Abstract
BACKGROUND
Basic research suggests that rapid increases in circulating inflammatory and hemostatic blood markers may trigger or indicate impending plaque rupture and coronary thrombosis, resulting in acute ischemic heart disease (IHD) events. However, these associations are not established in humans.
METHODS AND RESULTS
The Biomarker Risk Assessment in Vulnerable Outpatients (BRAVO) Study will determine whether levels of inflammatory and hemostatic biomarkers rapidly increase during the weeks prior to an acute IHD event in people with lower extremity peripheral artery disease (PAD). The BRAVO Study will determine whether biomarker levels measured immediately prior to an IHD event are higher than levels not preceding an IHD event; whether participants who experience an IHD event (cases) have higher biomarker levels immediately prior to the event and higher biomarker levels at each time point leading up to the IHD event than participants without an IHD event (controls); and whether case participants have greater increases in biomarkers during the months leading up to the event than controls. BRAVO enrolled 595 patients with PAD, a population at high risk for acute IHD events. After a baseline visit, participants returned every two months for blood collection, underwent an electrocardiogram to identify new silent myocardial infarctions, and were queried about new hospitalizations since their prior study visit. Mortality data were also collected. Participants were followed prospectively for up to three years.
CONCLUSIONS
BRAVO results will provide important information about the pathophysiology of IHD events and may lead to improved therapies for preventing IHD events in high-risk patients.
INTRODUCTION
Approximately 8 million men and women in the United States have lower extremity peripheral artery disease (PAD) and the prevalence of PAD is increasing world-wide (1, 2). People with PAD have a 2 to 4–fold increased rate of cardiovascular events compared to those without PAD, even after taking into account cardiovascular disease risk factors (3). Despite major treatment advances, current diagnostic methods and therapies are insufficient to prevent atherosclerotic disease progression. Many patients suffer cardiovascular events despite optimal medical therapy (4, 5). Thus, preventing cardiovascular morbidity and mortality in the large and growing number of people with PAD is an important public health goal.
Evidence from intra-vascular ultrasound, angiography, and pathology examinations indicates that ischemic heart disease (IHD) events often result from plaque rupture on areas of non-obstructive coronary atherosclerosis (6–11). Approximately 70% of IHD events are thought to result from plaque rupture and subsequent luminal thrombosis at arterial sites with minimally occlusive atherosclerosis (5). However, hemostatic and inflammatory protein triggers of acute plaque rupture and subsequent IHD events are not clearly identified
Animal studies and in-vitro models suggest that increases in circulating inflammatory and hemostatic biomarkers may trigger plaque rupture and coronary thrombosis, resulting in IHD events (12–14). Inflammatory and hemostatic blood markers are significantly elevated in people with PAD compared to those without PAD (15–16). Because people with PAD have higher rates of cardiovascular events than those without PAD and because of their increased levels of circulating inflammatory and hemostatic biomarkers, they are an optimal study population in which to assess associations of circulating biomarkers with cardiovascular events.
Establishing whether blood biomarkers increase shortly before an IHD event will help elucidate the pathophysiology of acute vascular events and determine whether acute increases in circulating biomarkers identify persons at high risk for a near-term (i.e. less than 60 days) IHD events. This information may lead to improved therapies for preventing and treating acute cardiovascular events in vulnerable populations, such as those with PAD.
The purpose of the Biomarker Risk Assessment in Vulnerable Outpatients (BRAVO) study is to assemble a cohort of patients at high-risk for IHD events and follow them prospectively with frequent follow-up visits in order to determine whether circulating levels of inflammatory and hemostatic factors increase acutely during the weeks and months leading up to an IHD event. The primary aim of the BRAVO Study is to determine whether among PAD participants who experience an IHD event, biomarker levels measured immediately prior to an IHD event are higher than levels not preceding an event. The second primary aim of the BRAVO Study is to determine whether participants who experience an IHD event (cases) have higher biomarker levels immediately prior to the event than participants who do not experience an event (controls). The second primary aim of the BRAVO Study will also determine whether case participants have greater increases in biomarkers during the months leading up to the IHD event, compared to controls (see Table 1). The biomarkers studied are D-dimer, C-reactive protein (CRP) and serum amyloid A (SAA).
Table 1.
Specific Aims of the BRAVO Study
| Specific Aim | Analyses Plan/Design | |
|---|---|---|
| Primary Aim #1 | Among participants who experience an acute ischemic heart disease event, we will determine whether biomarker levels measured immediately prior to the event are higher than levels not preceding the event. | Case-crossover design. Analyses will be performed within the subset of BRAVO participants who experience an event during follow-up. |
| Primary Aim #2a | We will determine whether participants who experience an acute ischemic heart disease event (cases) have higher biomarker levels immediately prior to the event than participants who do not experience an event (controls). | Case-control study design. Two controls will be randomly selected among those matched by age, sex, and length of time in the study. |
| Primary Aim #2b | We will determine whether case participants have greater increases in biomarkers during the months leading up to the event, compared to controls. | Case-control study design. Two controls will be randomly selected among those matched by age, sex, and length of time in the study. |
METHODS
Overview
The Institutional Review Board at Northwestern University and all participating sites approved the protocol. All participants provided written, informed consent. Enrollment took place between September 2009 and September 2012. Follow-up visits took place through January 2013.
