Abstract
We reported that IL-2 activated autologous NK cells can induce, but not maintain durable remissions in lymphoma patients. We hypothesized that allogeneic NK cells may overcome class I MHC-mediated inhibition of NK cell killing. In a pilot study, we evaluated infusion of haploidentical donor NK cells for antitumor efficacy. Six patients with advanced B cell non-Hodgkin lymphoma (NHL) received rituximab, cyclophosphamide, and fludarabine as immunosupression to permit homeostatic NK cell expansion, followed by CD3-depleted NK cell-enriched cell products followed by subcutaneous IL-2 administration (10 × 106 units every other day × 6 doses). At 2 months, four patients showed an objective clinical response. We observed early donor cell persistence in two patients (blood and in tumor-bearing node), but this was not detectable beyond 7 days. All patients demonstrated substantial increases in host-regulatory T cells (Treg) after NK cell and IL-2 therapy (180 ± 80 cells/µl vs. baseline: 58 ± 24 cells/µl, p = 0.04) which may have limited donor cell expansion in vivo. These findings suggest safety and feasibility of allogeneic NK cell therapy in patients with lymphoma; however host Treg and inadequate immunodepletion may contribute to a hostile milieu for NK cell survival and expansion. Cell therapy trials should incorporate novel strategies to limit Treg expansion.
Keywords: Allogeneic NK cells, Adoptive cell therapy, Lymphoma
Introduction
The success of allogeneic hematopoietic cell transplantation (HCT) in patients with lymphoma suggests that a potent graft versus lymphoma effect exerts protection from relapse [1] from donor-derived T lymphocytes and NK cells [2–5]. In humans, NK cell killing is partially regulated by inhibitory killer immunoglobulin receptors (KIRs) that limit autologous NK cell-induced tumor lysis by engagement of “self” major histocompatibility complex (MHC) class I alleles [6, 7]. Donor NK cell therapy has been explored for acute myelogenous leukemia with promising results [8, 9], but there is not much data on its activity in lymphoma.
After activation, NK cells acquire the capacity to lyse a broad array of fresh and cultured tumor targets not normally sensitive to NK lysis including lymphoma cell lines. High doses of IL-2 are synergistic with the monoclonal antibody rituximab against rituximab-resistant cell lines. This suggests that IL-2 provides a strong stimulus for effective NK-mediated antibody-dependent cellular cytotoxicity (ADCC) [10–13]. Several studies have supported a role for IL-2 in the therapy and remission maintenance of NHL, however, clinical efficacy has been limited [14–16] We hypothesized that haploidentical donor NK cells activated by IL-2 and targeted by rituximab would provoke an antitumor response in patients with refractory B cell NHL.
Methods
Patients and donors
Patients with relapsed or primary refractory CD20+ NHL with adequate organ function who had failed at least two salvage therapies were eligible. The protocol and consent procedures were approved by the University of Minnesota Institutional Review Board. Non-mobilized donor peripheral blood mononuclear cells were collected using a Fenwal CS-3000® Plus blood cell separator (#4R4538) with a granulocyte separation chamber and small volume collection chamber (SVCC) (Fenwal Division, Baxter Healthcare, Deerfield, IL).
Preparation of the NK cell product
The apheresis product was T cell- (CD3−) depleted using the Miltenyi Biotec CliniMACS® Cell Selection System and CD3 MicroBeads and reagent (Miltenyi Biotec, Auburn, CA) and cultured in serum-free media (X-VIVO 15, Cambrex BioScience, Walkersville, MD) supplemented with 10% human AB serum and 1,000 U/mL IL-2 (Chiron Corporation, Emeryville, CA) [17]. Following 8–16 h incubation, cells were washed twice with a 5% human serum albumin. An aliquot of the cell product was analyzed by flow cytometry to determine the number of T, B and NK cells (using FITC, APC, PE and perCP conjugated antibodies against CD3, CD56, CD19, KIR, NKG2A, NKG2D, CD16, CD69 [BD PharMingen, San Diego, CA]) and tested in a 4-h Cr-release cytotoxicity assay against K562 cell line pre and post IL-2 incubation [18].
