Abstract
The numbers of patients diagnosed with prostate cancers is increasing due to the widespread application of prostate-specific antigen screening and subsequent prostate biopsies. The methods of systemic administration of therapeutics are not target-specific and thus cannot efficiently destroy prostate tumour cells while simultaneously sparing the surrounding normal tissues and organs. Recent advances in imaging-guided minimally invasive therapeutic techniques offer considerable potential for the effective management of prostate cancers. An objective understanding of the feasibility, effectiveness, morbidity, and deficiencies of these interventional techniques is essential for both clinical practice and scientific progress. This review presents the recent advances in imaging-guided interventional techniques for the diagnosis and treatment of prostate cancers.
Keywords: prostatic neoplasms, minimally invasive, magnetic resonance imaging, image-guided intervention
1. Background
Prostate cancer is the cause of the most common malignant tumours and is the second leading cause of cancer death among American and European men[1,2]. As prostate-specific antigen (PSA) screening and prostate biopsies have become widespread, more patients have been diagnosed with low-grade and localised prostate malignancies[3]. In patients with low-grade prostate cancers, the role of traditional whole-gland radical therapies is being re-considered and re-evaluated. It is becoming clear that the measurements of PSA thresholds, Gleason grades, and clinical stage cut-off points for low-grade malignancies cannot reliably identify patients with unilateral or unifocal lesions[4,5] and lead to over-detection and over-treatment in more than 30% of cases of prostate cancers[6,7]. These clinical problems have motivated alternatives to traditional approaches that aim to diagnose prostate cancers precisely and treat them efficiently. Among these alternatives is the continuous exploration of imaging-guided, minimally invasive, interventional techniques that are specific for those patients with unifocal or unilateral prostate lesions[8].
2. Advances in imaging-guided interventions of prostate cancers
2.1. Imaging-guided prostate biopsy
Prostate biopsy has been widely used to facilitate the final diagnoses of prostate cancer[9]. Abnormal digital rectal examinations (DREs) and/or elevated PSA levels are currently used as indications for prostate biopsy. A recent study showed that some biological markers, such as urinary prostate cancer antigen 3(PCA3), may be helpful for the identification of positive biopsies and therefore reduce unnecessary biopsies[10]. Efforts have been made to improve the cancer detection rates of prostate biopsy; these efforts have primarily focused on the following two aspects: (i) optimising the biopsy core numbers from accurate locations; and (ii) exploring better imaging-guided techniques.
In 1989, Hodge first described the 6-core biopsy protocol, three of these cores are from the peripheral zone of each of the bilateral lobes along the anatomic parasagittal axis from the base to the apex of the prostate. This approach has improved prostate cancer detection rates over those achieved by finger-guided prostate biopsies[11]. Subsequently, this biopsy technique was modified to sample more tissues from the peripheral zones by laterally directing the needle and collecting 8–14 specimens; these modified biopsies are called “extended biopsies”[12]. Although such modified biopsy techniques result in higher cancer detection rate, excessive sampling of the prostate tissue leads to an increase in biopsy-related complications, such as haemorrhage, infection, and injury to the periprostatic structures [13]. To overcome this problem, scientists developed a method of transrectal ultrasound(TRUS) to guide prostate biopsies. TRUS guidance helps to localise the prostate. However, in many cases, this technique does not permit direct visualisation of the prostate lesions[9], and it is difficult to access the anterior and apical parts of the prostate under TRUS-guidance[13]. Additionally, many benign lesions, including benign prostatic hyperplasias (BPHs), prostate cysts, haematomas and infections, cannot be differentiated from prostate cancers with TRUS[14]. Thus, it is clear that the key to increasing prostate cancer detection rates is not simply reliance on increases in the numbers of biopsy cores; rather it is essential to increase the visibility and differentiability of prostate lesions by exploring optimal imaging-guided techniques that should be capable of accurate targeting and real-time monitoring[9]. Example techniques for such improvements included the refinements of TRUS-guided prostate biopsy via the use of contrast-enhanced power Doppler imaging and elastography. A retrospective single-centre study of 1776 patients demonstrated the benefits of the use of contrast-enhanced colour Doppler ultrasound to guide prostate biopsy over systematic biopsy[15]. Another study reported that fewer biopsy cores were needed in real-time elastography-targeted biopsies when the PSA level was ≥1.25ng/ml and ≤4ng/ml[16].