Recruitment
Participants with PAD were identified from computerized or manual lists of consecutive men and women diagnosed with PAD in non-invasive vascular laboratories or vascular surgery practices from the following six medical centers in Chicago: Northwestern Medical Center, Rush Medical Center, University of Chicago Medical Center, Mount Sinai Hospital, Saint Joseph Hospital and Jesse Brown Veterans Affairs Medical Center. Potential participants with PAD received a mailed recruitment letter, after permission to contact them was granted by their physician. Up to four recruitment letters were mailed, at least three weeks apart. If the potential participant did not respond within three weeks after the first recruitment letter was mailed, the potential participant was telephoned and invited to participate.
Inclusion and Exclusion Criteria
The inclusion criterion was an ankle brachial index (ABI) < 0.90 at the baseline study visit. Individuals with an ABI > 0.90 at their baseline visit who had documented evidence of PAD from a non-invasive vascular laboratory or documentation of prior lower extremity revascularization for PAD were also eligible. Exclusion criteria and justification for each criterion are listed in Table 2. Potential participants were first assessed for eligibility by telephone using a standardized interview. Those who remained eligible after the telephone screening were scheduled for a baseline study visit.
Table 2.
Exclusion criteria for the BRAVO Study.
| Exclusion criteria | Justification for exclusion criteria | Number of people excluded* |
|---|---|---|
| 1. ABI greater than 0.90 and no documented evidence PAD | This is a PAD-only study. Individuals with an ABI over 0.90 and no documented evidence of PAD were not eligible to be enrolled in the study. | 97 |
| 2. Mini-Mental Status Exam (MMSE) score lower than 23 out of 30, or other history of cognitive impairment | An MMSE score of < 23 is an exclusion criterion because these participants would be unlikely to provide accurate responses to study questionnaires. | 23 |
| 3. Refusal to have regular blood draws or inability to obtain a blood sample at baseline | Participants who refused blood draws or from whom we are unable to obtain baseline blood samples were excluded because they were unable to participate fully in the study. | 6 |
| 4. Coronary or cerebrovascular event during the previous six months | Persons with a recent cardiovascular event prior to baseline were excluded since their biomarker levels may have been acutely elevated from the recent events. | 1 |
| 5. History of inflammatory arthritis (rheumatoid arthritis, lupus erythematosus, or polymyalgia rheumatica, gout within the last 3 months) | Persons with inflammatory arthritis were excluded because inflammatory arthritis is associated with substantially elevated levels of inflammatory biomarkers. Associations of biomarker levels with acute coronary syndrome events may differ in PAD patients with vs. without inflammatory arthritis | 3 |
| 6. Not from an acceptable recruitment source | In order to be enrolled in the study, participants were to be recruited from specific medical centers at which the study has IRB approval. If not from those centers, these individuals were unable to join the study. | 1 |
| 7. Treatment for cancer other than non-melanoma skin cancer during the previous 2 years (those treated for non-invasive breast cancer or prostate cancer during the previous year were potentially eligible) | Persons treated for cancer in the two years prior to recruitment were excluded because they are less likely to survive for follow-up testing and because undiagnosed, recurrent cancer might affect their circulating blood marker levels. | 0 |
| 8. Unintentional weight loss of more than 7.5 pounds in the last six months | Persons with recent and significant unintentional weight loss were excluded because unintentional weight loss may signify undiagnosed cancer that could influence circulating biomarker levels. | 0 |
| 9. Communication difficulty due to language barriers | Study staff members are not fluent in non-English languages, preventing accurate data collection from potential participants who did not speak English. | 0 |
| 10. Residence more than 40 miles away from the medical center and unwillingness to travel to the medical center for every two month blood collection | Individuals who lived more than 40 miles from the medical center were excluded, unless they were willing to return to Northwestern medical center for bi-monthly blood draws. | 0 |
| 11. Unable to return for follow up testing for ≥ a consecutive six month period in the next two years | Individuals who were unable to return for a consecutive 6-month or greater period were excluded because they would not be able to participate fully in the study. | 0 |
| 12. Heart transplant surgery | Individuals who have had heart transplant surgery were excluded because their ECG tracing after surgery and other characteristics of the transplanted heart preclude identification of a new myocardial infarction. | 0 |
| 13. Major surgery within the past 3 months | Persons who had a recent major surgery have increased levels of biomarkers. This would interfere with the study data. | 0 |
| 14. Currently enrolled in a clinical research trial or another study with the same principal investigator (MMM) | Persons who are enrolled in a clinical trial or another trial with the study’s principal investigator were not eligible to join the study so that the study outcomes would not be affected by this (MMM). | 0 |
Numbers shown for scheduled participants who were excluded at baseline only (n=131).
Overview of baseline and follow-up data collection
Table 3 shows data collected at baseline and at each follow-up visit. Baseline measures consisted of the ankle brachial index (ABI), standardized questionnaire administration to obtain medical history and information about leg symptoms, phlebotomy, a resting 12-lead electrocardiogram, a six-minute walk test, four-meter walking velocity, and height and weight for measurement of body mass index (BMI).
Table 3.
