (LPS). Previously, we have showen that mice with a targeted deletion of the tyrosine kinase (TK) signaling domain of the Ron receptor exhibited more severe lung injury in response to intranasal LPS administration as evidenced by increased leakage of albumin in the lungs and a greater thickening of the alveolar septae compared to wild-type mice. In addition, lung injury in the Ron TK deficient (TK−/−) mice was associated with increased activation of the transcription factor, nuclear factor kappaB (NF-κB) and significantly increased intrapulmonary expression of tumor necrosis factor alpha (TNFα). TNFα, a multifunctional pro-inflammatory cytokine, is a central mediator in several disease states including rheumatoid arthritis and sepsis. Based on the observation that TNFα production is increased in the Ron TK−/− mice and that macrophages are a major source of this cytokine, we hypothesized that the alterations observed in Ron TK−/− mice may be due, in part, to Ron signaling specifically in alveolar macrophages. To test this hypothesis, wild-type and Ron TK−/− primary alveolar macrophages and the murine alveolar macrophage cell line, MH-S, were used to examine the effects of Ron activation on LPS-induced TNFα production and NK-κB activity. Here, we report that Ron is expressed on alveolar macrophages and MH-S cells. Activation of Ron by its ligand, hepatocyte growth factor-like protein (HGFL), decreases TNFα production in alveolar macrophages following LPS challenge. Decreased TNFα is associated with HGFL-induced decreases in NF-κB activation and increases in the NF-κB inhibitory protein, IκB. We also provide the first evidence for Ron as a negative regulator of Adam17, the metalloprotease involved in TNFα processing. . These results indicate that Ron plays a critical role in regulation of alveolar macrophage signaling, and validates this receptor as a target in TNFα-mediated pulmonary pathologies.
Keywords: Inflammation, cell-signaling, cytokines, acute lung injury, LPS
INTRODUCTION
The usage of animal models of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) led to the discovery that macrophage activation triggered by the “early response cytokines” TNFα and IL-1, initiates the inflammatory cascade, which may perpetuate lung injury [1]. These cytokines initiate alterations in adhesion molecules and chemokine production resulting in the activation of macrophages and an influx of neutrophils, which are thought to contribute to lung injury through the generation of oxidants and the release of proteases [2–4]. Given that ALI/ARDS results in such a high degree of patient morbidity and may occur secondary to an initial systemic insult, the identification and potential modulation of key regulatory components involved in this inflammatory response is highly desirable [5, 6]. The identification of TNFα as a pro-inflammatory host tissue damaging cytokine has been well established both experimentally in animal models of disease and clinically, as a molecular therapeutic target in human disease [7, 8]. Differential regulation of TNFα in various disease states has been attributed to a wide assortment of control mechanisms including regulation resulting from promote–specific polymorphisms and in response to increased levels of the TNFα ectodomain shedding protease Adam17 [9]. The identification of a regulatory paradigm for TNFα in acute lung injury, however, remains poorly understood.
Ron, a member of the Met family of receptor tyrosine kinases, is widely expressed in epithelial cells and has been found in select macrophage populations [10–13]. The ligand for Ron is hepatocyte growth factor-like protein, HGFL, which is secreted from hepatocytes into the circulation as an inactive precursor . The active form of the ligand is generated through cleavage by endogenous proteases that can be found at sites of tissue injury, resulting in a disulfide linked heterodimeric ligand which can bind to and activate Ron [14]. We have previously demonstrated that the Ron receptor regulates TNFα production in the lungs of mice during LPS-induced acute lung injury [15]. This study demonstrated that the increased NF-κB activation and TNFα production in whole lung tissue following LPS challenge in wild-type mice was further enhanced in Ron tyrosine kinase domain-deficient animals and associated with worsened lung injury. Furthermore, these findings are consistent with other animal models of inflammatory injury where Ron-deficient animals exhibit an enhanced inflammatory response that results in increased tissue damage [15, 16]. The select cell populations, namely macrophages, and mechanisms by which Ron acts to regulate inflammation during the lung injury process remain unclear. In this report, we focus on the role of Ron specifically in alveolar macrophages. Through the use of MH-S cells, an alveolar monocyte cell line, and primary alveolar macrophages isolated from mice deficient in the Ron tyrosine kinase domain, Ron TK−/− mice, and wild-type control mice (TK+/+), we examine the molecular consequences of Ron activation in alveolar monocytes following LPS challenge.
MATERIALS AND METHODS
Mice
All experiments were performed using adult male mice between 8 and 16 weeks of age. Mice were age-matched within 2 weeks internally in each experiment. Ron tyrosine kinase (TK) deficient mice (TK−/−) were generated as previously described and have been backcrossed at least 10 generations into a purified C57Bl/6 background [17]. C57Bl/6 mice containing wild-type Ron (TK+/+) were used as controls for all experiments. All mice were maintained under specific pathogen-free conditions and were treated in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Cincinnati.
Alveolar Macrophages and Macrophage Cell Lines
The mice were euthanized by intraperitoneal injections of 200 mg/kg body weight Nembutol (pentobarbitol hydrochloride, Ovation Pharmaceuticals, Deerfield, IL). Immediately thereafter a midline neck incision was made and the trachea was cannulated. The lungs were lavaged twice with 0.7 ml sterile phospho-buffered saline (PBS). The recovered Bronchoalveolar-lavage fluid (BALF) was centrifuged at 500 × g for 5 minutes at 4°C, and resuspended in 200 µl sterile RPMI 1640 medium with 2 mM L-glutamine, 0.05 mM 2-mercaptoethanol, and 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA). Cells were plated at 5×104 cells/well in 24 well plates. The mouse alveolar macrophage cell line MH-S (American Type Culture Collection (ATCC); Manasas, VA) was used for in vitro studies. Cells were routinely passed in RPMI 1640 medium with 2 mM L-glutamine, 0.05 mM 2-mercaptoethanol, and 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA). Cells were seeded, at 0.5 × 106 cells/well, in 12 well plates for the analysis of cell culture supernatants and RNA isolation. Cells were seeded at 1.0 × 106 cells/well in 6 well plates and grown to approximately 75% confluence for NF-κB and western analyses. In designated experiments, culture media was pretreated with a range of HGFL (R&D systems, Minneapolis, MN) from 100 to 400ng/ml for 18 hours prior to stimulation with 1µg/ml LPS (E. coli serotype 0111:B4; Sigma, St Louis, MO) and/or HGFL.
Lentiviral shRNA Constructs and Establishment of Ron Knockdown Cell Lines
Lentiviral expression vectors carrying shRNAs (short hairpin RNAs) specific for Ron were purchased from Open Biosystems, Inc. (Huntsville, AL), and lentiviral stocks were prepared as recommended by the supplier. The Ron specific shRNA used in this study had the following sequence: CCGGCGAGTCATTCACAGTCAAGGTCTCGAGACCTTGACTGTGAATGACTCGTTTTTG. Scrambled control shRNA constructs (referred to as nonsense control), which did not target the endogenous Ron mRNA for degradation, were also purchased. All transcript-specific and control shRNAs were expressed by viral infection of the pLKO1-puro vector. The mouse alveolar macrophage cell line MH-S was utilized for the Ron knockdown studies. For these experiments, cells were placed in 10cm plates at 50% confluency. Cells were washed and infected with 4 ml of virus plus 8µg/ml polybrene for 6 hours. The virus was removed and complete media was added to the cells. One day after infection with either a Ron-specific shRNA or a nonsense control, the cells were selected with Puromycin (3 µg/ml) for 10 to 12 days. Independent drug-resistant clones were isolated and expanded. Knockdown of the target gene was assessed by performing western analyses with Ron-specific antibodies.
