Abstract
Microglia are the resident immune cells and phagocytes of our central nervous system (CNS). While most work has focused on the rapid and robust responses of microglia during CNS disease and injury, emerging evidence suggests that these mysterious cells have important roles at CNS synapses in the healthy, intact CNS. Groundbreaking live imaging studies in the anesthetized, adult mouse demonstrated that microglia processes dynamically survey their environment and interact with other brain cells including neurons and astrocytes. More recent imaging studies have revealed that microglia dynamically interact with synapses where they appear to serve as “synaptic sensors,” responding to changes in neural activity and neurotransmitter release. In the following review, we discuss the most recent work demonstrating that microglia play active roles at developing and mature synapses. We first discuss the important imaging studies that have led us to better understand the physical relationship between microglia and synapses in the healthy brain. Following this discussion, we review known molecular mechanisms and functional consequences of microglia-synapse interactions in the developing and mature CNS. Our current knowledge sheds new light on the critical functions of these mysterious cells in synapse development and function in the healthy CNS, but has also incited several new and interesting questions that remain to be explored. We discuss these open questions, and how the most recent findings in the healthy CNS may be related to pathologies associated with abnormal and/or loss of neural circuits.
Keywords: Glia, Pruning, Plasticity, Neurotransmission, Cortex, Hippocampus, Retinogeniculate
Introduction
Nearly a century has passed since the concept of microglia was originally described in the healthy mammalian CNS by Pio del Rio Hortega (del Rio-Hortega 1932). Surprisingly, there is still very little known regarding the role of these mysterious cells in the normal, healthy CNS. A vast majority of studies have focused on the role of microglia in the context of disease, in which they display an extraordinary ability to respond rapidly and perform a broad range of functions such as shielding injury sites, phagocytosing cellular material, and releasing inflammatory signals to initiate and/or propagate the immune response. While these rapid and robust responses to CNS injury and disease make microglia interesting candidates for developing diagnostic and therapeutic strategies, these qualities also make microglia very difficult to study in the context of the healthy brain. Culture models such as dissociated cells and brain slices can mimic injury resulting in ‘activation’ of microglia, a shift toward a graded cellular state most associated with injury and characterized by morphological characteristics more closely resembling peripheral macrophages (amoeboid, rod-like, etc.) as well as functional changes such as increased phagocytic capacity, chemotaxis, proliferation, and expression of pro-inflammatory molecules (Kettenmann et al. 2011; Ransohoff and Perry 2009). Thus, while in vitro studies are valuable for understanding some of the basic cellular biology, in vivo strategies must also be utilized to understand the function of these cells in the context of the healthy, intact CNS.
In 2000, a transgenic mouse was engineered that enabled visualization of microglia by EGFP (CX3CR1+/EGFP)(Jung et al. 2000). These mice, combined with new technology to image the brain in a live, anesthetized mouse by 2-photon microscopy, resulted in groundbreaking findings in 2005. Two studies used a thin-skulled transcranial approach and demonstrated that, in contrast to their name, ‘resting’ microglia in the healthy, adult cerebral cortex continuously survey their surrounding extracellular environment (Davalos et al. 2005; Nimmerjahn et al. 2005). Time-lapse imaging revealed that microglia rapidly extend and retract their processes on the order of minutes while their cell bodies remain stationary. As a result, within a few hours, microglia processes have the capacity to sample the entire brain parenchyma and physically interact with other cortical cells including astrocytes and neurons (Nimmerjahn et al. 2005). These key findings beg the question, what is the function of microglia in the healthy brain? The following review will focus on the most recent studies that suggest that microglia play important roles at synapses in the developing and mature CNS and that synaptic activity may concomitantly modulate microglia function.
I. Imaging activity-dependent interactions between microglia and synaptic circuits
The pioneering imaging studies conducted in 2005 laid a foundation for more work focused on the interactions between microglia and synapses in response to spontaneous and sensory experience-driven changes in neuronal activity. The following sections will discuss key imaging studies demonstrating that microglia have the capacity to rapidly respond to changes in neurotransmitters and physically interact with synapses in an activity-dependent manner.
Imaging the effects of neurotransmission on microglial dynamics
In vitro preparations of microglia demonstrate that microglia have the capacity to express receptors for and respond to neurotransmitters such as acetylcholine, gamma-aminobutyric acid (GABA), glutamate, and purinergics including adenosine-5’-triphosphate (ATP). Such studies have demonstrated that neurotransmitters can affect microglia in vitro in numerous ways, including changes in membrane potential, intracellular calcium, cytokine release, and overall cellular motility, reviewed in (Biber et al. 2007; Kettenmann et al. 2011; Pocock and Kettenmann 2007). Since these original studies, several live imaging studies have demonstrated the capacity of microglia to rapidly respond and change their dynamics in response to neurotransmitters.
Of the many neurotransmitters known to elicit an effect, in vitro and in vivo preparations alike have demonstrated that ATP is one of the most potent signals to elicit a microglial response (Farber and Kettenmann 2006; Inoue et al. 2007). In 2005, Davalos et al. demonstrated that purinergic signaling increased basal microglia motility in vivo. In the anesthetized mouse, the authors applied either apyrase, which hydrolyzes ATP and ADP, or pharmacological blockers of gap junction hemichannels (carbenoxolone or flufenamic acid) to the cortical surface followed by live imaging (Davalos et al. 2005). In all instances, disrupting ATP-dependent signaling resulted in reduced basal velocity of microglial processes. More recently, using mice harboring a deletion in the purinergic receptor P2Y12 or A2A, it has been demonstrated in vitro and in vivo that purinergic signaling through these receptors can mediate microglial process extension and chemotaxis or retraction, respectively, in response to local nucleotide application or lipopolysaccharide (LPS)-induced activation (Haynes et al. 2006; Orr et al. 2009). However, it remains to be determined whether purinergic signaling can regulate microglia under more basal, physiological conditions in the absence of local nucleotide or LPS application. Interestingly, a more recent live imaging studying using ex vivo retinal explants revealed that glutamatergic transmission increased microglia motility indirectly via increased ATP release upon neuronal excitation, data suggesting a role for ATP in regulating microglia dynamics under more basal conditions (Fontainhas et al. 2011).
Similar to purinergic and glutamatergic signaling, inhibitory neurotransmission has also been implicated in regulating microglial dynamics. In the same ex vivo study in the retina described above, which demonstrated effects of glutamate and ATP, the inhibitory neurotransmitter, GABA, decreased microglia process motility and overall velocity, while the GABAA receptor antagonist, bicuculline, increased motility and velocity. These data are similar to the in vivo work by Nimmerjahn et al. (2005) that showed a similar increase in motility upon bicuculline application; however, overall velocity was unchanged, data that may reflect differences between ex vivo and in vivo preparations and/or region analyzed (retina vs. cortex).
Overall, the studies discussed above suggest that increased excitatory neurotransmission and purinergic signaling can cause an increase in microglial process motility, while inhibitory neurotransmission may decrease this motility. However, one recently published study by Grinberg et al. (2011) used an in vitro hippocampal slice preparation to assess microglial dynamics in response to spreading depression and have results which suggest a seemingly opposite result. When long term potentiation (LTP) was induced by increasing cyclic adenosine monophosphate (cAMP), enhanced neural transmission induced a decrease in microglial movement (Grinberg et al. 2011). Conversely, blockade of activity with tetrodotoxin (TTX) resulted in increased movement, and restoration of activity with glutamate or ATP subsequently decreased movement. However, these results may reflect the in vitro nature of this sample preparation, regional differences in neurotransmission (e.g., hippocampus vs. retina or cortex), regional microglial heterogeneity, and/or differences in stimulation paradigms (e.g., cAMP or TTX ± glutamate or ATP vs. direct application of neurotransmitters or antagonists).
Despite discrepancies, it is clear that microglia are responding to changes in neurotransmission and that neurotransmitters can have direct and rapid effects on the overall dynamics of these cells. Future in vivo work will be required to further dissect the nature of microglia responses to excitatory and inhibitory neurotransmission as well as identify whether these effects have regional specificity.