Patients received fludarabine 25 mg/m2/day intravenously (IV) daily (days −6 to −2) and cyclophosphamide 60 mg/kg/days IV day −6 to induce immunosupression and lymphodepletion required to facilitate homeostatic expansion of allogeneic donor NK cells. Rituximab 375 mg/m2 IV was administered on day −8 and then weekly for 3 more doses (days −1, +6, +15) to enhance ADCC. Cell products were administered by intravenous infusion on day 0 followed by subcutaneous IL-2 10 × 106 units starting 4 h after NK cell infusion and given every other day ×6 doses to facilitate NK cell survival and expansion in vivo. Patients’ lymphoma was re-staged at day 28, months 2 and 3 by PET/CT and responses determined according to the IWG 2007 criteria for NHL. Patients in complete remission had a bone marrow biopsy performed at 2 months. We monitored donor cell persistence by short tandem repeats (STRs) analysis of whole peripheral blood (PB) at days 7, 14 and fine-needle aspirates from enlarged lymph nodes obtained between days 8 and 14. Toxicity and adverse events were classified according to National Cancer Institute Common Terminology Criteria for Adverse Events V 3.0 (CTCAE).
Phenotyping, cytotoxicity, ELISA
Patients’ blood was analyzed by flow cytometry pre-chemotherapy, days 0, +7, +14 and +28 with multicolor fluorescent control or specific antibodies directed at CD45, CD56, CD3, CD4, CD25, CD19, CD25, CD127, and Foxp3 (BP PharMingen, San Diego, CA). The frequency of regulatory T cells were analyzed pre-chemotherapy and on days 0 and 14. Plasma IL-15, IL-7, tumor necrosis factor receptor 1 (TNFR1) and IL-10 concentrations were determined by commercial enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN).
Statistical analysis
The prospectively established primary endpoint of this study was to achieve donor NK cell expansion to an absolute level of >100 cells/μL at day 14 after NK cell infusion. Cell frequencies were summarized using descriptive statistics. Statistical analysis was performed using Graphpad Prism software (ElCamino, CA) using a paired Student’s t test. A p < 0.05 was considered statistically significant.
Results
Patient, NK cell product characteristics and clinical outcomes
Six patients with advanced NHL were enrolled. All patients were resistant to rituximab-containing salvage chemotherapy regimens and three patients were refractory to fludarabine. They were ineligible for autologous or allogeneic HCT due to progressive disease. Three out of six haploidentical donor-recipient pairs were KIR ligand (HLA) mismatched in the GvH direction (Table 1).
Table 1.