Protein Isolation and Assays
Cells were lysed in buffer (50 mM TrisHCl pH7.4, 150 mM NaCl, 2 mM EDTA, 1% NP-40, 0.1% SDS) containing protease inhibitor (Complete Mini, EDTA-free, Roche Diagnostics, Indianapolis, IN) and 1 mmol/L Na3VO4. Proteins were separated by SDS-PAGE and transferred to Immobilon-P membranes (Millipore, Billerica, MA). After transfer, the membranes were probed with a rabbit polyclonal anti-Ron antibody (1:500; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), or a rabbit polyclonal anti-TACE antibody (1:400; Abcam, Cambridge, MA). Specific binding was detected using an anti-rabbit peroxidase conjugated secondary antibody. The membrane was developed using ECL Plus Western detection reagent (GE Health Care Life Sciences, Piscataway, NJ) and the images were developed on film. Membranes were stripped and reprobed with anti-actin antibody C4 as a loading control. As a positive control for both Ron and Adam17 expression, lysates from the murine prostate cancer cell line, TRAMP-C1, were used [18, 19]. Ron and Adam17 have been shown to be overexpressed in most human prostate cancers [18, 19] and our laboratory has extended this finding to murine prostate cancer cell lines (TRAMP-C1) established from the transgenic adenocarcinoma of the mouse prostate (TRAMP) model.
RNA Isolation and Quantitative Real Time PCR Analysis
For quantitative real-time transcript analysis, RNA was extracted from MH-S cells or isolated alveolar monocytes using Trizol (Invitrogen, Carlsbad, CA) reagent as per the manufacturer’s instructions. One microgram of total RNA from MH-S cells or 0.05µg of total RNA from primary alveolar cells was reverse transcribed to cDNA using Applied Biosystems high capacity cDNA reverse transcription kit, according to the manufacturer’s instructions. Ron, TNFα, and Adam17 were quantified using real-time PCR with the ABI 7300 instrument using 4 µl of cDNA in 25µl of Power SYBR green master mix (Applied Biosystems, Foster City, CA). The cycling parameters for this reaction were: 50 degrees for 2 minutes, 95 degrees for 10 minutes and then 40 cycles of 95 degrees for 15 seconds and 60 degrees for 1 minute. This reaction was followed by a dissociation stage to examine probe specificity. The following primers were used to amplify the cDNA: murine Ron (forward-tcccattgcaggtctgtgtaga, reverse-cggaagctgtatcgttgatgtc), murine TNFα (forward-catcttctcaaaattcgagtgacaa, reverse-tgggagtagacaaggtacaaccc), murine Adam17 (forward-ggtgtgcagtgcagtgatagg, reverse-gcgccgtctcaaactgaca). Gene expression values were normalized to 18S (forward-agtccctgcccttgtacaca; reverse-gatccgagggcctcactaaac) or normalized to endogenous β-glucuronidase expression (forward-ttgagaactggtataagacgcatcag; reverse-tctggtggtactcctcactgaacatgc) as internal controls. Relative gene expression results are reported. Real-time analyses were repeated in duplicate with similar results using samples from three independent experiments.
ELISA Measurements
Supernatant TNFα levels were measured by ELISA according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN).
EMSA Analysis
Cells were lysed in buffer (50 mM TrisHCl pH7.4, 150 mM NaCl, 2 mM EDTA, 1% NP-40, 0.1% SDS) containing protease inhibitor and 1 mmol/L Na3VO4. Protein concentrations were determined by bicinchoninic acid (BCA) assay as per the manufacturer’s instructions (Pierce, Rockford, IL). Double stranded NF-κB consensus oligonucleotide (5’-AGTGAGGGGACTTTCCCAGGC-3’; Promega, Madison, WI) was end-labeled with γ[32P] ATP. Binding reactions (total volume 15 µL) with equal amounts of whole cell lysates (15 µg), oligonucleotide (35fmol), and binding buffer containing 20% glycerol (vol/vol), 50 mM Tris · HCl, pH 7.9, 2.5 mM EDTA, 2.5 mM DTT, 5 mM MgCl2, 250 mM NaCl and 0.25 µg/µl poly[d(I-C)] (USB Corp, Cleveland, OH) were incubated at room temperature for 30 minutes. Samples were subjected to electrophoretic separation on a nondenaturing 5% polyacrylamide gel. Gels were dried at 53°C for 3 h and analyzed by autoradiography utilizing a Typhoon 9400 variable mode phosphorimager (GE Healthcare, Piscataway, NJ).
NF-κB Luciferase Reporter Assay
2 ×105 cells were plated in RPMI containing 10% FBS. The next day, cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) with either a NF-κB reporter (pNF-κBluc) or empty vector (pTAL luc) construct and a control plasmid expressing Renilla (pRL-TK). Fourteen hours after transfection, the cells were pretreated with or without HGFL overnight, followed by stimulation in the presence or absence of LPS (1µg/ml) for 4 hours. The cells were lysed and subjected to a dual-luciferase assay according to manufacturer’s protocol (Dual-Luciferase Reporter Assay System, Promega, Madison, WI). Samples were read using the GloMax® 96 Microplate Luminometer with Dual Injectors (Promega, Madison, WI). NF-κB luminescence in each sample was normalized to the extent of Renilla expression and the results displayed are relative NF-κB activity.
Statistical Analysis
Data are expressed as Mean ± Standard Error . Statistical significance comparing different experimental groups was determined by Student’s t-test for pairwise comparisons, or ANOVA for comparison of multiple groups using SigmaPlot 11.0 software (Systat Software, Inc., San Jose, CA). Differences between groups were accepted as significant when p<0.05.
