Synopsis
Cell adhesion and migration are tightly controlled by regulated changes in the actin cytoskeleton. Previously we reported that the TGF-β superfamily coreceptor, the type III TGF-β receptor (TβRIII/betaglycan), regulates cell adhesion, migration and invasion and suppresses cancer progression in part, through activation of the small GTPase, Cdc42, and Cdc42-dependent alterations to the actin cytoskeleton. Here we demonstrate that TβRIII specifically promotes filopodial formation and extension in MCF10A and HMEC mammary epithelial cells. Mechanistically, cell surface TβRIII and Cdc42 colocalize to filopodial structures and co-complex in a β-arrestin2 dependent, and a TβRI/TβRII independent manner. The β-arrestin2-mediated interaction between TβRIII and Cdc42 increases complex formation between the Cdc42 effectors, IRSp53 with N-WASP, to increase filopodial formation. We demonstrate a function link between filopodial structures and epithelial cell adhesion as regulated by the TβRIII-Cdc42 interaction. These studies identify TβRIII as a novel regulator of IRSP53/NWASP via Cdc42 to regulate filopodial formation and cell adhesion.
Keywords: TβRIII, filopodia, Cdc42, β-arrestin2, IRSp53
Introduction
Adhesion and migration of both tumor cells and host cells are essential components of the metastatic process. Both cell adhesion and migration are accompanied by changes in the actin cytoskeleton during epithelial to mesenchymal transition, intravasation and heterotypic cell interaction with blood cells and platelets during circulation, extravasation and colonization [1].
Filopodia are thin, actin-rich plasma-membrane protrusions that function as antennae for cells to probe their environment and have an important role in cell migration [2]. The Rho (Ras homology) family small guanosine triphosphatase (GTPase) Cdc42 is a well-known regulator in the formation of filopodia [3]. Deregulation of Cdc42 has been demonstrated in several pathogenic processes including cancer, cardiovascular disease, and neuronal degenerative disease [4]. Several Cdc42 effectors N-WASP, IRSp53, PAK and MRCK have been implicated in filopodia formation. [5]. The Wiskott-Aldrich syndrome protein and neuronal-Wiskott-Aldrich syndrome protein (WASP/N-WASP) are scaffolds that link upstream signals to the activation of the ARP2/3 complex, leading to actin polymerization [6]. IRSp53 was identified as an effector for Rac1 and Cdc42, participating in filopodia and lamellipodia production. [7]. Recent studies have demonstrated that N-WASP is an essential mediator of IRSp53-induced filopodia formation. The SH3 domain of IRSp53 can bind N-WASP directly, with IRSP53 failing to induce filopodia in N-WASP KO fibroblast, while still maintaining the ability to induce lamellipodia formation and membrane ruffling [8]. IRSP53 can also generate filopodia by coupling membrane protrusion through its I-BAR domain with actin dynamics through SH4 domain binding partners including N-WASP and Mena [5]. Upstream mechanisms for regulating Cdc42:IRSP53: NWASP-mediated filopodial formations remain to be defined.
The ubiquitously expressed type III TGF-β receptor (TβRIII/betaglycan) is the most abundantly expressed TGF-β superfamily receptor [9]. TβRIII is classically thought to function as a coreceptor, presenting TGF-β superfamily ligands to their respective signaling receptors [10]. TβRIII mediates TGF-β superfamily ligand dependent as well as ligand independent signaling to both Smad and non-Smad signaling pathways [9]. TβRIII has essential roles in murine and chick development [11, 12], and has been defined as a suppressor of cancer progression/metastasis suppressor, with loss of TβRIII expression correlating with disease progression, advanced stage, and/or a poorer prognosis for patients [13–15].
TβRIII/betaglycan suppresses cancer progression primarily by regulating cancer cell motility and invasion [16–19]. Mechanistically, TβRIII suppresses motility of epithelial derived cancer cells via activation of Cdc42 in a β-arrestin2 dependent manner [18] and by regulating cell adhesion via integrin α5β1 trafficking [20], which can act upstream of Cdc42 as well [21]. Here we examine the mechanism by which TβRIII regulates Cdc42 function in MCF10A and HMEC mammary epithelial cells.
Materials and Methods
Cell Culture and reagents
Immortalized but non-tumorigenic MCF10A and HMEC cells were cultured as described by ATCC or as previously described [22]. DMEM/F12 (1:1) media with 5% horse serum, EGF (epidermal growth factor, 20ng/ml), hydrocortisol (0.5 mg/ml), cholera toxin (100 ng/ml) and insulin (10μg/ml) for MCF10A and DMEM media with 10% fetal bovine serum (FBS) and insulin (10μg/ml) for HMEC’s. COS7 and MEF cell lines were cultured in DMEM with 10% FBS. All cell lines were cultured at 37°C in a humidified incubator. Antibodies used were TβRIII (R&D System, Minneapolis, MN), Cdc42 (Cell Signaling Technology, Danvers, MA), Rac 1 (Santa Cruz Biotechnology, Santa Cruz, CA), β-arrestin 2 (Cell Signaling Technology), IRSp53 (Santa Cruz Biotechnology) and NWASP (Cell Signaling Technology).
Transfection and Adenoviral Infection
Transient transfection was performed as described in transfection reagent manufacture’s protocol. For TβRII and rat TβRIII transient transfection we used FuGENE (Roche Applied Science, Indianapolis, IN) and for siRNA to Control, Cdc42, Rac1 and IRSp53 (all from Santa Cruz Biotechnology) we used Lipofectamine (Invitrogen Life Technology, Carlsbad, CA). Cells were transfected for 48 hours for TβRII and 96 hours for shRNA and Control, Cdc42, Rac1 and IRSp53 before harvesting. Adenoviral infections were performed as previously described (2). For NTC and shTβRIII, adenoviral infections were done at multiplicity infection (MOI) of 50–75 in both MCF10A and HMEC and incubated for 96 hours before harvesting. For GFP-tagged CA/DN Cdc42 adenoviral infections were done at MOI of 100 and incubated for 36 hours before harvesting. shRNA to TβRIII was as described previously [20]. WASP constructs pRK5myc:WASP and pRK5myc:WASPH256ΔSH were kind gifts from Dr. Alan Hall.
Immunofluorescence
For actin staining, cells were plated at a low density to observe filopodia formation and serum depleted for indicated times. Cell were fixed in 4% paraformaldehyde and permeabilized with 0.1% Triton X for 3 minutes. Blocking was performed with 1% BSA and then incubated with 1:50 dilution of phalloidin conjugated to Alexa Fluor 488 or Alexa Fluor 660 for 20 minutes. For TβRIII and Cdc42 staining, cells were fixed in 4% paraformaldehyde and permeabilized with 0.3% Triton X for 5 minutes, blocked with 1% BSA and then incubated with primary antibodies (antiCdc42 and anti-TβRIII, both 1:200 dilution) overnight. Secondary antibodies were used with donkey anti-goat conjugated to Alexa Fluor 555 and donkey anti- rabbit conjugated to Alexa Fluor 488 (Invitrogen Life Technology). Immunofluorescence images were obtained either using a Nikon inverted microscope or a Leica SP5 confocal microscope.
GST-PAK-CRIB pull-down assay and TGF-β Binding and Cross-Linking
GST-PAK-CRIB pull-down assay and TFG-β Binding and Cross-Linking were performed as previously described [13, 16, 18, 23] under the same conditions as for immunofluorescence.