Imaging physical interactions between microglia and synapses
The imaging studies discussed above suggest that microglia can rapidly change their dynamics in response to neurotransmission. In order to better understand how microglia may be physically interacting with synapses in paradigms known to change neural activity and induce synaptic remodeling, recent work has taken advantage of 2-photon live imaging in the anesthetized mouse, in which microglia and neurons are fluorescently labeled (Tremblay 2012). The first imaging study to characterize physical interactions between microglia and synaptic elements in vivo used 2-photon time-lapse imaging and transgenic mice expressing EGFP in both microglia and neurons (Iba-1-EGFP/Thy1-EGFP M line) (Feng et al. 2000; Hirasawa et al. 2005; Wake et al. 2009). This study revealed that microglial processes briefly (~5 min) contacted synaptic elements in layers II-III of the somatosensory and visual cortices at a rate of ~1 structure per hour and that microglial processes appeared to enlarge once presynaptic terminals were contacted. Following these baseline measurements, the authors investigated how changes in neural activity might affect physical interactions between microglia and synaptic elements. Using 3 independent methods to decrease neural activity (enucleation, retinal TTX administration, or lowered body temperature), the authors observed that microglial processes retracted and made fewer contacts with less active presynaptic terminals within the cortex. These data are in discordance with previously published work demonstrating no change in microglial motility in response to direct cortical application of TTX in vivo (Nimmerjahn et al. 2005) or increased microglial movement in response to TTX administration in an in vitro slice preparation (Grinberg et al. 2011). However, these discrepancies may easily be explained by method of TTX administration, regional differences, and/or in vivo vs. in vitro preparations. To further assess interactions between microglia and synapses, Wake et al. (2009) induced ischemic injury by photochemical occlusion of the middle cerebral artery (MCA) and demonstrated that, under these conditions, microglia contact with presynaptic structures was prolonged and was often accompanied or followed by a disappearance of these structures. These data suggest a potential function for microglia in the removal of synapses undergoing, albeit injury-induced, remodeling.
In order to address whether microglia were indeed playing a role at remodeling synapses under more physiological conditions, Tremblay et al. published a recent study assessing in vivo changes in microglia-synapse interactions in the developing primary mouse visual cortex (V1)(Tremblay et al. 2010). This study was able to attain better spatial resolution than previous studies by combining high resolution 3-D serial electron microscopy (3-D serial EM) with 2-photon, transcranial imaging. In addition, in contrast to Wake et al. (2009), Tremblay et al. (2010) were better able to distinguish processes belonging to microglia versus neurons by using a transgenic mouse line expressing EGFP in microglia and YFP in neurons (CX3CR1+EGFP/Thy-1 YFP). This study utilized the critical period in the mouse visual system to assess sensory experience-induced changes in microglia-synapse interactions (Gordon and Stryker 1996; Tremblay et al. 2010). During this particular period in visual cortex development (postnatal day 21-30 (P21-30) in the mouse), several aspects of visual perception develop (e.g., direction selectivity, ocular dominance, etc.) and are associated with changes in spine dynamics, size, and number (Bence and Levelt 2005; Hooks and Chen 2007; Majewska and Sur 2003). During the peak of the critical period (P28), high resolution imaging demonstrated that microglia in layer II of V1 normally contacted spines, synaptic terminals and synaptic clefts (Tremblay et al. 2010). Importantly, this landmark study revealed for the first time that spines often changed size upon microglia contact, suggesting that microglia may be key regulators of structural spine plasticity. Consistent with this idea, the authors noted that spines which changed size upon microglial contact tended to be smaller and, during later imaging sessions, were often eliminated. These data raise the intriguing possibility that microglia may mediate the elimination of spines in response to sensory experience and suggest one potential mechanism could be phagocytosis.
The idea that glial cells could be phagocytosing circuits undergoing active remodeling is substantiated by earlier work in the Drosophila mushroom body and mammalian neuromuscular junction (NMJ) (Freeman 2006; Mallat et al. 2005). In developing Drosophila, glial cells engulf the axons of mushroom body γ neurons undergoing anatomical pruning. In addition, in the mammalian system, Schwann cells phagocytose axonal arbor remnants (i.e., “axosomes”) undergoing pruning at the developing NMJ (Bishop et al. 2004). Consistent with these data, glia have been shown to engulf destabilized synaptic boutons and presynaptic debris at the developing Drosophila NMJ (Fuentes-Medel et al. 2009). However, except for early work suggesting that microglia and astrocytes could be engulfing axonal debris during large-scale cortical pruning (Berbel and Innocenti 1988), very little was known regarding glial-mediated phagocytosis of mammalian CNS synapses undergoing local, small-scale remodeling. Thus, to further assess physical interactions between microglia and remodeling CNS synapses, Tremblay et al. used a dark adaptation paradigm in which juvenile mice were placed in the dark for 6 days during the visual system critical period (Tremblay et al. 2010). In some cases, mice were then re-exposed to light for 2 days, a paradigm known to elicit synaptic remodeling in V1 (Mower et al. 1983; Philpot et al. 2001; Tropea et al. 2010; Viegi et al. 2002). Although microglia in V1 contacted synapses in a similar manner in dark-adapted mice ± light exposure as compared to normal, light exposed mice, the authors provided exciting new evidence that microglia had more phagocytic inclusions upon dark adaptation + light exposure, which resembled pre- and postsynaptic elements. Consistent with these data, a more recent study by the same group demonstrated an age-dependent increase in phagocytic inclusions resembling pre- and postsynaptic elements in either V1 or primary auditory cortex (A1) in association with age-dependent loss of vision or hearing, respectively (Tremblay et al. 2012). Furthermore, another recent study used stimulated emission depletion (STED) microscopy and post-embedding EM to assess microglia-mediated phagocytosis of synapses in the developing hippocampus (Paolicelli et al. 2011), data that further suggest that microglia may engulf remodeling synaptic elements.
The data described above suggest that microglia are phagocytosing synaptic elements and that this interaction may underlie synaptic remodeling in response to changes in sensory experience. In order to more directly address whether microglia are phagocytosing synapses undergoing activity-dependent synaptic remodeling, we recently (Schafer et al. 2012) assessed phagocytosis in a system in which activity-dependent synaptic remodeling has been very well characterized: the postnatal retinogeniculate system. In this system, retinal ganglion cells (RGCs) form synapses on relay neurons residing in the dorsal lateral geniculate nucleus (dLGN) of the thalamus and these synapses are known to undergo activity-dependent remodeling during a very small developmental window (see Section III for details regarding retinogeniculate remodeling) (Guido 2008; Hong and Chen 2011; Huberman 2007; Sretavan and Shatz 1986). Using this system, we developed a high throughput in vivo phagocytic assay to demonstrate that microglia phagocytose presynaptic RGC inputs during a peak period in retinogeniculate synaptic remodeling (P5 in mouse), data that were validated by several different light and ultrastructural assays (Schafer et al. 2012). By EM, this phagocytosed material had characteristic features of presynaptic terminal machinery such as 40 nm vesicles; however, engulfment of axonal arbors cannot be excluded. Unlike previous work, this study observed little evidence suggesting engulfment of postsynaptic machinery, which may be due to regional differences, particularly given that RGCs synapse on the cell body and proximal dendrites of postnatal dLGN relay neurons (Guido 2008). We further demonstrated that engulfment and synaptic remodeling were temporally correlated such that as remodeling was nearly complete, engulfment of RGC inputs was also dramatically reduced.
In addition to assessing developmental regulation, we developed an in vivo assay to test whether microglia synapse-interactions are affected by activity-dependent synaptic competition. Activity-dependent competition was established by injecting one eye with either TTX to silence firing or forskolin, a cAMP analog, to increase firing (Dunn et al. 2006; Stellwagen and Shatz 2002; Stellwagen et al. 1999). The other eye was injected with vehicle. After pharmacologically decreasing (TTX) or increasing (forskolin) firing in one eye, data revealed that microglia preferentially engulfed inputs originating from the ‘weaker’ or less active eye (Schafer et al. 2012). Importantly, these particular pharmacological manipulations are known to disrupt normal synaptic remodeling such that inputs from the ‘weaker’ or less active eye lose territory and inputs from the ‘stronger’ or more active eye gain territory (Cook et al. 1999; Del Rio and Feller 2006; Huberman et al. 2008; Penn et al. 1998; Shatz 1990; Shatz and Stryker 1988; Stellwagen and Shatz 2002). Thus, these data suggest that microglia are dynamic sensors during activity-dependent synaptic remodeling and may be actively engulfing synapses destined for elimination.
Taken together, these provocative studies demonstrate that microglia, indeed, interact with and engulf synaptic elements and that microglia-synapse interactions are dependent upon neural activity and sensory experience. Importantly, these studies raise new and interesting questions including: 1) What are the molecular mechanisms underlying microglia-synapse interactions? and 2) What are the physiological consequences of these interactions at remodeling synapses?
