Abstract
Reduced intensity conditioning (RIC) extends the curative potential of allogeneic hematopoietic cell transplantation (HCT) to patients with hematologic malignancies unable to withstand myeloablative conditioning. We prospectively analyzed the outcomes of 123 patients, median age of 57 (range 23-70), with hematologic malignancies treated with a uniform RIC regimen of cyclophosphamide, fludarabine, and total body irradiation (200 cGy) with or without anti-thymocyte globulin (ATG) followed by related donor allogeneic HCT at the University of Minnesota from 2002-2008. Forty-five patients had acute myelogenous leukemia (AML) or myelodysplastic syndrome (MDS), 27 patients had aggressive non-Hodgkin lymphoma (NHL), 8 indolent NHL, 10 Hodgkin Lymphoma (HL), 10 myeloma and the remaining 23 had acute lymphocytic leukemia (ALL), chronic myelogenous leukemia (CML), other leukemias, or myeloproliferative disorders. Probability of four year overall survival (OS) was 73% for patients with indolent NHL, 58% for aggressive NHL, 67% for HL, 30% for AML/MDS, and only 10% for those with myeloma. Corresponding outcomes for relapse were 0%, 32%, 50%, 33%, and 38% and for progression free survival (PFS) were 73%, 45%, 27%, 27%, and 10%, respectively. The incidence of treatment related mortality (TRM) was 14% at day +100 and 22% at 1 year. The incidence of grade II-IV acute graft-versus-host disease (aGVHD) at day +100 was 38% and chronic GVHD at 2 years was 50%. Multivariate analysis revealed superior OS and PFS in patients with both indolent and aggressive NHL compared with AML/MDS, HL, or myeloma. Worse 1 year TRM was observed with hematopoietic cell transplant comorbidity index (HCT-CI) score ≥ 3 and CMV seropositive recipients. These results suggest that: 1) RIC conditioning was well tolerated by an older, heavily pre-treated population; 2) indolent and aggressive NHLs respond well to RIC conditioning highlighting the importance of the graft versus lymphoma (GVL) effect; and 3) additional peri-transplant manipulations are needed to improve outcomes for patients with AML/MDS or myeloma undergoing RIC conditioning.
Keywords: Reduced Intensity Conditioning (RIC), hematopoietic stem cell transplantation (HCT), AML, MDS, Hodgkin Lymphoma, aggressive and indolent non-Hodgkin lymphoma
INTRODUCTION
Allogeneic hematopoietic cell transplants (HCT) are standard therapy for a wide range of hematologic malignancies. Advanced age, medical comorbidities, and prior treatment history can preclude the use of more toxic myeloablative conditioning and limit the applicability of this potentially curative therapy. Consequently, reduced intensity conditioning (RIC) regimens have been developed to limit transplant-related mortality (TRM) and broaden the use of HCT. RIC regimens have the added benefits of shorter duration of neutropenia and thrombocytopenia, decreased hospitalization time, and potential improvements in long term survival due to decreased TRM. Prior publications have suggested that outcomes with RIC are impacted by underlying disease type, disease stage at transplantation, comorbidity and the degree of conditioning intensity reduction [1-8].
We report here on our experience with a consistent RIC platform of cyclophosphamide, fludarabine, and low dose total body irradiation (TBI) evaluating engraftment and toxicity and present multi-year follow-up to assess risk of late relapse and long term survival.
PATIENTS AND METHODS
Patients and inclusion criteria
All consecutive adult patients (age 18-70) undergoing RIC allogeneic HCT from adult related donors at the University of Minnesota from 2002-2008 were enrolled on this single center trial and included in the analysis. Disease eligibility included: 1) AML: high risk complete remission (CR) 1 or CR2 or greater; 2) ALL: high risk CR1 or CR2 or greater; 3) CML: all phases except blast crisis; 4) NHL, HL, chronic lymphocytic leukemia (CLL) or myeloma demonstrating chemosensitive disease; 5) MDS of all subtypes if severe pancytopenia or transfusion dependent and blasts < 5%; and 6) chronic myeloproliferative disorders (MPD).
To be eligible for this analysis, patients were required to be ≤ 70 with HLA 5/6 or 6/6 related donor match. Younger patients were enrolled on myeloablative protocols when possible but were eligible if they had evidence of organ dysfunction, were heavily pre-treated or had a recent fungal infection as previously described [9]. Minimum required organ function was: cardiac ejection fraction ≥ 35%; no decompensated heart failure or uncontrolled arrhythmia; DLCO ≥ 30% predicted, no oxygen requirements; transaminases < 5 upper limit of normal (ULN) and bilirubin < 3 ULN; creatinine ≤ 2 mg/dl or clearance > 40 ml/min; Karnofsky performance status (KPS) > 60; mold infections treated and responding after a minimum of 30 days of therapy, and albumin of > 2.5 g/dl. Patients with an active serious infection, previous TBI precluding use of 200 cGy of TBI, CML in refractory blast crisis, or chemoresistant lymphoma or myeloma were not eligible for enrollment in this trial.