Immunoprecipitation was performed essentially as described previously [20] and under the similar cell density and serum depletion conditions as described for immunoflourescence. Briefly cells were lysed in buffer containing 20mM Hepes pH7.4, 150mM NaCl, 2mM EDTA, 10% glycerol, 10mM NaF and 0.5% NP40 in the presence of protease inhibitors. Cells were lysed in buffer, incubated for 20 min on ice and then centrifuged at 15 000 g, 20 min at 4 °C. Equivalent amounts of protein were immunoprecipitated overnight with the antibody of interest and immune complexes were recovered on either protein G-Sepharose or protein A-Sepharose (GE Healthcare). Immunoprecipitates were washed four times with lysis buffer, twice with the same buffer without Tween-20, and then separated by SDS-PAGE. Proteins were then transferred to a Hybond-C extra nitrocellulose membrane (Amersham), probed with antibodies of interest, and detected by an enhanced chemiluminescence technique. For IP with TβRIII, anti-TβRIII antibody was incubated with protein G-agarose beads. For IP with IRSp53 or NWASP, antibodies were incubated with protein A-agarose beads.
Western Blot Analysis
Membranes were incubated with primary antibodies overnight at 4 °C, washed, and incubated either with horseradish peroxidase-conjugated (1:2,000) or fluorescently tagged secondary antibodies (1:5,000) for 1 h at room temperature. Loading controls were performed using primary antibodies as indicated. Proteins were detected either by the ECL Plus chemiluminescence system (Amersham Biosciences) or the Odyssey infrared imaging system (Li-Cor Biotechnology, Lincoln, NE). Immunoreactive signals were captured on Hyperfilm ECL film and quantified by computer-assisted densitometry.
For immunoprecipitation using binding and crosslinking followed by IP with GST-CRIB beads, cells were first subjected to I-125 TGF-β binding crosslinking as described previously [13, 16, 18, 23]. Cells were then lysed in standard RIPA buffer with protease inhibitors and incubated with GST-PAK-CRIB beads. Immunoprecipitated samples were subject to SDS PAGE and exposed on phosphoimager to detect I-125 TGF-β bound, GST-CRIB immunoprecipitated receptors.
Live Cell imaging
MCF10A cells were infected with adenovirus expressing NTC or shTβRIII followed by transfection with 1μg of GFP tagged actin using Lipofectamine 48 hours prior to imaging. Cells were collected and replated in 35-mm glass bottom dishes (MatTek Corporation) in regular culture medium and placed in a temperature- and CO2-controlled chamber. Zeiss Axio Observer Microscope equipped with 100x objective lens and 488nm filter was used for imaging. Images were taken every 15 seconds for 10 minutes with cooled charge-coupled device (CCD) video camera (Coolsnap ES high resolution CCD camera) operated by Metamorph image analysis software. Filopodia were counted manually for both live cell and fixed IF assays. For live cell analyses, NIH ImageJ’s count function was used, starting at the top of the cell as if it were a clock and moving clockwise around the cell. Ten filopodia were measured for each of seven cells in each of the two conditions (shNTC and shTβRIII). For live-cell imaging, selected filopodia that persisted through at least 3 frames (not a focus error) were measured. The speed of extension started from the first frame (0 sec), and length of a given filopodium was measured at each 15 sec frame until maximal extension (the average was around 45 sec or 3 frames). A variety of relative filopodia sizes were selected within each condition, as well as a variety of the number of frames the filopodium persisted through, to minimize the influence of these factors. Speed of extension calculated by subtracting original filopodium length from final (fully extended) length and dividing by time to maximal extension. For fixed cell analyses all extensions were counted and number of cells for each condition is presented in the text.
Adhesion Assays were performed essentially as described previously [20]. Briefly, 96 well microtiter plates were pre-coated with 2.5 μg/mL of fibronectin and saturated with 1% bovine serum albumin. Adherent cells were detached using 5 mM EDTA and resuspended at a cell density of 25,000 cells/well (depending on the cell line) in serum free media for 60 minutes. Cells were washed in PBS and fixed in 4% paraformaldehyde and then stained with crystal violet for 20 min. The stain was washed, dried and color solubilized using 2% SDS for 20 min. Absorbance was read at 595 nm on the Wallac Victor Counter (PerkinElmer Life Sciences) and values normalized to BSA only controls
Statistical analysis
Significance of results was assessed using either standard student t-test, or ANOVA analyses followed by post hoc analysis of either a 1 sample t-test to compare the experimental condition to the control or a 2 –tailed unpaired Student’s t test, with a minimum of a 95% confidence interval. These are indicated in legends.
Results
TβRIII promotes filopodia formation and extension
We have previously demonstrated that restoring TβRIII expression can regulate cell adhesion and migration via effects on the actin cytoskeleton [18, 20]. To determine the mechanism by which TβRIII regulates the actin cytoskeleton in epithelial cells, we silenced endogenous TβRIII expression in the immortalized, but non-tumorigenic MCF10A and HMEC human mammary epithelial cells, and examined effects on serum starvation induced filopodia formation. shRNA-mediated silencing of TβRIII expression (shTβRIII) in MCF10A and HMEC cells (Fig. 1A and Supp. Fig. 1) significantly decreased both the number (Fig. 1C, Supp. Fig. 1) and length (Fig. 1E) of the filopodia relative to non-targeting shRNA control (NTC) (Fig. 1B). The effect of shTβRIII was specific as we were able to rescue the effects of shRNA-mediated silencing of TβRIII expression on filopodia with shTβRIII-resistant rat TβRIII (Fig. 1C, Supp. Fig. 1), which effectively restored TβRIII expression (Fig. 2A).
Figure 1. TβRIII promotes filopodial formation and extension in human mammary epithelial cells.
(A) MCF10A mammary epithelial cells adenovirally infected with either shRNA to TβRIII (shTβRIII) or non-targeted control shRNA (shNTC) were harvested 96 hours after infection and TβRIII expression levels assessed either by I-125 TGF-β binding and crosslinking followed by immunoprecipitation for TβRIII to examine cell surface levels (upper panel) or by western blot in the total cell lysates. β-actin was used as a loading control. (B) MCF10A cells either shNTC or shTβRIII, or shTβRIII followed by rescue with rat TβRIII (rTβRIII) cells were serum starved to induce filopodia, fixed and stained for actin using phalloidin-conjugated to Alexa 488. Only adenovirus expressing cells were examined. Representative examples of the spectrum observed are presented with the number in each panel indicating the number of filopodia for the presented cell (C) Average filopodial number per cell was quantitated (Methods), and presented. Data represent the mean ± SE of three independent experiments with >150 cells per condition counted. *,p<0.005, **, p<0.05 (D–F) Filopodial dynamics of shNTC and shTβRIII MCF10A cells. Cells were imaged using actin-GFP for 10 minutes as described in Methods, with images acquired every 15 seconds, Bar= 1μm. Average length (E) and extension speed (F) of 40 filopodia/condition were quantified and presented here. Arrows indicate filopodia. **, p<0.05.
Figure 2. TβRIII activates Cdc42 in human mammary epithelial cells.
(A, B) MCF10A (left) and HMEC (right) cells infected with shNTC, shTβRIII, and shTβRIII followed by rescue with rat TβRIII (rTβRIII), were assayed after serum starvation to mimic filopodia-inducing conditions for either Cdc42-GTP (A) or Rac1-GTP levels (B) as described in Methods. 5% of total cell lysate was used for input. Representative experiment of 3 replicates is presented. (C–D) MCF10A were infected with shNTC or shTβRIII, followed by infection with adenovirus expressing GFP (control), GFP tagged constitutively active Cdc42 (CACdc42) or dominant-negative Cdc42 (DNCdc42), serum starved for 14–16 hours then fixed and stained for actin using with phalloidin conjugated Alexa Fluor 660. Cells with both dsRed (to select cells expressing shNTC or shTβRIII) and GFP (to detect cells expressing CACdc42 or DNCdc42) were examined using confocal microscopy and counted for filopodia. Examples of cells under each condition are presented with the number in each panel indicating the number of filopodia for the presented cell. Average number of filopodia per cell of 3 independent experiments is shown. *p<0.05
Filopodia grow and retract after their initiation, exhibiting rich dynamical behaviors. To investigate the mechanism by which TβRIII was regulating filopodial number and length we used sequential time-lapse wide field fluorescence microscopy on control MCF10A-shNTC and MCF10A-shTβRIII cells expressing actin–GFP to follow dynamics of the actin cytoskeleton as described in detail in Methods. The live cell analyses (Fig. 1D–F) focused on cytoskeletal structures in dynamic flux between microspikes and filopodia, with the majority of the structures returning to a short microspike-like state. Filopodia selected for quantification were cycling through elongation and retraction, commonly observed for filopodial structures. We find that shRNA-mediated silencing of TβRIII expression decreased the rate of filopodial extension (Fig. 1F), suggesting that TβRIII regulates filopodial number and length through regulation of the rate of filopodial extensions.
