Xenotransplantation (heterotransplantation) exemplifies how history repeats itself. Attempts at whole organ transplantation were made between 1906 and 1923 using pig, sheep, goat, and subhuman primate kidney donors.1 The first of these efforts were in France and Germany,2,3 and in some cases, the blood vessels coming to and leaving these organs were sewn to recipient blood vessels in much the same way as today. None of the kidneys functioned for long, if at all, and the human recipients died anywhere from a few hours to 9 days later.
The Chimpanzee Donor
The demonstration in 1962 and 1963 that renal allotransplantation was routinely feasible with azathioprine-prednisone therapy4 created an organ shortage crisis and reawakened interest in xenotransplantation. Cadaveric brain death was 5 years in the future, the number of living donors was limited, and renal dialysis programs as an option were few in number. Desperate potential kidney recipients were piling up faster than places could be found for them in the few existing dialysis facilities.
As the crisis deepened, xenotransplantation was re-explored. On February 16, 1963, Dr. Claude Hitchcock of the Hennepin County Hospital, in Minneapolis, transplanted the kidney of a baboon to a 65-year-old woman. The organ functioned for 4 days before its artery clotted.5 The case was not made public until it was learned later in 1963 of a far more encouraging experience by Dr. Keith Reemtsma of Tulane University using the closer-to-human chimpanzee donor.6 One of Reemtsma’s first six chimpanzee kidney grafts functioned for 9 months. The second longest survival time was 2 months, but in subsequent trials, neither of these longevity landmarks could be reached again. Reemtsma also transplanted the liver of a Rhesus monkey, and it was fiercely rejected.
Later, Cortesini of Rome7 and probably others who did not report their cases accumulated chimpanzee xenotransplantation experience that generally confirmed Reemtsma’s findings. Hardy attempted a chimpanzee-to-human heart transplant that failed intraoperatively,8 and liver transplantation with chimpanzee donors was attempted three times by us, 19 or more years ago, with a maximum survival time of 9 days.9 The histopathologic findings at autopsy were not distinguishable from those in hepatic allografts at comparable times. No further attempts at chimpanzee-to-human xenotransplantation have been made using modern immunosuppression (with cyclosporine or FK 506), and future attempts are not likely because of the endangered status of these animals.
The Baboon Donor
Beginning with Hitchcock’s case,5 much also was learned about baboon-to-human transplantation during this period. In December 1963 and January 1964, six additional patients were given baboon kidneys at the University of Colorado.10,11 All of the organs functioned promptly and maintained dialysis-free life for 10 to 60 days. However, the necessary doses of azathioprine and prednisone were very high, and eventually, the grafts were rejected. The rejection was midway in severity between that of the chimpanzee kidney and that of the Rhesus monkey kidney, but it was not qualitively different than had been observed in allografts.12 The same events were recapitulated two decades later in the Baby Fae baboon-to-human heart transplantation, in spite of heavy cyclosporine-steroid immunosuppression.13 It was clear that the use of baboon organs would have to wait for better and possibly fundamentally different immunosuppression. The more distant phylogenetic separation of the baboon from the human compared with the chimpanzee-human relationship was reflected in the pathologic findings in these xenografts. Spotty necrosis or uneven regional infarctions developed in all of the baboon kidneys (and Baby Fae’s heart). It was unfortunate that there were not better humoral antibody studies in the early days. It already was known that anti-graft ABO isoagglutinins could cause hyperacute rejection,14 but the use of serologic techniques beyond this for the detection of preformed anti-graft antibodies had not yet been applied in transplantation. For example, the role of lymphocytotoxic antibodies as a cause for hyperacute humoral allograft rejection was not recognized until 1965.15,16
Nevertheless, in the 1963 baboon cases, we showed that heterospecific antibodies bound to the grafts by showing titer declines in the patients’ sera with confirmatory electron micographic studies.10,11 The pathologist K. A. Porter concluded that:
“in the resulting [xenograft] rejection process, cellular infiltration and peritubular capillary destruction are prominent early pathologic features, but by nine days the vasculonecrotic element is marked. There is circumstantial evidence to suggest that, whereas the peritubular capillary damage is mediated by cell-bound antibody, the fibrinoid necrotic vascular lesions are caused by circulating antibody.”12
Interdiction of Antibodies
The antibody (humoral) component of rejection has been the central issue of xenotransplantation since that time.17–19 The interrelation of all performed anti-allograft and anti-xenograft antibody syndromes was recognized from the outset,20–22 and in fact, xenograft models using disparate donor and recipient species have been used to evaluate treatment strategies that are designed to prevent the hyperacute rejection of ABO-incompatible allografts or allografts transplanted to sensitized human recipients. The justifiable assumption has been that the mechanism of antibody destruction of allografts and xenografts is by the same process. Techniques to prevent humoral rejection have been summarized elsewhere.20 They include plasmapheresis; antibody removal with a Staphylococcus A column; transplantation of serial grafts to reduce the antibody titer; infusion of the chelating agent citrate, which is an anticoagulant and an effective preventer of complement activation; and the use of prostanoids and other inhibitors of the inflammatory response.
Of these approaches, it is interesting that one of the most promising, prostaglandin therapy, has received the least attention. Prostaglandin compounds can mitigate the xenograft rejection of hamster-to-rat,23,24 cat-to-dog,25 and pig-to-dog28 organs. Quagliata et al. published evidence in 1973 that prostaglandin had a specific effect on B cells and concluded that such drugs would be valuable for xenotransplantation.27
Most importantly, it was shown recently that PGE1 and a short course of high-dose steroids as part of FK 506-based drug cocktails permitted the transplantation of liver allografts to patients with lymphocytotoxic antibodies, with no increased risk of immediate antibody rejection. The long-term prognosis in these high-risk patients was converted to the same as that of crossmatch-negative recipients.28 This discovery was one of the prime justifications for the recent baboon-to-human liver xenotransplantation trial. Although prostaglandins are inherently but weakly immunosuppressive,29 their unique value for xenografting was via the antibody arm of the xenograft reaction, possibly by modifying the cytokine inflammatory response.24,30 In addition to their immunosuppressive effect, prostaglandins also reduce the nephrotoxicity of cyclosporine31,32 and FK 506.28,33 This latter property has made us recommend PGE1–along with FK 506 plus prednisone and PGE1–as one of the three constituents of our current immunosuppressive cocktail for all liver recipients.33 This was the baseline treatment for a recent human recipient of a baboon liver.