Data Collection at baseline and follow-up in BRAVO.
| Measurement | Baseline | Every Two Months |
Every Six Months |
Every Year |
|---|---|---|---|---|
| Ankle Brachial Index | X | |||
| Seated Blood Pressure | X | |||
| LDL and HDL cholesterol, hemoglobinA1C* | X | |||
| Fasting glucose level** | X | |||
| Height | X | |||
| Weight | X | X | ||
| Blood Collection and Storage | X | X | ||
| Patient reported medical history*** | X | X | ||
| Patient reported hospitalizations | X | X | ||
| Electrocardiogram | X | X | ||
| Six-minute and four-meter walking tests | X | X | ||
| Primary care physician questionnaires | X | X |
Will be measures for case and matched control samples only
Glucose is measured at baseline for non-diabetic participants only
Questionnaires address many issues including medication lists/updates and febrile illnesses
Participants were asked to return every two months for follow-up testing. At each follow-up visit, participants underwent repeat blood collection (phlebotomy), an electrocardiogram, and weight measurement to identify significant changes in body weight. Participants also were administered detailed study questionnaires to identify new cardiovascular events, signs or symptoms of infection, new venous thromboembolic events, and any hospitalizations since the last study visit. The six-minute walk test and measurement of four-meter walking velocity were repeated every six months. Details about each measurement are provided below.
Measures
Baseline comorbidities
Baseline comorbidities were ascertained and confirmed using patient-report obtained from questionnaire administration, medical record review, patient medication use, and results of a primary care physician questionnaire (17). These data are entered into comorbidity algorithms, developed and validated by the Women’s Health and Aging Study, to ascertain and confirm the presence of baseline comorbidities including diabetes, angina, heart failure, pulmonary disease, history of myocardial infarction, cancer, spinal stenosis, disk disease, and knee or hip osteoarthritis (17).
Questionnaire administration
At each bi-monthly follow-up visit, questionnaires were administered to identify hospitalizations and emergency room visits, febrile illnesses, and any new antibiotics prescribed since the last study visit. A current list of medications was obtained at each visit. Medical records were ordered for all new hospitalizations or emergency department visits reported at each follow-up visit.
Ankle-brachial index
A hand-held Doppler probe (Nicolet Vascular Pocket Dop II, Golden, CO) was used to measure systolic blood pressures after the participant rested supine for five minutes. Measured pressures were: right brachial, dorsalis pedis, and posterior tibial arteries; left dorsalis pedis, posterior tibial, and brachial arteries. Each pressure was measured twice. The ABI was calculated by dividing average pressures in each leg by the average of the four brachial pressures (18–19).
Phlebotomy
Blood samples were obtained in the morning between 7 AM and noon whenever possible. Specimens were immediately iced and transported to the laboratory for processing and storage. Specimens were processed and prepared for long-term storage within 90 minutes of collection.
Electrocardiogram
Because as many as 20% to 30% of myocardial infarctions are considered asymptomatic (20, 21), we performed electrocardiograms at baseline and at each follow-up visit to identify new silent myocardial infarctions. We used methods and equipment (General Electric’s MAC1200 portable ECG units) from the Multi-Ethnic Study of Atherosclerosis (MESA) and Atherosclerotic Risk in Communities (ARIC) cohorts for ECG measures (22, 23). Methods from the Cardiovascular Health Study were used to diagnose new silent myocardial infarctions during follow-up (24).
Biomarkers for study
We used objective criteria to select the biomarkers most likely to be associated with near-term risk for acute coronary syndrome events. First, we developed four criteria to rank the relative merits of each potential marker and to identify those most likely to be markers of imminent acute coronary events, based on available evidence. These four criteria consisted of a) evidence from in-vitro studies and animals suggesting that the biomarker is likely to increase prior to an IHD event; b) evidence from epidemiologic studies suggesting that the biomarker is likely to increase prior to an IHD event; c) evidence that the biomarker is elevated in the setting of an IHD event; and d) stability and validity of the biomarker assay. Second, we performed an exhaustive search of the English-language scientific literature to identify studies related to the four criteria and the potential biomarkers. Third, each identified biomarker was assigned a score (ranging from 0 to 3, where 3 indicated the most favorable score) for each of the four criteria. These scores were summed to obtain a total score for each blood marker. The three biomarkers with the highest scores were D-dimer, CRP, and SAA. Thus, these three biomarkers were selected for study. Changes in levels of these biomarkers during the weeks and months leading up to our primary outcome (ischemic heart disease events) were the independent variables of interest.
Biomarker measurement methods
Serum Amyloid A and CRP were measured using an immunotechnique on the Behring BN II analyzer (Dade Behring, Wilmington, DE). An Asserachrom D-Di kit (STA-Liatest D-Di kit, Diagnostica Stago, Parsippany, NJ) was used to measure D-dimer using an immune-turbidimetric assay.
Primary outcome
The primary outcome (dependent variable) for the BRAVO study is the combined outcome of fatal and non-fatal IHD events. Non-fatal IHD events were defined as acute myocardial infarction (MI), hospitalizations for unstable angina, and new ECG findings consistent with MI. Records for hospitalizations identified during follow-up were obtained. Study adjudicators reviewed any medical records that mentioned angina or chest pain, reported elevated coronary enzymes, or had a discharge diagnosis consistent with angina or myocardial infarction. Photocopied packets of the hospital discharge summary, laboratory results, admission history and physical, electrocardiograms, and the discharge diagnoses (ICD-10 codes) were reviewed separately and independently by two adjudicators to determine whether the participant met criteria for myocardial infarction or unstable angina, based on previously established criteria (22–23). When there was disagreement between the two primary adjudicators, a third adjudicator reviewed the case and the outcome was determined by consensus. Adjudicators were blinded to the biomarkers of interest for case and control participants.