TβRIII is required for maximal activation of Cdc42 in human mammary epithelial cells to regulate filipodia formation
The small Rho-subfamily GTPase, Cdc42, is a well-established regulator of filopodia formation. We have previously demonstrated that restoring TβRIII expression in cancer cells constitutively activated Cdc42 and Rac1 [18]. Accordingly, we investigated the effects of silencing endogenous TβRIII expression on Cdc42 and Rac1 activity in human mammary epithelial cell lines under conditions that stimulated filopodia formation (serum starvation). In both MCF10A and HMEC human mammary epithelial cell lines, shRNA-mediated silencing using two independent shRNA’s to TβRIII decreased Cdc42 activation (Fig. 2A, Supp. Fig. 2), and this effect was specific as the shRNA-mediated silencing decrease in Cdc42 activation could be rescued with shRNA resistant rat TβRIII (Fig. 2A). Interestingly, rescue of TβRIII expression in shTβRIII cells did not further enhance CDC42 activation in part due to high basal Cdc42 activation under serum-starved conditions in shNTC cells. In contrast, shRNA-mediated silencing of TβRIII had no effect on Rac1 activation in either MCF10A or HMEC cells (Fig. 2B) consistent with our prior observation on TβRIII’s effects on Rac1 in cancer cells [18]. These support a specific effect of TβRIII on Cdc42 in human mammary epithelial cells and indicate that while TβRIII is required for maximal activation, it may not further enhance activation of cells that have constitutively high active Cdc42 levels as observed for the MEC’s under serum free conditions (Fig. 2A, shNTC)
To investigate whether Cdc42 was downstream of TβRIII in regulating filopodia formation, we expressed either dominant negative Cdc42 (DN-Cdc42) or constitutively active Cdc42 (CA-Cdc42) in the presence or absence (shTβRIII) of TβRIII expression (Fig. 2C). Similar to the effect of shRNA-mediated silencing of TβRIII expression, DN-Cdc42 decreased filopodial number to the same level as shTβRIII (Fig. 2C,D). Importantly, in the presence of DN-Cdc42, shRNA-mediated silencing of TβRIII expression was unable to further decrease filopodial formation, suggesting that TβRIII was functioning through Cdc42 to mediate its cellular effects (Fig. 2C,D). Consistent with a role for Cdc42 downstream of TβRIII, CA-Cdc42 was able to rescue 50% of the phenotype of shRNA-mediated silencing of TβRIII expression on filopodial formation (Fig. 2C, D), without altering basal filopodial formation. Taken together, these data indicate that TβRIII regulates filopodia formation at least in part through activation of Cdc42 in human mammary epithelial cells.
TβRIII co-complexes specifically with Cdc42 and co-localize to the cell edge and filopodial structures
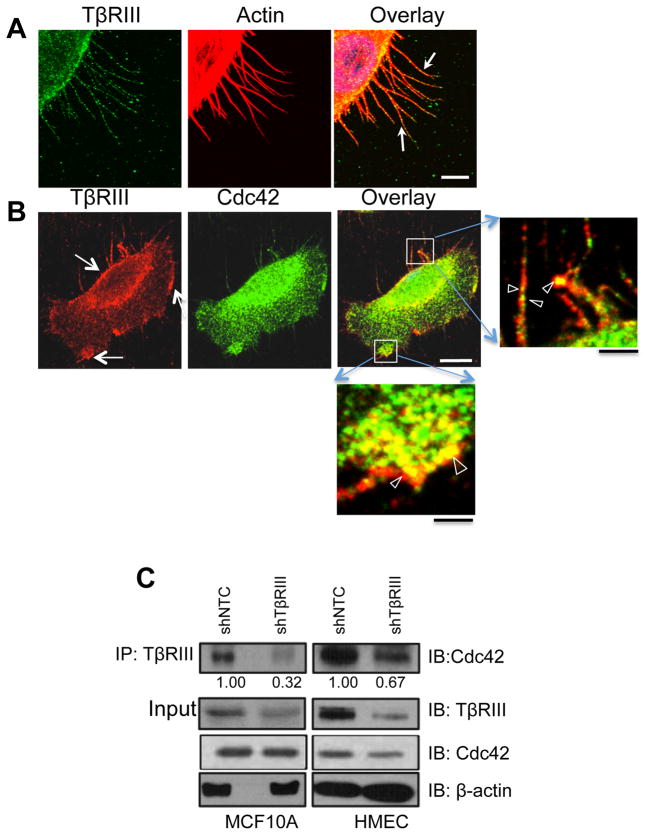
The localization of small Rho-GTPases is thought to have an important role in regulating their function. As TβRIII activates Cdc42 to regulate filopodia formation, we first examined the localization of endogenous TβRIII upon stimulation of filopodia in MCF10A cells using serum starvation. Endogenous TβRIII localized in a punctate fashion to filopodial structures and at the base of the filopodial structures (Fig. 3A). Upon examining the spatial relationship between endogenous Cdc42, TβRIII and the actin cytoskeleton in mammary epithelial cells, endogenous TβRIII localized along the cell membrane, and along the lengths of filopodia (Fig. 3A, B), while endogenous Cdc42 localized along the cell membrane and on filopodial structures (Fig. 3B). Perinuclear localization for TβRIII, as have been noted for other internalized receptors [24] and nuclear localization for Cdc42 was also observed. Endogenous Cdc42 and TβRIII co-localized at the cell membrane and along filopodial extensions (Fig. 3B). To determine whether TβRIII and Cdc42 existed in a complex, we performed co-immunoprecipitation analysis of the endogenous proteins. In both MCF10A and HMEC cells, immunoprecipitating endogenous TβRIII resulted in the co-immunoprecipitation of endogenous Cdc42 (Fig. 3C). shRNA-mediated silencing of endogenous TβRIII expression decreased the amount of Cdc42 immunoprecipitated by TβRIII antibody (Fig. 3C), supporting the specificity of the interaction.
Figure 3. Cell surface TβRIII interacts with and colocalizes with Cdc42 at the cell edge and on filopodial structures.
Confocal images of sub-confluent MCF10A cells serum starved for 14 hours before fixation and staining for (A) endogenous TβRIII (green) or actin (red) using phalloidin. Arrows indicate representative points of punctate localization of TβRIII at filopodial structures and at the base of filopodia. Bar=10μm or (B) endogenous TβRIII (red) and endogenous Cdc42 (green) were labeled using antibody to TβRIII and Cdc42. Closed arrows indicate representative areas where TβRIII and Cdc42 colocalize. Open arrows in magnified areas of boxed regions indicate areas where TβRIII and Cdc42 colocalize. White Bar= 3.3 μm, Black Bar=2.2μm. (C) Indicated MCF10A cells were immunoprecipitated with antibody to TβRIII and immunoblotted with antibody to endogenous Cdc42, with assessment of <5% of the cell lysate for TβRIII and Cdc42. Data are representative of >3 independent experiments. Quantitation relative to total input Cdc42 is presented below the immunoblot.