Clinical and disease characteristics
| Pt | Diagnosis | Age | Prior regimens | Stage | BM+ | Flu R | Rit R | Response | KIR-L MM | Time to next therapy (days) | Subsequent therapy | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Marginal zone lymphoma | 48 | 5 | IVB | Yes | Yes | Yes | CR | Yes Bw4 | 116 | MRD HCT | Alive in CR 6 + months |
| 2 | Follicular lymphoma | 49 | 4 | IVB | Yes | Yes | Yes | CR | Yes C2 | 70 | MRD HCT | Alive in CR 5 + months |
| 3 | Transformed lymphoma | 57 | 5 | IVB | Yes | Yes | Yes | PR | No | 62 | DUCB HCT | Alive, relapse at 6 months |
| 4 | DLBCL | 76 | 6 + XRT | IEA | No | N | Yes | PR | Yes C1 | 123 | Chemotherapy | Alive, relapse at 6 months |
| 5 | DLBCL | 48 | 3 | IVB | No | N | Yes | NR | No | 45 | Clinical trial | Dead |
| 6 | Transformed lymphoma | 35 | 3 + XRT | IIEA | No | N | Yes | NR | No | – | Palliative care | Dead |
DLBCL diffuse large B cell lymphoma, N not received, BM bone marrow, flu fludarabine, rit rituximab, XRT radiation therapy, CR complete remission, PR partial remission, NR no response, Flu R fludarabine refractory, Rit R rituximab refractory, MRD matched related donor, DUCB double umbilical cord blood, HCT hematopoietic cell transplantation, KIR-LMM killer immunoglobulin receptor-ligand (class I HLA mismatch) in graft versus host direction
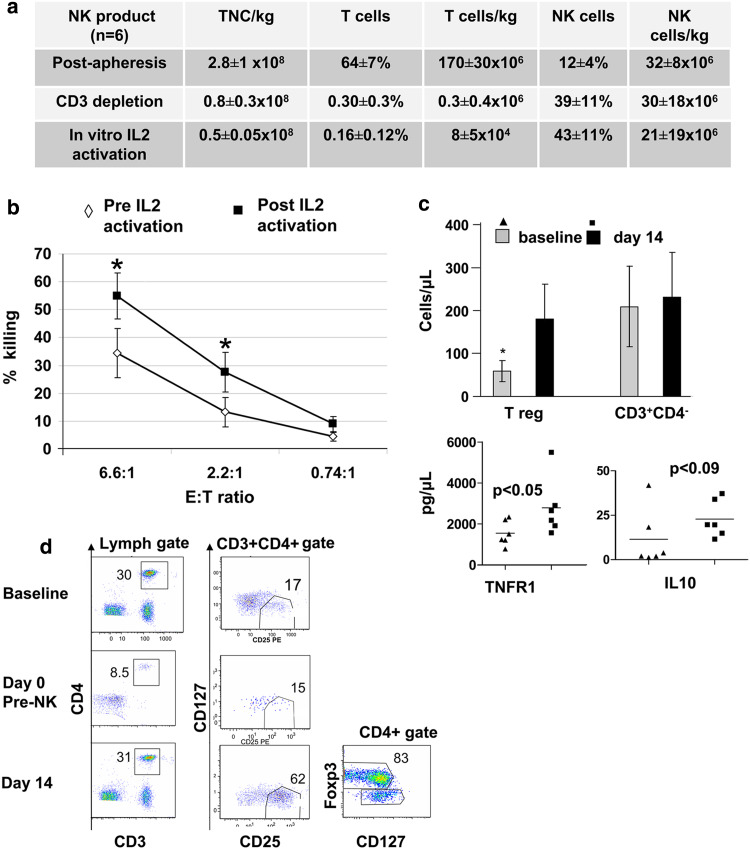
The final NK cell product contained 43 ± 11% NK cells yielding a dose of 21 ± 19 × 106 NK cells/kg. T cell depletion was efficient with only 0.16 ± 0.12% contaminating T cells (Fig. 1a). The activated NK cell product expressed high levels of FcRγIII CD16 (85 ± 6%), NKG2D receptors for stress-induced ligands (40 ± 5.4%) and CD69 (40 ± 24%) reflective of their pre-infusion activation. The expression of KIR and NKG2A was 80 ± 2 and 39 ± 1.8%, respectively. Ex vivo exposure to IL-2 had no impact on these levels. Following ex vivo IL-2 incubation NK cells were highly cytotoxic against K562 targets (Fig. 1b) and expressed twofold higher levels of the adhesion molecule CXCR4 (37 ± 9 vs. 17.6 ± 3%; p = 0.03).