Adjudication of acute coronary syndrome events
A hospitalization for myocardial infarction was adjudicated using criteria established for the ARIC and MESA studies (22, 23). Specifically, we required two of the following three criteria to adjudicate a hospitalized acute MI: a) chest pain, b) abnormal ECG consistent with an MI (ST segment elevation, new left bundle branch block, new Q waves), c) abnormal cardiac enzymes (troponin more than two times the upper limit of normal) consistent with an MI.
We used criteria from the MESA study (23) to adjudicate unstable angina. Unstable angina was defined as “non-elective admission to the hospital for acute angina that is not codable as definite or probable MI.” The admission face sheet, discharge summary, admission history and physical, laboratory results, and ECGs from relevant hospital admissions were used to adjudicate unstable angina. Clinical symptoms were required. Additional criteria used to support the diagnosis of unstable angina were a) treatment with nitrates, heparin, or beta-blockers; b) coronary artery bypass graft surgery or other coronary revascularization during the hospital stay; c) 70% or greater obstruction of any coronary artery per angiography performed during the hospital stay; d) an electrocardiogram (ECG) showing horizontal or down-sloping ST depression or abnormal ST elevation > 1 mm and these findings were present only during chest pain.
Ischemic heart disease death consisted of definite fatal myocardial infarction, definite coronary heart disease death, and possible coronary heart disease death (23). All three types of death require the absence of known non-ischemic or non-cardiac causes of death.
Definitions of cases and controls
Cases are participants who develop an acute IHD event (myocardial infarction, hospitalization for unstable angina, or coronary heart disease death) during follow-up. To achieve our study aims, case participants are censored from analyses after the date of their IHD event. Two control participants were randomly selected for each case from among participants without a coronary event as of the date of the coronary event for the corresponding case participant. Participants were not eligible to serve as a control if they experienced an IHD event at any time during follow-up in the BRAVO study.
As compared to case participants, matched control participants meet these criteria: i. They were the same age (within five years) of the case participant; ii. They were the same gender as the case participant; iii. They had at least the same length of time in the study as the case participant; iv. They had blood draws for the visits at which the case participant had blood draws.
Exploratory measures
Exploratory measures consisted of the six-minute walk test and four-meter walking velocity at usual and fastest pace (18). These measures will enable us to determine whether changes in biomarker levels are associated with changes in walking performance over time. In the six-minute walk, participants walk back and forth in a 100-foot hallway for six minutes, after receiving standardized instructions from a research coordinator. Participants are instructed to walk as far as possible in the six-minutes. In the usual-paced four-meter walking velocity test, participants are instructed to walk a four meter distance at their usual pace, as if they are walking down the street to go to the store. In the fast-paced four-meter walking velocity test, participants are instructed to walk a four-meter distance at their fastest pace. Second, we added exploratory measures of hospitalization for pulmonary outcomes including pneumonia and chronic obstructive pulmonary disease (COPD) or asthma exacerbations. Methods from the LIFE Study were used to adjudicate these outcomes (23). Pulmonary outcomes will allow us to determine whether hospitalizations for acute pulmonary disease may mediate associations of increasing biomarker levels with acute coronary events.
Statistical analyses
Table 4 illustrates an example time course of biomarker levels obtained during follow-up as well as the typical number of IHD events anticipated during each follow-up interval, assuming a relatively constant rate of IHD events during follow-up. Panel B in Table 4 illustrates an example of a participant who experienced an IHD event 13 months after enrollment.
Table 4.
Number of Acute Coronary Syndrome Events Anticipated during Follow-up and Example of a Participant Experiencing a Coronary Event 13 Months after Enrollment.
| PANEL A | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visit Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| Length of time in study (months) | Baseline | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | 22 | 24 |
| Approximate number of coronary events during follow-up. | 0 | 4 | 9 | 13 | 18 | 22 | 26 | 30 | 35 | 39 | 44 | 48 | 53 |
| PANEL B | |||||||||||||
| Example for a Participant who experiences a coronary event 13 months after baseline | |||||||||||||
| Visit Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| Length of time in study (months) | Baseline | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | 22 | 24 |
| Months before the coronary event | 13 | 11 | 9 | 7 | 5 | 3 | 1 | X | |||||
| Time point nomenclature | t6 | t5 | t4 | t3 | t2 | t1 | t0 | X | |||||
| Example time points for a control participant matched to the example participant | |||||||||||||
| Time point nomenclature. | t6 | t5 | t4 | t3 | t2 | t1 | t0 | No Event | |||||
Visit number” refers to the number of study visits completed. Time points ‘t0, t1, t2, t3, t4…’ refer to the bi-monthly biomarker measures obtained during months leading up to the coronary event, where ‘t0’ refers to the biomarker measurement obtained immediately before the event (i.e. ≤ 2 months prior to the event), t1 refers to biomarker measurements obtained two months prior to t0, t2 refers to biomarkers measured two months prior to t1 (four months prior to t0) and so on. Thus, for the example participant in Panel B who experiences a coronary event 13 months after baseline, time point ‘t6’ represents the first (baseline) biomarker levels obtained 13 months prior to the event date. Time point ‘t5’ represents the biomarkers measured at study visit #2, two months after baseline and 11 months prior to the event date. Time point ‘t0’ represents the biomarker levels measured at study visit #7, the final visit before the coronary event, which occurs 12 months after baseline and one month before the coronary event.