The Anti-metabolite Drugs
Although the duality of humoral and cellular mechanisms of xenograft rejection has become common knowledge, the antibody component has been refractory to treatment in many experimental models, even with the use of drug cocktails that include PGE1. For example, prostaglandin was effective only during its constant infusion at high doses in the cat-to-dog experiments,25 and in the hamster-to-rat model, its efficacy is even more limited (personal unpublished observations).23,24 A hamster organ is confronted in the rat recipient by a moderate titer (1:16 to 1:32) of preformed heterospecific cytotoxic antibodies of the IgM class and, subsequently, by a rapidly gathering antibody storm. The antibodies destroy abdominally placed cardiac grafts within 3 days in untreated recipients, before there is a trace histopathologically of immunocyte infiltration. Hamster livers are rejected in 7 days by combined cellular and humoral mechanisms. The antibody component is reflected in the occlusive endotheliolitis of the entire graft vasculature.
FK 506 prevents T cell activation and cytokine secretion. By itself, in doses of 2 mg/kg/day, FK 506 prolonged heart xenograft survival by only 1 day. After liver transplantation, it allowed survival of half of the liver recipients for longer than 30 days (Table 1), but none survived as long as 100 days. Monotherapy with either of two experimental “antiproliferative” drugs that suppress purine (RS 61443) or pyrimidine (Brequinar) ribonucleotide synthesis tripled or quadrupled the survival times of hamster-to-rat xenografts but did not permit consistent chronic survival. However, when either of the two anti-metabolite drugs or the conventional anti-cancer drug cyclophosphamide was added to FK 506 for the first 2 postoperative weeks, indefinite survival under continued administration of FK 506 alone became routinely possible after either heart or liver transplantation.34 The finding that a single large dose of cyclophosphamide 10 days before transplantation permitted nearly 100% success after either heart or liver transplantation with daily administration of FK 506 was particularly noteworthy (Table 1).
Table 1.
Graft Survival*
| Type of Graft | Group | Treatment+ | Without FK 506 | With FK 506 | ||
|---|---|---|---|---|---|---|
| n | Survival >30 days | n | Survival >30 days | |||
| Heart | 1 | None | 6 | 0 | – | – |
| 2 | FK 506 | 6 | 0 | – | – | |
| 3 | RS-61443 | 4 | 0 | 6 | 5 | |
| 4 | BQR | 6 | 1 | 5 | 5 | |
| 5 | Cyclophosphamide | 5 | 0 | 5 | 5 | |
| 6 | Cyclophosphamide | 5 | 0 | 5 | 5 | |
| Liver | 7 | None | 8 | 0 | – | – |
| 8 | FK 506 | 10 | 5 | – | – | |
| 9 | RS-61443 | ND | ND | 10 | 9 | |
| 10 | BQR | 7 | 1 | 7 | 6 | |
| 11 | Cyclophosphamide | ND | ND | 10 | 9 | |
| 12 | Cyclophosphamide | 5 | 0 | 15 | 12 | |
These experiments are a fraction of those performed. A full account of this work as well as the testing of numerous other compounds is provided in ref. 341
Daily dose (mg/kg): FK 506, 2.0 × 6, 1.0 × 25 (heart) or 1.0 × 30 (liver), and 0.5 on alternate days thereafter; RS-61443, 20.0 × 15 (14) starting day before Tx (heart) or day of Tx (liver); BQR, 4.0 × 3 and 3.0 × 12 starting day before Tx (heart) or 3.0 × 14 starting day of Tx (liver); cyclophosphamide, 7.5 × 15 (14) starting day before Tx (heart) or day of Tx (liver), except groups 6 and 12, to which one dose of 80 mg was given 10 days before operation.
Animals alive at 30 days survived as long as FK 506 was continued out of 100 days no matter what the adjuvant induction drug.
The conclusion from these studies by Murase et al34 was that prevention or mitigation of heterospecific antibody rejection by interdiction of the B cell proliferative response with a variety of anti-metabolite drugs for a surprisingly short period after transplantation or even beforehand was the essential first step to successful xenotransplantation. Thereafter, the potential of continuous therapy with T cell-directed immunosuppressants such as FK 506 was unmasked. Hasan et al35 showed the same kind of extraordinary synergism between cyclosporine and cyclophosphamide. Such combination therapy was predicted to be clinically applicable as long as the humoral antibodies did not act so rapidly that they caused hyperacute rejection in a few minutes or hours. This condition was known from the earlier clinical experience to pertain in the baboon-to-human species combination.9–13 The choice of cyclophosphamide as the anti-metabolite drug for the eventual clinical cocktail in preference to several other agents with the same general “anti-proliferative” mechanism34 hinged on this drug’s effectiveness, the fact that it was an accepted drug in the formulary, and the fact that we had previously used it extensively as a conventional immunosuppressant in the era preceding cyclosporine.36,37
The Liver as a Xenograft
Immunologic Advantages
In the research with hamster-to-rat xenotransplantation before the start of clinical trials, two organs were used forscreening.34 One was the heart, which is considered immunologically “difficult” because of its rejection within 3 days in unmodified recipients. In contrast, hamster liver xenografts were not rejected by rats until 7 days after transplantation.34 As a general principle, livers have an immunologic advantage relative to other organs, including a greater ease of inducing their acceptance as allografts38–40 or xenografts41 after a limited course of immunosupppression or, in swine42 and some rat strain combinations, with no treatment at all. In addition, the hepatic allograft and xenograft are relatively resistant to the preformed antigraft antibodies that cause hyperacute rejection of the kidney and heart41,43–45 Another quality of the liver is its unusual ability to induce a state of unresponsiveness to other tissues and organs transplanted concomitantly or subsequently from the donor or donor strain46,47 and even shield these organs from the hyperacute rejection caused by preformed allospecific48 or xenospecific49 anti-donor antibodies. Thus, the liver was the organ predicted to have the best prognosis for clinical xenotransplantation. It also was an organ for which there is great need, because unlike the kidney, there is no alternative of artificial organ support, and unlike the heart, there is no realistic prospect of developing an artificial alternative.