RESULTS
The Ron Receptor Tyrosine Kinase is Expressed on Primary Alveolar Macrophages and Negatively Regulates TNFα Production in Response to LPS Stimulation
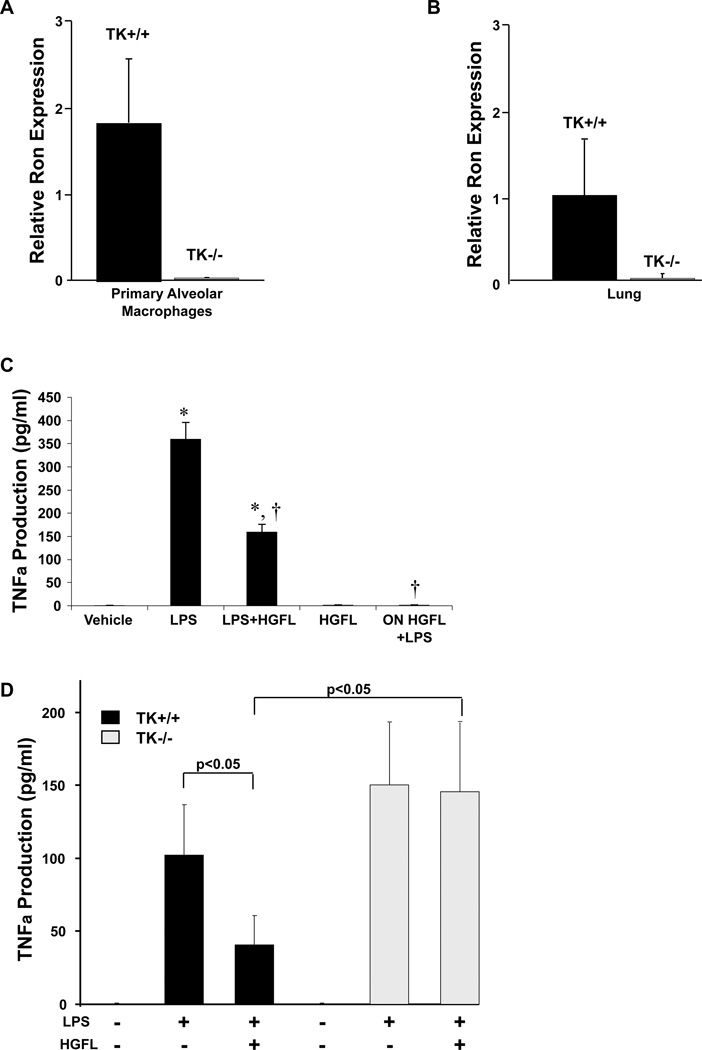
Previous studies in our laboratory demonstrated that mice with a targeted ablation of the tyrosine kinase signaling domain of Ron (TK−/−) mice exhibited an increase in the intrapulmonary production of the cytokine TNFα in response to LPS treatment[15]. Given that TNFα is predominately produced in macrophages, we postulated that Ron signaling may be modulating TNFα production by regulating the activity of pulmonary alveolar macrophages. Previous studies have shown Ron expression in murine peritoneal macrophages and Ron expression has been demonstrated in the lung epithelial cells by immunohistochemical staining [20, 21]. However, the expression and activity of Ron in primary pulmonary alveolar macrophages has not been characterized. To examine Ron expression in this compartment, Ron receptor transcript levels were evaluated in both isolated alveolar macrophages and in whole lung and by qRT-PCR. As illustrated in Figure 1A and 1B, Ron transcripts are readily detectable in both whole lung and in isolated primary alveolar macrophages. In wild-type mice, whole lung tissue contained 34-fold more Ron transcript expression than that observed in the alveolar macrophages supporting previously published studies also documenting Ron expression in the lung epithelium [20, 22]. To assess Ron function, primary wild-type alveolar macrophages were pretreated or concurrently treated with the Ron ligand, HGFL, and challenged with LPS. Following LPS stimulation of primary alveolar BAL cells, TNFα production is robust (Figure 1C). However, HGFL pretreatment (ON HGFL) abolished LPS-induced TNFα production (Figure 1C). Simultaneous treatment with HGFL and LPS (LPS+HGFL) reduced TNFα production, although not to the extent observed with overnight HGFL pretreatment (ON HGFL). These data suggest a delay in the onset of TNFα regulation following Ron activation by HGFL. Of note, the circulating physiologic concentration of HGFL in the serum is approximately 400ng/ml and this concentration was utilized in our analyses. However, based on titration experiments, we observed similar results when using HGFL concentrations between 100 and 400ng/ml. In similar experiments on TK−/− primary alveolar macrophages, HGFL had no impact on LPS-induced TNFα production (Figure 1D) attesting to the lack of ligand response in the Ron-deficient mice, which is consistent with previous observations [17]. While final TNFα concentrations varied from experiment to experiment based on the number of cells used in each assay with overall levels of TNFα production were consistently higher in the Ron deficient cells compared to controls. Overall, these data demonstrate that Ron activation in primary pulmonary macrophages is able to negatively regulate LPS-induced TNFα production.
Figure 1. The Ron receptor is expressed and functional in primary alveolar macrophages.
Ron transcript levels were examined from mRNA isolated from primary alveolar macrophages (A) and from whole lungs (B) from wild-type (TK+/+) and Ron tyrosine kinase-deficient (TK−/−) mice by qRT-PCR. Expression was normalized to an internal control as described in the Materials and Methods section and the relative gene expression changes are reported. Data is expressed as the mean ± the standard deviation and is representative of several independent experiments. (C) Alveolar macrophages were isolated and treated with LPS (1µg/ml) with or without HGFL (400ng/ml), HGFL alone, or overnight (ON) HGFL pretreatment followed by LPS. Cell culture supernatants were collected 24 hours after final stimulation and assayed for TNFα production. Results represent data from two independent experiments with n=8 per group. *p<0.05 compared to vehicle or HGFL treated groups. †p<0.05 compared to LPS treated group. HGFL treatment was able to significantly reduce the production of TNFα from wild-type alveolar macrophages following LPS stimulation. (D) Primary alveolar macrophages were isolated from TK+/+ and TK−/− mice and pretreated in the presence or absence of HGFL overnight. The next day the cells were stimulated in the presence or absence of LPS as indicated above. Culture supernatant was isolated after 4 hours and examined for TNFα production by ELISA analysis. Data is expressed as the mean ± the standard error and is representative of experiments from n=6–10 mice.
The Ron Receptor Tyrosine Kinase is Expressed in the Murine Alveolar Cell Line, MH-S, and Activation of Ron Leads to Decreased TNFα Transcript and Protein Production
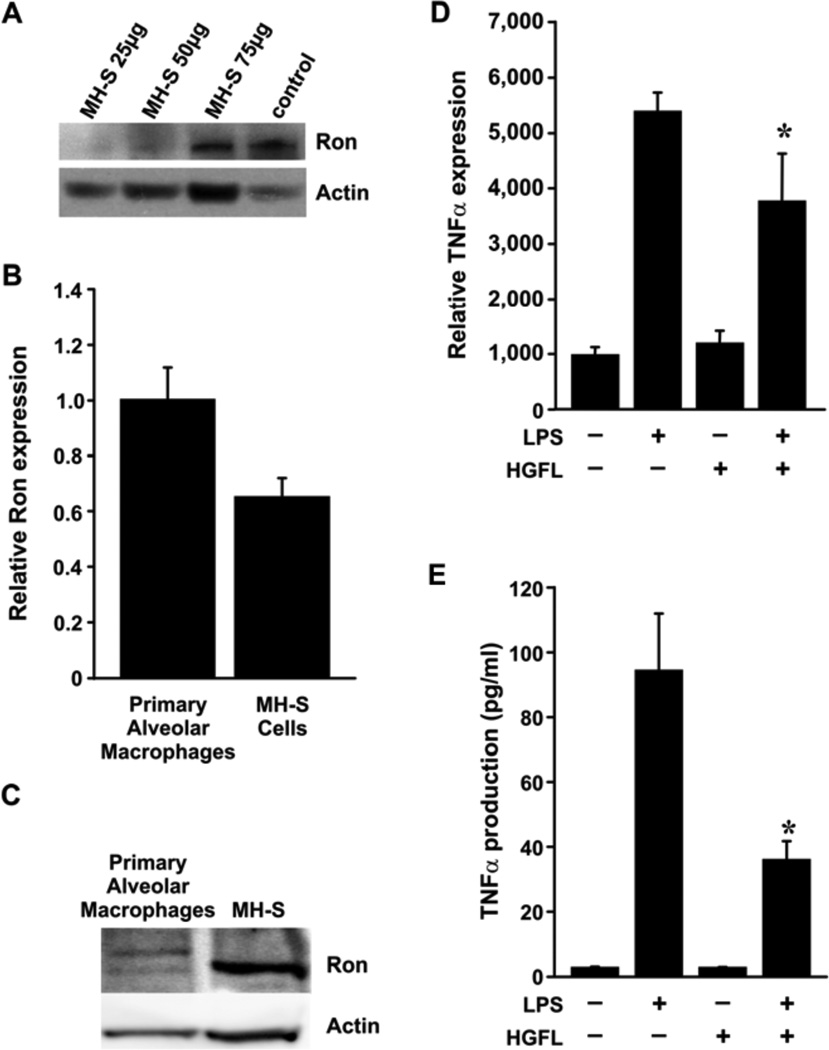
In order to examine the signaling mechanisms downstream of Ron that are important in the generation of pulmonary cytokine production, we sought to examine Ron expression and function in an alveolar macrophage cell line. MH-S cells are an SV40 transformed cell line derived from murine alveolar monocytes, which have been shown to display many of the same characteristics as wild-type derived alveolar macrophages including FC receptor dependent phagocytosis and IL-1 production [23, 24]. To use this as a model cell line to further examine Ron signaling, we examined Ron expression in MH-S cells by western analysis (Figure 2A). Our data demonstrates increasing Ron protein expression with increasing amounts of total protein lysate in MH-S cells. In addition, we compared the levels of Ron in the MH-S cells to that of primary murine pulmonary alveolar macrophages (Figure 2B). Ron transcript levels were very similar in the primary cells compared to the MH-S cell line based on qRT-PCR analyses (Figure 2B). We examined RON expression in broncho-alveolar lavage (BAL) cell lysates from pooled naïve mice (n=12). Our data provides evidence of Ron protein expression in primary alveolar cells (Figure 2C). To examine the outcome of Ron activation on LPS-induced cytokine production, the MH-S cells were pretreated with HGFL followed by LPS stimulation. Overnight pretreatment with HGFL followed by 4 hours of LPS stimulation led to a significant reduction in both TNFα transcript and protein levels compared to LPS stimulation alone (Figures 2D and 2E). HGFL treatment, in the absence of LPS stimulation, did not have an impact on TNFα levels compared to untreated control cells. Simultaneous treatment with HGFL and LPS reduced TNFα levels, but the extent of inhibition was variable and not significant in this cell line (data not shown). These data indicate that activation of Ron signaling by HGFL leads to repression of TNFα production following LPS-exposure at both the protein and transcript levels. Moreover, these results are consistent with the expression and activity of Ron observed in primary murine alveolar macrophages.