To investigate whether cell surface TβRIII formed a complex specifically with activated Cdc42, we utilized two distinct approaches. First, we examined the interaction between TβRIII and WASP, which binds with much higher affinity to Cdc42 than Rac1 [25] and depends on the nucleotide state of Cdc42 [26]. We find that endogenous TβRIII co-immunoprecipitated with WASP (Fig. 4A) in MCF10A cells. WASP interacts with Cdc42 via amino acids 201–321 [25]. To determine if the interaction was dependent on the ability of WASP to interact with Cdc42-GTP, we utilized the WASP mutant WASP H246ΔSH [25] and find that it was deficient at co-complexing with endogenous TβRIII (Fig. 4A). As an alternate method to examine TβRIII’s ability to interact with Cdc42 in its GTP bound state, we labeled cell surface TGFβ receptors with I125-TGFβ1, and performed a pulldown with GST beads conjugated to PAK-CRIB, which binds activated Cdc42 and Rac1 [27], or GST alone as control. GST-PAK-CRIB was able to pull down cell surface TβRIII, along with TβRII and TβRI in both MCF10A and HMEC cells (Fig. 4B), suggesting that perhaps, via TβRIII, all three TGFβ receptors may interact with activated Cdc42/Rac1(Fig. 4B,C), consistent with what has been reported for TβRII and TβRI previously [23]. Consistent with this hypothesis, the interaction of TβRIII and TβRII with activated Cdc42/Rac1 was decreased (Fig. 4B) by shRNA-mediated silencing of TβRIII expression (Fig. 4C), demonstrating the specificity of the TβRIII –Cdc42 interaction and suggesting that TβRIII may be required for the TβRII/TβRI -Cdc42/Rac1 interaction as well. Conversely, increasing TβRIII expression at the cell surface (Fig. 4C) in both MCF10A and HMEC cells resulted in increase in the amount of TβRIII that interacted with Cdc42.
Figure 4.
(A) MCF10A cells transiently transfected with either wild type WASP construct or WaspH246ΔSH were immunoprecipitated with anti-Myc antibody and immunoblotted for TβRIII or immunoprecipitated with TβRIII and immunoblotted for Myc as indicated. 5% of cell lysate was used to detect total TβRIII and expression of WASP constructs. Integrated density of the amount of TβRIII and MYC immunoprecipitated relative to inputs is presented using Odyssey infrared imaging system (LI-COR, Lincoln, NE) (B) MCF10A and HMEC cells expressing either shNTC, shTβRIII or full length TβRIII (FLTβRIII), were serum starved for 14 hours and subjected to 125I-TGF-β1 binding and cross-linking followed by pulldown (PD) with GST-CRIB beads (Methods) for active Cdc42. Parallel GST-PAK CRIB pull downs were performed to determine active Cd42 (GTP-Cdc42) levels as well. A representative experiment of 2 replicates of each cell line is presented with β-actin and Cdc42 as a loading control. For quantification of signal, Odyssey infrared imaging system (LI-COR, Lincoln, NE) was used and the quantification for the fold changes relative to total Cdc42 levels is presented (C) Parallel 125I-TGF-β1 binding and crosslinking studies of total cell lysates were performed to observe knockdown and overexpression of TβRIII at the cell surface. (D) MCF10A cells were subjected to siRNA mediated silencing with either non targeting control oligos (siCtl), Cdc42 (siCdc42) or Rac1 (siRac1). After 14 hours of serum starvation, cells were subjected to 125I-TGF-β1 binding and cross-linking followed by pulldown with GST-CRIB beads for active Cdc42 or Rac1. Immunoblots examining extent of Cdc42 and Rac1 knockdown and levels of TβRIII are presented below. A representative experiment of 3 replicates is presented. Graph of fold average TβRIII intensity relative to control of 3 independent experiments is presented (Right). * p<0.01
As GST-PAK-CRIB beads interact with activated Cdc42 and Rac1, to investigate the specificity of the interaction with cell surface TGFβ receptors, we individually silenced Cdc42 and Rac1 expression using siRNA, and performed GST-PAK-CRIB pulldown assays. While silencing of endogenous Cdc42 expression decreased GST-PAK-CRIB -mediated immunoprecipitation of cell surface TβRIII, silencing of endogenous Rac1 had no effect (Fig. 4D), supporting a specific interaction between cell surface TβRIII and activated Cdc42.
Taken together, these data indicate specific co-complex formation between TβRIII and Cdc42-GTP.
TβRIII can interact with activated Cdc42 independent of TβRII and TβRI
Although TβRIII functions as a co-receptor for TGFβ receptors, including TβRII and TβRI, TβRIII is present in abundance relative to TβRII and TβRI. Accordingly, we investigated whether the interaction of TβRIII with activated Cdc42 depends on TβRII or TβRI using a series of mink lung epithelial cell lines, wild type MV1Lu, which express all three TGF-β receptors, R1b, which lacks TβRI expression and DR, which lack TβRII and TβRI expression [28]. GST-PAK-CRIB pulled down cell surface iodinated TβRIII, along with TβRII and TβRI in wild type MV1Lu, TβRIII and TβRII in R1b cells and TβRIII in DR cells (Fig. 5A). DR cells with GST alone beads did not pull down any TβRIII (Fig. 5A). While the extent of the interaction with TβRIII was modestly diminished in DR cells (Fig. 5A), suggesting that TβRII might contribute to the interaction, TβRIII was still able to interact with activated Cdc42 in the absence of TβRII and TβRI.
Figure 5. Surface TβRIII interacts with GTP bound Cdc42 in a TβRII and TβRI independent manner.
(A) Mink lung epithelial cell lines MV1Lu (wild type), R1b (TβRI−/−) and DR (TβRI−/− and TβRII−/−) were serum starved for 14 hours and subjected to 125I-TGF-β1 binding and cross-linking followed by GST-CRIB pulldown assay (PD) with GST only as a control. Expression level of TβRI, TβRII and TβRIII of MV1Lu, R1b and DR cells were detected by 125I-TGF-β1 binding and cross-linking (right panel). A representative of two independent replicates is presented. β-actin was used as a loading control. Integrated density, normalized to total TβRIII, is presented below and fold integrated density of TβRIII for each cell line relative to MV1Lu cells is presented below. (B) COS7 cells infected with adenovirus expressing either GFP or TβRIII followed by transfection with empty vector, TβRII-HA or kinase dead TβRII (TβRIIKD-HA) were subjected to 125I-TGF-β1 binding and cross- linking followed by GST-CRIB pulldown assay. 5% of total cell lysate was used for input to immunoblot against TβRIII, β-actin and anti-TβRII using Odyssey infrared imaging system to determine expression of transfected receptors. Control TβRIII intensity was subtracted from each condition, normalized to total TβRIII and fold TβRIII intensity bound to GST-CRIB relative to TβRIII only condition is presented. Quantified data represent the average of five independent experiments. ANOVA; p<0.0001; one sample t-test and two sample t-test: *p<0.005
TβRIII forms a complex with TβRII, which subsequently phosphorylates TβRIII on its short cytoplasmic domain [29] and can also regulate signaling of other TGF-β superfamily ligands including activin and BMP, via their type II receptors [30]. To investigate the contribution of the kinase activity of TβRII, and other TGF-β superfamily type II receptors, ActRII and BMPRII, to TβRIII’s interaction with Cdc42, we expressed exogenous TβRIII with the wild type type II receptors and/or kinase dead TβRII, which functions as a dominant negative, in COS7 cells, labeled the cell surface receptors with I125TGFβ1 and pulled down labeled receptors with GST-PAK-CRIB beads. Consistent with our prior results, both endogenous TβRIII, which is present at low but detectable levels, and TβRII, and exogenously expressed TβRIII and TβRII were pulled down by GST-PAK-CRIB (Fig. 5B). Expression of kinase dead TβRII reduced the level of binding of TβRIII and TβRII to GST-PAK-CRIB (Fig. 5B). Increasing expression of ActRII and BMPRII had no effect on altering TβRIII’s interaction with GST-PAK-CRIB (Supp. Fig. 8). These data indicate that while TβRIII can interact with activated Cdc42 independently of the type II receptors TβRII, ActRII and BMPRII or TβRI, the kinase activity of TβRII enhances TβRIII’s interaction with activated Cdc42.