Disease status at the time of transplant was defined as the following: Early (CR1, refractory anemia (RA), refractory anemia with ringed sideroblasts (RARS), CML-chronic phase), intermediate (CR2, partial remission (PR)1, refractory anemia with excess blasts (RAEB), and advanced (≥ CR3, ≥ PR2, primary induction failure (PIF), minimally responsive or stable disease).
Hematopoietic comorbiditiy scores (HCT-CI) were calculated and assigned retrospectively.
TREATMENT and METHODS
Related Donor Graft
Peripheral blood stem cell (PBSC) were collected after priming with granulocyte stimulating factor (G-CSF) 10 micrograms/kg subcutaneously daily for 5 days. Donors were collected for 1-3 days with a target CD34+ cell dose of 5 × 106 CD34+ per kg recipient weight. Donors that failed to collect the minimum required cell dose of 2 × 106 CD34+/kg underwent bone marrow harvests with a target nucleated cell dose of 3 × 108 per kg recipient weight. Grafts were not manipulated and were infused by gravity without line filtration after pre-medication with acetaminophen and diphenhydramine. Patients receiving ABO incompatible grafts also received pre- and post-transplant hydration and RBC or plasma depletion as indicated.
Treatment Regimen
Conditioning for all patients consisted of Fludarabine 40mg/m2 intravenously (IV) day −6 through day −2 for a total dose of 200mg/m2 (reduced to 30 mg/m2/day for those with limited renal function defined as raw creatinine clearance less than 70 mg/min/m2 or for those with prior cranial radiation), Cyclophosphamide 50mg/kg IV day −6, a single dose of 200cGy total body radiation (TBI) on Day −1. Equine anti-thymocyte globulin (ATG) dosed at 15mg/kg IV every 12 hours for six doses on days −6, −5, and −4 with methylprednisolone 1mg/kg was administered to those not exposed to combination chemotherapy within the preceding 6 months.
Graft versus host disease (GVHD) prophylaxis consisted of cyclosporine (CSA), targeting a trough level 200-400 ng/ml, and mycophenolate mofetil (MMF), 2-3 g/day, beginning Day −3 until Day +30. CSA was continued through Day +100 and if no evidence of GVHD, was tapered off at a rate of 10% per week. G-CSF 5 micrograms/kg was administered beginning Day +1 and continued until absolute neutrophil count (ANC) was > 2.5 × 109/L for 2 consecutive days. Infectious prophylaxis was directed to include antibacterial, antifungal, and anti-viral therapies per institutional guidelines.
Study Endpoints
The primary clinical endpoint of this study was engraftment. Successful sustained engraftment was defined as primary neutrophil recovery by day +42 and 90% donor cells at day +100.
Additional endpoints for analysis were overall survival (OS), TRM, relapse, progression free survival (PFS), donor engraftment, and, acute and chronic GVHD.
Safety endpoints were included and defined as the development of severe adverse events totaling ≥ 30% transplant related mortality at day +100. There was continuous monitoring for stopping rules for transplant related mortality by day +100. In brief, early termination of the study was defined to occur if the following number of TRM deaths occurred prior to Day +100: if 3 of 4, 4 of 6, 5 of 8, 6 of 11, 7 of 13, 8 of 16, etc with a type I error rate of 0.05 for a rate of 30% and power of 80% to detect a rate of 50%
Measures of engraftment included neutrophil recovery to an absolute neutrophil count (ANC) of 0.5 × 109/L for 3 consecutive days and 7 days of untransfused platelet recovery > 20× 109/L. Diagnoses of acute and chronic GVHD were based on standard clinical criteria with histopathological confirmation where possible [10,11]. Diagnosis of relapse was based on hematologic, morphologic, and cytogenetic or molecular evaluation.
Probabilities of OS and PFS were estimated by the Kaplan-Meier method [12]. Cumulative incidence rates and 95% confidence intervals (CI) were estimated for neutrophil engraftment, relapse, TRM and GVHD. Non-event deaths (or relapse for TRM) were defined as competing risks [13]. The variables of age, sex, CD34 dose, KPS (≥ 90), HCT-CI (0-2 vs. 3+), cytomegalovirus (CMV) serostatus, disease group, ATG exposure were considered in multivariate analysis. Statistical comparison of time-to-event curves was completed by a log-rank test. The following factors were not considered in multivariate analysis for the corresponding reasons: 1) Stem cell and donor source were not included as a variable because nearly all were PBSC from 6/6 matched related donors, 2) prior transplantation was not included because the majority were autologous transplants for lymphoma and thus a surrogate marker of disease type, and lastly 3) disease stage was not included in analysis due to the heterogeneity of diseases and stages at transplant.