II. Microglia-synapse interactions in the developing CNS: Molecular mechanisms and functional consequences
The mature nervous system is characterized by a remarkably precise neural circuitry. However, in the developing nervous system, this circuitry is far less precise as neurons during early development form exuberant transient synapses. In a process termed synaptic pruning, many synapses are eliminated while the remaining synapses are maintained and strengthened. While it is clear that neural activity regulates pruning as well as synapse maturation (Hua and Smith 2004; Huberman et al. 2008; Katz and Shatz 1996; O'Leary and McLaughlin 2005; Sanes and Lichtman 1999; Torborg and Feller 2005), little is known regarding cellular and molecular mediators. Interestingly, several key papers published in the last decade have identified a critical role for molecules traditionally associated with immune function (MHC class I molecules and receptors, complement components and receptors, and neuronal pentraxins) as modulators of developmental synaptic pruning (Bjartmar et al. 2006; Boulanger 2009; Corriveau et al. 1998; Datwani et al. 2009; Goddard et al. 2007; Huh et al. 2000; Schafer and Stevens 2010; Stevens et al. 2007; Syken et al. 2006). Taken together with the emerging evidence that activity regulates microglia at synaptic sites (Schafer et al. 2012; Tremblay et al. 2010; Wake et al. 2009), it was hypothesized that microglia, the resident CNS immune cells, may be cellular mediators of activity-dependent synaptic pruning and maturation. To determine whether microglia-synapse interactions in the developing brain have physiological consequences and dissect molecular mechanisms underlying these interactions, recent studies have utilized mice harboring deletions of genes specific to microglia in the context of the healthy developing CNS (Paolicelli et al. 2011; Pascual et al. 2011; Roumier et al. 2004; Roumier et al. 2008; Schafer et al. 2012).
A role for microglia in developmental synaptic pruning
One molecular pathway that has been proposed as a potential mediator of microglia-synapse interactions and developmental synaptic pruning is the classical complement cascade (Alexander et al. 2012; Schafer and Stevens 2010; Stevens et al. 2007). Molecules belonging to the classical complement cascade, C1q and C3, are localized to synaptic compartments and mediate synaptic pruning in the developing retinogeniculate system (Stevens et al. 2007). In the innate immune system, C1q and/or C3 bind cellular material inducing its removal by several different mechanisms including phagocytic pathways (Gasque 2004; Lambris and Tsokos 1986; van Lookeren Campagne et al. 2007). Thus, an intriguing hypothesis has been proposed that complement proteins bind synapses, which are subsequently eliminated by the primary CNS phagocyte, microglia. Recently, we demonstrated in vivo that complement component C3, enriched in synaptic compartments, and its receptor, complement receptor 3 (CR3), expressed on the surface of microglia, mediated engulfment of presynaptic terminals in the developing retinogeniculate system during a peak period in synaptic remodeling (P5 mouse dLGN) (Schafer et al. 2012). Similarly, an in vitro study suggested that sialic acid localized to neurites regulates binding of complement components (C1q and C3) and CR3-dependent engulfment of neurites by microglia. (Linnartz et al. 2012). These data are consistent with the idea that complement proteins may be ‘tagging’ synapses for removal by complement-receptor expressing microglia (Figure 1, panel B). However, it remains to be determined in vivo whether complement components are regulated by activity and localized to synapses destined for elimination by microglia-mediated engulfment. However, because engulfment of RGC inputs was reduced by 50% in C3 and CR3 KO mice, data suggest that other phagocytic pathways must be involved (Schafer et al. 2012). Interesting candidates may be proteins belonging to the “find-me”, “eat-me,” and “don't-eat-me” signaling pathways traditionally associated with engulfment of apoptotic cells, for review see (Elward and Gasque 2003; Griffiths et al. 2009; Grimsley and Ravichandran 2003; Nagata et al. 2010; Ravichandran 2011). For example, the Drosophila Draper (CED-1 in C. elegans and MEGF10 in mammals) and CED-6 (GULP in mammals) pathways have been demonstrated to be involved in the removal of axonal debris during developmental axon pruning and following axonal injury (Awasaki et al. 2006; Fuentes-Medel et al. 2009; Logan et al. 2012; MacDonald et al. 2006; Ziegenfuss et al. 2008). It remains to be determined whether these pathways may also be involved in the mammalian system.
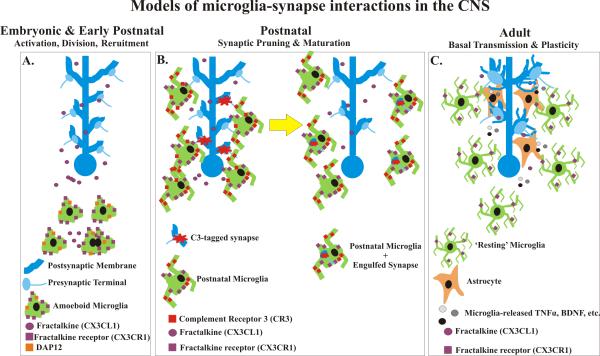
Figure 1. Models of microglia-synapse interactions in the CNS.
A. In the embryonic and early postnatal brain, microglia are of an amoeboid morphology resembling ‘activated’ cells associated with disease and injury. During this stage, they are actively dividing and recruited to regions throughout the CNS. Fractalkine (purple circles), which may be released by neurons, is proposed to act on fractalkine receptors (purple squares) expressed by microglia to regulate activation, cell number, and/or recruitment to synaptic-enriched regions. DAP12 (orange squares) expressed on the surface of microglia is also thought to affect synapse function during this period, perhaps, by regulating the ‘activation’ state of microglia. B. In the postnatal brain, during the first 3 weeks of postnatal life, microglia, which are still ‘activated’ but now have processes, participate in synaptic pruning. Along with the fractalkine receptor (purple squares) and DAP12 (orange squares), microglia express high levels of complement receptor 3 (CR3, red squares) on their surface. We propose that complement component C3 (red stars) is tagging synapses for removal and have demonstrated that synapses are engulfed by phagocytic microglia in a complement-dependent manner. Disruptions in any of these processes results in deficits in synaptic pruning or maturation. C. In the adult brain, evidence suggests that microglia are releasing soluble factors (grey and black circles) such as BDNF, TNFα, glycine, L-serine etc., which affect basal neurotransmission and synaptic plasticity (i.e., LTP) via direct action on neurons or indirectly via astrocytes (orange). In addition, fractalkine signaling via soluble fractalkine (purple circles), most likely released by neurons, and the fractalkine receptor (purple squares), expressed by microglia, modulates microglia-synapse interactions to affect LTP and behavior in the mature CNS.
Given that C3 and CR3 KO mice had a deficit in engulfment of remodeling RGC inputs, we directly addressed the role of microglia in developmental synaptic pruning by assessing C3 and CR3 KO mice for pruning defects in the developing retinogeniculate system. Early in development, RGCs in the retina project to and form exuberant, transient synaptic connections within the early dLGN of the thalamus (Guido 2008; Hong and Chen 2011; Huberman 2007; Sretavan and Shatz 1986). Within the first week of rodent postnatal development, RGC synaptic inputs compete for territory, resulting in the elimination of many transient synaptic connections and the maintenance and strengthening of those synapses that remain. This competition can occur between synapses originating from the same eye (monocular) as well as between synapses originating from different eyes (binocular). One gross assessment for deficits in retinogeniculate pruning is eye specific segregation, in which inputs from both eyes compete for territory, ultimately resulting in the termination of ipsilateral and contralateral synaptic inputs in distinct non-overlapping domains in the mature dLGN (Godement et al. 1984; Guido 2008; Huberman et al. 2008; Jaubert-Miazza et al. 2005; Sretavan and Shatz 1986; Ziburkus and Guido 2006). Using this system, pharmacological (minocycline) or more specific genetic (C3 or CR3 KO) disruptions in microglia function resulted in deficits in eye specific segregation as well as an increased density of structurally intact synapses (Schafer et al. 2012; Stevens et al. 2007). Importantly, these effects were sustained into adulthood and, in the case of the CR3 KO, could be specifically attributed to microglia in the context of the healthy developing brain. One question that remains unanswered is whether microglia-dependent pruning effects represent an active or passive process. That is, while C3/CR3-mediated engulfment and pruning data are temporally correlated, it remains to be determined whether microglia mediate pruning by actively engulfing intact terminals destined for elimination via C3/CR3 signaling or whether C3/CR3-mediated engulfment represents a ‘clean-up’ process independent of C3/CR3-dependent pruning. In addition, future work should aim to assess whether these effects are specific to the retinogeniculate system or have broader implications throughout the CNS and whether other immune pathways previously identified to play a role in retinogeniculate pruning and plasticity throughout the CNS (e.g., MHC class I molecules) may interact with complement and/or microglia to mediate synaptic pruning (Corriveau et al. 1998; Datwani et al. 2009; Goddard et al. 2007; Huh et al. 2000).