β-arrestin2 is essential for TβRIII’s interaction with GTP-Cdc42
To determine the structural determinants on TβRIII required for the interaction between TβRIII and Cdc42, we overexpressed either GFP control, TβRIII (wild type full-length TβRIII) or TβRIIIΔCyto (TβRIII lacking cytoplasmic domain) in MCF10A cells. Using GST-PAK-CRIB pulldown we determined that, interaction between GST-PAK-CRIB and GFP expressing cells was below detectable levels. However increasing TβRIII levels resulted in co-complex formation between TβRIII and GST-PAK-CRIB (Fig. 6A, top). In contrast, TβRIIIΔCyto was pulled down much less efficiently, with a 60% decrease relative to TβRIII (Fig. 6A, B), suggesting that the cytoplasmic domain of TβRIII mediated the interaction of TβRIII with active Cdc42. Aside from interaction with and phosphorylation by TβRII, the cytoplasmic domain of TβRIII has been demonstrated to interact with two scaffolding proteins, β-arrestin2, via threonine 841, and GIPC, via a class I PDZ binding motif comprised of the last three amino acids of TβRIII. To examine the contribution of TβRIII’s interaction with β-arrestin2 and GIPC, we utilized TβRIII-T841A (β-arrestin2 binding deficient) and TβRIII-DEL, (GIPC binding deficient) [31]. While TβRIII-DEL was pulled down by GTP bound Cdc42 (Fig. 6A,B) TβRIII-T841A was pulled down much less efficiently, with a 70% decrease relative to TβRIII (Fig. 6A, B), suggesting that the interaction of TβRIII with Cdc42 was mediated by the interaction of TβRIII with β-arrestin2.
Figure 6. β-arrestin2 is required for TβRIII’s interaction with and activation of Cdc42.
(A–B) MCF10A cells infected with adenovirus expressing GFP (control), TβRIII (wild type full length TβRIII), TβRIIIΔCyto (TβRIII lacking cytoplasmic domain), TβRIII-Del (cannot bind to GIPC) and TβRIII-T841A (cannot bind to β-arrestin2) were serum starved for 14 hours and subjected to pulldown (PD) with GST-PAK-CRIB beads. Pulldowns were subject to immunoblotting against TβRIII. 5% of lysate was used as inputs and immunoblotted against TβRIII, Cdc42 and β-actin using Odyssey infrared imaging system. (B) TβRIII pulled down with GST-PAK CRIB for each condition is normalized to total TβRIII for each condition then fold relative to TβRIII is presented. For quantification of signal, Odyssey infrared imaging system (LI-COR, Lincoln, NE) was used and the quantification for the fold changes for four independent experiments is presented. ANOVA; p<0.005; one sample t-test *p<0.05 C) MCF10A cells were subjected to siRNA mediated silencing with either non targeting control oligos (siCtl), or β-arrestin2 (si β-arr2). After 14 hours of serum starvation, cells were subjected to 125I-TGF-β1 binding and cross-linking followed by pulldown with GST-CRIB beads for active Cdc42 or Rac1. Top panel represents cell surface TβRIII in siCtl and sib-arr2 cell interacting with GST-PAK-CRIB. As input TβRIII, β-arrestin2 and β-actin levels are presented (D) Mouse embryonic fibroblasts (MEF cell lines), wild type (β-arr2 +/+) and β-arrestin2 knock out (βarr2 −/−) were subjected to 125I-TGF-β1 binding and cross-linking and then to GST-PAK-CRIB pulldown assays. Top panel represents cell surface TβRIII in WT and βarr2 KO MEF pulled down with GST-PAK-CRIB. As input TβRIII β-arrestin2 and β–actin are shown. Data represent one of three independent experiments. (E–F) MCF10A cells infected with adenovirus expressing shNTC or shTβRIII, were transfected with control empty vector (EV), rat TβRIII (rTβRIII, column 3), rat TβRIIIΔcyto (rTβRIIIΔcyto), rat TβRIIIDel (rTβRIIIDel) and rat TβRIIIT845A (rTβRIIIT845A, TβRIII mutant which cannot bind to β-arrestin2, column 6). After 48 hours, cells were re-plated on the coverslips and serum starved for 14 hours. Cells were fixed and actin stained with phalloidin. Arrows indicate filopodia (E). (F) Filopodia in each condition (n>50) were counted as described in Methods. Data represent the average of three independent experiments. ANOVA test was used to determine difference between groups followed by post hoc t- tests. * 1 sample t- test, ** standard 2 tailed t-test. Both * and ** have p<0.05.
To investigate the role of β-arrestin2 in the interaction of TβRIII and activated Cdc42, we utilized two distinct approaches. First, we used siRNA to β-arrestin2 in MCF10A cells. Compared to siRNA control cells, the interaction of cell surface TβRIII with activated Cdc42 was significantly reduced in si-β-arr2 MCF10A cells (Fig. 6C). As an alternative approach, we used mouse embryonic fibroblasts (MEF’s) derived from β-arrestin2 knock out mice [32]. Compared to MEFs obtained from wild type (WT) littermate controls, the interaction of cell surface TβRIII with activated Cdc42 was significantly reduced in β-arrestin2 −/− MEFs (Fig. 6D). In addition, activation levels of active Cdc42 were also reduced in β-arrestin2 −/− MEF cells compared to β-arrestin2 +/+ cells (Supp. Fig. 4), consistent with our previously published observations demonstrating a role for β-arrestin2 in Cdc42 activation (Mythreye and Blobe 2009). Taken together, these data suggest that the interaction of TβRIII with Cdc42 is mediated via β-arrestin2 and is required for efficient Cdc42 activation.
To investigate the role of the interaction of TβRIII with Cdc42 via β-arrestin2 on filopodial formation, we transiently knocked down TβRIII expression and rescued with shRNA resistant rat wild type TβRIII, TβRIIIΔCyto, TβRIII-DEL and TβRIII-T845A (Supp. Fig. 5). Consistent with our prior results, shRNA-mediated silencing of TβRIII expression decreased filopodial formation (Fig. 6E, F), which was rescued by wild type TβRIII. In addition, TβRIII-DEL was able to effectively rescue filopodial formation, while TβRIIIΔCyto and TβRIII-T845A were unable to do so (Fig. 6E, F). These data strongly support a role for TβRIII via β-arrestin2 interacting with Cdc42 in regulating filopodial formation in human mammary epithelial cells.