Magnetic resonance(MR) technology has progressed rapidly in recent decades. The application of advanced MR techniques, such as diffusion-weighted imaging and MR spectroscopy, has improved the sensitivity and specificity of the visualisation and differentiation of prostate diseases. The development of endorectal coils further enhanced prostate cancer visualisation with MRI, and contrast-enhanced diffusion-weighted imaging combined with MR spectroscopy is a promising approach for the more accurate detection of prostate cancer with MRI[17]. It has also been reported that the combination of diffusion-weighted imaging with T2-weighted imaging can further increase the sensitivity (84.1% vs. 61.9%) and specificity(72.0% vs. 36.0%) of predicting unilateral prostate cancer compared to T2-weighted magnetic resonance alone[18].
The development of MR-compatible implements has made MRI-guided prostate biopsies possible, and such biopsies are becoming a frontier in imaging-guided interventions involving prostates(Fig. 1)[19]. A study of 98 patients with MRI-guided prostate biopsies found an overall higher Gleason-grade detection of prostate cancers with MRI-guided biopsy than with TRUS-guided biopsy(88% vs. 55%). It has also been reported that MRI-guided biopsy significantly improves the pretreatment risk stratification by obtaining biopsies that are representative of the true Gleason grades[20]. Another group of authors reported that prostate cancer was detected with MRI-guided biopsy in 52/100 (52%) patients with previous negative TRUS-guided biopsies and persistently rising serum PSA levels[21]. The shortcoming of the long times required for MR scans may potentially be overcome by the fusion of MR imaging with TRUS. Preprocedural MR images can be fused with real-time TRUS monitoring images to realise real-time monitoring of the location of the needle and targeting of the lesion. This technique combines the advantages of the two techniques and is less expensive and less time consuming[9,22]. Although the fused MR imaging and TRUS-guiding method is regarded as a promising imaging-guided technique for prostate biopsies, multicentre studies are needed to evaluate the effectiveness of the technique[23].
Fig. 1.
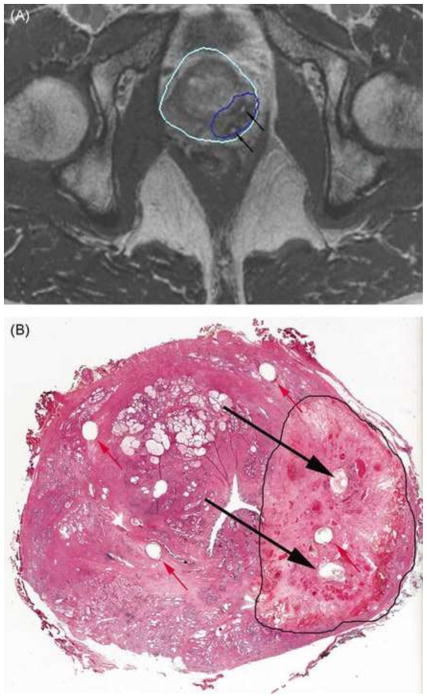
MR imaging–guided prostate biopsy. Photographs (top) and computer screens (bottom) showing the use of an MR imaging–compatible biopsy device. The device (top left) has an endorectal probe (arrow), a needle guide (arrowhead), and a set of dials (D) that which allow the needle to be directed to the target on the basis of input from the targeting software (bottom left). The software provides the necessary angles for probe rotation, needle angulation, and needle depth (bottom right). The dials are adjusted manually by the operator based on the software’s calculations, which are derived from pre-biopsy MR images. The patient is placed in the prone position (top right), and the biopsy probe is placed endorectally (arrow at bottom right). Reprinted with permission from (19).
In addition to ultrasound imaging, the first clinical trial with a MRI-guided transrectal robotic prostate biopsy system was initiated at the U.S. National Cancer Institute[24]. A subsequent retrospective study of the same system has further improved this approach by applying motion compensation for organ displacement, which increases targeting accuracy [23]. Although these pioneer works have established the “proof-of-principle” of these robotically assisted prostate biopsy systems, the continued technical improvements and larger-scale clinical trials are warranted prior the wide applications of these techniques in routine clinical practice.