Figure 2. The Ron receptor is expressed in the murine MH-S cell line and negatively regulates TNFα production following LPS challenge.
(A) Western analysis of Ron protein levels from increasing amounts of MH-S cell lysates. Lysates from the murine prostate cancer cell line, TRAMP-C1, was used as a positive control. (B) Ron transcript levels were measured by qRT-PCR from RNA isolated from primary alveolar macrophages and MH-S cells. Similar levels of Ron were observed in both cell types. Expression was normalized to an internal control as described in the Materials and Methods section. (C) Western analysis of Ron protein levels from primary broncho-alveolar lavage cells (BAL) and MH-S cell lysates (150µg). (D, E) MH-S cells were pretreated with or without HGFL (400ng/ml) overnight followed by LPS (1µg/ml) stimulation. Cells and culture supernatants were collected 4 hours after final stimulation and assayed for TNFα transcript (D) and protein levels (E), respectively. Ligand-induced Ron signaling blocks the production of TNFα transcripts (D) and protein TNFα (E). Data are expressed as the mean ± standard error performed in triplicate. The data is representative of three independent experiments with similar results. *, p<0.05 compared to the corresponding LPS alone treated group.
Ron Activation Regulates IκB Protein Levels and NF-κB Activity Following LPS Exposure
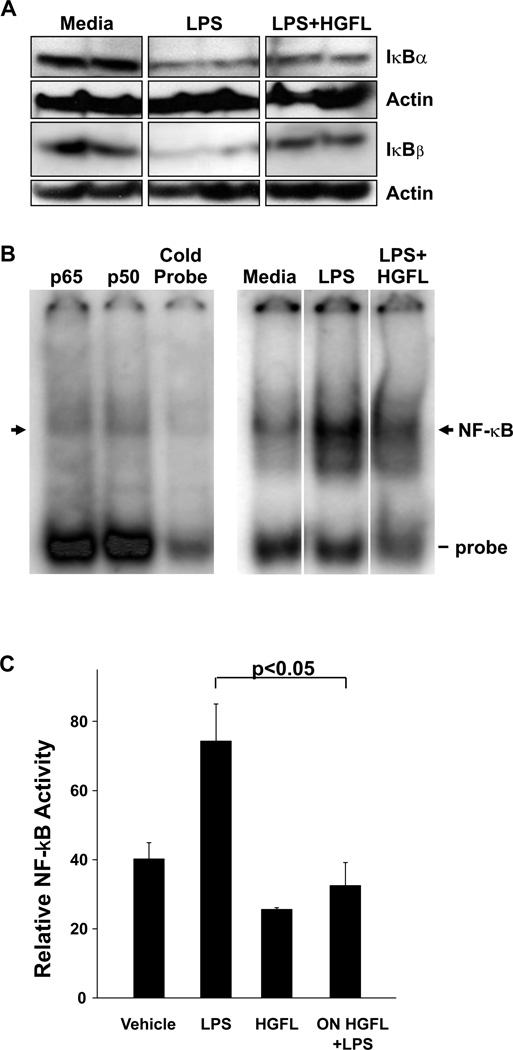
Previously we demonstrated that whole lung homogenates from Ron-deficient mice treated with LPS exhibited augmented activation of the transcription factor NF-κB and decreased expression IκBα and IκBβ proteins. To extend upon these observations and to mechanistically determine if Ron signaling specifically in macrophages contributes to this in vivo effect, we generated cell lysates from MH-S cells that pretreated with or without HGFL overnight followed stimulation in the presence or absence of LPS for 4 hours. Western analyses of these lysates revealed that IκBα and IκBβ protein levels were increased following Ron activation by HGFL treatment, compared to LPS treatment alone (Figure 3A). Consistent with the Western analyses, electrophoretic mobility shift analyses on whole cell lysates from the MH-S cells showed reduced NF-κB DNA binding activity following similar treatment with LPS and HGFL compared to LPS alone (Figure 3B). Specificity of NF-κB binding was performed by competition analysis with an excess of unlabeled NF-κB consensus sequence DNA, while the composition of the NF-κB complex was determined by supershift analyses. The NF-κB complexes in the MH-S cells consisted largely of the p65 subunit of NF-κB (Figure 3B) and are consistent with the NF-kB composition found in our prior report utilizing whole lung lysates from LPS treated mice [15]. A loss of the specific protein/DNA complex was not observed with other antibodies to different NF-κB proteins or with a nonspecific competitor (data not shown). To substantiate the NF-κB-DNA binding studies, we also examined the ability of HGFL to the transcriptional activity of NF-κB in response to LPS stimulation in MH-S cell utilizing reporter assays (Figure 3C). Pretreatment with HGFL was able to significantly reduce the amount of NF-κB reporter activity in response to LPS stimulation.
Figure 3. HGFL treatment impacts IκB protein levels and inhibits NF-κB activation in MH-S cells following LPS stimulation.