TβRIII regulates IRSp53 and NWASP interaction via Cdc42 activation to regulate cell adhesion
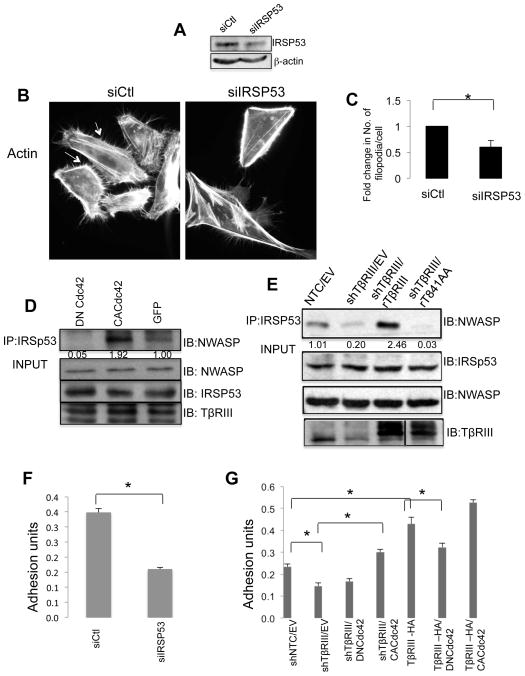
The Rho GTPase, Cdc42, has a number of downstream effectors. One of these effectors, IRSp53 has a specific role in mediating filopodial formation in a context dependent manner [7, 33]. In addition, increasing IRSp53 in most cell types induces extensive actin-containing cell protrusions that are dependent on Cdc42 and Rac1 [7]. IRSp53 has been demonstrated to directly interact with N-WASP as well, with this interaction being required for IRSp53-induced filopodial formation [5]. To investigate whether IRSp53 has a role in filopodial formation in human mammary epithelial cells, we silenced IRSp53 expression and examined the actin cytoskeleton of MCF10A cells. Compared to the control, siRNA-mediated silencing of IRSp53 expression decreased filopodial formation in MCF10A cells (Fig. 7A–C). As we have established a role for Cdc42 in regulating filopodial formation in human mammary epithelial cells, we investigated whether altering Cdc42 activation was able to alter the interaction of IRSp53 and NWASP in mammary epithelial cells. Constitutively activate Cdc42 (CACdc42) increased the interaction of IRSp53 and NWASP (Fig. 7D, Supp. Fig. 6A), while dominant negative Cdc42 (DNCdc42) decreased the interaction of IRSp53 and NWASP compared to GFP control cells (Fig. 7D, Supp. Fig. 6A). To determine the functional link between TβRIII- β-arrestin2 and the IRSp53 and N-WASP complex that regulates filopodial formation, we determined whether TβRIII was required for the IRSP53-NWASP interaction. We silenced TβRIII expression, rescued with shRNA resistant wild type TβRIII or TβRIII-T845A, and assessed the interaction of IRSp53 and N-WASP in MCF10A cells. Consistent with a role for TβRIII, shRNA-mediated silencing of TβRIII expression decreased the interaction of IRSp53 with N-WASP (Fig. 7E, Supp. Fig. 6B). Importantly, the effect of shRNA-mediated silencing of TβRIII expression on the interaction of IRSp53 with N-WASP could be rescued by wild type TβRIII but not by TβRIIIT845A (Fig. 7E, Supp. Fig. 6B). These data demonstrate that the TβRIII-β-arrestin2 interaction is important for filopodia formation in human mammary epithelial cells, with TβRIII mediating the interaction of IRSp53 and NWASP in a β-arrestin2 and Cdc42 dependent manner.
Figure 7. TβRIII enhances IRSp53 and NWASP interaction to regulate filopodia formation and cell adhesion in epithelial cells.
(A–C) MCF10A cells transfected with either control siRNA (siCtrl) or siRNA to IRSp53 (siIRSp53) were either (A) immunoblotted against IRSP53 to determine extent of knockdown (B) fixed and stained for actin with phalloidin conjugated with Alexa Fluor 488. (C) >20 cells/condition in two experiments were counted for filopodia and average number of filipodia is compared in both conditions. *, p<0.01. (D) MCF10A cells infected with either control GFP adenovirus (control), constitutively active Cdc42 (CACdc42) or dominant-negative Cdc42 (DNCdc42) and subjected to anti-IRSp53 immunoprecipitation (IP) and probed for NWASP. NWASP, IRSP53 and TβRIII are shown as input controls. Integrated density of immunoprecipitated WASP, normalized to total WASP, is presented below (E) shNTC or shTβRIII MCF10A cells transfected with empty vector, rTβRIII or rTβRIIIT845A were immunoprecipitated-using antibody to endogenous IRSp53 and immunoblotted against endogenous NWASP. Inputs were probed for IRSp53, NWASP and TβRIII as input controls. Integrated density relative to input WASP is presented. For quantification of signal of D and E, Odyssey infrared imaging system (LI-COR, Lincoln, NE) was used. (F–G) MCF10A cells transfected with (F) either control siRNA (siCtrl) or siRNA to IRSp53 (siIRSp53) or (G) MCF10A cells with shNTC and shTβRIII transfected with empty vector (EV), rTβRIII or rTβRIIIT845A, overexpressing TβRIII (TβRIII-HA) alone or with DNCdc42 and CACdc42 as indicated were plated on fibronectin coated 96 wells for 1 hr, and adhesion levels determined as described in Methods. ANOVA test was used to determine difference between groups followed by post hoc unpaired 2 tailed student t- tests between indicated groups. *, p<0.05
We have recently demonstrated a role for TβRIII in regulating epithelial cell adhesion that depends on β-arrestin2 [20]. In addition, IRSP53 has recently been shown to regulate cell-ECM adhesion signaling in epithelial cells [34]. Therefore, to examine the functional link between filopodia formation and cell adhesion in mammary epithelial cells, we silenced IRSp53 expression and examined the effect on adhesion to fibronectin (FN) in MCF10A cells. Compared to the control, siRNA-mediated silencing of IRSp53 expression decreased cell adhesion in MCF10A cells (Fig. 7F). To further determine if TβRIII effects cell adhesion via Cdc42, which in turn regulates the IRSp53-NWASP interaction, we examined the effect of altering CDC42 upon altering TβRIII expression. As we previously reported [20], shTβRIII significantly reduced cell adhesion to FN, while increasing TβRIII expression significantly enhanced cell adhesion (Fig. 7G, Supp. Fig. 7). Consistent with a role for Cdc42 downstream of TβRIII, CA-Cdc42 was able to rescue the adhesion defect in shTβRIII cells (Fig. 7G, Supp. Fig. 7). Conversely, DNCdc42 was able to significantly diminish TβRIII induced cell adhesion (Fig. 7G). These data demonstrate a functional link between the TβRIII-Cdc42 complex in downstream IRSP53-NWASP dependent filopodia formation and cell adhesion in mammary epithelial cells.
Discussion
The ability of TβRIII to suppress cancer progression is due, in part, to inhibition of migration and invasion in cancer cells [9]. We have reported specific effects of TβRIII on directional migration of epithelial-derived cancer cells in a Cdc42 dependent manner [18]and effects of TβRIII on cell adhesion via integrin α5β1 [20], which can function upstream of Cdc42 [21]. Here, we demonstrate that in mammary epithelial cells TβRIII is required for filopodial formation, via direct co-complexing with active Cdc42 to regulate Cdc42 activation. Further, we demonstrate an essential role for IRSP53 in filopodial structure organization to regulate cell adhesion in epithelial cells and find that TβRIII is required for the interaction of IRSp53 and NWASP to regulate filopodia formation downstream of Cdc42.
The mechanism by which Cdc42 specifically promotes filopodia formation is controversial and an active area of investigation. Over 30 target proteins have been identified that interact with either or both Cdc42 and Rac1[35] [7]. Several studies implicate a WASP/NWASP interaction with Arp2/3 downstream of Cdc42 in filopodia formation in vitro [36] and in vivo [37, 38]. IRSP53, which is a target of Cdc42 [33], has been shown to bind Mena and Ena/VASP family proteins to promote filopodia formation [7]. IRSp53 has also been shown to be a downstream effector of Rac1 [37]. Here we tested the interactions of NWASP-ARP2/3 and IRSp53-Mena in the presence and absence of TβRIII in mammary epithelial cells but were unable to detect interaction of the endogenous proteins (data not shown). Thus the mechanisms by which Cdc42 regulates filopodia formation are likely to be context dependent.
Cdc42 regulates multiple cellular functions including motility, proliferation, apoptosis and cell morphology. To perform these diverse roles, the timing and location of Cdc42 activation must be tightly controlled [39]. Spatiotemporal dynamic studies of Cdc42 localization revealed localized activation of Cdc42 in the cell periphery at cell edges extending filopodia but not within the filopodia themselves [39]. However, EGF stimulated filopodia have been shown to contain active Cdc42 [40]. IRSp53 has been localized to the very edge or tips of filopodia [41] and is activated by an interaction with Cdc42. NWASP has also been found to be active on extending filopodia [40], implying the presence of activated Cdc42 in filopodial structures. We observe Cdc42 and TβRIII colocalization both at the cell periphery and in extended filopodia, and demonstrate that the TβRIII –Cdc42 interaction is essential for Cdc42 activation and filopodial formation in epithelial cells. Our data suggest that TβRIII activates Cdc42 by interactions on the cell periphery and possibly in the filopodia as well. Our studies also demonstrate specifically the ability of cell surface TβRIII to co-complex with active Cdc42. Further studies will focus on the dynamics of the interaction in a spatio-temporal fashion.