Fig. 1.
a Allogeneic NK cell products and ex vivo expansion. Total nucleated cell (TNC), CD56+/CD3− NK cell and CD3+ T cell frequencies (mean ± SEM) in six apheresis products, after CD3 depletion and overnight activation with 1,000 IU/ml IL-2. b CD3 depleted clinical products (n = 6) were tested in Cr51 release cytotoxic assay against K562 targets before and after ex vivo incubation with 1,000 IU/ml IL-2 at E:T ratio as shown (*p < 0.05). c Regulatory T cells increase after NK cell and IL-2 therapy. Patients with refractory lymphoma received pre-infusion chemotherapy, haploidentical donor NK cell infusion and IL-2 × 6 doses. Absolute numbers of regulatory T cells, cytotoxic CD3+CD4− lymphocytes, serum levels of tumor necrosis factor receptor 1 and IL-10 are compared at baseline and at day 14. Mean cell frequencies ± SEM of five evaluable patients are shown (*p < 0.05). d Flow cytometric detection of T lymphocytes subsets and Treg in peripheral blood at baseline, days 0 (prior to allogeneic NK cells infusion) and 14 (following IL-2 therapy) are compared. Representative plot from patient #6 is shown
The high-dose chemotherapy and NK cell infusion did not induce unexpected toxicity. 5 of 6 patients completed all prescribed IL-2 doses. No patient developed prolonged marrow aplasia or GVHD. Non-hematologic toxicities were NCI CTCAE grade 1–3 rigors and fevers with the NK cell infusion (4 patients), skin redness/swelling at IL-2 injection site (4 patients), fatigue (3 patients), sepsis (1 patient), hypertension (1 patient), bigeminy related to electrolyte disturbance (1 patient) and tumor-induced airway obstruction (1 patient). In 1 patient, IL-2 was stopped after the fourth dose due to dyspnea with pulmonary infiltrates. The patients’ condition rapidly improved with antifungal therapy although no fungal infection was documented. We observed grade 3 neutropenia and thrombocytopenia in all patients. Mean time to neutrophil and platelet recovery was 12 (range 10–16 days) and 14 days (range 3–38 days), respectively. One patient had delayed hematologic recovery associated with human herpes virus-6 infection, which responded to the treatment with foscarnet. This patient was not evaluable for PB cell analysis due to prolonged lymphopenia until day 30.
Clinical outcomes are summarized in Table 1. Four patients achieved objective remissions (2 complete remissions, 2 partial remissions) on day 28 which were maintained at the 2 months post-therapy evaluation. Two patients (#1 and #2) with complete resolution of lymphoma on PET/CT also cleared their bone marrow of lymphoma at 2 months. Three responding patients underwent allogeneic donor HCT at 116, 70 and 62 days post-NK cell therapy with no evidence of disease progression at the time of transplant. The PR in the fourth responding patient lasted for 123 days.
Lack of donor NK cell persistence in blood and tissues
We hypothesized that transient donor NK cell in vivo expansion and persistence is a prerequisite to clinical efficacy. One patient demonstrated PB donor cell expansion (43% circulating donor cells) at day 7 following NK cell infusion, which were replaced by all host cells at later time points. At days 14 and 28, none of the patients had detectable donor cells. NK cells did not significantly change after IL-2 therapy (percentage of lymphocytes and absolute NK cells [mean (range) pre-chemotherapy vs. day 14: 31 ± 12 vs. day 28 ± 11%; 94 NK cells/µl (range 0–129) vs. 137 NK cells/µl (range 0–520); p = NS).
To investigate the possibility of NK cell trafficking to affected lymph nodes, we performed fine-needle aspiration of palpable tumor in three patients, but obtained a sufficient quantity of cells from only one patient. This needle aspirate (at day 14 after NK infusion) showed 2.1% donor cells by STR and 3% of lymphocytes were NK cells. Given the absence of donor NK cells in the blood of this patient, the future study of donor NK cells homing to tumor-bearing lymph nodes is warranted.