2.2. Imaging-guided brachytherapy
TRUS-guided transperineal iodine-125 (125I) seed implantation was first reported by Holm et al. in 1983[25]. After two decades of evolution, this technique has become an effective interventional approach for the treatment of localised prostate cancers and has the primary advantages of being capable of intraprostatically delivering a high radiation dose while sparing the surrounding normal tissues. Currently, there are two general types of prostate brachytherapy techniques: low-dose rate (LDR) brachytherapy, and high-dose rate (HDR) brachytherapy. Several studies have shown that HDR brachytherapy is a valuable approach for achieving prostatic dose escalation, particularly when combined with external beam radiation therapy(EBRT), which results in impressive outcomes for the treatment of intermediate and high-risk patients with prostate cancers. It is believed that these patients should receive high-dose radiation seeds to achieve satisfactory and long-term disease control.
Currently, both types of LDR and HDR brachytherapies are performed with the transperineal approach under TRUS guidance. However, the TRUS-guided approach has several drawbacks, including unsatisfactory localisation ability of the implanted seeds and poor image quality due to the variable anatomical deformities of the patients or faeces and air in the rectum[26]. MRI can overcome these limitations through its advantages of providing a three-dimensional dataset, multiplanar reconstruction, more accurate volume measurement, and satisfactory soft-tissue resolution[9]. A recent study reported results of MRI-guided prostate brachytherapy in 318 patients; this therapy was performed with MR-compatible iodine-125 seeds in a an intraoperative 0.5 Tesla MRI unit[27]. Via an MR-compatible catheter, iodine-125 seeds were placed transperineally under MRI guidance, while an MRI-based real-time dosimetry technique was used to monitor the delivered dose at the target(Fig. 2). MRI-guided prostate brachytherapy offers the improved treatment of prostate cancers, and the PSA failure-free survival rates at 5 and 8 years are 95.6% and 90.0% for low-risk cases and 73.0% and 66.4% for intermediate-risk cases, respectively. However, the current use of MRI as a tool for the guidance of prostate brachytherapy has certain limitations that are primarily due to the suboptimal availability of intraoperative MR units and the relatively long MRI scanning times[28].
Fig. 2.
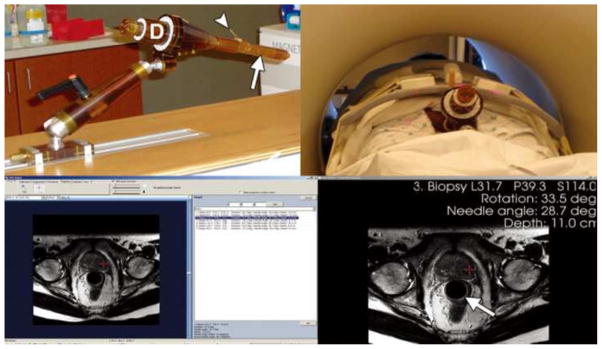
Example of a contoured peripheral zone target volume (A) and post-implant dosimetry (B). Red represents the contoured peripheralzone, and blue represents the urethra and the anterior rectal wall. One hundred percent or more of the prescription dose is shown in yellow, 150% or more of the prescription dose is shown in red, 63% or more of prescription dose is shown in blue, and no colour indicates less than 63% of the prescription dose after the implantation of the seeds. Reprinted with permission from (27).
2.3. Imaging-guide cryotherapy
Cryotherapy was first described in 1972 and can cause tissue coagulative necrosis and cellular death resulting from extremely cold temperatures(<−140°C)[29]. Cryotherapy is based on the following two mechanisms of: (i) intracellular ice crystal formation that leads to mechanical trauma to the cells; and (ii) cellular dehydration that is related to osmotic changes due to such cold temperatures and results in subsequent cell apoptosis [30].