Cox regression was used for engraftment, survival and PFS and the method of Fine and Gray was used in multiple variable regression for the competing risk endpoints, relapse, TRM, and GVHD [14]. Final multivariate models were selected by backward step wise method using variables with p= 0.2 retained in the model. Due to the small numbers of patients within the disease categories of ALL, myeloproliferative disorders, CML, and “other leukemia” these subsets were not included in multivariate analysis.
Analyses were performed using SAS 9.2 software. All P-values were two sided. Groups with p values of ≤ 0.05 were considered to be statistically different.
This trial was a prospective clinical study, which was reviewed and approved by the Masonic Cancer Center Protocol Review Committee and Human Subjects Institutional Review Board (IRB) at the University of Minnesota. All patients signed IRB approved informed consent in accordance with the Declaration of Helsinki. The trial was registered under the clinicaltrials.gov website NCT00303719.
RESULTS
Patients
One hundred and twenty-three consecutive patients received allogeneic related RIC transplantation for hematologic malignancies (Table 1). Most had AML/MDS (37%) and aggressive NHL (22%). Indolent lymphomas, HL, myeloma, chronic leukemias, myeloproliferative disorders, and ALL represented the remainder.
Table 1. Patient Characteristics.
| Factor | N (%) |
|---|---|
| All | 123 |
| Age at Tx (years) | |
| Median (range) | 57 (23-70) |
| <40 | 10 (8%) |
| 40-49 | 19 (15%) |
| 50-59 | 55 (45%) |
| >= 60 | 39 (32%) |
| Gender | |
| Male | 80 (65%) |
| Female | 43 (35%) |
| Prior HCT | |
| No | 106 (86%) |
| Yes | 17 (14%) |
| ALLO | 3 (18%) |
| AUTO | 14 (82%) |
|
Disease Groups
A + Disease Status at HCT |
|
| AML + MDS | 45 (37%) |
| Early: n=21 | |
| Intermediate: n=14 | |
| Advanced: N=10 | |
| Aggressive NHL | 27 (22%) |
| Early: n=2 | |
| Intermediate: n = 2 | |
| Advanced: n=23 | |
| Indolent NHL | 8 (7%) |
| Early: n=1 | |
| Advanced: n=7 | |
| Hodgkins | 10 (8%) |
| Advanced: n=10 | |
| Myeloma | 10 (8%) |
| Intermediate: n=3 | |
| Advanced: n=7 | |
| Other | 23 (18%) |
| Early: n=3 | |
| Intermediate: n=16 | |
| Advanced: n=4 | |
| Time from diagnosis to HCT | |
| Median , months (range) | 23.6 (2.5 - 154) |
|
Comorbidity Score at HCT
(HCT-CI) |
|
| 0 | 20 (16%) |
| 1-2 | 38 (31%) |
| ≥ 3 | 65 (53%) |
| CD34 × 106/kg | |
| Median (range) | 5.79 (0.64-21.84) |
| ATG use in conditioning | |
| No | 93 (76%) |
| Yes | 30 (24%) |
| Recipient CMV status | |
| Negative | 55 (45%) |
| Positive | 68 (55%) |
| CMV status | |
| D−R− | 38 (31%) |
| D+R− | 17 (14%) |
| D−R+ | 32 (26%) |
| D+R+ | 36 (29%) |
| Missing | |
| Year of HCT | |
| 2002-2003 | 34 (27.5%) |
| 2004-2005 | 35 (28.5%) |
| 2006-2008 | 54 (44%) |
| Cell Source | |
| MarrowB | 5 (4%) |
| PBSC | 118 (96%) |
| HLA Matching C | |
| Matched Related (6/6) | 113 (92%) |
| Related Mismatch (5/6) | 10 (8%) |
|
Follow up time of Survivors
(months) |
|
| Median (range) | 30.6 months (3.3 -81) |
- AML (n=33) and MDS (n=12)
- Aggressive Lymphoma: Diffuse Large Cell (n=10), Other Aggressive NHL (n=12), Mantle (n=4), Burkitts (n=1)
- Other: “Other Leukemia” (n=10), CML (n=4), ALL (n=3), Myeloproliferative Disease (n=6)
Of the 5 marrow sources, 4 included marrow + PBSC for those who didn’t adequately collect peripherally and required bone marrow harvest to achieve minimum cell dose
-
-Matched related includes: Siblings with 6/6 or 8/8 match (n =112) and Cousin with 6/6 match (n=1)
-
-Related mismatch includes: Sibling with 5/6 match (n=9) and Offspring mismatched (5/6) (n=1)
HCT = Hematopoietic Cell Transplantation
D= Donor
R= Recipient
M = Marrow
AML = Acute Myelogenous Leukemia
MDS = Myelodysplastic Syndrome
NHL = Non-Hodgkins Lymphoma
P= Peripheral Blood
Their median age was 57 (range 23-70) with a median follow-up of 2.5 years (range 0.3-6.6 years). Median time from diagnosis to transplant was 24 months (range 2.5 - 154), 65% were male, and 58% had a HCT-CI of 0-2. High comorbidity score (HCT-CI 3+) was noted in a majority of patients who would otherwise have qualified for myeloablative conditioning based on age. Fourteen percent had a prior transplant (3 allogeneic, 14 autologous). PBSC were used in 96% and 92% had matched related donors (6/6 HLA matched) with the remaining 8% mismatched related donors (5/6 HLA matched).