Cell lysates from the murine alveolar macrophage cell line, MH-S, were obtained after overnight pretreatment in the presence or absence of HGFL (400ng/ml) followed by 4 hours of treatment with or without LPS. (A) Western analysis was performed on the MH-S cell lysates to examine IκBα and IκBβ protein expression levels. Actin served as a loading control. Each lane represents an independent sample and is representative of at least two independent experiments. (B) Electrophoretic mobility shift assays on whole cell extracts from MH-S cells were performed to examine NF-κB DNA binding activity in response to LPS alone or LPS plus HGFL pretreatment. The specific NF-κB DNA complex is denoted by the arrow with free probe also indicated. Competition and supershift analyses for NF-κB-DNA binding proteins were also performed. The protein-DNA complexes were competed with either 100-fold excess of cold probe or with antibodies directed against the p50 and p65 subunits of NF-κB. (C) MH-S cells were transfected with an NF-κB reporter plasmid along with a control plasmid. After transfection, the cells were pretreated in the presence or absence of HGFL overnight and the next day stimulated with or without LPS. Relative NF-κB activity for each sample is depicted. Data are expressed as the mean ± standard error.
Adam17 is Expressed on Primary Alveolar Macrophages and in MH-S Cells
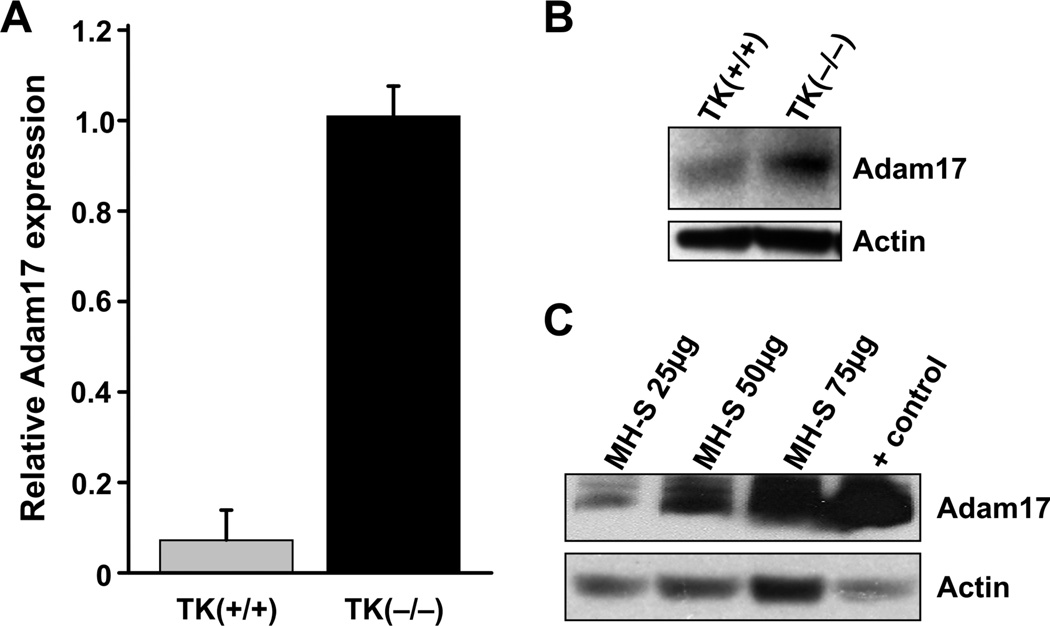
Given that TNFα levels are regulated both at the transcriptional level by NF-κB and at the protein level by Adam17, an enzyme responsible for cleaving TNFα from the surface of the cells, we sought to determine if Ron signaling might also regulate TNFα production by impacting TNFα release through Adam17 modulation. To investigate this potential mechanism, Adam17 levels were first evaluated in alveolar macrophages isolated from wild-type and Ron tyrosine kinase-deficient mice (TK−/−). Strikingly, Adam17 levels were found to be significantly increased in the alveolar macrophages from Ron TK−/− mice compared to Ron TK+/+ primary cells when examined by qRT-PCR (Figure 4A). Western analyses also demonstrate significant Adam17 levels in primary alveolar macrophages isolated from Ron TK−/− mice compared to Ron TK+/+ controls (Figure 4B). Furthermore, Adam17 expression in MH-S cells was confirmed by western analysis of whole cell lysates (Figure 4C). These studies suggest that Ron is an endogenous negative regulator of Adam17 production in the absence of any treatment.
Figure 4. Adam17 levels in primary alveolar macrophages and in MH-S cells.
(A) Adam17 transcript levels were measured by qRT-PCR from RNA isolated from primary alveolar macrophages of wild-type TK(+/+) and Ron TK(−/−) mice. Data is expressed as the mean ± the standard deviation and is representative of several independent experiments. Expression was normalized to an internal control as described in the Materials and Methods section. (B) Western analysis of Adam17 protein levels from alveolar macrophages isolated from wild type TK(+/+) and Ron TK(−/−) mice. Five µg of total cell lysates were loaded per lane with Actin serving as a loading control. (C) Western analysis of Adam17 protein levels from MH-S cell lysates. The murine prostate cancer cell line, TRAMP-C1, was used as a positive control for Adam17 expression and Actin expression is provided as a loading control.
Ron Expression and Activation Negatively Regulates Adam17 Levels in Alveolar Macrophages
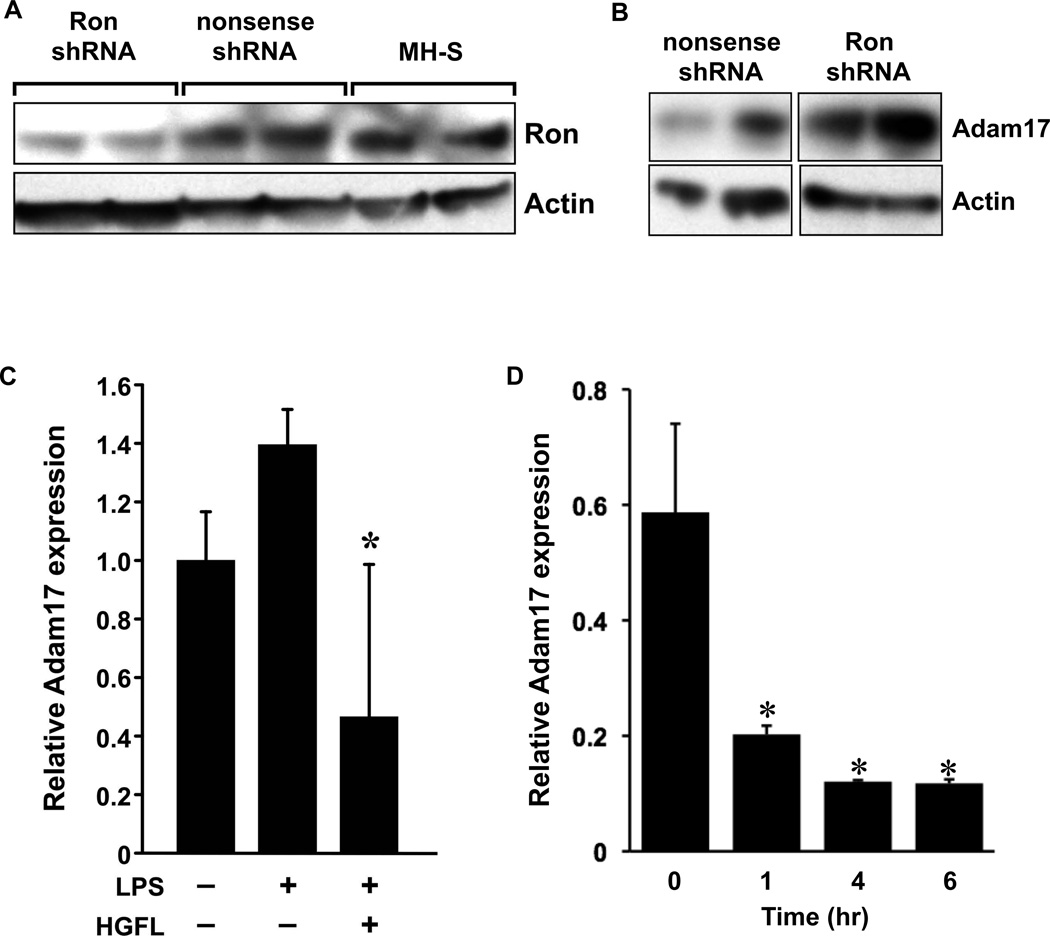
Given that we observed elevated levels of Adam17 in the primary alveolar macrophages isolated from the mice with a de novo loss of Ron compared to Ron expressing macrophages (Figure 4A), we next sought to determine if the levels of Adam17 might be modulated in response to changes in Ron expression and/or activation. To investigate if Ron and Adam17 expression levels are coupled, we infected MH-S cells with a lentiviral construct carrying either a shRNA sequence to knockdown Ron expression or with a control (nonsense) shRNA. Figure 5A demonstrates that Ron levels were significantly reduced on average by about 60% following infection with a Ron shRNA construct compared to the control nonsense shRNA or uninfected MH-S cells. Multiple independent infections were performed with results from two independent experiments shown. Next, Western analysis was performed to examine Adam17 protein levels in the control and Ron knockdown MH-S cells. Figure 5B clearly demonstrates that a Ron knockdown leads to increased Adam17 protein levels, consistent with our data presented in Figure 4B documenting increased levels of Adam17 in primary alveolar macrophages isolated from Ron TK−/− mice compared to wild-type alveolar macrophages. These data suggest that Ron levels are inversely correlated with Adam17 levels.