For Primary Aim #1, we will determine whether, among participants who experience an IHD event, biomarker levels obtained at t0 (the final visit prior to the event) are significantly higher than previous biomarker levels (Table 1). Among all participants, such as the example Participant #1 in Panel B of Table 4, we will use a one-sided paired t-test to determine whether biomarker levels at t0 are significantly greater than biomarker levels at t1 (measured three months prior to the event), significantly greater than biomarkers measured at t2 (five months prior to the event), significantly greater than biomarkers measured at t3, and so on. Our primary comparison of interest is between biomarkers measured at t0 and t1. Because of the three biomarkers we will study, our a priori selected level of statistical significance is alpha=0.0167. Because we are evaluating the overall pattern of the associations, we did not adjust for multiple comparisons of t1 vs.t0, t2 vs. t0, etc for each biomarker. We will also perform mixed effect regression analyses with the time from the biomarker measurement to the time of the IHD event as the independent variable and blood marker level as the response. For each biomarker, all of the available longitudinal biomarker data for individual cases will enter the regression and the regression coefficient of time reflects the change rate and direction of the biomarker level prior to the IHD event.
In Primary Aim #2a, we will use a one-sided two-sample t-test to determine whether participants experiencing an IHD event (cases) have higher biomarker levels immediately prior to the event than participants without an IHD event (controls) (Table 1). P values less than 0.0167 will be considered statistically significant to adjust for the multiple comparisons with respect to the three biomarkers.
In our Primary Aim #2b, we will use a one-sided two-sample t-test with alpha = 0.0167 to determine whether increases in biomarker levels at the time points leading up to the event (i.e. at t0, t1, t2, t3, etc) are higher in case participants than control participants. We hypothesize that increases in biomarker levels at the time points leading up to the IHD event will be significantly higher for the case than for the control participants. In addition, we will use a paired t-test with alpha = 0.0167 to determine whether the changes in biomarkers during the months leading up to the event are higher in case than in control participants. Specifically, average differences in biomarker levels between time points ‘t0 and t1’, between ‘t0 and t2’, between ‘t0 and t3’, and so on will be compared between case and control participants (Table 4). We hypothesize that differences in biomarkers over these intervals will be greater in case than control participants. In addition, we will perform similar mixed effect regression analyses as Specific Aim #1 with the case indicator as an additional independent variable. The regression coefficient of the case indicator represents the case vs. control difference in the changes in biomarkers during the months leading up to the event.
Cases and controls will be matched on age, sex, race, duration in the study, and number of blood draws. In addition, for our Primary Aim #2, we may adjust for additional covariates including race, hypertension, smoking, BMI, diabetes, cholesterol levels, relevant medications (statins, ACE inhibitors, aspirin), month of the blood draw, history of angina, heart failure, myocardial infarction, stroke, cancer, deep venous thrombosis, pulmonary embolism, and chronic obstructive pulmonary disease. To limit covariate number, while selecting the most appropriate covariates, we will first examine univariate associations of each potential confounders with our primary outcome of IHD events. Variables significantly associated with coronary events at p <0.10 will be considered for inclusion in our final analyses. We will select covariates a priori from among those with p values < 0.10 based on the strength of scientific evidence regarding their association with biomarkers and coronary events.
In exploratory analyses, we will determine whether pulmonary events may mediate associations of elevated or increasing biomarker levels with IHD events, by determining whether the associations of elevated and increasing biomarker levels with IHD are attenuated after adjusting for pulmonary hospitalizations.
The statistical analysis for all the specific aims will be performed independently. To address the risk of false positives, we will report both positive and negative findings for each set of analyses so that the results can be interpreted with appropriate caution. On the other hand, we anticipate positive findings for a large proportion of hypotheses such as the comparisons between t0 and t1 and between cases and controls and thus the overall false discovery rate can still be reasonably low even though the family-wise error for the entire study is not formally controlled.
Power Calculations
Based on mortality rates in our prior research, we anticipated 47 PAD participants would die during follow-up (25). Based on published literature (26–27), we anticipated that six silent myocardial infarctions would be identified during follow-up (i.e. incidence of 0.5% per year). Because people with PAD have higher cardiovascular event rates than people without PAD (28–29), we anticipated that silent coronary event rates in our PAD cohort would be at least comparable to that of community dwelling individuals. Thus, we anticipated a total of 53 coronary events during follow-up. Since we hypothesized that biomarkers would be higher prior to an IHD event, our power calculations were constructed using a one-tailed test. Because three biomarkers will be studied, our power calculation is based on a significance level of 0.0167.
For primary aim #1, our primary comparison of interest is the difference in biomarker levels between the final two blood draws before the date of the IHD event (Table 4). We anticipated that 48 participants who experience a coronary event would have data for both visits immediately preceding an IHD event. We will have 80% power to detect a difference of 0.43 standard deviations (SD) for the comparison of biomarkers between t0 and t1 for these 48 participants.
For primary aim #2a, we will determine whether participants with an IHD event (cases) have higher biomarker levels immediately prior to the event than participants without an acute IHD event (controls). This power calculation is based on a one-tailed two-sample t-test at alpha=0.0167, which provides a conservative estimation of statistical power. With the sample sizes of 53 cases and 106 controls, we will have 80% power to detect a difference of 0.50 SD of the biomarker levels between cases and controls.