For the treatment of prostate tumours, three generations of cryotherapy techniques have been developed thus far. The first generation of cryotherapy involved transurethral or transperineal placement of a cryo-probe to freeze the target tissues with liquid nitrogen. Because the first generation of prostate cryotherapy was performed without any imaging guidance, complications were quite common. The second generation of prostate cryotherapy was developed in the 1990s and used 3-mm cryoprobe cooling with liquid nitrogen. At that time, the placement of the small-sized cryoprobe was monitored by TRUS. However, TRUS could create severe artefacts around the freezing ball of the cryoprobe that could interrupt the visualisation of the target lesions. The third generation of prostate cryotherapy has been applied in clinical practice since 2000; this cryotherapy involves the use of an even smaller cryoprobe that is as thin as 1.5mm (17-gauge). The thinner cryoprobes not only facilitate more accurate percutaneous access to precisely target the lesions but also greatly reduce technique-related complications. The latest prostate cryotherapy is based on the mechanisms of freezing the tissues with argon and thawing the tissues with helium gas. Repeating the freeze-thaw cycle leads to remarkable cell killing due to recrystallisation, cellular swelling and bursting [30]. Some authors have reported that they use this third-generation prostate cryotherapy as the primary treatment for low-risk and localised prostate cancers and achieve satisfactory early clinical outcomes [31]. Nerve-sparing cryotherapy is expected to decrease the high erectile dysfunction (ED) rates, while use of a urethral warming device can protect the urethra from damage due to over-freezing [32]. Cryotherapy has also been used for salvage treatment of local recurrent prostate cancer and has led to an acceptable 10-year disease-free survival rate (39%) [33].
One of the current scientific focuses on prostate cryotherapy is the exploration of imaging-guided cryotherapy. TRUS was the first imaging modality that was used to guide cryotherapy for prostate cancers. However, most prostate tumour masses are not visualised well with ultrasound, and it is sometimes difficult to distinguish malignancies from either normal prostatic tissues or benign prostatic hyperplasia. Additionally, technically, up to 99% of the acoustic waves are reflected from the surface of the ice-ball closest to the TRUS probe, which results in difficulty in real-time monitoring and assessment of the adequacy of the cryoablation zone during the procedure [34]. Thus, TRUS is not an optimal imaging modality for precisely guiding prostate cryotherapy[9].
In recent years, the multiparametric 3T MR technique became available. This technique not only has high specificity and sensitivity as an effective differential diagnostic tool for prostate cancers [35,36], but is also an accurate method for the quantitative measurement of prostate tumour sizes [37]. Compared to other imaging modalities, MRI has excellent advantages for ice ball visualisation and cryotherapy monitoring [38,39]. Most interestingly, MRI has a uniquereal-time temperature mapping functionality that aids the precise and non-invasive temperature measurement of the cryoablation zone during prostate cryotherapy[40–42]. Under MRI, the ice-ball of the cryoablation zone can be clearly and sharply observed. Although the technique of MRI-guided cryotherapy still has challenges (such as ferrous material exclusion, space limitations in the operation room, challenging ergonomics, and relatively long scan sequences), the early results from this technique are promising.
2.4. Imaging-guided laser ablation
Laser ablation of prostate tumours is based on the application of external thermal energy that is transferred via a laser fibre placed into the prostate tumour in which the laser light induces irreversible coagulative necrosis of the tissue around the tip of the fibre[43–45]. A study reported on a phase I trial of12patients with low-risk prostate cancers who received laser ablation treatment under three-dimensional ultrasound imaging guidance[46]. During the laser ablation, the temperature probes were placed at the expected ablation boundaries and the surrounding critical structures, and the temperatures generated by the laser could be monitored in real time. Contrast-enhanced ultrasound has also been used to monitor necrosis formation due to the application of lasers.
A specific advantage of laser ablation is that the laser fibres are MRI-compatible and thus suitable for MRI guidance. As described above, MRI has the advantages of high-spatial resolution and multi-plane imaging, which provides more accurate information about the target lesion and the ablated area relative to the surrounding crucial organs. The use of MR-thermometry further enables the real-time visualisation of laser temperature distribution and thereby allows for the precise monitoring of the range of the ablation zone. Additionally, the fibres used in the laser ablation do not distort the electromagnetic field, and thus avoid MRI artefacts emerge in the ablation area (Fig. 3). Recently, some authors have validated the feasibility of the use of 3T MRI to guide laser ablation with cadaveric prostates[47]. Subsequently, the same group of authors reported a case of a patient who received salvage 3T MRI-guided laser ablation after a radical prostatectomy and presented with no adverse events during the early follow-up[48]. MRI-guided laser ablation is considered to be a promising technique for accurate tumour targeting and real-time monitoring of ablation temperature with high spatial and temporal resolutions and unique MR-thermometry.