A role for microglia in synapse maturation in the developing CNS
Consistent with microglia having a broader role in synapse development, recent work in the developing cortex and hippocampus has suggested a role for microglia in remodeling and/or maturation of synaptic circuits (Paolicelli et al. 2011; Roumier et al. 2004; Roumier et al. 2008; Tremblay et al. 2010). For example, Paolicelli et al. (2011) demonstrated a role for the fractalkine receptor (CX3CR1), expressed specifically on the surface of microglia in the developing CNS (Harrison et al. 1998), in hippocampal synapse development. In the context of disease, CX3CR1 has the capacity to modulate microglia number, activation, and recruitment to sites of injury by binding its ligand fractalkine (CX3CL1), expressed by injured neurons (Cardona et al. 2006; Jung et al. 2000). In the healthy, developing hippocampus, Cx3cr1KO mice exhibited an increase in spine density and PSD-95 immunoreactivity, enhanced hippocampal long-term depression (LTD), and decreased duration of and latency to pentylenetetrazol (PTZ)-induced seizure response, characteristics associated with less mature synapses and possibly associated with abnormal pruning (Paolicelli et al. 2011). However, postnatal (P15) Cx3cr1KO mice had no deficits in engulfment of postsynaptic elements. In contrast, the authors demonstrated that fewer microglia were present in the postnatal Cx3cr1KO hippocampus as compared to age-matched wild-type controls. Importantly, abnormalities in microglia number and synapse density in postnatal Cx3cr1KO mice returned to normal levels by adulthood. Thus, in the context of the developing CNS, it is most likely that fractalkine signaling regulates microglia activation, number, and/or recruitment to synaptic sites in the early postnatal brain (Figure 1, panels A&B). It remains to be determined if microglia play any sustained functional role at these synapses. In contrast to Paolicelli et al. (2011), another recent study demonstrated different defects in microglia and synaptic function in the mature (3 month old) hippocampus of Cx3cr1KO mice (i.e., increased microglia numbers and reduced LTP; see Section III for more details) raising the possibility that fractalkine signaling may play different roles in the developing and mature brain (Figure 1, panels A-C) (Rogers et al. 2011).
Consistent with microglia playing a role in synapse maturation in the hippocampus, earlier in vitro studies demonstrated that maturation of hippocampal synapses was altered in mice harboring a mutation in KARAP/DAP12 (DAP12KI), a transmembrane receptor expressed by microglia from birth and a known regulator of macrophage activation in the immune system (Hamerman et al. 2005; Roumier et al. 2004; Roumier et al. 2008; Tomasello et al. 2000; Tomasello et al. 1998; Turnbull et al. 2005). Acute hippocampal slices prepared from P22 DAP12KI mutant mice had increased NR2B-containing N-Methyl-D-aspartate (NMDA) receptors as assessed by ifenprodil sensitivity and increased 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl) propanoic acid (AMPA) receptor calcium permeability, characteristics indicative of less mature synapses (Roumier et al. 2004). In a later study, acute hippocampal slices prepared from P18-P25 DAP12KI mice had increased AMPA/NMDA ratios and, while most synapses appeared normal by EM, there was an observed increase in perforated synapses in the stratum radiatum of CA1 (Roumier et al. 2008). In order to address whether this effect was a prenatal developmental defect, the authors prepared near-pure dissociated hippocampal neuron cultures from P0 DAP12KI mice and from mice subjected to in utero inflammation with LPS. After 14 days in culture (DIV 14), functional calcium imaging revealed significantly reduced calcium fluctuations in the presence of the AMPAR blocker 6-cyano-7 nitroquinoxline-2 (CNQX) in cultures prepared from P0 mutant and LPS-treated mice as compared to wild-type (WT) or vehicle-treated mice. In addition, there was an increase in immunohistochemical colocalization of pre- and postsynaptic markers in cultures prepared from DAP12KI mice. Because DAP12 expression is specific to microglia and cultures were presumably void of microglia, these data suggest that the physiological and structural deficits are the result of a developmental defect in synapse maturation that originates prenatally (Figure 1, panel A) (Roumier et al. 2008). However, because the oldest age at which synapse function was assessed was P25 and most experiments were performed in vitro, it remains to be determined whether deficits in DAP12KI hippocampal synapse maturation represent a developmental delay and are later recovered.
These new insights offer significant advancements in our understanding of molecular mechanisms and functional consequences of microglia-synapse interactions in the developing brain and have provoked interest in identifying more microglia-related molecular pathways and functions throughout the CNS. Indeed, identification of such mechanisms and functions will have tremendous impact on the way we think about synapse development and plasticity and may also greatly increase our understanding of diseases associated with abnormal brain wiring (e.g., autism, schizophrenia, etc.) and/or synaptic degeneration (e.g., Alzheimer's disease, Parkinson's, etc.) (See Section IV for discussion of disease relevance).
III. Microglia-synapse interactions in the mature CNS: Molecular mechanisms and functional consequences
The previously discussed studies suggest that interactions between microglia and synapses play an important role in the pruning and/or maturation of synaptic connectivity in the developing CNS. Could microglia play a role in the plasticity and function of synaptic circuits in the mature CNS? In addition to roles at developing synapses, several studies have now described potential functions for microglia at synapses in the healthy, adult CNS, such as regulating LTP, synaptic scaling, and basal glutamatergic and GABAergic transmission (Figure 1, panel C) (Ben Achour and Pascual 2010; Bessis et al. 2007; Kettenmann et al. 2011). The following section will review several key findings.
Microglia-dependent synaptic plasticity in the mature CNS
While studies in the developing brain have demonstrated that physical interactions between microglia and synaptic elements can regulate plasticity, most of the work in the mature CNS has suggested that microglia can function to modulate the plasticity of neural circuits by paracrine signaling. In vitro studies have demonstrated that when either cultured microglia or conditioned media from cultured microglia was added to cortical slice cultures, NMDA-receptor mediated excitatory postsynaptic currents (EPSCs) were larger in amplitude and longer in duration (Moriguchi et al. 2003). Work in hippocampal neuron cultures confirmed this finding and demonstrated that microglia conditioned media also enhanced LTP induction (Hayashi et al. 2006). The same study identified glycine and L-serine secreted by microglia as molecular mediators of this effect. Consistent with a role for microglia in hippocampal LTP, a recent study found that LTP induction was reduced in organotypic hippocampal slices prepared from adult (3 month) Cx3cr1KO mice as compared to WT littermates (Rogers et al. 2011). Importantly, these in vitro deficits were coincident with in vivo impairments in learning and memory as assessed by Morris water maze and contextual and cued fear conditioning. Because these mice also had impaired adult neurogenesis in vivo, it remains to be determined whether CX3CR1 signaling in microglia has a direct or indirect effect on synapse function. Interestingly, unlike postnatal hippocampus, which was characterized by a transient decrease in microglia density (Paolicelli et al. 2011), microglia density was increased in adult Cx3cr1KO hippocampus (Rogers et al. 2011). These data raise the intriguing possibility that fractalkine signaling could have differential effects on microglia depending on the context (Figure 1, panels A-C).
In addition to synaptic plasticity associated with LTP, microglia have also been suggested to play a role in synaptic scaling, a homeostatic mechanism that promotes long-term stability of neural networks (Stellwagen and Malenka 2006; Turrigiano 2008; Turrigiano and Nelson 2004). Using cultured hippocampal neurons, glial-derived TNFα was shown to be necessary for synaptic scaling in excitatory and inhibitory neurons. Because both astrocytes and microglia produce TNFα, the relative contribution of these two cell types to in vitro and in vivo synaptic scaling is an interesting and important area for future investigation.
Microglia-mediated effects on basal synaptic transmission
Besides synaptic plasticity, it has been suggested that microglia could regulate basal glutamatergic and GABAergic synaptic transmission (Coull et al. 2005; Pascual et al. 2011; Tsuda et al. 2003). For example, when acute organotypic hippocampal slices were exposed to the pro-inflammatory molecule LPS, microglia became more ‘activated’ and AMPA receptor-mediated spontaneous EPSC frequency was increased in CA1 neurons (Pascual et al. 2011). Pascual et al. (2011) then demonstrated that this effect was attenuated in slices prepared from PU.1 null mice that lack several cells types belonging to the lymphoid and myeloid lineages (McKercher et al. 1996; Scott et al. 1994), data suggesting that the effect was specific to microglia in the context of the CNS. The authors further demonstrated that the microglia-specific effect was, most likely, indirect and suggested that LPS induces microglia to release ATP that binds P2Y1 receptors on astrocytes. Astrocytes subsequently mediate an increase in excitatory transmission via a metabotropic glutamate receptor 5 (mGluR5)-dependent mechanism. While this study was performed in vitro and may be more disease relevant as LPS is a proinflammatory agent thought to more closely mimic inflammation, it raises the intriguing question as to whether microglia have the capacity to regulate basal glutamatergic transmission under less pathological conditions in vivo.
In addition to affecting basal glutamatergic signaling, microglia have the capacity to modulate GABAergic synaptic transmission; however, this effect has only been demonstrated in the context of injury (Coull et al. 2005; Tsuda et al. 2003). Briefly, upon injury, ATP is released, which, in turn, stimulates microglia to release brain-derived neurotrophic factor (BDNF). Microglia-derived BDNF induces a depolarizing shift in the anion reversal potential that results in an inversion of current polarity activated by GABA. Excitatory transmission via GABA receptor activation results in hyperactivity and increased allodynia. It remains to be determined whether similar microglia-mediated mechanisms may be involved in modulating GABAergic neurotransmission in the healthy CNS.