Host-regulatory T cells proliferate following IL-2 therapy
Pre-infusion chemotherapy led to lymphodepletion and an endogenous release of IL-7 and IL-15 as shown previously [8]. However, lymphodepletion was incomplete and transient as demonstrated by persistence of recipient T cells immediately before NK cell infusion (Fig. 1d) and rapid recipient T lymphocyte recovery at day 14 with a mean absolute lymphocyte count of 695 ± 83 cells/µl (mean 63 ± 25% T lymphocytes, 28 ± 11% NK cells). To understand this prompt immune reconstitution after high-dose therapy, we examined the phenotype of T cells for the presence of the regulatory T cell (Treg) subset. We observed a threefold increase in CD4+CD127−/lowCD25+ cells following 14 days of IL-2 therapy compared with baseline (31.1 ± 13% vs. 9.82 ± 4%; n = 5, p = 0.003) which lead to an absolute Treg cell increase ([mean ± SEM] 180 ± 80 cells/µl vs. 58 ± 24 cells/µl, p = 0.04; Fig. 1c). The Treg expressed high levels of Foxp3 (Fig. 1d). Phenotypic and functional characteristics of these cells suggest modest immunosuppressive activity (not shown). Expansion of Tregs correlated with twofold increase in plasma levels of TNFR1 and IL-10 at day 14 as compared to pre-NK infusion levels (Fig. 1c). In contrast, no differences in absolute cytotoxic T cells (CD3+CD4−) were observed after therapy as compared to pre-chemotherapy (Fig. 1c). These findings suggest that inadequate lymphodepletion followed by IL-2 administration can permit enhanced host Tregs. By active suppression, secretion of inhibitory cytokines or possibly by competition for exogenous IL-2 the Tregs may have interfered with donor NK cell proliferation and activation. Since the primary endpoint of NK cell expansion was not met, we adhered to prospectively designed stopping rules and closed the trial after enrolling six patients.
Discussion
In this pilot trial, we demonstrated feasibility and safety of haploidentical NK cells infusions in NHL patients. Our findings suggest that in vitro IL-2 activation improves donor NK cells cytotoxicity and can impact on homing to bone marrow by increased expression of CXCR4 on NK cells. We observed objective clinical responses in four patients, but without sustained donor NK cell expansion in peripheral blood. Therefore, the objective clinical responses observed cannot be directly associated with NK cell therapy. NK cell responses cannot be separated from the pre-infusion chemo-immunotherapy and IL-2 despite their clinical tumor progression following similar chemotherapy agents used previously. It is also not known what level of NK cell expansion will result in clinical efficacy. In fact, one study using adoptive T cell therapy reported major tumor responses without evidence of sustained donor chimerism so the threshold may be very low [19].
Importantly, our observations led to the identification of host factors that may have interfered with donor NK cell expansion, particularly host Treg proliferation. Transient lymphodepletion following high-dose chemotherapy spared regulatory T cells and exogenously administered IL-2 allowed their proliferation. Although immune properties of IL-2 induced Tregs are not well understood, functional testing and cytokine profiles suggest immunosuppressive potential that could interfere with donor NK cells survival, expansion and activation [20, 21]. Our findings are particularly relevant in the context of recent animal observations, which demonstrated that endogenous Treg interfered with NK cell proliferation and function and combination therapy using IL-2 and anti-CD25 results in augmented NK cell-mediated antitumor responses [22]. In addition, depletion of Tregs by IL-2 diphtheria toxin facilitated expansion of transferred cytotoxic T lymphocytes and significantly enhanced leukemia killing and prolonged survival of animals with AML when compared with T cell infusion alone [23].
The conditions required for survival and expansion of adoptively transferred lymphocytes in humans are not known and remain an area of active clinical research. Development of optimal chemotherapy to prolong survival and homing of infused NK cells are beginning to be understood. Our results suggest that future adoptive donor cell transfer chemotherapies may benefit from specific agents to eliminate or prevent Treg expansion.