We also observed that while the cytoplasmic domain of TβRIII significantly diminished co-complex formation between TβRIII and GTP-Cdc42, it was not completely abrogated. We hypothesize that the transmembrane domain of TβRIII, which can interact with TβRII or TβRI, may contribute to the interaction, as TβRII and TβRI can also interact with Cdc42 [23].
TGF-β binds either to TβRIII which presents the ligand to dimeric TβRII or directly to TβRII. Ligand binding to TβRII leads to recruitment of TβRI and activates TβRI kinase activity which can initiate Smad and non-Smad signaling pathways [42]. TGF-β induces actin cytoskeleton mobilization in cells, and these effects appear to be dependent upon non-Smad signaling [43, 44]. TGF-β can also activate Cdc42 [43] [18]. Here we demonstrate a specific interaction between active Cdc42 and TβRIII/TβRII/TβRI. Interestingly, TβRIII’s interaction with active Cdc42 is only partially dependent on the presence of TβRII or TβRI (Fig. 5A, B). Interactions of TβRII and TβRI with Cdc42 have been reported [23]. Interestingly, we find in both MCF10A and HMEC cells, loss of TβRIII resulted in a reduction in the binding of TβRII to active Cdc42 (Fig. 4) indicating a requirement for TβRIII in forming a functional complex between the TGF-β receptors and Cdc42. In addition, reduction of TβRI binding in shTβRIII cells was also observed (Fig. 4B), which could either be due to reduced ligand presentation in the absence of TβRIII or due to decreased TβRIII-mediated TGF-β receptor complex formation with Cdc42-GTP. We did observe some variability in total Cdc42 levels upon increasing TβRIII expression, but this was not consistent. Conversely, as TβRIII can interact with Cdc42GTP in the absence of TβRI (Fig. 5A, 5B) overexpression of TβRIII may sequester Cdc42-GTP reducing the amount of Cdc42-GTP available to complex with TβRI (Fig. 4B, C). The role of activated Cdc42/Rac1 in interacting with distinct TGF-β receptors remains to be explored.
Efforts to define the mechanisms mediating TβRIII’s roles in cell migration and invasion to regulate cancer progression have revealed several mechanisms including TβRIII dependent down regulation of NFκB via β-arrestin2 and activation of Cdc42 via β-arrestin2 and regulation of integrin trafficking also via β-arrestin2 [20] [18, 45]. These studies have defined β-arrestin2 as a key regulator for facilitating these interactions. TβRIII–Cdc42 interaction via β-arrestin2 may be required for proper transport of Cdc42 to sites of actin nucleation. As such, we have recently demonstrated that TβRIII can regulate integrin α5β1 trafficking via β-arrestin2 [20] and hence similar trafficking consequences might be observed in the context of Cdc42 as well. Here we demonstrate a novel role for TβRIII in regulating filopodial formation via activation of Cdc42 and effects on the downstream Cdc42 effectors, IRSp53 and NWASP. The activation of Cdc42 in response to upstream signaling is regulated by guanine nucleotide exchange factors (GEFs) [46, 47] and GTPase activating proteins. Current studies are directed at identifying the GEF’s specifically coupling the TGF-β receptors to Cdc42 to regulate epithelial cell filopodial formation.
Supplementary Material
Acknowledgments
We thank Alan Hall for the kind gifts of the WASP constructs.
Funding: This work was supported in part by NIH Grants R01-CA135006 and R01-CA136786 (GCB), Komen for the Cure Grants KG090154 and SAC100002 (GCB) and Department of Defense grant W81 XWH-09-1-0265 (KM).
References
- 1.Moncharmont C, Levy A, Gilormini M, Bertrand G, Chargari C, Alphonse G, Ardail D, Rodriguez-Lafrasse C, Magne N. Targeting a cornerstone of radiation resistance: Cancer stem cell. Cancer Lett. 2012 doi: 10.1016/j.canlet.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Meng W, Xia Q, Wu L, Chen S, He X, Zhang L, Gao Q, Zhou H. Downregulation of TGF-beta receptor types II and III in oral squamous cell carcinoma and oral carcinoma-associated fibroblasts. BMC Cancer. 2011;11:88. doi: 10.1186/1471-2407-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 4.Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70:9621–9630. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 5.Fonsatti E, Vecchio LD, Altomonte M, Sigalotti L, Nicotra MR, Coral S, Natali PG, Maio M. Endoglin: An Accessory Component of the TGF-beta-Binding Receptor-Complex with Diagnostic, Prognostic, and Bioimmunotherapeutic Potential in Human Malignancies. J Cell Physiol. 2001;188:1–7. doi: 10.1002/jcp.1095. [DOI] [PubMed] [Google Scholar]
- 6.Ochiumi T, Kitadai Y, Tanaka S, Akagi M, Yoshihara M, Chayama K. Neuropilin-1 is involved in regulation of apoptosis and migration of human colon cancer. Int J Oncol. 2006;29:105–116. [PubMed] [Google Scholar]
- 7.Krugmann S, Jordens I, Gevaert K, Driessens M, Vandekerckhove J, Hall A. Cdc42 induces filopodia by promoting the formation of an IRSp53:Mena complex. Curr Biol. 2001;11:1645–1655. doi: 10.1016/s0960-9822(01)00506-1. [DOI] [PubMed] [Google Scholar]
- 8.Lacal PM, Failla CM, Pagani E, Odorisio T, Schietroma C, Falcinelli S, Zambruno G, D’Atri S. Human melanoma cells secrete and respond to placenta growth factor and vascular endothelial growth factor. J Invest Dermatol. 2000;115:1000–1007. doi: 10.1046/j.1523-1747.2000.00199.x. [DOI] [PubMed] [Google Scholar]
- 9.Gatza CE, Oh SY, Blobe GC. Roles for the type III TGF-beta receptor in human cancer. Cell Signal. 2010;22:1163–1174. doi: 10.1016/j.cellsig.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Casillas F, Wrana JL, Massague J. Betaglycan presents ligand to the TGF beta signaling receptor. Cell. 1993;73:1435–1444. doi: 10.1016/0092-8674(93)90368-z. [DOI] [PubMed] [Google Scholar]
- 11.Brown CB, Boyer AS, Runyan RB, Barnett JV. Requirement of type III TGF-beta receptor for endocardial cell transformation in the heart. Science. 1999;283:2080–2082. doi: 10.1126/science.283.5410.2080. [DOI] [PubMed] [Google Scholar]
- 12.Stenvers KL, Tursky ML, Harder KW, Kountouri N, Amatayakul-Chantler S, Grail D, Small C, Weinberg RA, Sizeland AM, Zhu HJ. Heart and liver defects and reduced transforming growth factor beta2 sensitivity in transforming growth factor beta type III receptor-deficient embryos. Mol Cell Biol. 2003;23:4371–4385. doi: 10.1128/MCB.23.12.4371-4385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong M, How T, Kirkbride KC, Gordon KJ, Lee JD, Hempel N, Kelly P, Moeller BJ, Marks JR, Blobe GC. The type III TGF-beta receptor suppresses breast cancer progression. J Clin Invest. 2007;117:206–217. doi: 10.1172/JCI29293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hempel N, How T, Dong M, Murphy SK, Fields TA, Blobe GC. Loss of betaglycan expression in ovarian cancer: role in motility and invasion. Cancer Res. 2007;67:5231–5238. doi: 10.1158/0008-5472.CAN-07-0035. [DOI] [PubMed] [Google Scholar]
- 15.Howe JR, Haidle JL, Lal G, Bair J, Song C, Pechman B, Chinnathambi S, Lynch HT. ENG mutations in MADH4/BMPR1A mutation negative patients with juvenile polyposis. Clin Genet. 2007;71:91–92. doi: 10.1111/j.1399-0004.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- 16.Gordon KJ, Dong M, Chislock EM, Fields TA, Blobe GC. Loss of type III transforming growth factor beta receptor expression increases motility and invasiveness associated with epithelial to mesenchymal transition during pancreatic cancer progression. Carcinogenesis. 2008;29:252–262. doi: 10.1093/carcin/bgm249. [DOI] [PubMed] [Google Scholar]