Metabolic Questions
In spite of its immunologic advantages, serious further questions were raised about liver xenografts. After hepatic transplantation, liver allografts continue to produce donor-phenotype proteins and other synthetic products, allowing this operation to be used to correct numerous liver-based inborn errors of metabolism.50 Because the same retention of donor specificity was expected after successful hepatic xenotransplantation, the consequence of successfully engrafting a liver xenograft could be the imposition on the recipient of an interspecies metabolic incompatibility. This would be equivalent to transplanting an inborn error of metabolism.
We examined this possibility in the hamster-to-rat liver replacement model by studying clotting factors known to besynthesized in the liver as metabolic markers. Although hamsters and rats are both rodents, the phylogenetic distance by paleontologic and genetic evidence has been estimated at 15 to 40 million years.51 Results of clotting tests showed great disparity between the two species, the most striking being protein C, which was always present in normal hamsters but was undetectable in normal rats. In rats that had been transplanted with hamster livers, the coagulation profile of the rat recipient quickly changed to that of the hamster range of values.52 Neither bleeding nor clotting was observed clinically more than after rat liver allotransplantation.
The replacement of other metabolic moieties was obvious; for example, circulating hamster albumin was found in rat recipients of hamster livers.52 These results and the results of other metabolic studies suggested that the donor-specific products of hepatic synthesis in significantly disparate species combinations such as hamster to rat did not present an insurmountable or even an important metabolic barrier to hepatic xenotransplantation.
Beyond the Baboon
Generally speaking, the humoral antibody barrier becomes more extreme roughly in proportion to the degree of species disparity, so that with widely divergent species, humoral (hyperacute) rejection is expected within a few minutes.17–19,53–57 However, trial and error has been the only way to rise above speculation with any given animal-to-human combination, providing an example that “all triumphs in medicine are the forgotten sorrows of past days.”53 Thus, the earlier failed xenotransplantation efforts9–13 yielded information about the extent of the human barrier to the baboon species that was the background for the 1992 trial.
Because the pig is often mentioned as a possible clinical organ donor, it is important to recount an unreported attempt by Rene Kuss, the pioneer French transplant surgeon, at pig-to-human renal transplantation in the early 1960s under azathioprine and prednisone administration (personal conversation with Professor Kuss of Paris and Dr. Jacques Poisson of Nice, November 1990). The kidney functioned well for approximately 30 minutes, but then it underwent hyperacute rejection. The dominant finding was widespread thrombosis of the microvasculature, concentrated in the venules. Kuss’ willingness to share this experience almost three decades later was important because this kind of vitally needed information would be difficult to obtain in the clinical research climate of today. As a donor, the pig will not be easy. Platt et al.56 have studied the details of the hyperacute rejection seen in discordant species combinations, with particular emphasis on the complement-mediated injury to the graft vascular endothelial cells. Efforts to definitively prevent the resulting destruction of the vasculature have been uniformly unsuccessful to date. Consequently, the use of genetic engineering to humanize the organ blood supply endothelium in pigs or other discordant species may be necessary before there is hope of clinical application. This strategy and others have been discussed in recent reviews and editorials.56,57,59
Cell Migration, Repopulation, and Chimerism
After breaking through the antibody barrier, the process of xenograft acceptance involves the cell migration and consequent systemic chimerism that were recently delineated in humans after hepatic and other allotransplantation.60–62 One hundred days after hamster-to-rat heart or liver xenotransplantation, we showed that rat recipient dendritic and lymphoid cells migrated into hamster heart or liver xenografts, where they become part of genetically composite transplants.93 The displaced cells going out from the xenografts can be detected in recipient tissues with polyclonal anti-hamster antisera and confirmed with polymerase chain reaction techniques (Fig. 1). In these experiments, the chimerism was most obvious and frequent in the spleen or heart of the rat recipients. It was unequivocal after liver transplantation and occurred at a low level after heart transplantation. This means that successful clinical xenotransplantation must be visualized along the same lines of donor-recipient cellular intimacy, which we perceive to be the fundamental means of xenograft and allograft acceptance (Fig. 2).
Fig. 1.
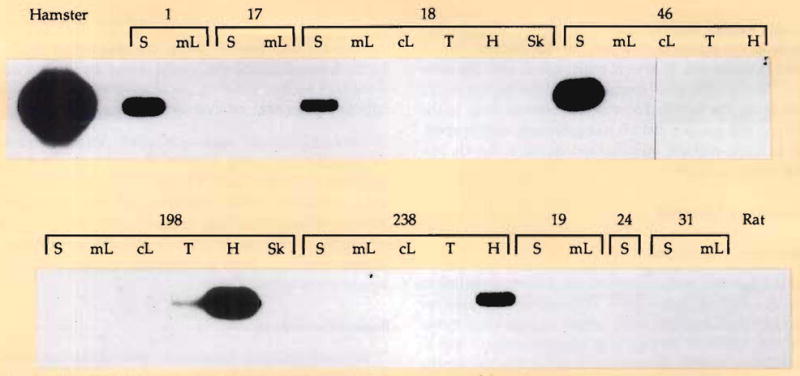
For molecular detection of chimerism in the rat tissues after xenotransplantation of hamster hearts or livers, 1 μg of genomic DNA extracted from each tissue was polymerase chain reaction amplified for 30 cycles with hamster-specific oligonucleotides. One fifth of the volume of each reaction was size separated on an agarose gel, transferred on a nylon membrane (hybond–N+, Amersham, Arlington Heights, IL), and probed with a hamster hypoxanbne phosphoribosyltransferase exon 9 probe. The experiments were performed by Drs. Noriko Murase and Luis Valdivia, and the polymerase chain reaction examinations were done by Drs. Massimo Trucco and Roberto Giorda. S, spleen; mL, mesenteric lymph node; cL, cervical lymph node; T, thymus; H, heart; Sk, skin. Rats 1, 17, 18, 46, 198, and 238 received hamster livers 104 to 141 days previously; rats 19, 24, and 31 received hearts 111 to 135 days earlier.
Fig. 2.