For primary aim #2b, we will compare changes in biomarker levels from t1 to t0, from t2 to t0, from t3 to t0, from t4 to t0, from t5 to t0, and from t6 to t0 between cases and controls. Our primary comparison of interest is the difference between cases and controls in the change of biomarker levels between t0 and t1. Assuming that the 53 events occur at an approximately constant rate during follow-up, we anticipate the following: 48 cases vs. 96 controls for comparisons between t1 and t0, 44 cases vs. 88 controls for comparisons between t2 and t0, 39 cases vs. 78 controls for comparisons between t3 and t0, 35 cases vs. 70 controls for comparisons between t4 and t0, 30 cases vs. 60 controls for comparisons between t5 and t0, and 26 cases for 52 controls for comparisons between t6 and t0. We have 80% power to detect differences of 0.52 SD, 0.55 SD, 0.58 SD, 0.61 SD, 0.66 SD, and 0.71 SD for these comparisons. Our power calculations are based on one-tailed tests and a p value of 0.0167, adjusting for each of the three biomarkers we will study. We did not adjust our alpha for each individual comparison within each biomarker, because we are looking for patterns of differences within each biomarker.
RESULTS
Of 950 participants with a scheduled baseline visit, 131 met exclusion criteria and 224 did not arrive for their appointments or refused participation after scheduling their baseline visit. A total of 595 participants were enrolled. Mean (standard deviation (SD)) follow-up was 1.56 years (0.81). Median (inter-quartile range) follow-up was 1.64 years (0.87, 2.05).
Table 5 shows baseline characteristics of study participants. The mean (SD) age of the population is 68.6 years (10.1), 64% were male, and the mean (SD) ABI value is 0.79 (0.33). At baseline, nearly 80% of participants were taking anti-platelet therapy, and 73.9% were taking statins. Eight percent had Q waves on their baseline electrocardiogram.
Table 5.
Characteristics of cases, controls, and the entire cohort in the BRAVO Study.
| Group | Total (N= 595) | Case (N= 50) | Control (N=100) |
|---|---|---|---|
| AGE | 68.62 (10.12) | 69.78(11.11) | 69.69(10.01) |
| Ankle-brachial index | 0.79 (0.33) | 0.80(0.36) | 0.79(0.29) |
| Body mass index | 29.67 (6.20) | 30.70(6.50) | 29.93(6.67) |
| Male sex | 64.20 n=382 | 60.00 n= 30 | 60.00 n= 60 |
| African American (%) | 36.64 n=218 | 40.00 n= 20 | 34.00 n= 34 |
| Current/former smoker (%) | 86.39 n=514 | 82.00 n= 41 | 88.00 n= 88 |
| Diabetes (%) | 42.93 n=255 | 44.00 n= 22 | 37.00 n= 37 |
| Angina (%) | 16.44 n=97 | 22.45 n= 11 | 19.00 n= 19 |
| MI (%) | 24.11 n=143 | 38.00 n= 19 | 21.00 n= 21 |
| Stroke (%) | 16.36 n=97 | 22.00 n= 11 | 16.00 n= 16 |
| Heart disease (%) | 13.95 n=82 | 24.49 n= 12 | 12.24 n= 12 |
| Hypertension (%) | 82.15 n=488 | 90.00 n= 45 | 81.00 n= 81 |
| Cancer (%) | 20.71 n=123 | 22.00 n= 11 | 16.00 n= 16 |
| Pulmonary disease (%) | 31.48 n=187 | 48.00 n= 24 | 34.00 n= 34 |
During follow-up, 48 participants experienced one or more acute IHD events. The first IHD events experienced by participants were 8 cardiac deaths, 24 hospitalizations for acute MI, 15 hospitalizations for unstable angina, and 1 hospitalization for a resuscitated cardiac arrest. In addition, two participants developed new Q waves on their ECGs during follow-up.
Two control participants who did not experience an IHD event during follow-up and were matched by age, sex, duration in study, and number of blood draws to the corresponding case were randomly selected for each case participant. Among the 512 participants who did not experience an IHD event during follow-up, 473 (93%) were eligible to serve as a control for one or more cases, based on the matching criteria. Of the 473 eligible, 100 were randomly selected.
DISCUSSION
Available evidence supports a key role for inflammation and hemostatic disorders in the initiation and progression of atherosclerosis, including rupture of vulnerable plaques resulting in IHD events. Proposed theoretical models of atherosclerotic disease progression underscore the role of inflammation and thrombosis in triggering IHD events (5–7, 12–14). Early in atherogenesis, circulating monocytes and lymphocytes are recruited to the vascular intima where they mediate the inflammatory response and promote plaque growth. Ultimately, a combination of cellular, local, and humoral processes can destabilize atherosclerotic plaque, resulting in intra-plaque hemorrhage, fibrous cap erosion, or rupture of the fibrous cap (12–14). Any of these latter events can expose plaque contents to platelets and circulating prothrombotic elements, leading to platelet aggregation, thrombus formation, and an IHD event (5–7, 12–14). Data from studies incorporating pathologic, angiographic and intra-vascular ultrasound evidence indicate that IHD events usually result from plaque rupture on areas of relatively insignificant coronary atherosclerosis (9, 11–15). Inflammatory and hemostatic blood markers have been implicated in plaque instability and rupture (5–8). However, these associations have not been clearly established in human populations. Identifying biomarkers that are elevated before an imminent IHD event could provide important prognostic information and elucidate mechanisms of these acute events for the development of future treatment targets.