IV. Microglia-synapse interactions in the healthy CNS: Disease Relevance
The studies discussed in previous sections demonstrate roles for microglia in the remodeling and maturation of synaptic circuits in the developing CNS as well as in contributing to basal transmission and plasticity in the adult. In addition to elucidating mechanisms of synapse development and function in the healthy brain, these studies have important implications for understanding mechanisms underlying synapse pathology in disease. In the following section, we will briefly touch on some key studies suggesting a function for microglia at diseased synapses.
Microglial pathological functions have been traditionally associated with diseases in which they are known to perform several functions, ranging from removal of debris and shielding injury sites to initiating and propagating immune responses (Hanisch and Kettenmann 2007; Kreutzberg 1996; Prinz et al. 2011; Ransohoff and Perry 2009). In the context of models of neuropathic pain, one of the most intriguing synaptic functions is their role in the reversal of GABA currents resulting in pain hypersensitivity (discussed in previous section). In neurodegenerative diseases of the CNS (e.g., Multiple Sclerosis, Alzheimer's disease, Huntington's disease, glaucoma, etc.), abnormal synapse function and loss are increasingly being recognized as hallmarks of early stages of disease progression (Alexander et al. 2012; Coleman et al. 2004; Mandolesi et al. 2010; Milnerwood and Raymond 2010). While there are instances in which synapse loss associated with neurodegeneration (i.e., Prion disease) is a neuron-autonomous event (Perry and O'Connor 2010; Siskova et al. 2009), data in other contexts suggest that microglia are contributing to early synapse loss and/or dysfunction. (Alexander et al. 2012; Alexander et al. 2008; Beggs and Salter 2010; Rosen and Stevens 2010; Schafer and Stevens 2010). For example, in a mouse model of tauopathy (P301S), in vivo hippocampal synapse loss and microglial activation were coincident and occurred as early as 3 months of age, while significant atrophy is not observed until 9-12 months of age (Yoshiyama et al. 2007). Thus, data place microglia at the right time and place to contribute to early synapse loss and/or dysfunction. In addition, microglia are thought to be involved in synaptic stripping following axotomy (Cullheim and Thams 2007; Kreutzberg 1993; Perry and O'Connor 2010; Trapp et al. 2007). These experiments were first performed in the context of the injured facial nerve where microglial processes were shown to intercalate between pre- and postsynaptic elements within the facial nerve nucleus following injury, which effectively “stripped” away presynaptic terminals from their postsynaptic targets within the facial nerve nucleus (Blinzinger and Kreutzberg 1968). Interestingly, a recent study in the aging brain demonstrated an increase in phagocytic inclusions within microglia resembling pre- and postsynaptic elements that was concommittant with age-dependent loss of vision and hearing independent of any signficant neuronal cell loss (Tremblay et al. 2012). Future work will be necessary to better define the role of microglia at dysfunctional synapses associated with neurodegenerative disease and aging and to determine whether these cells, indeed, contribute to early synapse loss.
In addition to synaptic dysfunction and loss associated with neurodegenerative disorders and acute neuronal injury, particularly timely and relevant is the potential contribution of microglia to synaptic abnormalities associated with psychiatric disorders such as autism spectrum disorder (ASD), obsessive compulsive disorder (OCD), and schizophrenia (Chen et al. 2010; Hashimoto 2008; Havik et al. 2011; Monji et al. 2009a; Morgan et al. 2010; Pardo et al. 2005; Vargas et al. 2005). Several groups have published interesting studies demonstrating that early infection, a risk factor for many psychiatric disorders, can result in abnormalities in synapses, microglia, and/or behavior, particularly after a second immune or stress challenge (Bilbo 2010; Bilbo et al. 2006; Bitanihirwe et al. 2010; Ito et al. 2010; Shi et al. 2003). One of the most relevant and exciting new studies that implicates microglia in the abnormal brain wiring associated with ASD is a very recent study by Derecki et al. (2012). Using a genetic model of Rett syndrome, the Mecp2-null, that has behavioral and synaptic phenotypes resembling those associated with ASD (Chen et al. 2001; Guy et al. 2001), Derecki et al. demonstrated that replenishing an Mecp2-null with wild-type microglia resulted in the attenuation of several behavioral and physiological deficits (e.g., body weight, breathing rate, locomotion, etc.). Furthermore, the beneficial effects of wild-type microglia on Mecp2-null mice were diminished when phagocytic activity was blocked pharmacologically with annexin-V. However, it remains unclear precisely how abnormal phagocytic activity may be contributing to the phenotype and whether and how microglia may be contributing to synaptic abnormalities associated with ASD. Interestingly, one in vitro study using dissociated hippocampal neuron cultures demonstrated that cells treated with conditioned media isolated from Mecp2-null mice had delayed and abnormal dendritic morphology, signs of microtubule disruption and damage to postsynaptic glutamatergic components due to toxic levels of glutamate released by the microglia (Maezawa and Jin 2010). Given that deficits in synaptic circuit development are emerging as important underlying correlates of behavioral outcomes (Belmonte et al. 2004; LeBlanc and Fagiolini 2011; Melom and Littleton 2011; Rubenstein and Merzenich 2003; Waites and Garner 2011) and the new data that microglia are participating in synaptic pruning via phagocytic immune pathways (Schafer et al. 2012), it is a highly speculative yet provocative hypothesis that microglia are contributing to ASD symptoms, in part, through aberrant neural-immune signaling at developing synapses.
V. Summary and Remaining Questions
In summary, the recent attention focused on the role of microglia in the healthy brain has elicited several exciting new findings suggesting that microglia play dynamic roles at developing and mature synapses (see Table I). The interactions between microglia and synapses are dependent upon direct, physical contact as well as signaling via soluble factors, and some molecular mediators of these contact-dependent and independent interactions have been identified. On a functional level, it is now clear that microglia interact with and/or engulf synaptic elements in a manner dependent upon neural activity, mediate synaptic pruning in at least one region of the developing CNS, regulate synapse maturation, and modulate plasticity (LTP and synaptic scaling) and basal transmission in the mature CNS. As a result of these important first studies, several questions have arisen and remain unanswered in the field. First, in the context of the developing CNS, while it is clear that complement-dependent phagocytic signaling is one pathway underlying physical interactions between microglia and remodeling synapses, other, yet to be identified, pathways must also be involved. Second, at this point, data demonstrate that phagocytosis of synaptic elements and pruning are temporally correlated. It remains to be determined whether engulfment of synaptic elements is, indeed, an underlying mechanism of plasticity and an active process by which microglia selectively engulf intact synapses destined for elimination. Furthermore, while current imaging data in the cortex and hippocampus suggest a role for microglia in activity-dependent synaptic remodeling or maturation (Paolicelli et al. 2011; Tremblay et al. 2010), it is still unclear whether microglia are necessary for bona fide synaptic pruning in these other brain regions, and if so, what are the underlying mechanisms? In the context of the mature adult CNS, while several molecular pathways have been identified to contribute to synaptic plasticity and basal transmission in slice and cultured cell preparations, in vivo contributions are still relatively unclear with only a few behavioral correlates. In addition, in the mature CNS, it is unknown whether and how microglia-specific pathways may interact with one another to affect neurotransmission and plasticity and whether many of these molecular pathways have similar or different functions in the context of microglia in the developing brain (Figure 1, panels A-C).
Table I.