Engraftment
All patients achieved neutrophil recovery by Day +42 with a median time to ANC recovery of 8 days (range 0-15). Seventy-five percent of patients (95% CI, 67-82%) had platelet recovery by day 42 at a median of 16.5 (range 0-37) days. At Day +100, 110 patients (89.4%) had >90% donor chimerism in the marrow. One patient lost donor engraftment at day 309 with no evidence of recurrent small lymphocytic lymphoma but marrow morphology and chimerism studies demonstrating recipient-derived MDS.
Survival
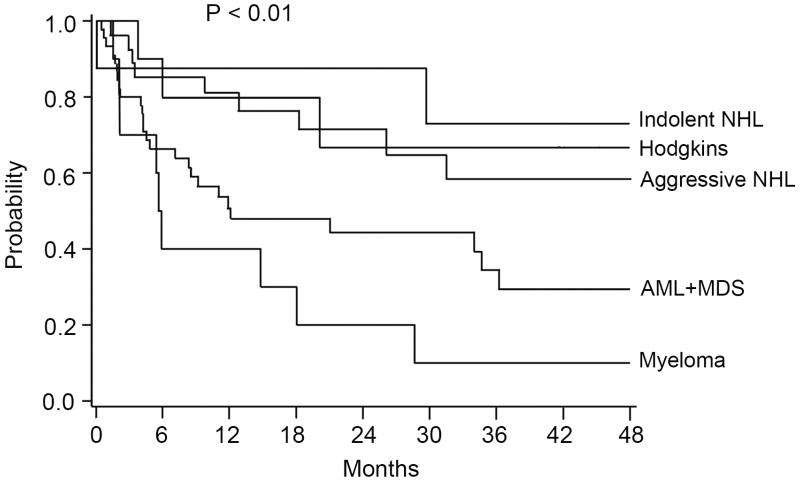
After a median follow-up of 2.5 years (range 0.3-6.75), 64 patients survive for a 4 year overall survival (OS) of 45% (95% CI, 35-55%). The underlying diagnosis significantly impacted overall survival with the best survival in those with indolent NHL (73%, 95% CI 28-93%), HL (67%, 95% CI 27-88%), or aggressive NHL (58%, 95% CI 34-77%) compared with AML/MDS (30%, 95% CI 14-47%) or myeloma (10%, 95% CI 1-36%, p=<0.01) (Figure 1) (Table 2).
Figure 1. Four Year Overall Survival by Disease Group.
Table 2. 1 and 4 year Univariate Outcomes by Disease Group.
| Disease Group | OS % (95% CI) |
PFS % (95% CI) |
Relapse % (95% CI) |
TRM % (95% CI) |
|||
|---|---|---|---|---|---|---|---|
| 1 yr | 4yr | 1yr | 4yr | 1yr | 4yr | 1 year | |
|
Indolent NHL
n=8 |
88% (39-98) |
73% (28-93) |
88% (39-98) |
73% (28-93) |
0% | 0% | 13% (0-34) |
|
Aggressive NHL
n=27 |
81% (60-92) |
58% (34-77) |
70% (49-84) |
45% (21-66) |
15% (2-29) |
32% (9-54) |
7% (0-17) |
|
HL
n=10 |
80% (41-95) |
67% (27-88) |
40% (12-67) |
27% (5-56) |
50% (19-81) |
50% (19-81) |
10% (0-27) |
|
AML + MDS
n=45 |
51% (35-65) |
30% (14-47) |
40% (26-55) |
27% (13-44) |
29% (15-43) |
33% (17-49) |
28% (14-42) |
|
Myeloma
n=10 |
40% (12-67) |
10% (1-36) |
20% (3-47) |
10% (1-36) |
38% (0-48) |
38% (7-69%) |
34% (4-63) |
NHL = Non-hodgkins lymphoma
HL = Hodgkins Lymphoma
AML = Acute Myelogenous Leukemia
MDS = Myelodysplastic Syndrome
PFS = Progression Free Survival
OS=Overall Survival
CI = Confidence Interval
In multivariate analysis only disease group significantly impacted OS. Compared to AML/MDS survival was improved in aggressive NHL (RR 0.41 (95% CI 0.19 −0.89), indolent NHL (RR 0.25 (95% CI, 0.06-1.09), and HL (RR 0.32 (95% CI, 0.09 −1.06) and considerably worse in those with myeloma (RR 1.69 (95% CI, 0.78-3.65) (p < 0.01). Interestingly, age and HCT-CI did not impact OS (Table 3).