Several prior studies support the hypothesis that inflammatory and hemostatic biomarkers may increase acutely during the weeks prior to an acute coronary event. First, investigators in the Cardiovascular Health Study studied the association of baseline and follow-up biomarker levels with acute coronary syndrome events in 146 cases and 146 matched controls. The odds ratio for the association of an elevated D-dimer level for IHD events was 5.0 (95% Confidence Interval =0.60, 42.8) for acute IHD events occurring during the first year of follow-up but only 1.8 (95% CI=0.80, 4.0) for acute IHD events occurring after the first year of follow-up (30). Second, an analysis from the Cardiovascular Health Study of 5,828 men and women age 65 and older reported that among women, elevated levels of CRP were associated with increased cardiovascular disease death rates during the first three years of follow-up, but not thereafter (31). Among men, elevated levels of CRP were more strongly associated with cardiovascular disease death that occurred during the first three years of follow-up, compared to cardiovascular deaths that occurred later. Third, the Quebec Cardiovascular Study of 2,037 community dwelling men and women reported that elevated levels of CRP were associated with an increased risk of IHD events during the first two-years of follow-up but not during subsequent follow-up (32). However, all of these studies are based on only a single, baseline biomarker measurement. Vidula et al previously reported that elevated biomarker levels were more strongly associated with near-term than later-term mortality in a cohort of PAD participants who underwent annual biomarker measurements (25). In this study, greater increases in levels of CRP, D-dimer, and SAA were associated with increased mortality compared to lesser increases or declines in these biomarkers. However, to our knowledge, no prior studies have assembled a large cohort of patients at high-risk of IHD events and measured biomarker levels more frequently than annually to determine whether levels of inflammatory biomarkers increase during the weeks leading up to IHD events.
The BRAVO study extends prior work by enrolling a cohort of 595 PAD participants and collecting blood every two months for up to three years. Identifying blood markers that are elevated shortly before an IHD event is expected to help clarify mechanisms by which chronic subclinical atherosclerosis transitions into acute coronary syndrome events. Elucidating this mechanism will improve the ability to identify high-risk patients at risk of near-term IHD events and lead to improved therapies for prevention and treatment of coronary events.
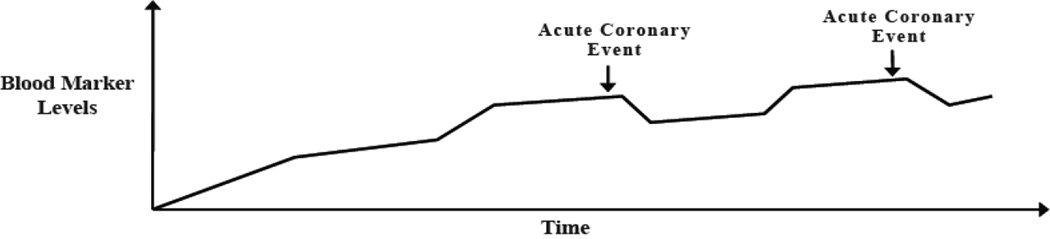
Figure 1. Model for Temporal Associations between Blood Markers and Acute Coronary Events.
Theoretical model for the association of short-term increases in inflammatory biomarkers and D-dimer with short-term risk for acute ischemic heart disease events. Marker levels increase shortly before and decline soon after an event.
Acknowledgments
Funded by: R01 HL089619
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES: There are no conflicts of interest to disclose.
REFERENCES
- 1.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, Criqui MH. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32:328–333. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Fowkes FGR, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013 Jul 31; doi: 10.1016/S0140-6736(13)61249-0. (EPub ahead of print) [DOI] [PubMed] [Google Scholar]
- 3.Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrrell K, Fowkes FG, Lee AJ, Price JF, d’Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Maskai KH, Rodríguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J, McDermott MM Ankle Brachial Index Collaboration. Ankle Brachial Index combined with Framingham risk score to predict cardiovascular events and mortality. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Shreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013 Jan 1;127(1):143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 5.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Juhani Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 6.Falk E, Shah PK, Fuster V. Pathogenesis of plaque disruption. In: Ross R, Fuster V, Topol EJ, editors. Atherosclerosis and Coronary Artery Disease. Vol 1. Philadelphia: Lippincott-Raven; 1996. pp. 491–507. [Google Scholar]
- 7.Ross R, Fuster V. The pathogenesis of atherosclerosis. In: Fuster V, Ross R, Topol EJ, editors. Atherosclerosis and Coronary Artery Disease. Vol 1. Philadelphia: Lippincott-Raven; 1996. pp. 441–474. [Google Scholar]
- 8.Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336:1276–1282. doi: 10.1056/NEJM199705013361802. [DOI] [PubMed] [Google Scholar]
- 9.Mann J, Davies MJ. Mechanisms of progression in native coronary artery disease: Role of healed plaque disruption. Heart. 1999;82:265–268. doi: 10.1136/hrt.82.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambrose JA, Tannenbaum MA, Alexopoulos D, Hjemdahl-Monsen CE, Leavy J, Weiss M, Borrico S, Gorlin R, Fuster V. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol. 1988;12:56–62. doi: 10.1016/0735-1097(88)90356-7. [DOI] [PubMed] [Google Scholar]
- 11.Rioufol G, Finet G, Ginon I, Andre-Fouet X, Rossi R, Vialle E, Desjoyaux E, Convert G, Huret JF, Tabib A. Multiple atherosclerotic plaque rupture in acute coronary syndrome: A threevessel intravascular ultrasound study. Circulation. 2002;106:804–808. doi: 10.1161/01.cir.0000025609.13806.31. [DOI] [PubMed] [Google Scholar]