Figure 5. The Ron receptor negatively regulates Adam17 levels in MH-S cells.
(A) Ron protein levels were measured by western analysis of cell lysates from MH-S cells, MH-S cells infected with an shRNA construct to knockdown Ron expression (Ron shRNA) or a control shRNA construct (nonsense shRNA). Each lane represents a separate independent experimental sample. Actin expression is shown as a loading control. These studies demonstrate knockdown of Ron expression in Ron shRNA infected cells. (B) Adam17 protein levels were measured by Western analysis from MH-S cells following infection with a Ron-specific shRNA (Ron shRNA) or with a nonsense shRNA control (nonsense shRNA) construct. Actin expression is shown as a loading control. Of note, following Ron knockdown in MH-S cells, the levels of Adam17 are increased. (C) Adam17 transcript levels were measured by qRT-PCR from RNA isolated from MH-S cells pretreated with HGFL (400ng/ml) or media alone, followed by stimulation with (+) or without (-) LPS (1 µg/ml). (D) Adam17 transcript levels were measured by qRT-PCR from RNA isolated from MH-S cells treated with HGFL (100ng/ml) or media alone. Expression was normalized to an internal control as described in the Materials and Methods section. In response to LPS, Adam17 RNA levels are increased and this induction can be significantly blunted by HGFL pretreatment. Data is expressed as the mean ± the standard error and is representative of several independent experiments.
We next sought to determine if HGFL treatment might also regulate Adam17 production and ensuing TNFα secretion following LPS exposure. To test this, MH-S cells were pretreated in the presence or absence of HGFL followed by stimulation with LPS. Following LPS treatment, Figure 5C demonstrates that Adam17 levels are increased. Importantly, pretreatment with HGFL significantly reduced Adam17 transcripts. Given the time frame that pretreatment with HGFL prior to LPS stimulation we were interested in determining whether HGFL stimulation directly affected Adam17 expression. To test this, a kinetic analysis of MH-S cells treated with or without HGFL was undertaken. Following HGFL treatment Figure 5D demonstrates that Adam17 expression is significantly decreased within 1 hour of HGFL treatment. These results suggest that HGFL-induced downregulation of Adam17 during LPS exposure may also be an important factor in blocking TNFα production. While we observed a significant decrease in Adam17 levels in the Ron knockdown cells, we observed a trend toward increased levels of TNFα in these cells following LPS stimulation (data not shown). However, this trend was not significant and is likely due to the incomplete knockdown of Ron in these cells.
DISCUSSION
Mice challenged with intrapulmonary LPS develop experimental acute lung injury characterized by increased levels of the pro-inflammatory cytokine TNFα which triggers an inflammatory cascade resulting in pulmonary edema, tissue damage, and death [25] We have previously demonstrated that the Ron receptor tyrosine kinase plays a regulatory role in down-modulating the inflammatory response as measured by TNFα release in response to intrapulmonary LPS treatment [15]. Furthermore, we have identified a protective role for Ron in a Nickel-mediated pulmonary model of acute lung injury [16, 26]. The data presented in this manuscript identify an important regulatory role for Ron specifically in primary alveolar macrophages. Our results have uncovered several principles concerning Ron signaling and acute lung injury. First, the Ron receptor tyrosine kinase is expressed on primary alveolar macrophages isolated from mouse lungs as well as the murine alveolar cell line, MH-S. Interestingly, our findings suggest that Ron may be expressed in its slightly larger pro-form in primary alveolar macrophages when compared to MH-S cells. Ron expression was previously examined by immunohistochemistry in murine lungs whereby Ron was detected in the lung epithelium surrounding the airways [16]. In humans, Ron expression has also been identified in normal lung at the apical surface of ciliated epithelia in the airways and oviduct, and in human alveolar macrophages [20, 22, 27, 28]. Second, pretreatment of macrophages expressing Ron with its natural ligand HGFL, prior to stimulation with LPS, is able to decrease TNFα production. This finding is consistent with previous studies that described a regulatory role for Ron in primary macrophages isolated from the peritoneum where loss of Ron receptor results in increased activation of macrophages following LPS stimulation and that treatment with HGFL is able to down regulate iNOS production and cell mediated immune responses [29–31]. Third, the reduction in TNFα production following Ron activation correlates with decreased NF-κB activation and increased IκB levels following LPS challenge in alveolar macrophage cell lines. Fourth, we demonstrate that partial knockdown of the Ron receptor in MH-S cells is sufficient to increase Adam17 levels and that alveolar macrophages from Ron-deficient mice also exhibit a dramatic increase in Adam17. Finally, we identify the downstream regulation of Adam17 by Ron as a further potential mechanism by which Ron regulates TNFα levels following LPS challenge. Significantly, down regulation of Adam17 at the transcript level occurs within 1 hour following Ron activation with HGFL. Our studies provide strong evidence that the early regulation of acute lung injury in vivo by Ron is mediated primarily through early activation events in the immune compartment of the lung.
We have previously shown that the loss of the Ron receptor tyrosine kinase domain results in an augmented TNFα response to LPS in both liver and lung models of injury [15, 32]. In this study, we were interested in determining whether activation of the Ron receptor de novo would decrease TNFα production following LPS challenge. Our results indicate that Ron activation by HGFL pretreatment prior to the LPS challenge is able to decrease TNFα production in both primary alveolar macrophages and in MH-S cells in vitro. This finding identifies that Ron levels in alveolar macrophages are sufficient for potential regulation of the inflammatory response in vitro. This raises the possibility of Ron as an early therapeutic target in the initiation cascade of the inflammatory response in acute lung injury in vivo.