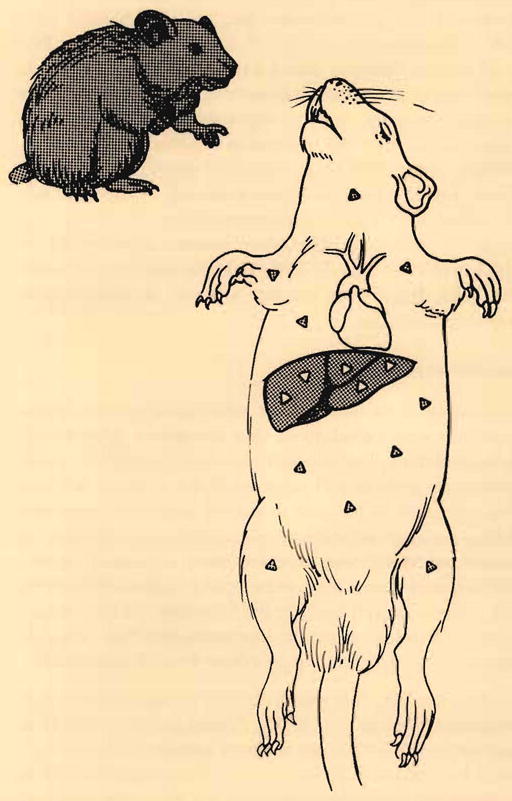
Schematic view of the kind of chimerism that is documented in Figure 1. We believe that this mixed chimerism is necessary for either allograft or xenograft acceptance.
Peer Review
This background provided our personal knowledge base for xenotransplantation. Convinced from our own work by November 1991 that baboon-to-human liver grafting could be performed successfully, we notified officials at the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (Drs. Jay Hoofnagle and Philip Gordon), the Food and Drug Administration (Drs. Ron Lieberman and Gregory Burke), and the head of the Department of Health and Human Services (Dr. Louis Sullivan) of this conclusion. The next 8 months were spent in discussions with members of these government agencies, the University of Pittsburgh Institutional Review Board, a variety of ethics committees and, toward the end, members of Congress who have had a special interest in health care problems. In March 1992, an outside peer review board of six members from centers in the United States and Europe (chaired by Dr. Keith Reemtsma of Columbia University, New York) met in Pittsburgh with the University Institutional Review Board to evaluate the evidence, the proposal to go forward clinically, and the informed consent They recommended unanimously that the trial proceed, providing that certain nonsubstantive changes were made or that ancillary experimental data be acquired. These recommendations or suggestions were followed.
A baboon-to-human liver transplantation was scheduled on June 28, 1992. Consensus was reached with all parties consulted. At 5:00 A.M., Jeffrey Romoff (President of the University Medical Center), Professor Luigi Fassati of Milan (one of our European collaborators), and I flew to Washington to address a joint meeting of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health and the New York Academy of Sciences. There, at the end of an account of the supporting research, I announced that:
“the decision was made to proceed with the baboon-to-human liver transplantation that will take place there after our return this afternoon. There is no more appropriate forum to make these plans known in advance and to provide their justifications as I have tried to do than at this remarkable meeting of scientists.”
We returned immediately to Pittsburgh, where the operation began.
Baboon-to-Human Liver Transplantation
The donor and recipient operations were performed in Pittsburgh on June 28, 1992, by Drs. Andreas Tzakis, John Fung, and Satoru Todo, with subsequent intensive care provided by Drs. Ignazio R. Marino and Howard R. Doyle. The recipient was a 35-year-old man with end-stage chronic active hepatitis caused by B virus that is thought incapable of infecting the baboon liver.64 Although he also was a carrier of the human immune deficiency virus (HIV), he was immune competent as judged by normal responsiveness of his lymphocytes to phytohemagglutin, concavalin A, baboon lymphocyte stimulation, and third-party lymphocyte stimulation.
The four-drug immunosuppressive cocktail of FK 506, prednisone, PGE1, and cyclophosphamide (Fig. 3) was remarkably effective in preventing the fierce cellular rejection seen in previous baboon-to-human renal or heart xenografts under the administration of azathioprine and prednisone or cyclosporine and prednisone.9–13 Most importantly, the deadly occlusive endotheliolitis of xenospecific humoral rejection was completely avoided. Preformed lymphocytotoxic anti-baboon antibodies of class IgM that were present in low titer preoperatively in the recipient serum did not increase after transplantation, and no circulating IgG antibodies ever were measurable. A postperfusion biopsy of the baboon liver from a 53-pound donor showed neutrophils in the sinusoids, but at a time when there was no clinical evidence of hyperacute rejection. Diffuse IgM and IgG antibodies shown in the xenograft biopsy specimen with immunofluorescence at 12 days had largely disappeared by 24 days.
Fig. 3.
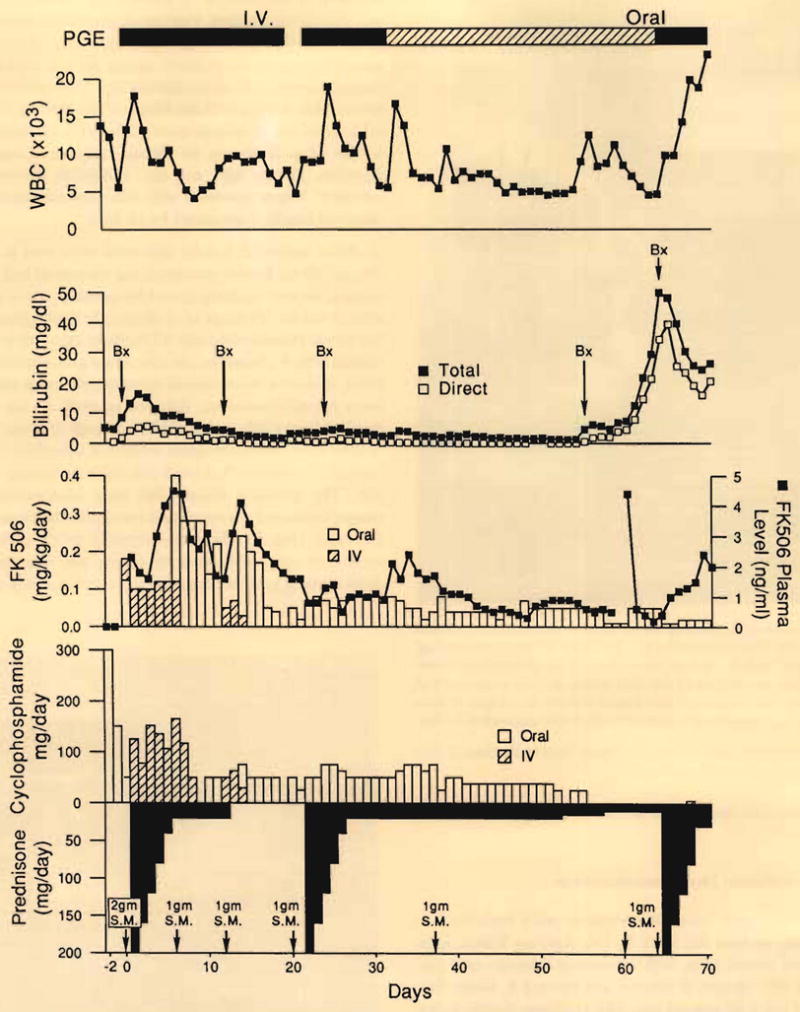
Course of the patient after receipt of baboon liver. PGE, prostaglandin E; SN, solumedrol (methylprednisolone).