Fig. 3.
Registration of ablation in magnetic resonance (MR) imaging and pathology. (A) MR image showing the with the damage contouring. The black arrows indicate the placements of the laser fibre. (B) The same pathologic slice as in (A). The black arrows indicate the placement of the laser fibres. The red arrows indicate the placements of the fiducial markers. Reprinted with permission from (43).
2.5. Imaging-guided magnetic nanoparticle hyperthermia
Magnetic nanoparticle-mediated hyperthermia is a novel concept for interstitial thermal therapy. After the systemic administration of biocompatible and magnetic iron-oxide nanoparticles (1–100 nm), the patient is placed into an alternating magnetic field in which the thermal energy caused by the specific absorption rate (SAR) of the nanoparticles in the magnetic field is released to the target tissues and thereby kills the tumour cells[49].
A few prospective phase-I studies have validated the feasibility of magnetic nanoparticle thermotherapy in patients with locally recurrent prostate cancers[50,51]. The mean core size of the nanoparticles used in this study was 15 nm, and the particles were coated with an aminosilane-type shell (MFLAS, MagForce® Nanotechnologies, Berlin, Germany). A magnetic applicator (MFH®300F, MagForce Nanotechnologies) was used to generate the alternating magnetic field with a frequency of 100 kHz at variable field strengths of 2.5–18.0 kA/m, which enabled the achievement of a maximum temperature of 55°C within the prostate. At the median follow-up period of 17.5 months, the patients’ PSA levels had declined. Alternating magnetic field strengths of 4–5 kA/m were tolerated by all patients, although higher field strengths resulted in discomfort in the perineal regions of the patients.
Subsequent efforts were made to combine magnetic nanoparticle thermotherapy with radiotherapy or brachytherapy to treat prostate cancers[52,53]. In one such effort, the nanoparticle suspensions were transperineally injected, and 125I seeds were simultaneously implanted under TRUS and fluoroscopy guidance, which led to a 100% technical success.
The main limitations that hinder the widespread clinical application of nanoparticle thermotherapy include irregular nanoparticle distributions in the prostate and local discomfort caused by high magnetic field strength[49]. It is believed that once these disadvantages are overcome, magnetic nanoparticle thermotherapy should offer satisfactory efficacy in the treatment of prostate tumours, either as a monotherapy or in combination with other treatments.
2.6. Imaging-guided photodynamic therapy
Photodynamic therapy (PDT) is a type of focal ablation therapy that uses photosensitising drugs. Photosensitising drugs are activated in the targeted organ or tissue via fibre optic illumination at a specific wavelength and form reactive oxygen species that lead to the loss of endothelial integrity and vascular thrombosis and subsequent tissue destruction around the optical fibre (Fig. 4)[54–57]. In 1990, a group of authors demonstrated the first photodynamic therapy for prostate cancers by delivering a photosensitising agent via a transurethral approach[58]. The later studies confirmed that photosensitising drugs can be either orally or intravenously administered. In advanced photodyanamic therapy of prostate cancers, the activation light energy is generated by a low-power laser and delivered to the lesion through optical fibre via a transperineal approach under TRUS guidance.
Fig. 4.
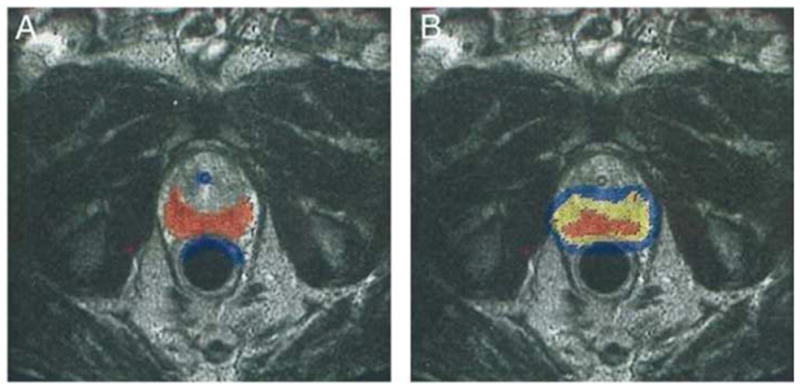
During photodynamic therapy (PDT), a photosensitising agent is injected intravenously and is distributed throughout the body. Small energy-delivering probes deliver the appropriate wavelength of light energy. To treat localised prostate cancer, these probes can be positioned to deliver PDT to either a portion of or the entire gland. Reprinted with permission from (57).