Overview of synapse-related microglia functions
| Microglia function | Age of analysis | Molecules mediating function | Method of study | Region studied | References |
|---|---|---|---|---|---|
| Activity-dependent synaptic pruning | First postnatal week | C3, CR3, C1q | in vivo: Engulfment assay, EM, anatomical tracing, array tomography | Retinogeniculate system | Schafer et al. 2012 |
| Phagocytic removal of neurites | Cultures prepared from embryonic mice | C3, CR3, C1q, Sialic acids | in vitro: Sialidase treatment of dissociated neuron cultures, IHC, co-culture of neurons and microglia | Hippocampus | Linnartz et al. 2012 |
| Regulation of synapse maturation | Second and third postnatal weeks | CX3CL1, CX3CR1 | in vivo: IHC, EM, and chemical seizure induction ex vivo: Physiology in acute slice | Hippocampus | Paolicelli et al. 2011 |
| Regulation of synapse maturation | Third postnatal week | KARAP/DAP12 | ex vivo: Physiology in acute slice, calcium imaging in vitro: Physiology in dissociated neuron cultures, IHC | Hippocampus | Roumier et al. 2004, Roumier et al. 2008 |
| Activity-dependent structural remodeling | Adulthood | Unknown | in vivo: Transcranial imaging, EM, calcium imaging, alteration of sensory experience, ischemic injury | Somatosensory and visual cortex | Wake et al. 2009 |
| Sensory experience driven structural remodeling | Third and fourth postnatal weeks | Unknown | in vivo: Transcranial imaging, EM, IHC. alteration of sensory experience | Visual cortex | Tremblay et al. 2010 |
| Age-dependent structural remodeling | Adulthood | Unknown | in vivo: EM, behavioral studies, IHC | Visual and auditory cortex | Tremblay et al. 2012 |
| Extracellular surveillance | Adulthood | Unknown | in vivo: Transcranial imaging, pharmacology, targeted injury | Cerebral cortex | Davalos et al. 2005. Nimmerjahn et al. 2005 |
| Enhancement of NMDA receptor mediated responses, LTP | Adulthood | Glycine, L-serine | in vitro: Physiology in organotypic slice and dissociated neuron cultures, preparation of microglia conditioned medium | Cortex, Hippocampus | Moriguchi et al. 2003, Hayaslii et al. 2006 |
| Regulation of LTP, learning and memory | Adulthood | CX3CL1, CX3CR1 | in vivo: Behavioral assays, pharmacology ex vivo: Physiology in acute slice | Hippocampus | Rogers et al. 2011 |
| Synaptic scaling | Adulthood | TNFci | ex vivo: Physiology in acute slice in vitro: Physiology in dissociated neuron cultures | Hippocampus | Stellwagen & Malenka 2006 |
| Modulation of GABAergic transmission | Adulthood | BDNF, ATP | in vivo: Pharmacology, behavioral assays for allodynia ex vivo: Physiology in acute slice | Spinal cord | Tsuda et al. 2003, Coull et al. 2005 |
| Modulation of basal glutamatergic signaling | Adulthood | ATP | ex vivo: Physiology in acute slice, pharmacology in vitro: Physiology in organotypic slice and dissociated neuron cultures | Hippocampus | Pascual et al. 2011 |
| Unknown | Third postnatal week to adulthood | ATP, Glutarmate,GABA | ex vivo.Time lapse imaging in explants, pharmacology, physiology | Retina | Fontainhas et al. 2011 |
| Unknown | Cultures prepared from postnatal mice | ATP, Glutamate | in vitro: Live imaging in slice cultures, pharmacology, physiology | Hippocampus | Grinberg et al. 2011 |
Summary of studies discussed in this review. IHC- Immunohistochemistry, EM- Electron Microscopy, CR3- Complement receptor 3, CX3CL1-Fractalkine, CX3CR1- Fractalkine receptor, TNFα- Tumor necrosis factor-alpha, BDNF- Brain derived neurotrophic factor, ATP- Adenosine triphosphate, GABA- Gamma-aminobutyric acid
Imperative to our understanding of these mysterious cells is the use of in vivo strategies. Microglia are highly reactive cells that respond within minutes to manipulation (Davalos et al. 2005; Hanisch and Kettenmann 2007; Kreutzberg 1996; Nimmerjahn et al. 2005; Ransohoff and Perry 2009). Thus, while in vitro preparations (e.g., isolated cells, acute slice, slice culture, etc.) are important and necessary strategies for dissecting mechanism and function, the physiological relevance and molecular mechanisms identified must be confirmed in vivo. Future in vivo studies using a combinatorial approach of live imaging and molecular biology, including the use of newly derived cre lines (Parkhurst 2011), should significantly advance our understanding of the function of microglia at synapses and the molecular mechanisms underlying these interactions. Given that several prevalent psychiatric and developmental disorders (e.g., schizophrenia, obsessive compulsive disorder, autism, etc.) have recently been linked to deficits in synapse development and/or function, as well as a growing body of evidence suggesting abnormalities in microglia (Derecki et al. 2012; Maezawa and Jin 2010; Monji et al. 2009b; Morgan et al. 2010; Steiner et al. 2008; Vargas et al. 2005; Yang and Lu 2011), understanding functions and molecular pathways underlying microglia-synapse interactions in the healthy brain becomes imperative. This knowledge of molecules and function is important for advancing our understanding of basic biological mechanisms as well as for the promise of developing novel diagnostic and therapeutic strategies associated with CNS disease and injury.
Bibliography
- Alexander A, Barres B, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annual Review of Neuroscience. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Anderson AJ, Barnum SR, Stevens B, Tenner AJ. The complement cascade: Yin-Yang in neuroinflammation--neuro-protection and -degeneration. J Neurochem. 2008;107:1169–87. doi: 10.1111/j.1471-4159.2008.05668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasaki T, Tatsumi R, Takahashi K, Arai K, Nakanishi Y, Ueda R, Ito K. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron. 2006;50:855–67. doi: 10.1016/j.neuron.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Beggs S, Salter MW. Microglia-neuronal signalling in neuropathic pain hypersensitivity 2.0. Curr Opin Neurobiol. 2010;20:474–80. doi: 10.1016/j.conb.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–31. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Achour S, Pascual O. Glia: the many ways to modulate synaptic plasticity. Neurochem Int. 2010;57:440–5. doi: 10.1016/j.neuint.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Bence M, Levelt CN. Structural plasticity in the developing visual system. 2005;147:125–139. doi: 10.1016/S0079-6123(04)47010-1. [DOI] [PubMed] [Google Scholar]
- Berbel P, Innocenti GM. The development of the corpus callosum in cats: a light- and electron-microscopic study. J Comp Neurol. 1988;276:132–56. doi: 10.1002/cne.902760109. [DOI] [PubMed] [Google Scholar]
- Bessis A, Bechade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia. 2007;55:233–8. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Bilbo SD. Early-life infection is a vulnerability factor for aging-related glial alterations and cognitive decline. Neurobiol Learn Mem. 2010;94:57–64. doi: 10.1016/j.nlm.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Rudy JW, Watkins LR, Maier SF. A behavioural characterization of neonatal infection-facilitated memory impairment in adult rats. Behav Brain Res. 2006;169:39–47. doi: 10.1016/j.bbr.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Bishop DL, Misgeld T, Walsh MK, Gan WB, Lichtman JW. Axon branch removal at developing synapses by axosome shedding. Neuron. 2004;44:651–61. doi: 10.1016/j.neuron.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Bitanihirwe BK, Peleg-Raibstein D, Mouttet F, Feldon J, Meyer U. Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology. 2010;35:2462–78. doi: 10.1038/npp.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjartmar L, Huberman AD, Ullian EM, Renteria RC, Liu X, Xu W, Prezioso J, Susman MW, Stellwagen D, Stokes CC. Neuronal pentraxins mediate synaptic refinement in the developing visual system. J Neurosci. 2006;26:6269–81. doi: 10.1523/JNEUROSCI.4212-05.2006. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinzinger K, Kreutzberg GW. Displacement of Synaptic Terminals from Regenerating Motoneurons by Microglial Cells. Z Zellforsch Mikrosk Anat. 1968;85:145–157. doi: 10.1007/BF00325030. [DOI] [PubMed] [Google Scholar]
- Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–24. doi: 10.1038/nn1715. others. [DOI] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, Tudor M, Jaenish R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nature Genetics. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- Chen SK, Tvrdik P, Peden E, Cho S, Wu S, Spangrude G, Capecchi MR. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141:775–85. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman P, Federoff H, Kurlan R. A focus on the synapse for neuroprotection in Alzheimer disease and other dementias. Neurology. 2004;63:1155–62. doi: 10.1212/01.wnl.0000140626.48118.0a. [DOI] [PubMed] [Google Scholar]
- Cook P, Prusky G, Ramoa A. The role of spontaneous retinal activity before eye opening in the maturation of form and function in the retinogeniculate pathway of the ferret. Visual Neuroscience. 1999;16:491–501. doi: 10.1017/s0952523899163107. [DOI] [PubMed] [Google Scholar]
- Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–20. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–21. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Cullheim S, Thams S. The microglial networks of the brain and their role in neuronal network plasticity after lesion. Brain Res Rev. 2007;55:89–96. doi: 10.1016/j.brainresrev.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Datwani A, McConnell MJ, Kanold PO, Micheva KD, Busse B, Shamloo M, Smith SJ, Shatz CJ. Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009;64:463–70. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- del Rio-Hortega P. Microglia. In: W Penfield., editor. Cytology and cellular pathology of the nervous system. Hoeber; New York: 1932. [Google Scholar]