Minor periportal cellular infiltrates were seen in the 12-, 24-, and 65-day biopsy specimens, but the patient had continuously good liver function, except for jaundice, which recurred after 8 weeks. Findings of a diagnostic cholangiogram obtained on postoperative day 61 initially were interpreted as normal (Fig. 4). However, the procedure precipitated a septic crisis, including disseminated intravascular coagulation and acute hyperbilirubinemia, followed by death on day 70 that resulted from a subarachhnoid hemorrhage that was thought to have come from aspergillus erosion of a cerebral artery. At autopsy, the xenograft showed only minimal findings of rejection. The principal abnormalies were intrahepatic biliary sludge, rupture of the ducts, and consequent bile leakage into the tissue (Fig. 5). There was virtually no elevation in the results of tests of hepatocyte injury, nor was there any diminution of synthetic function up to the morning of death.
Fig. 4.
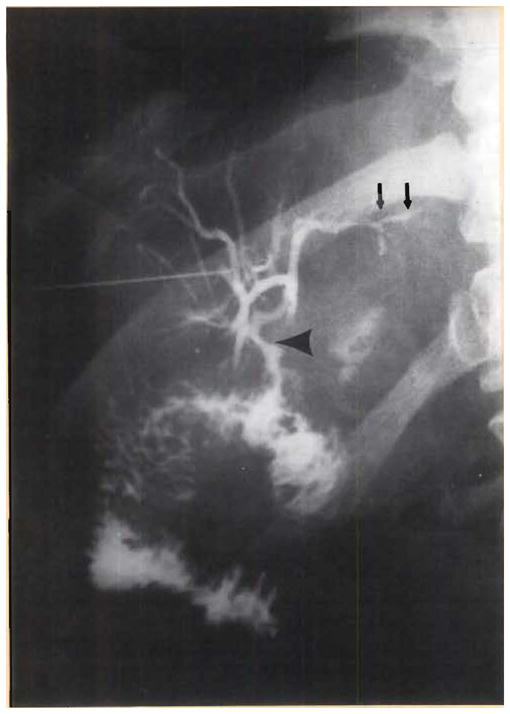
Transhepatic cholangiogram on postoperative day 6, several minutes after the injection of 5 mL dye. Although the initial reading was normal, with no obvious obstruction at the anastomosis (lower arrow), note the fullness of the duct system and the irregularity of the sludge-filled peripheral ducts (upper arrows). At autopsy 10 days later, the duct system was found to be filled with inspissated sludge.
Fig. 5.
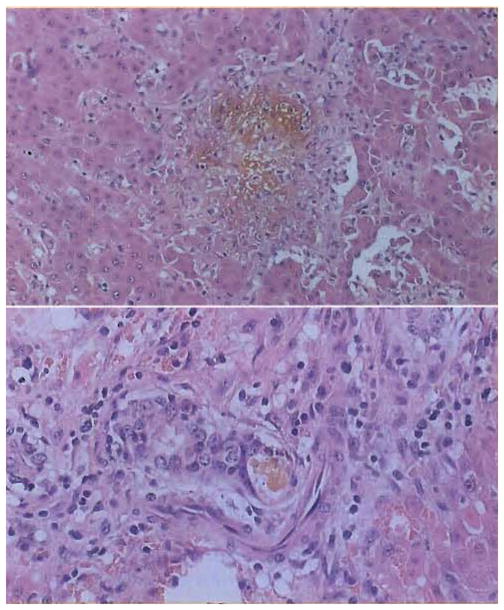
Xenograft biopsy at 65 days, 5 days before death. There was absence of rejection. The dominant finding was cholestasis. (A, top) A bile lake occupies the central part of the field. Note the absence of most of the epithelium. (B, bottom) Intrahepatic ducts showing discontinuity of epithelium at sites of rupture. H&E, original magnification ×250.
The primary explanation for the fatal outcome was unrecognized partial biliary obstruction with bacterial translocation through the compromised ducts. Ironically, the repeated failure to diagnosis this same complication impeded the early development of liver allotransplantation more than any other single factor.65–68 Then, as in the current case, the elevated levels of alkaline phosphatase and other cannulicular enzymes and, ultimately, the jaundice caused by biliary stasis were systematically ascribed to rejection and treated as such in spite of the lack of histopathologic support for this diagnosis. In future trials, biliary stasis should be preventable by stenting the biliary anastomosis with an exteriorized catheter that also can be used to perform cholangiography postoperatively or to irrigate at will.
Aside from stimulating improvements in surgical management, the candid acknowledgement that failure in this case was fundamentally technical and, thus, avoidable allows easy interpretation of the other observations that were generally encouraging and supportive of further cautious trials. With a cocktail that included nonmyelotoxic quantities of Cytoxan (cyclophosphamide), the doses of conventional immunosuppressive agents were not remarkably different from those used for allotransplantation. How much this patient’s HIV carrier status contributed to the ease and completeness with which the xenograft rejection was controlled and to the complex terminal sepsis is open to speculation. However, because the patient was immunocompetent at the outset, the principal immunologic depression postoperatively was clearly iatrogenic, not derivative from HIV.
In addition, the recipient appeared to have started the transformation to the same state of mixed chimerism observed in rats after their receipt of hamster livers. Thirty-five days after the transplantation, baboon DNA blood chimerism was identified in the patient’s blood with baboon chorionic gonadotropin B subunit genes.69 At autopsy, chimerism was shown unequivocally in the lungs, heart, skin, lymph nodes, and numerous other host tissues.70 The extent of the chimerism was far greater than that seen in the hamster-to-rat model and has emphasized the degree of donor-recipient cellular intimacy that can be expected with successful xenotransplantation (Fig. 6).