Table 3. Multivariate Analysis.
| Outcome | Factor | RR (95% CI) | P value |
|---|---|---|---|
|
| |||
|
Overall Survival at
4 years |
Disease Group: | ||
| AML/MDS | 1.0 | <0.01 | |
| Aggressive NHL | 0.41 (0.19-0.89) | ||
| Indolent NHL | 0.25 (0.06–1.09) | ||
| HL | 0.32 (0.09–1.06) | ||
| Myeloma | 1.69 (0.78-3.65) | ||
|
| |||
| PFS at 4 years | Disease Group: | ||
| AML/MDS | 1.00 | 0.02 | |
| Aggressive NHL | 0.49 (0.25-0.97) | ||
| Indolent NHL | 0.26 (0.06-1.10) | ||
| HL | 1.14 (0.49-2.63) | ||
| Myeloma | 1.87(0.87-4.02) | ||
|
| |||
| TRM at 1 year | HCT-CI: | ||
| 0-2 | 1.0 | 0.04 | |
| 3+ | 2.41 (1.02-5.70) | ||
|
Recipient CMV
Status: |
|||
| Negative | 1.0 | 0.03 | |
| Positive | 2.71 (1.08-6.79) | ||
Only significant Outcomes and Factors shown:
--Relapse at 4 years showed no significant variables and thus not shown
--Variables of age, sex, CD34 dose, KPS, HCT-CI, CMV status, disease group, ATG exposure evaluated
RR = Relative Risk
CI= Confidence Interval
PFS = Progression Free Survival
TRM = Treatment Related Mortality
Progression Free Survival (PFS)
Four year PFS for the entire cohort was 29% (95% CI, 20-38%). Underlying diagnosis was the only feature that significantly impacted PFS. Patients with indolent and aggressive NHL had the best 4 year PFS at 73% (95% CI, 28-93%) and 45% (95% CI, 21-66%), respectively (p=0.02). In contrast, four year PFS was only 27% (95% CI, 5-56%) for those with HL, 27% (95% CI, 13-44%) for those with MDS/AML, and 10% (95% CI, 1-36%) for those with myeloma. (Table 2)
Impact of disease group on 4 year PFS was substantiated in multivariate analysis. Compared to AML/MDS, indolent and aggressive NHL had improved outcomes (RR of 0.26 [95% CI, 0.06-1.10] and 0.49 [95% CI, 0.25-0.97] respectively) while outcomes were worse with myeloma (RR 1.87 [95% CI, 0.87-4.02]) (p=0.02). No other factors in the multivariate analysis significantly impacted PFS.
Relapse
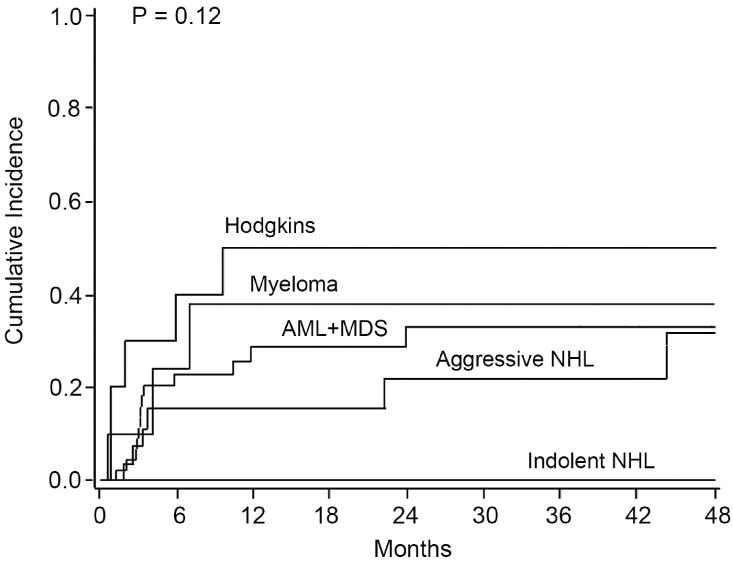
The 4 year cumulative incidence of relapse for the entire cohort was 36% (95% CI, 25-47%). In univariate analysis the relapse rate varied depending on the underlying disease. Notably, no patients with indolent NHL relapsed. Patients with AML/MDS, aggressive NHL, and myeloma had similar rates of relapse at 33% (95% CI, 17-49%), 32% (95% CI, 9-54%), and 38% (95% CI, 7-69%), respectively (p=0.12). The majority of relapses for AML/MDS and myeloma patients occurred within the first year while those with aggressive NHL had relapses occurring out to 3-4 years. Those with HL had the highest incidence of relapse at 50% (95% CI, 19-81%) with all relapses occurring within the first year (Figure 2). Multivariate analysis for relapse at 4 years showed no significantly different outcomes based on disease group or any other variable tested likely related to the timing of relapse within each disease group.