- 12.Moss A, Goldstein RE, Marder VJ, Sparks CE, Oakes D, Greenberg H, Weiss HJ, Zareba W, Brown MW, Liang CS, Lichstein E, Little WC, Gillespie JA, Van Voorhees L, Krone RJ, Bodenheimer MM, Hochman J, Dwyer EM, Jr, Arora R, Marcus FI, Watelet LF, Case RB. Thrombogenic factors and recurrent coronary events. Circulation. 1999;99:2517–2522. doi: 10.1161/01.cir.99.19.2517. [DOI] [PubMed] [Google Scholar]
- 13.Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104:365–372. doi: 10.1161/01.cir.104.3.365. [DOI] [PubMed] [Google Scholar]
- 14.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 15.McDermott MM, Guralnik J, Corsi A, Albay M, Macchi C, Bandinelli S, Ferrucci L. Patterns of inflammation associated with peripheral arterial disease: The inchianti study. Am Heart J. 2005;150:276–281. doi: 10.1016/j.ahj.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 16.McDermott MM, Green D, Greenland P, Liu K, Criqui M, Chan C, Guralnik J, Pearce W, Ridker P, Taylor L, Rifai N, Schneider J. Relation of levels of hemostatic factors and inflammatory markers to the ankle brachial index. Am J Cardiol. 2003;92:194–199. doi: 10.1016/s0002-9149(03)00537-x. [DOI] [PubMed] [Google Scholar]
- 17.Guralnik JM, Fried LP, Simonsick EM, et al. The Women’s Health and Aging Study: health and social characteristics of older women with disability. Bethesda, MD: National Institute on Aging; 1995. NIH publication No. 95-4009, Appendix E. [Google Scholar]
- 18.McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, Chan C, Martin GJ, Schneider J, Pearce WH, Taylor LM, Clark E. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–883. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 19.McDermott MM, Criqui MH, Liu K, Guralnik JM, Greenland P, Martin GJ, Pearce W. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164–1171. doi: 10.1067/mva.2000.108640. [DOI] [PubMed] [Google Scholar]
- 20.Medalie JH, Goldbourt U. Unrecognized myocardial infarction five-year incidence, mortality, and risk factors. Ann Intern Med. 1976;84:526–531. doi: 10.7326/0003-4819-84-5-526. [DOI] [PubMed] [Google Scholar]
- 21.Yano K, MacLean CJ. The incidence and prognosis of unrecognized myocardial infarction in the Honolulu Heart Program. Arch Intern Med. 1989;149:1528–1532. [PubMed] [Google Scholar]
- 22.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study: Methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 23.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: Objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 24.Sheifer SE, Gersh BJ, Yanez D, et al. Prevalence, predisposing factors, and prognosis of clinically unrecognized myocardial infarction in the elderly. (The CHS Study) J Am Coll Cardiol. 2000;35:119–126. doi: 10.1016/s0735-1097(99)00524-0. [DOI] [PubMed] [Google Scholar]
- 25.Vidula H, Tian L, Criqui MH, Ferrucci L, Pearce WH, Greenland P, Green D, Tan J, Garside DB, Guralnik J, Ridker PM, Rifai N, McDermott MM. Biomarkers of inflammation and thrombosis as predictors of near-term mortality in patients with peripheral arterial disease: a cohort study. Ann Intern Med. 2008;148(2):85–93. doi: 10.7326/0003-4819-148-2-200801150-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Torbal A, Boersma E, Kors JA, van Herpen G, Deckers JW, van der Kuip DA, Stricker BH, Hofman A, WItteman JC. Incidence of recognized and unrecognized myocardial infarction in men and women aged 55 and older: The Rotterdam Study. Eur Heart J. 2006;27:729–736. doi: 10.1093/eurheartj/ehi707. [DOI] [PubMed] [Google Scholar]
- 27.Aguilar D, Goldhaber SZ, Gans DJ, Levey AS, Porush JG, Lewis JB, Rouleau JL, Berl T, Lewis EJ, Pfeffer MA the Collaborative Study Group. Clinically unrecognized Q-wave myocardial infarction in patients with diabetes mellitus, systemic hypertension, and nephropathy. Am J Cardiol. 2004;94:337–339. doi: 10.1016/j.amjcard.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 28.Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrell K, Fowkes FG, Lee AJ, Price JF, d’Agostino RB, Muravito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodriguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J, McDermott MM Ankle Brachial Index Collaboration. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008 Jul 9;300(2):197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326(6):381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 30.Cushman M, Lemaitre RN, Kuller LH, Psaty BM, Macy EM, Sharrett AR, Tracy RP. Fibrinolytic activation markers predict myocardial infarction in the Elderly: The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1999;19:493–498. doi: 10.1161/01.atv.19.3.493. [DOI] [PubMed] [Google Scholar]
- 31.Tracy R, Lemaitre Rozenn N, Psaty BM, Ives DG, Evans RW, Cushman M, Meilahn EN, Kuller LH. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly. Arteriosclerosis, Thrombosis, and Vascular Biology. 1997;17:1121–1127. doi: 10.1161/01.atv.17.6.1121. [DOI] [PubMed] [Google Scholar]
- 32.Pirro M, Bergeron J, Dagenais GR, Bernard P-M, Cantin B, Despres J-P, Lamarche B. Age and duration of follow-up as modulators of the risk for ischemic heart disease associated with high plasma C-reactive protein levels in men. Arch Intern Med. 2001;161:2474–2480. doi: 10.1001/archinte.161.20.2474. [DOI] [PubMed] [Google Scholar]