Padoporfin (WST-09, Tookad®; Steba Biotech, The Hague, The Netherlands) is a new palladium bacteriopheophorbide photosensitiser that functions as an avascular-acting photosensitising drug when an activating light at a wavelength of 763 nm is used. A phase I/II study reported on the treatment of 24 patients with recurrent prostate cancer using Padoporfin that were followed up with MRI[56,59]. The minimum light dose required to identify a therapeutic response with MRI was 23 J/cm2 in at least 90% of the prostate volumes. Currently, the heterogeneity of the responses, which is possibly due to the heterogeneous uptake of photosensitisers, is the primary disadvantage of photodynamic therapy. The development of imaging-guided local delivery approaches may help to overcome this limitation.
3. Conclusions
Developments on different image-guided interventional techniques have improved the capability of accurately diagnosing and effectively treating prostate malignancies. Among these advanced techniques, CT and ultrasound-guided brachytherapy and cryotherapy have generated encouraging clinical outcomes. Compared to TRUS, MRI-guided interventions have demonstrated additional advantages in tumour lesion detection, pre-treatment planning, monitoring of interventional procedures, and assessment of treatment outcomes. However, the widespread clinical application of MRI-guided interventions still requires continuous technical refinements that include shortening of the long scanning times, enhancing the real-time imaging capabilities, and increasing the cost-effectiveness.
Footnotes
Disclosures: None of the authors have any conflicts of interests to disclose.
Conflict of Interest Statement
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled “Recent advances on imaging-guided interventions of prostate cancers”.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Xia Wu, Email: wuxa_0@126.com.
Feng Zhang, Email: fengz@uw.edu.
Ran Chen, Email: dlcr_cr@126.com.
Weiliang Zheng, Email: ZWLtree@163.com.
Xiaoming Yang, Email: xmyang@uw.edu.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA: a cancer journal for clinicians. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Polascik TJ, Mayes JM, Sun L, Madden JF, Moul JW, Mouraviev V. Pathologic stage T2a and T2b prostate cancer in the recent prostate-specific antigen era: implications for unilateral ablative therapy. The Prostate. 2008;68:1380–1386. doi: 10.1002/pros.20804. [DOI] [PubMed] [Google Scholar]
- 4.Polascik TJ, Mayes JM, Schroeck FR, Sun L, Madden JF, Moul JW, Mouraviev V. Patient selection for hemiablative focal therapy of prostate cancer: variables predictive of tumor unilaterality based upon radical prostatectomy. Cancer. 2009;115:2104–2110. doi: 10.1002/cncr.24258. [DOI] [PubMed] [Google Scholar]
- 5.Iczkowski KA, Hossain D, Torkko KC, Qian J, Lucia MS, Wheeler TM, Rewcastle JC, Bostwick DG. Preoperative prediction of unifocal, unilateral, margin-negative, and small volume prostate cancer. Urology. 2008;71:1166–1171. doi: 10.1016/j.urology.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Scattoni V, Zlotta A, Montironi R, Schulman C, Rigatti P, Montorsi F. Extended and saturation prostatic biopsy in the diagnosis and characterisation of prostate cancer: a critical analysis of the literature. European urology. 2007;52:1309–1322. doi: 10.1016/j.eururo.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Stattin P, Holmberg E, Johansson JE, Holmberg L, Adolfsson J, Hugosson J. Outcomes in localized prostate cancer: National Prostate Cancer Register of Sweden follow-up study. Journal of the National Cancer Institute. 2010;102:950–958. doi: 10.1093/jnci/djq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mouraviev V, Johansen TE, Polascik TJ. Contemporary results of focal therapy for prostate cancer using cryoablation. Journal of endourology/Endourological Society. 2010;24:827–834. doi: 10.1089/end.2009.0546. [DOI] [PubMed] [Google Scholar]