Fig. 6.
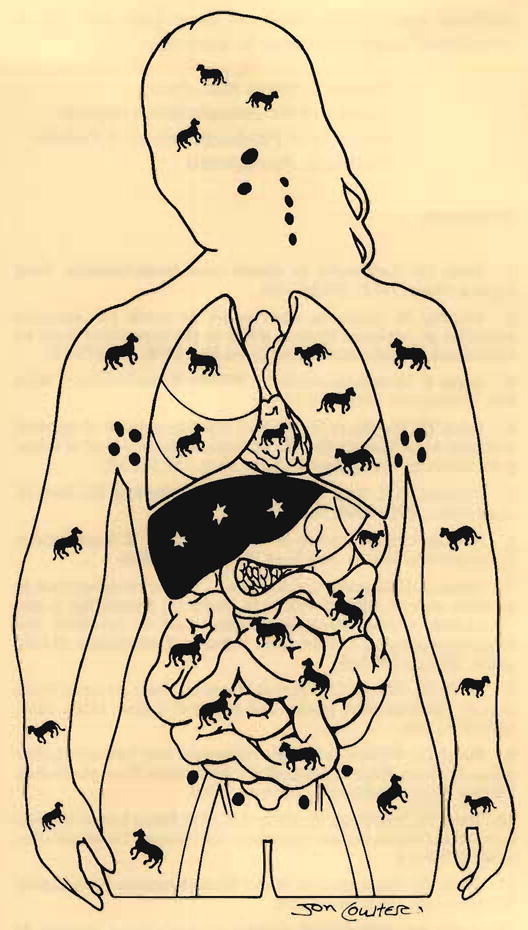
The nature of chimerism 70 days after clinical xenotransplantation.
An additional question largely answered by this single case was whether elaboration by a liver xenograft of donor phenotype proteins and other synthetic products would in essence impose on the recipient as serious or lethal incompatibility of metabolism. As had been predicted from the hamster-to-guinea pig experience,52 the serum protein pattern of the recipient was rapidly “baboonized,” including the albumin, C3 complement, and other moieties involved in either immune reactions or blood coagulation.69 The fall of the patient’s serum uric acid level postoperatively to the nearly undetectable level that is normal for the baboon was another particularly dramatic demonstration of the recreation by the xenograft of its own chemical environment with no apparent adverse effects. Such observations support the conclusion from the hamster-to-rat experiments that donor-specific products of hepatic synthesis do not present a fundamental barrier to liver xenotransplantation.
Death at 70 days occurred too soon to allow a conclusion that the transplanted baboon liver could successfully resist infection with hepatitis B virus. However, there was no evidence of the hepatitis B virus surface or core antigen in the transplant at autopsy, after a time in which infection of allografts has been frequently recorded.71
Aftermath
On Friday, September 11, 1992, 5 days after the patient’s death, a meeting organized by Dr. Keith Reemtsma of New York City was convened under the sponsorship of the New York Academy of Medicine. A clinicopathologic analysis of this crucial case was provided by the xenotransplantation teams from the University of Pittsburgh and Columbia University, with discussion by other experts from four European and numerous American centers. The consensus of the participants was that cautious further xenotransplantations were justified with baboon donors, but with emphasis on the extremely experimental nature of these trials and with a commitment to open disclosure in every case.
References
- 1.Groth CG. Landmarks in clinical renal transplantation. Surg Gynecol Obstet. 1972;134:323–328. [PubMed] [Google Scholar]
- 2.Jaboulay M. Greffe du reins au pli du conde par soudures arterielles et veineuses (Kidney grafts in the antecubital fossa by arterial and venous anastomosis) Lyon Med. 1906;107:575–577. [Google Scholar]
- 3.Unger E. Nierentransplantation (Kidney transplantation) Wien Klin Wochenschr. 1910;47:573–578. [Google Scholar]
- 4.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385–395. [PMC free article] [PubMed] [Google Scholar]
- 5.Hitchcock CR, Kiser JC, Telander RL, Seljoskog EL. Baboon renal grafts. JAMA. 1964;189:934–937. doi: 10.1001/jama.1964.03070120056013. [DOI] [PubMed] [Google Scholar]
- 6.Reemtsma K, McCracken BH, Schlegel JU, et al. Renal heterotransplantation in man. Ann Surg. 1964;160:384–410. doi: 10.1097/00000658-196409000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortesini R, Casciani C, Cucchiara G. Heterotransplantation in primates: current state of affairs. In: Balner H, Bewedridge J, editors. Proceedings of the International Symposium on Infections and Immunosuppression in Subhuman Primates. Copenhagen: Munksgaard; 1970. pp. 239–248. [Google Scholar]
- 8.Hardy JD, Chavez CM, Kurrus FD, et al. Heart transplantation in man: developmental studies and report of a case. JAMA. 1964;188:1132–1140. [PubMed] [Google Scholar]
- 9.Starzl TE. Baboon and renal chimpanzee liver heterotransplantation. In: Hardy MA, editor. Xenograft 25. Amsterdam: Excerpta Medica, Elsevier Science Publishers; 1989. pp. 17–28. [Google Scholar]
- 10.Starzl TE, Marchioro TL, Peters GN, et al. Renal homotransplantation from baboon to man: experience with 6 cases. Transplantation. 1964;2:752–776. doi: 10.1097/00007890-196411000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starzl TE. Experience In Renal Transplantation. Philadelphia: WB Saunders; 1964. pp. 262–298. [Google Scholar]
- 12.Porter KA. Pathological changes in transplanted kidneys. In: Starzl TE, editor. Experience in Renal Transplantation. Philadelphia: WB Saunders; 1964. pp. 346–357. [Google Scholar]
- 13.Bailey L, Nehlsen-Cannarella S, Concepcion W, Jolley W. Baboon to human cardiac xenotransplantation in a neonate. JAMA. 1985;254:3321–3329. [PubMed] [Google Scholar]
- 14.Starzl TE. Patterns of permissible donor-recipient tissue transfer in relation to ABO blood groups. In: Starzl TE, editor. Experience in Renal Transplantation. Philadelphia: WB Saunders; 1964. pp. 37–47. [Google Scholar]
- 15.Terasaki PI, Marchioro TL, Starzl TE. Sero-typing of human lymphocyte antigens: preliminary trials on long-term kidney homograft survivors. In: Russell PS, Winn HJ, Amos DB, editors. Histocompatibility Testing 1965. Washington, DC: National Academy of Sciences; 1965. pp. 83–96. [Google Scholar]
- 16.Kissmeyer-Neilsen F, Olsen S, Peterson VP, Fjeldborg O. Hyperacute rejection of kidney allografts, associated with preexisting humoral antibodies against donor cells. Lancet. 1966;11:662–665. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- 17.Perper RJ, Najarian JS. Experimental renal heterotransplantation. I. In widely divergent species. Transplantation. 1966;3:377–388. doi: 10.1097/00007890-196607000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Perper RJ, Najarian JS. Experimental renal tenterotransplantation. II. Closely related species. Transplantation. 1966;4:700–712. doi: 10.1097/00007890-196611000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Cortesini R, Casciani C. Histocompatibility Testing 1967. Washington, DC: National Academy of Sciences; 1967. Xenograft across strong histocompatibility barriers; pp. 281–284. [Google Scholar]