Acknowledgments
The clinical trial was supported in part by a gift from the patient advocate group, CLL Topics, Inc. The authors declare they have no conflict of interest. We would like to thank our dedicated research staff, nurse practitioner Megan Whitmore, research nurses Dixie Lewis and Roby Nicklow and clinical trial office staff, Jill Aughey, for their outstanding support and invaluable contributions in conducting the trial. We want to thank Michael Franklin and Carol Taubert for editorial assistance. We want to acknowledge the University of Minnesota Masonic Cancer Center Translational Therapy Core for their excellent assistance.
References
- 1.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, Rimm AA, Ringden O, Rozman C, Speck B. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 2.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 3.Novitzky N, Thomas V. Allogeneic stem cell transplantation with T cell-depleted grafts for lymphoproliferative malignancies. Biol Blood Marrow Transplant. 2007;13:107–115. doi: 10.1016/j.bbmt.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalandon Y, Roosnek E, Mermillod B, Waelchli L, Helg C, Chapuis B. Can only partial T-cell depletion of the graft before hematopoietic stem cell transplantation mitigate graft-versus-host disease while preserving a graft-versus-leukemia reaction? A prospective phase II study. Biol Blood Marrow Transplant. 2006;12:102–110. doi: 10.1016/j.bbmt.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Perales MA, Jenq R, Goldberg JD, Wilton AS, Lee SS, Castro-Malaspina HR, Hsu K, Papadopoulos EB, van den Brink MR, Boulad F, Kernan NA, Small TN, Wolden S, Collins NH, Chiu M, Heller G, O’Reilly RJ, Kewalramani T, Young JW, Jakubowski AA (2010) Second-line age-adjusted International Prognostic Index in patients with advanced non-Hodgkin lymphoma after T-cell depleted allogeneic hematopoietic SCT. Bone Marrow Transplant [DOI] [PMC free article] [PubMed]
- 6.Wagtmann N, Biassoni R, Cantoni C, Verdiani S, Malnati MS, Vitale M, Bottino C, Moretta L, Moretta A, Long EO. Molecular clones of the p58 NK cell receptor reveal immunoglobulin-related molecules with diversity in both the extra- and intracellular domains. Immunity. 1995;2:439–449. doi: 10.1016/1074-7613(95)90025-X. [DOI] [PubMed] [Google Scholar]
- 7.Colonna M, Samaridis J. Cloning of immunoglobulin-superfamily members associated with HLA-C and HLA-B recognition by human natural killer cells. Science. 1995;268:405–408. doi: 10.1126/science.7716543. [DOI] [PubMed] [Google Scholar]
- 8.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 9.Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, Pui CH, Leung W (2010) NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol [DOI] [PMC free article] [PubMed]
- 10.Lopes de Menezes DE, Denis-Mize K, Tang Y, Ye H, Kunich JC, Garrett EN, Peng J, Cousens LS, Gelb AB, Heise C, Wilson SE, Jallal B, Aukerman SL. Recombinant interleukin-2 significantly augments activity of rituximab in human tumor xenograft models of B-cell non-Hodgkin lymphoma. J Immunother. 2007;30:64–74. doi: 10.1097/01.cji.0000211315.21116.07. [DOI] [PubMed] [Google Scholar]
- 11.Hooijberg E, Sein JJ, van den Berk PC, Hart AA, van der Valk MA, Kast WM, Melief CJ, Hekman A. Eradication of large human B cell tumors in nude mice with unconjugated CD20 monoclonal antibodies and interleukin 2. Cancer Res. 1995;55:2627–2634. [PubMed] [Google Scholar]