Figure 2. Four Year Incidence of Relapse by Disease Group.
TRM
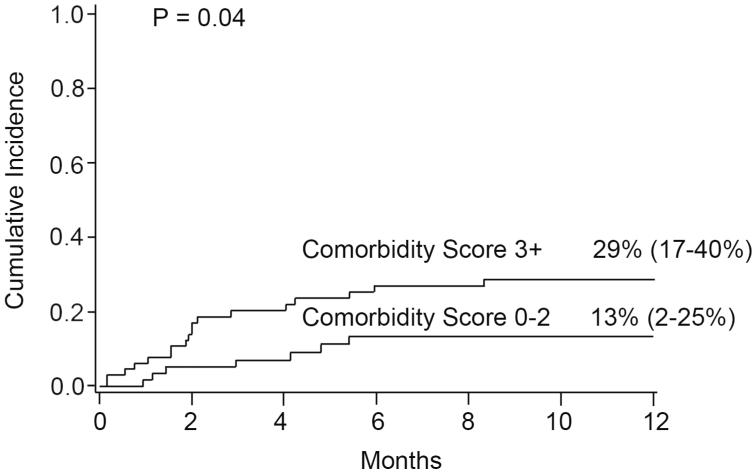
Treatment related mortality for the entire cohort at day +100 was 14% (95% CI, 8-20%). In univariate analysis Day +100 TRM was 7% (95% CI, 0-13%) for those with a HCT-CI score of 0-2 versus 20% (95% CI, 10-30%) with a score of 3 or above (p=0.04). One year TRM was 22% (95% CI, 14-29%) for the entire cohort. For those with a HCT-CI of 0-2 it was 13% (2-25%) versus 29% (17-40%) for those with a score of 3 or above (p=0.04) (Figure 3). For CMV seronegative recipients one year TRM was only 13% (95% CI, 4-22%) versus 29% (95% CI, 17-41%) in seropositive recipients (p=0.04).
Figure 3. One Year Treatment Related Mortality by Comorbidity Score.
In multivariate analysis, TRM at 1 year was impacted significantly by recipient CMV serologic status with a RR of 2.71 (95% CI, 1.08-6.79, p=0.03) in those CMV seropositive recipients and by HCT-CI with a RR of 2.41 (95% CI, 1.02-5.70, p=0.04) in those with HCT-CI ≥ 3. Age, sex, CD34, KPS, ATG exposure, and disease type were also included as factors in the multivariate analysis and had no significant impact on outcomes (Table 3).
GVHD
At 100 days, the cumulative incidence of acute GVHD (aGVHD) grades II-IV was 38% (95% CI 29-47%) and 20% (95% CI, 13-27%) grade III-IV. Rates of day +100 aGVHD grade II-IV were decreased in more recent years with an incidence of only 24% (95% CI, 13-36%) in 2006-2008 versus 52% (95% CI, 33-70%) in 2002-2003 (p=0.05). ATG use increased in latter years with only 6% (n=2) of patients receiving ATG in the 2002-2003 time period compared with 31% (n=11) and 31% (n=17) during 2004-2005 and 2006-2008 respectively. At 6 months, incidence of aGVHD grades II-IV was 47% (95% CI, 37-56%) and 26% (95% CI, 18-34%) grade III-IV. Interestingly, the prior trend of decreased aGVHD in more recent years documented with day +100 aGVHD rates was no longer present at 6 months. Rates of aGVHD were not influenced by disease status at transplant, underlying disease, cell source or degree of HLA matching given that the majority of patients were a 6/6 or 8/8 match.
The two year cumulative incidence of chronic GVHD (cGVHD) was 50% (95% CI, 39-61%) for the entire cohort. Patients transplanted in 2006-2008 had an incidence of 42% (95% CI, 27-57%) versus 68% (95% CI, 47-88%) during 2002-2003 (p=0.01). No other factors significantly impacted rates of cGVHD.