- Del Rio T, Feller MB. Early retinal activity and visual circuit development. Neuron. 2006;52:221–2. doi: 10.1016/j.neuron.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Derecki NC, Cronk JC, Lu Z, Xu E, Abbott SB, Guyenet PG, Kipnis J. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484:105–9. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn TA, Wang CT, Colicos MA, Zaccolo M, DiPilato LM, Zhang J, Tsien RY, Feller MB. Imaging of cAMP levels and protein kinase A activity reveals that retinal waves drive oscillations in second-messenger cascades. J Neurosci. 2006;26:12807–15. doi: 10.1523/JNEUROSCI.3238-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elward K, Gasque P. “Eat me” and “don't eat me” signals govern the innate immune response and tissue repair in the CNS: emphasis on the critical role of the complement system. Molecular Immunology. 2003;40:85–94. doi: 10.1016/s0161-5890(03)00109-3. [DOI] [PubMed] [Google Scholar]
- Farber K, Kettenmann H. Purinergic signaling and microglia. Pflugers Arch. 2006;452:615–21. doi: 10.1007/s00424-006-0064-7. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging Neuronal Subsets in Transgenic Mice Expressing Multiple Spectral Variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Fontainhas AM, Wang M, Liang KJ, Chen S, Mettu P, Damani M, Fariss RN, Li W, Wong WT. Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PLoS One. 2011;6:e15973. doi: 10.1371/journal.pone.0015973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MR. Sculpting the nervous system: glial control of neuronal development. Current opinion in neurobiology. 2006;16:119–25. doi: 10.1016/j.conb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Fuentes-Medel Y, Logan MA, Ashley J, Ataman B, Budnik V, Freeman MR. Glia and muscle sculpt neuromuscular arbors by engulfing destabilized synaptic boutons and shed presynaptic debris. PLoS Biol. 2009;7:e1000184. doi: 10.1371/journal.pbio.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol. 2004;41:1089–98. doi: 10.1016/j.molimm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Goddard CA, Butts DA, Shatz CJ. Regulation of CNS synapses by neuronal MHC class I. Proc Natl Acad Sci U S A. 2007;104:6828–33. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godement P, Salaun J, Imbert M. Prenatal and postnatal development of retinogeniculate and retinocollicular projections in the mouse. J Comp Neurol. 1984;230:552–75. doi: 10.1002/cne.902300406. [DOI] [PubMed] [Google Scholar]
- Gordon J, Stryker M. Experience-Dependent Plasticity of Binocular Responses in the Primary Visual Cortex of the Mouse. Journal of Neuroscience. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths MR, Gasque P, Neal JW. The Multiple Roles of the Innate Immune System in the Regulation of Apoptosis and Inflammation in the Brain. Journal of Neuropathology and Experimental Neurology. 2009;68:217–226. doi: 10.1097/NEN.0b013e3181996688. [DOI] [PubMed] [Google Scholar]
- Grimsley C, Ravichandran KS. Cues for apoptotic cell engulfment: eat-me, don't eat-me and come-get-me signals. Trends in Cell Biology. 2003;13:648–656. doi: 10.1016/j.tcb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Grinberg YY, Milton JG, Kraig RP. Spreading depression sends microglia on Levy flights. PLoS One. 2011;6:e19294. doi: 10.1371/journal.pone.0019294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido W. Refinement of the retinogeniculate pathway. J Physiol. 2008;586:4357–62. doi: 10.1113/jphysiol.2008.157115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nature Genetics. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol. 2005;6:579–86. doi: 10.1038/ni1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Harrison J, Jiang Y, Chen S, Xia Y, Maciejewski D, Mcnamara R, Streiti W, Salafranca M, Adhikari S, Thompson D. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Microglial activation in schizophrenia and minocycline treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1758–9. doi: 10.1016/j.pnpbp.2008.06.012. author reply 1760. [DOI] [PubMed] [Google Scholar]
- Havik B, Le Hellard S, Rietschel M, Lybaek H, Djurovic S, Mattheisen M, Muhleisen TW, Degenhardt F, Priebe L, Maier W. The Complement Control-Related Genes CSMD1 and CSMD2 Associate to Schizophrenia. Biol Psychiatry. 2011;70:35–42. doi: 10.1016/j.biopsych.2011.01.030. others. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Ishibashi H, Hashimoto K, Nakanishi H. Potentiation of the NMDA receptor-mediated responses through the activation of the glycine site by microglia secreting soluble factors. Glia. 2006;53:660–8. doi: 10.1002/glia.20322. [DOI] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–9. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- Hirasawa T, Ohsawa K, Imai Y, Ondo Y, Akazawa C, Uchino S, Kohsaka S. Visualization of microglia in living tissues using Iba1-EGFP transgenic mice. J Neurosci Res. 2005;81:357–62. doi: 10.1002/jnr.20480. [DOI] [PubMed] [Google Scholar]
- Hong YK, Chen C. Wiring and rewiring of the retinogeniculate synapse. Curr Opin Neurobiol. 2011;21:228–37. doi: 10.1016/j.conb.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Critical periods in the visual system: changing views for a model of experience-dependent plasticity. Neuron. 2007;56:312–26. doi: 10.1016/j.neuron.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nat Neurosci. 2004;7:327–32. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- Huberman AD. Mechanisms of eye-specific visual circuit development. Curr Opin Neurobiol. 2007;17:73–80. doi: 10.1016/j.conb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–9. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Koizumi S, Tsuda M. The role of nucleotides in the neuron--glia communication responsible for the brain functions. J Neurochem. 2007;102:1447–58. doi: 10.1111/j.1471-4159.2007.04824.x. [DOI] [PubMed] [Google Scholar]
- Ito HT, Smith SE, Hsiao E, Patterson PH. Maternal immune activation alters nonspatial information processing in the hippocampus of the adult offspring. Brain Behav Immun. 2010;24:930–41. doi: 10.1016/j.bbi.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaubert-Miazza L, Green E, Lo FS, Bui K, Mills J, Guido W. Structural and functional composition of the developing retinogeniculate pathway in the mouse. Vis Neurosci. 2005;22:661–76. doi: 10.1017/S0952523805225154. [DOI] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–14. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L, Shatz C. Synaptic activity and the constuction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch U, Noda M, Verkhratsky A. Physiology of Microglia. Physiological Reviews. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Dynamic changes in motoneurons during regeneration. Restorative Neurology and Neuroscience. 1993;5:59–60. doi: 10.3233/RNN-1993-5115. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–8. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lambris J, Tsokos G. The biology and pathophysiology of complement receptors. Anticancer Research. 1986;6:515–23. [PubMed] [Google Scholar]
- LeBlanc JJ, Fagiolini M. Autism: a “critical period” disorder? Neural Plast. 2011;2011:921680. doi: 10.1155/2011/921680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnartz B, Kopatz J, Tenner AJ, Neumann H. Sialic Acid on the neuronal glycocalyx prevents complement c1 binding and complement receptor-3-mediated removal by microglia. J Neurosci. 2012;32:946–52. doi: 10.1523/JNEUROSCI.3830-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan MA, Hackett R, Doherty J, Sheehan A, Speese SD, Freeman MR. Negative regulation of glial engulfment activity by Draper terminates glial responses to axon injury. Nat Neurosci. 2012;15:722–30. doi: 10.1038/nn.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;50:869–81. doi: 10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Maezawa I, Jin LW. Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. J Neurosci. 2010;30:5346–56. doi: 10.1523/JNEUROSCI.5966-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska A, Sur M. Motility of dendritic spines in visual cortex in vivo: changes during the critical period and effects of visual deprivation. Proc Natl Acad Sci U S A. 2003;100:16024–9. doi: 10.1073/pnas.2636949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat M, Marin-Teva JL, Cheret C. Phagocytosis in the developing CNS: more than clearing the corpses. Curr Opin Neurobiol. 2005;15:101–7. doi: 10.1016/j.conb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Mandolesi G, Grasselli G, Musumeci G, Centonze D. Cognitive deficits in experimental autoimmune encephalomyelitis: neuroinflammation and synaptic degeneration. Neurol Sci. 2010;31:S255–9. doi: 10.1007/s10072-010-0369-3. [DOI] [PubMed] [Google Scholar]
- McKercher S, Torbett B, Anderson K, Henkel G, Vestal D, Baribault H, Klemsz M, Feeney A, Wu G, Paige C. Targeted disruption of the PU. 1 gene results in multiple hematopoietic abnormalities. The EMBO Journal. 1996;15:5647–5658. others. [PMC free article] [PubMed] [Google Scholar]
- Melom JE, Littleton JT. Synapse development in health and disease. Curr Opin Genet Dev. 2011;21:256–61. doi: 10.1016/j.gde.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Milnerwood AJ, Raymond LA. Early synaptic pathophysiology in neurodegeneration: insights from Huntington's disease. Trends Neurosci. 2010;33:513–23. doi: 10.1016/j.tins.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Monji A, Kato T, Kanba S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin Neurosci. 2009a;63:257–65. doi: 10.1111/j.1440-1819.2009.01945.x. [DOI] [PubMed] [Google Scholar]
- Monji A, Kato T, Kanba S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry and Clinical Neurosciences. 2009b;63:257–265. doi: 10.1111/j.1440-1819.2009.01945.x. [DOI] [PubMed] [Google Scholar]
- Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, Courchesne E, Everall IP. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry. 2010;68:368–76. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Moriguchi S, Mizoguchi Y, Tomimatsua Y, Hayashia Y, Kadowaki T, Kagamiishi Y, Katsube N, Yamamoto K, Inoue K, Watanabe S. Potentiation of NMDA receptor-mediated synaptic responses by microglia. Molecular Brain Research. 2003;119:160–169. doi: 10.1016/j.molbrainres.2003.09.007. others. [DOI] [PubMed] [Google Scholar]
- Mower G, Christen W, Caplan C. Very Brief Visual Experience Eliminates Plasticity in the Cat Visual Cortex. Science. 1983;221:178–180. doi: 10.1126/science.6857278. [DOI] [PubMed] [Google Scholar]
- Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–30. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- O'Leary DDM, McLaughlin T. Mechanisms of retinotopic map development: Ephs, ephrins, and spontaneous correlated retinal activity. 2005;147:43–65. doi: 10.1016/S0079-6123(04)47005-8. [DOI] [PubMed] [Google Scholar]
- Orr AG, Orr AL, Li XJ, Gross RE, Traynelis SF. Adenosine A(2A) receptor mediates microglial process retraction. Nat Neurosci. 2009;12:872–8. doi: 10.1038/nn.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science. 2011 doi: 10.1126/science.1202529. others. [DOI] [PubMed] [Google Scholar]
- Pardo CA, Vargas DL, Zimmerman AW. Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry. 2005;17:485–95. doi: 10.1080/02646830500381930. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Littman DN, Gan W. Generation of characterization of a CX3CR1-CreER for the study of microglia function in the CNS. Society for Neuroscience. 2011 2011 Abstract 664.16/I10. [Google Scholar]
- Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1111098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn A, Riquelme P, Feller MB, Shatz C. Competition in retinogeniculate patterning driven by spontaneous activity. Science. 1998;279:2108–2112. doi: 10.1126/science.279.5359.2108. [DOI] [PubMed] [Google Scholar]
- Perry VH, O'Connor V. The role of microglia in synaptic stripping and synaptic degeneration: a revised perspective. ASN Neuro. 2010;2 doi: 10.1042/AN20100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot B, Sekhar A, Shouval H, Bear M. Visual Experience and Deprivation Bidirectionally Modify the Composition and Function of NMDA Receptors in Visual Cortex. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–35. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci. 2011;14:1227–35. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–45. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Ravichandran KS. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 2011;35:445–55. doi: 10.1016/j.immuni.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JT, Morganti JM, Bachstetter AD, Hudson CE, Peters MM, Grimmig BA, Weeber EJ, Bickford PC, Gemma C. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J Neurosci. 2011;31:16241–50. doi: 10.1523/JNEUROSCI.3667-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen AM, Stevens B. The role of the classical complement cascade in synapse loss during development and glaucoma. Adv Exp Med Biol. 2010;703:75–93. doi: 10.1007/978-1-4419-5635-4_6. [DOI] [PubMed] [Google Scholar]
- Roumier A, Bechade C, Poncer JC, Smalla KH, Tomasello E, Vivier E, Gundelfinger ED, Triller A, Bessis A. Impaired synaptic function in the microglial KARAP/DAP12-deficient mouse. J Neurosci. 2004;24:11421–8. doi: 10.1523/JNEUROSCI.2251-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumier A, Pascual O, Bechade C, Wakselman S, Poncer JC, Real E, Triller A, Bessis A. Prenatal activation of microglia induces delayed impairment of glutamatergic synaptic function. PLoS One. 2008;3:e2595. doi: 10.1371/journal.pone.0002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–67. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annual Review of Neuroscience. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Stevens B. Synapse elimination during development and disease: immune molecules take centre stage. Biochem Soc Trans. 2010;38:476–81. doi: 10.1042/BST0380476. [DOI] [PubMed] [Google Scholar]
- Scott E, Simon M, Anastasi J, Singh H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science. 1994;265:1573–1577. doi: 10.1126/science.8079170. [DOI] [PubMed] [Google Scholar]
- Shatz C. Competitive Interactions between Retinal Ganglion Cells during Prenatal Development. Journal of Neurobiology. 1990;21:197–211. doi: 10.1002/neu.480210113. [DOI] [PubMed] [Google Scholar]
- Shatz C, Stryker M. Prenatal Tetrodotoxin Infusion Blocks Segregation of Retinogeniculate Afferents. Science. 1988;242:87–89. doi: 10.1126/science.3175636. [DOI] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal Influenza Infection Causes Marked Behavioral and Pharmacological Changes in the Offspring. The Journal of Neuroscience. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskova Z, Page A, O'Connor V, Perry VH. Degenerating synaptic boutons in prion disease: microglia activation without synaptic stripping. Am J Pathol. 2009;175:1610–21. doi: 10.2353/ajpath.2009.090372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sretavan DW, Shatz CJ. Prenatal development of retinal ganglion cell axons: segregation into eye-specific layers within the cat's lateral geniculate nucleus. J Neurosci. 1986;6:234–51. doi: 10.1523/JNEUROSCI.06-01-00234.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–7. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–9. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Shatz C. An Instructive Role for Retinal Waves in the Development of Retinogeniculate Connectivity. Neuron. 2002;33:357–367. doi: 10.1016/s0896-6273(02)00577-9. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Shatz C, Feller M. Dynamics of Retinal Waves Are Controlled by Cyclic AMP. Neuron. 1999;24:673–685. doi: 10.1016/s0896-6273(00)81121-6. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–78. doi: 10.1016/j.cell.2007.10.036. others. [DOI] [PubMed] [Google Scholar]
- Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313:1795–800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- Tomasello E, Desmoulins P, Chemin K, Guia S, Cremer H, Ortaldo J, Love P, Kaiserlian D, Vivier E. Combined Natural Killer Cell and Dendritic Cell Functional Deficiency in KARAP/DAP12 Loss-of-Function Mutant Mice. Immunity. 2000;13:355–364. doi: 10.1016/s1074-7613(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Tomasello E, Olcese L, Vely F, Geourgeon C, Blery M, Moqrich A, Gautheret D, Djabali M, Matteii M, Vivier E. Gene Structure, Expression Pattern, and Biological Activity of Mouse Killer Cell Activating Receptor-associated Protein (KARAP)/DAP-12. The Journal of Biological Chemistry. 1998;273:34115–34119. doi: 10.1074/jbc.273.51.34115. [DOI] [PubMed] [Google Scholar]
- Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog Neurobiol. 2005;76:213–35. doi: 10.1016/j.pneurobio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Wujek JR, Criste GA, Jalabi W, Yin X, Kidd GJ, Stohlman S, Ransohoff R. Evidence for synaptic stripping by cortical microglia. Glia. 2007;55:360–8. doi: 10.1002/glia.20462. [DOI] [PubMed] [Google Scholar]
- Tremblay M-È. The role of microglia at synapses in the healthy CNS: novel insights from recent imaging studies. Neuron Glia Biology FirstView. 2012:1–10. doi: 10.1017/S1740925X12000038. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Zettel ML, Ison JR, Allen PD, Majewska AK. Effects of aging and sensory loss on glial cells in mouse visual and auditory cortices. Glia. 2012;60:541–58. doi: 10.1002/glia.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea D, Majewska AK, Garcia R, Sur M. Structural dynamics of synapses in vivo correlate with functional changes during experience-dependent plasticity in visual cortex. J Neurosci. 2010;30:11086–95. doi: 10.1523/JNEUROSCI.1661-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter M, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Turnbull IR, McDunn JE, Takai T, Townsend RR, Cobb JP, Colonna M. DAP12 (KARAP) amplifies inflammation and increases mortality from endotoxemia and septic peritonitis. J Exp Med. 2005;202:363–9. doi: 10.1084/jem.20050986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–35. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- van Lookeren Campagne M, Wiesmann C, Brown EJ. Macrophage complement receptors and pathogen clearance. Cell Microbiol. 2007;9:2095–102. doi: 10.1111/j.1462-5822.2007.00981.x. [DOI] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Viegi A, Cotrufo T, Berardi N, Mascia L, Maffei L. Effects of dark rearing on phosphorylation of neurotrophin Trk receptors. European Journal of Neuroscience. 2002;16:1925–1930. doi: 10.1046/j.1460-9568.2002.02270.x. [DOI] [PubMed] [Google Scholar]
- Waites CL, Garner CC. Presynaptic function in health and disease. Trends Neurosci. 2011;34:326–37. doi: 10.1016/j.tins.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–80. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XW, Lu XH. Molecular and cellular basis of obsessive-compulsive disorder-like behaviors: emerging view from mouse models. Curr Opin Neurol. 2011;24:114–8. doi: 10.1097/WCO.0b013e32834451fb. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–51. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Ziburkus J, Guido W. Loss of binocular responses and reduced retinal convergence during the period of retinogeniculate axon segregation. J Neurophysiol. 2006;96:2775–84. doi: 10.1152/jn.01321.2004. [DOI] [PubMed] [Google Scholar]
- Ziegenfuss JS, Biswas R, Avery MA, Hong K, Sheehan AE, Yeung YG, Stanley ER, Freeman MR. Draper-dependent glial phagocytic activity is mediated by Src and Syk family kinase signalling. Nature. 2008;453:935–9. doi: 10.1038/nature06901. [DOI] [PMC free article] [PubMed] [Google Scholar]