- 20.Starzl TE, Tzakis A, Makowka L, et al. The definition of ABO factors in transplantation: relation to other humoral antibody states. Transplant Proc. 1987;19:4492–4497. [PMC free article] [PubMed] [Google Scholar]
- 21.Starzl TE, Demetris AJ, Todo S, et al. Evidence for hyperacute rejection of human liver grafts: the case of the canary kidneys. Clin Transplant. 1989;3:37–45. [PMC free article] [PubMed] [Google Scholar]
- 22.Demetris AJ, Nakamura K, Yagihasihi A, et al. A clinicopathological study of human liver allograft recipients harboring preformed IgG lymphocytotoxic antibodies. Hepatology. 1992;16:671–681. doi: 10.1002/hep.1840160310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kakita A, Blanchard J, Fortner JG. Effectiveness of prostaglandin E1 and procarbazine hydrochloride in prolonging the survival of vascularized cardiac hamster-to-rat xenograft. Transplantation. 1975;20:439–442. doi: 10.1097/00007890-197512000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Shaw JRL. Role of prostaglandins in transplantation. In: Cohen MM, editor. Biological Protection with Prostaglandins. Vol. 1. Boca Raton: CRC Press; 1985. pp. 111–128. [Google Scholar]
- 25.Mundy AR. Prolongation of cat to dog renal xenograft survival with prostacyclin. Transplantation. 1980;30:226–228. [PubMed] [Google Scholar]
- 26.Makowka L, Miller C, Chapchap P, et al. Prolongation of pig-to-dog renal xenograft survival by modification of the inflammatory mediator response. Ann Surg. 1987;206:482–495. doi: 10.1097/00000658-198710000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quagliata F, Lawrence VJW, Phillips-Quagliata JM. Short communication: prostaglandin E as a regulator of lymphocyte function selective action on B lymphocytes and synergy with procarbazine in depression of immune responses. Cell Immunol. 1972;6:457–465. doi: 10.1016/0008-8749(73)90044-0. [DOI] [PubMed] [Google Scholar]
- 28.Takaya S, Iwaki Y, Starzl TE. Value of high dose steroids and prostaglandin E1 for liver transplantation in positive cytotoxic crossmatch cases. Transplantation. doi: 10.1097/00007890-199211000-00031. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strom TB, Carpenter CB. Prostaglandin as an effective antirejection therapy in rat renal allograft recipients. Transplantation. 1983;35:279–281. doi: 10.1097/00007890-198304000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Rappaport RS, Dodge GR. Prostaglandin E inhibits the production of human interleukin 2. J Exp Med. 1982;155:943–948. doi: 10.1084/jem.155.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makowka L, Lopatin W, Gilas T, et al. Prevention of cyclosporine (CyA) nephrotoxicity by synthetic prostaglandins. Clin Nephrol. 1986;25 (Suppl 1):S89–S94. [PubMed] [Google Scholar]
- 32.Moran M, Mozes MF, Maddux MS, et al. Prevention of acute graft rejection by the prostaglandin E1 analogue misoprostol in renal-transplant recipients treated with cyclosporine and prednisone. N Engl J Med. 1990;322:1183–1188. doi: 10.1056/NEJM199004263221703. [DOI] [PubMed] [Google Scholar]
- 33.Takaya S, Bronsther O, Abu-Elmagd K, et al. The use of prostaglandin E1 in crossmatch negative liver transplant recipients treated with FK 506. Transplant Proc. (in press) [PMC free article] [PubMed] [Google Scholar]
- 34.Murase N, Starzl TE, Demetris AJ, et al. Hamster to rat heart and liver xenotransplantation with FK 506 plus antiproliferative drugs. Transplantation. doi: 10.1097/00007890-199304000-00003. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasan RIR, Bogaerde van den J, Forty J, et al. Prolonged survival of hamster to rat xenografts with cyclophosphamide therapy. Transplant Proc. 1992;24:517–518. [PubMed] [Google Scholar]
- 36.Starzl TE, Putnam CW, Halgrimson CG, et al. Cyclophosphamide and whole organ transplanation in human beings. Surg Gynecol Obstet. 1971;133:981–991. [PMC free article] [PubMed] [Google Scholar]
- 37.Starzl TE, Groth CG, Putnam CW, et al. Cyclophosphamide for clinical renal and hepatic transplantation. Transplant Proc. 1973;5:511–516. [PMC free article] [PubMed] [Google Scholar]
- 38.Starzl TE, Marchioro TL, Porter KA, et al. Factors determining short- and long-term survival after orthotopic liver homotransplantation in the dog. Surgery. 1965;58:131–155. [PMC free article] [PubMed] [Google Scholar]
- 39.Starzl TE. Experience in Hepatic Transplantation. Philadelphia: WB Saunders; 1969. pp. 198–201. [Google Scholar]
- 40.Murase N, Kim DG, Todo S, et al. FK 506 suppression of heart and liver allograft rejection. II. The induction of graft acceptance in rat. Transplantation. 1990;50:739–744. doi: 10.1097/00007890-199011000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valdivia LA, Fung JJ, Demetris AJ, Starzl TE. Differential survival of hamster-to-rat liver and cardiac xenografts under FK 506 immunosuppression. Transplant Proc. 1991;23:3269–3271. [PMC free article] [PubMed] [Google Scholar]
- 42.Garnier H, Clot J, Bertrand M, et al. Liver transplantation in the pig: surgical approach. C R Acad Sci [III] 1965;260:5621–5623. [PubMed] [Google Scholar]