DISCUSSION
Reduced intensity conditioning extends the potentially curative therapy of HCT to older patients or those otherwise ineligible for full myeloablative therapy. We studied a cohort of patients with high risk and advanced hematologic malignancies that received a uniform conditioning regimen and found that: 1) RIC was tolerated well by a heavily pre-treated cohort of older patients with advanced disease and that low HCT-CI correlated with low TRM; 2) Our uniform RIC platform produced successful engraftment and donor chimerism; 3) PFS was significantly influenced by primary disease with indolent and aggressive NHL having superior outcomes; 4) Poorer outcomes were seen with myeloid malignancies, HL, and myeloma highlighting the need for additional peri-HCT manipulations.
We observed low TRM even in older patients. Similar to younger patients, adverse comorbidity scores and CMV seropositive status identified patients at higher risk. Observed reasonable rates of severe grade III/IV acute GVHD and prompt neutrophil engraftment with high donor chimerism at day 100 possibly contributed to lower TRM. Interestingly, neither underlying hematologic malignancy nor disease stage at transplant (advanced versus early) significantly impacted TRM. These data support other findings that chronologic age should not be the primary deciding factor on HCT eligibility [15] and support the use of HCT-CI as a powerful prognostic tool.
Patterns of outcomes in OS, PFS, and relapse based on underlying hematologic malignancy highlighted important findings of our study. In disease-specific subsets we observed trends consistent with the natural history of these diseases and their potential responsiveness to RIC HCT. Patients with NHL, both indolent and aggressive, showed promising PFS and encouraging long term OS despite being heavily pre-treated with a moderate number of patients receiving prior autologous transplantation. Our results with aggressive and indolent NHL compare favorably to recent analyses [5,6] and are supported by other findings that conditioning intensity is less important for disease control in lymphomas and may contribute more TRM in those patients who enter transplant with underlying comorbidities [7,8]. In those with indolent NHL we observed no relapses perhaps demonstrating the sensitivity of this disease to GVL reactions. Relapse in those with aggressive NHL were modest and primarily occurred early; however, some later relapses occurred at 3-4 years indicating no relapse plateau in the observation period. As the majority of the NHL patients had advanced disease at the time of transplant these data underscore the point that myeloablative conditioning may not be needed in these diseases and support the concept that immunological eradication of lymphoma may be the precedent for long-term survival.
The outcomes in patients with AML/MDS highlight a very different natural history and responsiveness to GVL. Notably, all enrolled MDS patients had low volume blasts (<5%) and the majority of AML patients were in CR1 or CR2 at that time of transplant. Despite good disease control at transplant, rates of relapse were substantial and corresponding PFS and OS were reduced. However, if post transplant remission was obtained and maintained for 1-2 years, late relapse was not observed and extended survival was maintained. These data suggest that even with optimal disease status at transplant, GVL may not be adequate to control disease and conditioning intensity may be critical in certain myeloid malignancies.
Numerous non-randomized studies have addressed the importance of conditioning intensity in myeloid malignancies with conflicting data. Some data suggest minimal increased benefit to MA conditioning if entering transplant in CR [1-3], some suggest that MA conditioning is important for disease control when entering transplant with active disease [4], while other studies suggest that MA conditioning is optimal even in those patients in CR or <5% blasts [16]. Despite this controversy, the majority stress that when MA conditioning is not an option, RIC conditioning is a reasonable alternative that may have slightly higher rates of relapse frequently offset by reduced TRM. Comparison between these trials is challenging due to a heterogeneous patient population, disease burden at transplant, diversity in degree of conditioning intensity, and variable follow-up. Randomized trials addressing this question are crucial and are in development. While our 4 year relapse incidence of 33% was considerable, it is comparable to other studies which site relapse rates of 21-61% [1,4,17,18] and highlights the need for therapeutic adjustments pre or post HCT for myeloid malignancies.
The rates of severe aGVHD and chronic GVHD were acceptable and comparable to other series [4,5,19]. Interestingly we did observe a decrease in day +100 aGVHD rates and cGHVD in more recent years (2006-2008 compared with 2002-2003). While the majority of supportive care measures have not changed during this time period and all patients were treated with a uniform conditioning regimen platform, MMF dosing transitioned from 2gm/day to 3 gm/day in 2005. ATG use also increased slightly in the 2004-2005 and 2006-2006 time periods potentially due to more defined criteria for use and a slight increase in MDS patients transplanted during that time period. Both of these variables (MMF dosing and ATG use) could potentially explain this decrease in both day +100 aGVHD and cGVHD in more recent years. We also observed a slightly higher rate of cGVHD in indolent lymphomas similar to other series [5] which seemed to correlated with the improved disease control in that cohort.