To examine the impact of Ron activation on downstream signaling cascades, our data demonstrate ligand induced Ron activation in MH-S cells leads to decreased levels of NF-κB and increased IκB levels. NF-κB activation has been shown to be important for TNFα production and our results show a corresponding decrease in TNFα [33]. The reduction in TNFα is expected to correlate to decreased inflammation in vivo and in decreased levels of lung damage following intrapulmonary LPS challenge [34–36]. These data correlate with previous results in our laboratory indicating that the loss of the Ron receptor tyrosine kinase domain results in increased pro-inflammatory cytokine production in vivo [15, 16, 26].
In this study, we show that alveolar macrophages isolated from Ron-deficient mice exhibit increased levels of Adam17 de novo at both the transcript and protein levels compared to wild-type controls. Additionally, we demonstrate that MH-S cells express basal levels of Adam17 which are upregulated in response to LPS treatment. Interestingly, HGFL treatment of MH-S cells is able to attenuate induction of Adam17 by LPS. Furthermore, Adam17 levels are increased in MH-S cells following a partial knockdown of Ron in the absence of LPS treatment. These data taken together identify Adam17 as a potential target of regulation by Ron. Previous studies have linked increased levels of Adam17 to increases in TNFα production in osteoarthritis [9, 37]. The role of Adam17 as the cause or consequence remains a subject of continuing study. The direct upregulation of Adam17 levels by protein kinase C (PKC) or by treatment with LPS and TNFα remains only partially understood . Analysis of the Adam17 promoter has identified the region necessary for basal transcription of Adam17 but identification of the regulatory regions of this promoter remains elusive [38]. Sequence analysis of the promoter region has been shown to contain canonical NF-κB binding sequences which might suggest a direct role for NF-κB activation in Adam17 upregulation, but this has yet to be demonstrated experimentally.
The results of this study indicate a regulatory role for Ron receptor tyrosine kinase in alveolar monocytes following LPS challenge. Ligand-dependent activation of Ron results in decreased NF-κB activation leading to decreased early cytokine production as measured by TNFα production following LPS challenge. Furthermore we have identified a potential regulatory role for Ron in Adam17 expression both without, and following LPS challenge, although the direct mechanism by which Adam17 is regulated remains unclear. Further studies examining the role of Adam17 in macrophage signaling are warranted to determine if Adam17 is required for Ron regulation of the acute inflammatory response.
Acknowledgments
FUNDING This work was supported by Public Health Services Grants DK-073552 (S.E.W.), and the Digestive Diseases Research Development Center grant DK-064403 (S.E.W.) from the National Institutes of Health, and by grant project #8950 (S.E.W.) from Shriners Hospital for Children.
ABBREVIATIONS
- HGFL
hepatocyte growth factor-like protein
- LPS
lipopolysaccharide
- TK
tyrosine kinase
- TNFα
Tumor necrosis factor alpha
- NF-κB
nuclear factor kappa B
- Adam17
a disintegrin and metalloprotease
REFERENCES
- 1.Lukacs NW, Ward PA. Inflammatory mediators, cytokines, and adhesion molecules in pulmonary inflammation and injury. Adv Immunol. 1996;62:257–304. doi: 10.1016/s0065-2776(08)60432-0. [DOI] [PubMed] [Google Scholar]
- 2.Shanley TP, Warner RL, Ward PA. The role of cytokines and adhesion molecules in the development of inflammatory injury. Mol Med Today. 1995;1(1):40–45. doi: 10.1016/1357-4310(95)80019-0. [DOI] [PubMed] [Google Scholar]
- 3.Strieter RM, Kunkel SL, Keane MP, Standiford TJ. Chemokines in lung injury: Thomas A. Neff Lecture. Chest. 1999;116(1 Suppl):103S–110S. doi: 10.1378/chest.116.suppl_1.103s. [DOI] [PubMed] [Google Scholar]
- 4.Warren JS, Kunkel SL, Cunningham TW, Johnson KJ, Ward PA. Macrophage-derived cytokines amplify immune complex-triggered O2-. responses by rat alveolar macrophages. Am J Pathol. 1988;130(3):489–495. [PMC free article] [PubMed] [Google Scholar]
- 5.Fantini GA, Conte MS. Pulmonary failure following lower torso ischemia: clinical evidence for a remote effect of reperfusion injury. Am Surg. 1995;61(4):316–319. [PubMed] [Google Scholar]
- 6.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151(2 Pt 1):293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 7.Hehlgans T, Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: players, rules and the games. Immunology. 2005;115(1):1–20. doi: 10.1111/j.1365-2567.2005.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Windsor AC, Walsh CJ, Mullen PG, Cook DJ, Fisher BJ, Blocher CR, Leeper-Woodford SK, Sugerman HJ, Fowler AA., 3rd Tumor necrosis factor-alpha blockade prevents neutrophil CD18 receptor upregulation and attenuates acute lung injury in porcine sepsis without inhibition of neutrophil oxygen radical generation. J Clin Invest. 1993;91(4):1459–1468. doi: 10.1172/JCI116351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amin AR. Regulation of tumor necrosis factor-alpha and tumor necrosis factor converting enzyme in human osteoarthritis. Osteoarthritis Cartilage. 1999;7(4):392–394. doi: 10.1053/joca.1998.0221. [DOI] [PubMed] [Google Scholar]
- 10.Gaudino G, Follenzi A, Naldini L, Collesi C, Santoro M, Gallo KA, Godowski PJ, Comoglio PM. RON is a heterodimeric tyrosine kinase receptor activated by the HGF homologue MSP. Embo J. 1994;13(15):3524–3532. doi: 10.1002/j.1460-2075.1994.tb06659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwama A, Okano K, Sudo T, Matsuda Y, Suda T. Molecular cloning of a novel receptor tyrosine kinase gene, STK, derived from enriched hematopoietic stem cells. Blood. 1994;83(11):3160–3169. [PubMed] [Google Scholar]
- 12.Iwama A, Wang MH, Yamaguchi N, Ohno N, Okano K, Sudo T, Takeya M, Gervais F, Morissette C, Leonard EJ, Suda T. Terminal differentiation of murine resident peritoneal macrophages is characterized by expression of the STK protein tyrosine kinase, a receptor for macrophage-stimulating protein. Blood. 1995;86(9):3394–3403. [PubMed] [Google Scholar]
- 13.Quantin B, Schuhbaur B, Gesnel MC, Doll'e P, Breathnach R. Restricted expression of the ron gene encoding the macrophage stimulating protein receptor during mouse development. Dev Dyn. 1995;204(4):383–390. doi: 10.1002/aja.1002040405. [DOI] [PubMed] [Google Scholar]
- 14.Waltz SE, McDowell SA, Muraoka RS, Air EL, Flick LM, Chen YQ, Wang MH, Degen SJ. Functional characterization of domains contained in hepatocyte growth factor-like protein. J Biol Chem. 1997;272(48):30526–30537. doi: 10.1074/jbc.272.48.30526. [DOI] [PubMed] [Google Scholar]