- 12.Eisenbeis CF, Grainger A, Fischer B, Baiocchi RA, Carrodeguas L, Roychowdhury S, Chen L, Banks AL, Davis T, Young D, Kelbick N, Stephens J, Byrd JC, Grever MR, Caligiuri MA, Porcu P. Combination immunotherapy of B-cell non-Hodgkin’s lymphoma with rituximab and interleukin-2: a preclinical and phase I study. Clin Cancer Res. 2004;10:6101–6110. doi: 10.1158/1078-0432.CCR-04-0525. [DOI] [PubMed] [Google Scholar]
- 13.Miller JS, Tessmer-Tuck J, Pierson BA, Weisdorf D, McGlave P, Blazar BR, Katsanis E, Verfaillie C, Lebkowski J, Radford J, Jr, Burns LJ. Low dose subcutaneous interleukin-2 after autologous transplantation generates sustained in vivo natural killer cell activity. Biol Blood Marrow Transplant. 1997;3:34–44. [PubMed] [Google Scholar]
- 14.Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S, Linehan WM, Robertson CN, Lee RE, Rubin JT. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987;316:889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- 15.Khan KD, Emmanouilides C, Benson DM, Jr, Hurst D, Garcia P, Michelson G, Milan S, Ferketich AK, Piro L, Leonard JP, Porcu P, Eisenbeis CF, Banks AL, Chen L, Byrd JC, Caligiuri MA. A phase 2 study of rituximab in combination with recombinant interleukin-2 for rituximab-refractory indolent non-Hodgkin’s lymphoma. Clin Cancer Res. 2006;12:7046–7053. doi: 10.1158/1078-0432.CCR-06-1571. [DOI] [PubMed] [Google Scholar]
- 16.Burns LJ, Weisdorf DJ, DeFor TE, Vesole DH, Repka TL, Blazar BR, Burger SR, Panoskaltsis-Mortari A, Keever-Taylor CA, Zhang MJ, Miller JS. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I/II trial. Bone Marrow Transplant. 2003;32:177–186. doi: 10.1038/sj.bmt.1704086. [DOI] [PubMed] [Google Scholar]
- 17.McKenna DH, Kadidlo DM, Miller JS, Orchard PJ, Wagner JE, McCullough J. The Minnesota Molecular and Cellular Therapeutics Facility: a state-of-the-art biotherapeutics engineering laboratory. Transfus Med Rev. 2005;19:217–228. doi: 10.1016/j.tmrv.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Miller JS, Oelkers S, Verfaillie C, McGlave P. Role of monocytes in the expansion of human activated natural killer cells. Blood. 1992;80:2221–2229. [PubMed] [Google Scholar]
- 19.Colvin GA, Berz D, Ramanathan M, Winer ES, Fast L, Elfenbein GJ, Quesenberry PJ. Nonengraftment haploidentical cellular immunotherapy for refractory malignancies: tumor responses without chimerism. Biol Blood Marrow Transplant. 2009;15:421–431. doi: 10.1016/j.bbmt.2008.12.503. [DOI] [PubMed] [Google Scholar]
- 20.Barao I, Hanash AM, Hallett W, Welniak LA, Sun K, Redelman D, Blazar BR, Levy RB, Murphy WJ. Suppression of natural killer cell-mediated bone marrow cell rejection by CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci USA. 2006;103:5460–5465. doi: 10.1073/pnas.0509249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmer J, Andres E, Hentges F. NK cells and Treg cells: a fascinating dance cheek to cheek. Eur J Immunol. 2008;38:2942–2945. doi: 10.1002/eji.200838813. [DOI] [PubMed] [Google Scholar]
- 22.Hallett WH, Ames E, Alvarez M, Barao I, Taylor PA, Blazar BR, Murphy WJ. Combination therapy using IL-2 and anti-CD25 results in augmented natural killer cell-mediated antitumor responses. Biol Blood Marrow Transplant. 2008;14:1088–1099. doi: 10.1016/j.bbmt.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Q, Bucher C, Munger ME, Highfill SL, Tolar J, Munn DH, Levine BL, Riddle M, June CH, Vallera DA, Weigel BJ, Blazar BR. Depletion of endogenous tumor-associated regulatory T cells improves the efficacy of adoptive cytotoxic T-cell immunotherapy in murine acute myeloid leukemia. Blood. 2009;114:3793–3802. doi: 10.1182/blood-2009-03-208181. [DOI] [PMC free article] [PubMed] [Google Scholar]