In summary, our platform of RIC was well tolerated in elderly patients, produced successful engraftment, yielded promising clinical outcomes in indolent and aggressive NHL, but highlights a need for further anti-tumor approaches in AML/MDS and myeloma. Maintenance therapy post transplant with agents such as azacitidine or decitabine for myeloid malignancies [20,21] or rituximab for CD20+ malignancies might further improve these outcomes.
Footnotes
Financial Disclosure statement: No financial conflict of interest related to this study.
REFERENCES
- 1.Martino R, Iacobelli S, Brand R, et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood. 2006;108:836–846. doi: 10.1182/blood-2005-11-4503. [DOI] [PubMed] [Google Scholar]
- 2.Martino R, Valcarcel D, Brunet S, Sureda A, Sierra J. Comparable non-relapse mortality and survival after HLA-identical sibling blood stem cell transplantation with reduced or conventional-intensity preparative regimens for high-risk myelodysplasia or acute myeloid leukemia in first remission. Bone Marrow Transplant. 2008;41:33–38. doi: 10.1038/sj.bmt.1705879. [DOI] [PubMed] [Google Scholar]
- 3.Shimoni A, Hardan I, Shem-Tov N, Yerushalmi R, Nagler A. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: long-term follow-up. Leukemia. 2010;24:1050–1052. doi: 10.1038/leu.2010.12. [DOI] [PubMed] [Google Scholar]
- 4.Alyea EP, Kim HT, Ho V, et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol.Blood Marrow Transplant. 2006;12:1047–1055. doi: 10.1016/j.bbmt.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Armand P, Kim HT, Ho VT, et al. Allogeneic transplantation with reduced-intensity conditioning for Hodgkin and non-Hodgkin lymphoma: importance of histology for outcome. Biol.Blood Marrow Transplant. 2008;14:418–425. doi: 10.1016/j.bbmt.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corradini P, Dodero A, Farina L, et al. Allogeneic stem cell transplantation following reduced-intensity conditioning can induce durable clinical and molecular remissions in relapsed lymphomas: pre-transplant disease status and histotype heavily influence outcome. Leukemia. 2007;21:2316–2323. doi: 10.1038/sj.leu.2404822. [DOI] [PubMed] [Google Scholar]
- 7.Sorror ML, Storer BE, Maloney DG, Sandmaier BM, Martin PJ, Storb R. Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative or myeloablative conditioning regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood. 2008;111:446–452. doi: 10.1182/blood-2007-07-098483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomblyn M, Brunstein C, Burns LJ, et al. Similar and promising outcomes in lymphoma patients treated with myeloablative or nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol.Blood Marrow Transplant. 2008;14:538–545. doi: 10.1016/j.bbmt.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 11.Akpek G, Zahurak ML, Piantadosi S, et al. Development of a prognostic model for grading chronic graft-versus-host disease. Blood. 2001;97:1219–1226. doi: 10.1182/blood.v97.5.1219. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 13.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat.Med. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 14.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 15.Koreth J, Aldridge J, Kim HT, et al. Reduced-intensity conditioning hematopoietic stem cell transplantation in patients over 60 years: hematologic malignancy outcomes are not impaired in advanced age. Biol.Blood Marrow Transplant. 2010;16:792–800. doi: 10.1016/j.bbmt.2009.12.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warlick ED, Cioc A, Defor T, Dolan M, Weisdorf D. Allogeneic stem cell transplantation for adults with myelodysplastic syndromes: importance of pretransplant disease burden. Biol.Blood Marrow Transplant. 2009;15:30–38. doi: 10.1016/j.bbmt.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Oran B, Giralt S, Saliba R, et al. Allogeneic hematopoietic stem cell transplantation for the treatment of high-risk acute myelogenous leukemia and myelodysplastic syndrome using reduced-intensity conditioning with fludarabine and melphalan. Biol.Blood Marrow Transplant. 2007;13:454–462. doi: 10.1016/j.bbmt.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gyurkocza B, Storb R, Storer BE, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J.Clin.Oncol. 2010;28:2859–2867. doi: 10.1200/JCO.2009.27.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aoudjhane M, Labopin M, Gorin NC, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT) Leukemia. 2005;19:2304–2312. doi: 10.1038/sj.leu.2403967. [DOI] [PubMed] [Google Scholar]
- 20.Jabbour E, Giralt S, Kantarjian H, et al. Low-dose azacitidine after allogeneic stem cell transplantation for acute leukemia. Cancer. 2009;115:1899–1905. doi: 10.1002/cncr.24198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Lima M, Giralt S, Thall PF, et al. Maintenance therapy with low-dose azacitidine after allogeneic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010 Jul 29; doi: 10.1002/cncr.25500. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]