- 15.Lentsch AB, Pathrose P, Kader S, Kuboki S, Collins MH, Waltz SE. The Ron receptor tyrosine kinase regulates acute lung injury and suppresses nuclear factor kappaB activation. Shock. 2007;27(3):274–280. doi: 10.1097/01.shk.0000239755.82711.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mallakin A, Kutcher LW, McDowell SA, Kong S, Schuster R, Lentsch AB, Aronow BJ, Leikauf GD, Waltz SE. Gene expression profiles of Mst1r-deficient mice during nickel-induced acute lung injury. Am J Respir Cell Mol Biol. 2006;34(1):15–27. doi: 10.1165/rcmb.2005-0093OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waltz SE, Eaton L, Toney-Earley K, Hess KA, Peace BE, Ihlendorf JR, Wang MH, Kaestner KH, Degen SJ. Ron-mediated cytoplasmic signaling is dispensable for viability but is required to limit inflammatory responses. J Clin Invest. 2001;108(4):567–576. doi: 10.1172/JCI11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karan D, Lin FC, Bryan M, Ringel J, Moniaux N, Lin MF, Batra SK. Expression of ADAMs (a disintegrin and metalloproteases) and TIMP-3 (tissue inhibitor of metalloproteinase-3) in human prostatic adenocarcinomas. Int J Oncol. 2003;23(5):1365–1371. [PubMed] [Google Scholar]
- 19.O'Toole JM, Rabenau KE, Burns K, Lu D, Mangalampalli V, Balderes P, Covino N, Bassi R, Prewett M, Gottfredsen KJ, Thobe MN, Cheng Y, Li Y, Hicklin DJ, Zhu Z, Waltz SE, Hayman MJ, Ludwig DL, Pereira DS. Therapeutic implications of a human neutralizing antibody to the macrophage-stimulating protein receptor tyrosine kinase (RON), a c-MET family member. Cancer Res. 2006;66(18):9162–9170. doi: 10.1158/0008-5472.CAN-06-0283. [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto O, Iwama A, Amitani R, Takehara T, Yamaguchi N, Yamamoto T, Masuyama K, Yamanaka T, Ando M, Suda T. Role of macrophage-stimulating protein and its receptor, RON tyrosine kinase, in ciliary motility. J Clin Invest. 1997;99(4):701–709. doi: 10.1172/JCI119214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang MH, Fung HL, Chen YQ. Regulation of the RON receptor tyrosine kinase expression in macrophages: blocking the RON gene transcription by endotoxin-induced nitric oxide. J Immunol. 2000;164(7):3815–3821. doi: 10.4049/jimmunol.164.7.3815. [DOI] [PubMed] [Google Scholar]
- 22.Willett CG, Wang MH, Emanuel RL, Graham SA, Smith DI, Shridhar V, Sugarbaker DJ, Sunday ME. Macrophage-stimulating protein and its receptor in non-small-cell lung tumors: induction of receptor tyrosine phosphorylation and cell migration. Am J Respir Cell Mol Biol. 1998;18(4):489–496. doi: 10.1165/ajrcmb.18.4.2978. [DOI] [PubMed] [Google Scholar]
- 23.Mbawuike IN, Herscowitz HB. MH-S, a murine alveolar macrophage cell line: morphological, cytochemical, and functional characteristics. J Leukoc Biol. 1989;46(2):119–127. doi: 10.1002/jlb.46.2.119. [DOI] [PubMed] [Google Scholar]
- 24.Sankaran K, Herscowitz HB. Phenotypic and functional heterogeneity of the murine alveolar macrophage-derived cell line MH-S. J Leukoc Biol. 1995;57(4):562–568. doi: 10.1002/jlb.57.4.562. [DOI] [PubMed] [Google Scholar]
- 25.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295(3):L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDowell SA, Mallakin A, Bachurski CJ, Toney-Earley K, Prows DR, Bruno T, Kaestner KH, Witte DP, Melin-Aldana H, Degen SJ, Leikauf GD, Waltz SE. The role of the receptor tyrosine kinase Ron in nickel-induced acute lung injury. Am J Respir Cell Mol Biol. 2002;26(1):99–104. doi: 10.1165/ajrcmb.26.1.4621. [DOI] [PubMed] [Google Scholar]
- 27.Gunella G, Bardelli C, Amoruso A, Viano I, Balbo P, Brunelleschi S. Macrophage-stimulating protein differently affects human alveolar macrophages from smoker and non-smoker patients: evaluation of respiratory burst, cytokine release and NF-kappaB pathway. Br J Pharmacol. 2006;148(4):478–489. doi: 10.1038/sj.bjp.0706751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunelleschi S, Penengo L, Lavagno L, Santoro C, Colangelo D, Viano I, Gaudino G. Macrophage stimulating protein (MSP) evokes superoxide anion production by human macrophages of different origin. Br J Pharmacol. 2001;134(6):1285–1295. doi: 10.1038/sj.bjp.0704356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen YQ, Fisher JH, Wang MH. Activation of the RON receptor tyrosine kinase inhibits inducible nitric oxide synthase (iNOS) expression by murine peritoneal exudate macrophages: phosphatidylinositol-3 kinase is required for RON-mediated inhibition of iNOS expression. J Immunol. 1998;161(9):4950–4959. [PubMed] [Google Scholar]
- 30.Liu QP, Fruit K, Ward J, Correll PH. Negative regulation of macrophage activation in response to IFN-gamma and lipopolysaccharide by the STK/RON receptor tyrosine kinase. J Immunol. 1999;163(12):6606–6613. [PubMed] [Google Scholar]
- 31.Morrison AC, Wilson CB, Ray M, Correll PH. Macrophage-stimulating protein, the ligand for the stem cell-derived tyrosine kinase/RON receptor tyrosine kinase, inhibits IL-12 production by primary peritoneal macrophages stimulated with IFN-gamma and lipopolysaccharide. J Immunol. 2004;172(3):1825–1832. doi: 10.4049/jimmunol.172.3.1825. [DOI] [PubMed] [Google Scholar]
- 32.Leonis MA, Toney-Earley K, Degen SJ, Waltz SE. Deletion of the Ron receptor tyrosine kinase domain in mice provides protection from endotoxin-induced acute liver failure. Hepatology. 2002;36(5):1053–1060. doi: 10.1053/jhep.2002.36822. [DOI] [PubMed] [Google Scholar]
- 33.Fan J, Ye RD, Malik AB. Transcriptional mechanisms of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2001;281(5):L1037–L1050. doi: 10.1152/ajplung.2001.281.5.L1037. [DOI] [PubMed] [Google Scholar]
- 34.Blackwell TS, Blackwell TR, Holden EP, Christman BW, Christman JW. In vivo antioxidant treatment suppresses nuclear factor-kappa B activation and neutrophilic lung inflammation. J Immunol. 1996;157(4):1630–1637. [PubMed] [Google Scholar]
- 35.Liu SF, Ye X, Malik AB. Inhibition of NF-kappaB activation by pyrrolidine dithiocarbamate prevents In vivo expression of proinflammatory genes. Circulation. 1999;100(12):1330–1337. doi: 10.1161/01.cir.100.12.1330. [DOI] [PubMed] [Google Scholar]
- 36.Liu SF, Ye X, Malik AB. Pyrrolidine dithiocarbamate prevents I-kappaB degradation and reduces microvascular injury induced by lipopolysaccharide in multiple organs. Mol Pharmacol. 1999;55(4):658–667. [PubMed] [Google Scholar]
- 37.Patel IR, Attur MG, Patel RN, Stuchin SA, Abagyan RA, Abramson SB, Amin AR. TNF-alpha convertase enzyme from human arthritis-affected cartilage: isolation of cDNA by differential display, expression of the active enzyme, and regulation of TNF-alpha. J Immunol. 1998;160(9):4570–4579. [PubMed] [Google Scholar]
- 38.Mizui Y, Yamazaki K, Sagane K, Tanaka I. cDNA cloning of mouse tumor necrosis factor-alpha converting enzyme (TACE) and partial analysis of its promoter. Gene. 1999;233(1–2):67–74. doi: 10.1016/s0378-1119(99)00155-9. [DOI] [PubMed] [Google Scholar]