- 17.Lee JD, Hempel N, Lee NY, Blobe GC. The type III TGF-beta receptor suppresses breast cancer progression through GIPC-mediated inhibition of TGF-beta signaling. Carcinogenesis. 2010;31:175–183. doi: 10.1093/carcin/bgp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mythreye K, Blobe GC. The type III TGF-beta receptor regulates epithelial and cancer cell migration through beta-arrestin2-mediated activation of Cdc42. Proc Natl Acad Sci U S A. 2009;106:8221–8226. doi: 10.1073/pnas.0812879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Criswell TL, Dumont N, Barnett JV, Arteaga CL. Knockdown of the transforming growth factor-beta type III receptor impairs motility and invasion of metastatic cancer cells. Cancer Res. 2008;68:7304–7312. doi: 10.1158/0008-5472.CAN-07-6777. [DOI] [PubMed] [Google Scholar]
- 20.Mythreye K, Knelson EH, Gatza CE, Gatza ML, Blobe GC. TbetaRIII/beta-arrestin2 regulates integrin alpha5beta1 trafficking, function, and localization in epithelial cells. Oncogene. 2012;32:1416–27. doi: 10.1038/onc.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nature reviews Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 22.Elenbaas B, Spirio L, Koerner F, Fleming MD, Zimonjic DB, Donaher JL, Popescu NC, Hahn WC, Weinberg RA. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 2001;15:50–65. doi: 10.1101/gad.828901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jelinek DF, RCT, Stolovitzky GA. Identification of a global gene expression signature of B-chronic lymphocytic leukemia. Mol Cancer Res. 2003;1:346–361. [PubMed] [Google Scholar]
- 24.Murthy U, Basu M, Sen-Majumdar A, Das M. Perinuclear location and recycling of epidermal growth factor receptor kinase: immunofluorescent visualization using antibodies directed to kinase and extracellular domains. J Cell Biol. 1986;103:333–342. doi: 10.1083/jcb.103.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aspenstrom P, Lindberg U, Hall A. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott-Aldrich syndrome. Curr Biol. 1996;6:70–75. doi: 10.1016/s0960-9822(02)00423-2. [DOI] [PubMed] [Google Scholar]
- 26.Parsons M, Monypenny J, Ameer-Beg SM, Millard TH, Machesky LM, Peter M, Keppler MD, Schiavo G, Watson R, Chernoff J, Zicha D, Vojnovic B, Ng T. Spatially distinct binding of Cdc42 to PAK1 and N-WASP in breast carcinoma cells. Mol Cell Biol. 2005;25:1680–1695. doi: 10.1128/MCB.25.5.1680-1695.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lebrin F, Goumans MJ, Jonker L, Carvalho RLC, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM, ten Dijke P. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J. 2004;23:4018–4028. doi: 10.1038/sj.emboj.7600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massague J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 29.Chen W, Kirkbride KC, How T, Nelson CD, Mo J, Frederick JP, Wang XF, Lefkowitz RJ, Blobe GC. Beta-arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science. 2003;301:1394–1397. doi: 10.1126/science.1083195. [DOI] [PubMed] [Google Scholar]
- 30.Kirkbride KC, Townsend TA, Bruinsma MW, Barnett JV, Blobe GC. Bone morphogenetic proteins signal through the transforming growth factor-beta type III receptor. J Biol Chem. 2008;283:7628–7637. doi: 10.1074/jbc.M704883200. [DOI] [PubMed] [Google Scholar]
- 31.Blobe GC, Liu X, Fang SJ, How T, Lodish HF. A novel mechanism for regulating transforming growth factor beta (TGF-beta) signaling. Functional modulation of type III TGF-beta receptor expression through interaction with the PDZ domain protein, GIPC. J Biol Chem. 2001;276:39608–39617. doi: 10.1074/jbc.M106831200. [DOI] [PubMed] [Google Scholar]
- 32.Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci U S A. 2001;98:1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duwel A, Eleno N, Jerkick M, Arevalo M, Blolanos JP, Bernabeu C, Lopez-Novoa JM. Reduced tumor growth and angiogenesis in endoglin-haploinsufficient mice. Tumour Biol. 2007;28:1–8. doi: 10.1159/000097040. [DOI] [PubMed] [Google Scholar]
- 34.Cohen D, Fernandez D, Lazaro-Dieguez F, Musch A. The serine/threonine kinase Par1b regulates epithelial lumen polarity via IRSp53-mediated cell-ECM signaling. J Cell Biol. 2011;192:525–540. doi: 10.1083/jcb.201007002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348(Pt 2):241–255. [PMC free article] [PubMed] [Google Scholar]
- 36.Tsujie M, Tsujie T, Toi H, Uneda S, Shiozaki K, Tsai H, Seon BK. Anti-tumor activity of an anti-endoglin monoclonal antibody is enhanced in immunocompetent mice. Int J Cancer. 2008;122:2266–2273. doi: 10.1002/ijc.23314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stephenson JM, Banerjee S, Saxena NK, Cherian R, Banerjee SK. Neuropilin-1 is differentially expressed in myoepithelial cells and vascular smooth muscle cells in preneoplastic and neoplastic human breast: a possible marker for the progression of breast cancer. Int J Cancer. 2002;101:409–414. doi: 10.1002/ijc.10611. [DOI] [PubMed] [Google Scholar]
- 38.Narazaki M, Tosato G. Ligand-induced internalization selects use of common receptor neuropilin-1 by VEGF165 and semaphorin3A. Blood. 2006;107:3892–3901. doi: 10.1182/blood-2005-10-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geretti E, Klagsbrun M. Neuropilins: Novel targets for anti-angiogenesis therapies. Cell Adh Migr. 2007;1:56–61. doi: 10.4161/cam.1.2.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finnson KW, Tam BY, Liu K, Marcoux A, Lepage P, Roy S, Bizet AA, Philip A. Identification of CD109 as part of the TGF-beta receptor system in human keratinocytes. FASEB J. 2006;20:1525–1527. doi: 10.1096/fj.05-5229fje. [DOI] [PubMed] [Google Scholar]
- 41.Babitt JL, Zhang Y, Samad TA, Xia Y, Tang J, Campagna JA, Schneyer AL, Woolf CJ, Lin HY. Repulsive guidance molecule (RGMa), a DRAGON homologue, is a bone morphogenetic protein co-receptor. J Biol Chem. 2005;280:29820–29827. doi: 10.1074/jbc.M503511200. [DOI] [PubMed] [Google Scholar]
- 42.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 43.Edlund S, Landstrom M, Heldin CH, Aspenstrom P. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell. 2002;13:902–914. doi: 10.1091/mbc.01-08-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung JS, Shiue LH, Duvic M, Pandya A, Cruz PD, Ariizumi K. Sezary syndrome cells overexpress syndecan-4 bearing distinct heparan sulfate moieties that suppress T-cell activation by binding DC-HIL and trapping TGF-beta on the cell surface. Blood. 2011;117:3382–3390. doi: 10.1182/blood-2010-08-302034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.You HJ, How T, Blobe GC. The type III transforming growth factor-beta receptor negatively regulates nuclear factor kappa B signaling through its interaction with beta-arrestin2. Carcinogenesis. 2009;30:1281–1287. doi: 10.1093/carcin/bgp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gulyas M, Hjerpe A. Proteoglycans and WT1 as markers for distinguishing adenocarcinoma, epithelioid mesothelioma, and benign mesothelium. J Pathol. 2003;199:479–487. doi: 10.1002/path.1312. [DOI] [PubMed] [Google Scholar]
- 47.Huang X, Xiao DW, Xu LY, Zhong HJ, Liao LD, Xie ZF, Li EM. Prognostic significance of altered expression of SDC2 and CYR61 in esophageal squamous cell carcinoma. Oncol Rep. 2009;21:1123–1129. doi: 10.3892/or_00000332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.