- 43.Starzl TE, Ishikawa M, Putnam CW, et al. Progress in and deterrents to orthotopic liver transplantation, with special reference to survival, resistance to hyperacute rejection, and biliary duct reconstruction. Transplant Proc. 1974;6:129–139. [PMC free article] [PubMed] [Google Scholar]
- 44.Kamada N, Davies HFFS, Roser B. Reversal of transplantation immunity by liver grafting. Nature. 1981;292:840–842. doi: 10.1038/292840a0. [DOI] [PubMed] [Google Scholar]
- 45.Hossin D, Gugenheim J, Bellon B, et al. Absence of hyperacute rejection of liver allografts in hypersensitized rats. Transplant Proc. 1985;17:293–295. [Google Scholar]
- 46.Starzl TE, Kaupp HA, Jr, Brock DR, et al. Homotransplantation of multiple visceral organs. Am J Surg. 1962;103:219–229. doi: 10.1016/0002-9610(62)90491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calne RY, Sells RA, Pena JR, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–474. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 48.Fung J, Makowka L, Tzakis A, et al. Combined liver-kidney transplantation: analysis of patients with preformed lymphocytotoxic antibodies. Transplant Proc. 1988;20 (Suppl 1):88–91. [PMC free article] [PubMed] [Google Scholar]
- 49.Valdivia L, Demetris AJ, Fung JJ, et al. Successful hamster to rat liver xenotransplantation under FK 506 immunosuppression induces unresponsiveness to hamster heart and skin. Transplantation. (in press) [PMC free article] [PubMed] [Google Scholar]
- 50.Starzl TE, Demetris AJ, Van Thiel DH. Medical progress: liver transplantation. N Engl J Med. 1989;321:1014–1022. doi: 10.1056/NEJM198910123211505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hartenberger JL. The order Rodentia: major questions on their evolutionary origin, relationships and suprafamilial systematics. In: Luckett WP, Hartenberger JL, editors. Evolutionary Relationships Among Rodents: A Multidisciplinary Analysis. New York: Plenum Press; 1985. p. 92. [Google Scholar]
- 52.Valdivia L, Lewis JH, Celli S, et al. Hamster coagulation protein activities in hamster-liver transplanted rats. Transplantation. (in press) [Google Scholar]
- 53.Giles GR, Boehmig JH, Lilly J, et al. Mechanism and modification of rejection of heterografts between divergent species. Transplant Proc. 1970;2:522–537. [PMC free article] [PubMed] [Google Scholar]
- 54.Boehmig HJ, Giles GR, Amemiya H, et al. Hyperacute rejection of renal homografts: with particular reference to coagulation changes, humoral antibodies, and formed blood elements. Transplant Proc. 1971;3:1105–1117. [PMC free article] [PubMed] [Google Scholar]
- 55.Kux M, Boehmig HJ, Amemiya H, et al. Modification of hyperacute canine renal homograft and pig-to-dog heterograft rejection by the intra-arterial infusion of citrate. Surgery. 1971;70:103–112. [PMC free article] [PubMed] [Google Scholar]
- 56.Piatt JL, Vercellotti GM, Delmasso AP, et al. Transplantation of discordant xenografts. Immunol Today. 1990;11:450–457. doi: 10.1016/0167-5699(90)90174-8. [DOI] [PubMed] [Google Scholar]
- 57.Auchincloss JH. Xenogeneic transplantation. Transplantation. 1988;46:1–20. doi: 10.1097/00007890-198807000-00001. [DOI] [PubMed] [Google Scholar]
- 58.Starzl TE. The Puzzle People. Pittsburgh: University of Pittsburgh Press; 1992. The day the string broke; p. 317. [Google Scholar]
- 59.Barker CF, Markmann JF. Xenografts: is there a future? Surgery. 1992;112:3–5. [PubMed] [Google Scholar]
- 60.Starzl TE, Demetris AJ, Murase N, et al. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Starzl TE, Demetris AJ, Trucco M, et al. Chimerism after liver transplantation for type IV glycogen storage disease and type I Gaucher’s disease. N Engl J Med. doi: 10.1056/NEJM199303183281101. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Starzl TE, Demetris AJ, Trucco M, et al. Systemic chimerism in human female recipients of male livers. Lancet. doi: 10.1016/0140-6736(92)93286-v. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valdivia LA, Demetris AJ, Langer AM, et al. Dendritic cell replacement in long-surviving liver and cardiac xenografts. Transplantation. doi: 10.1097/00007890-199308000-00048. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoofnagle J. Personal communication. Oct 28, 1991.
- 65.Martineau G, Porter KA, Corman J, et al. Delayed biliary duct obstruction after orthotopic liver transplantation. Surgery. 1972;72:604–610. [PMC free article] [PubMed] [Google Scholar]
- 66.Waldram R, Williams R, Calne RY. Bile composition and bile cast formation after transplantation of the liver in man. Transplantation. 1975;19:382–387. doi: 10.1097/00007890-197505000-00004. [DOI] [PubMed] [Google Scholar]
- 67.Starzl TE, Porter KA, Putnam CW, et al. Orthotopic liver transplantation in ninety-three patients. Surg Gynecol Obstet. 1976;142:487–505. [PMC free article] [PubMed] [Google Scholar]
- 68.Starzl TE, Putnam CW, Hansborough JF, et al. Biliary complications after liver transplantation: with special reference to the biliary cast syndrome and techniques of secondary duct repair. Surgery. 1977;81:212–221. [PubMed] [Google Scholar]
- 69.Starzl TE. Liver alio- and xenotransplantation. Transplant Proc. (in press) [Google Scholar]
- 70.Starzl TE, Fung JJ, Demetris AJ, et al. Baboon to human liver xenotransplantation. Lancet. (in press) [Google Scholar]
- 71.Todo S, Demetris AJ, Van Thiel D, et al. Orthotopic liver transplantation for patients with hepatitis B virus (HBV) related liver disease. Hepatology. 1991;13:619–626. [PMC free article] [PubMed] [Google Scholar]