- 9.Yacoub JH, Verma S, Moulton JS, Eggener S, Aytekin O. Imaging-guided prostate biopsy: conventional and emerging techniques. Radiographics: a review publication of the Radiological Society of North America, Inc. 2012;32:819–837. doi: 10.1148/rg.323115053. [DOI] [PubMed] [Google Scholar]
- 10.Kirby R, Fitzpatrick JM. Optimising repeat prostate biopsy decisions and procedures. BJU international. 2012;109:1750–1754. doi: 10.1111/j.1464-410X.2011.10809.x. [DOI] [PubMed] [Google Scholar]
- 11.Hodge KK, McNeal JE, Terris MK, Stamey TA. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. The Journal of urology. 1989;142:71–74. doi: 10.1016/s0022-5347(17)38664-0. discussion 74–75. [DOI] [PubMed] [Google Scholar]
- 12.Uno H, Nakano M, Ehara H, Deguchi T. Indications for extended 14-core transrectal ultrasound-guided prostate biopsy. Urology. 2008;71:23–27. doi: 10.1016/j.urology.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Ho H, Yuen JS, Cheng CW. Robotic prostate biopsy and its relevance to focal therapy of prostate cancer. Nature reviews Urology. 2011;8:579–585. doi: 10.1038/nrurol.2011.131. [DOI] [PubMed] [Google Scholar]
- 14.Punnen S, Nam RK. Indications and timing for prostate biopsy, diagnosis of early stage prostate cancer and its definitive treatment: a clinical conundrum in the PSA era. Surgical oncology. 2009;18:192–199. doi: 10.1016/j.suronc.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Mitterberger MJ, Aigner F, Horninger W, Ulmer H, Cavuto S, Halpern EJ, Frauscher F. Comparative efficiency of contrast-enhanced colour Doppler ultrasound targeted versus systematic biopsy for prostate cancer detection. European radiology. 2010;20:2791–2796. doi: 10.1007/s00330-010-1860-1. [DOI] [PubMed] [Google Scholar]
- 16.Aigner F, Pallwein L, Junker D, Schafer G, Mikuz G, Pedross F, Mitterberger MJ, Jaschke W, Halpern EJ, Frauscher F. Value of real-time elastography targeted biopsy for prostate cancer detection in men with prostate specific antigen 1.25 ng/ml or greater and 4.00 ng/ml or less. The Journal of urology. 2010;184:913–917. doi: 10.1016/j.juro.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Franiel T, Stephan C, Erbersdobler A, Dietz E, Maxeiner A, Hell N, Huppertz A, Miller K, Strecker R, Hamm B. Areas suspicious for prostate cancer: MR-guided biopsy in patients with at least one transrectal US-guided biopsy with a negative finding--multiparametric MR imaging for detection and biopsy planning. Radiology. 2011;259:162–172. doi: 10.1148/radiol.10101251. [DOI] [PubMed] [Google Scholar]
- 18.Jeong IG, Kim JK, Cho KS, You D, Song C, Hong JH, Ahn H, Kim CS. Diffusion-weighted magnetic resonance imaging in patients with unilateral prostate cancer on extended prostate biopsy: predictive accuracy of laterality and implications for hemi-ablative therapy. The Journal of urology. 2010;184:1963–1969. doi: 10.1016/j.juro.2010.06.136. [DOI] [PubMed] [Google Scholar]
- 19.Bonekamp D, Jacobs MA, El-Khouli R, Stoianovici D, Macura KJ. Advancements in MR imaging of the prostate: from diagnosis to interventions. Radiographics: a review publication of the Radiological Society of North America, Inc. 2011;31:677–703. doi: 10.1148/rg.313105139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hambrock T, Hoeks C, Hulsbergen-van de Kaa C, Scheenen T, Futterer J, Bouwense S, van Oort I, Schroder F, Huisman H, Barentsz J. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. European urology. 2012;61:177–184. doi: 10.1016/j.eururo.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 21.Roethke M, Anastasiadis AG, Lichy M, Werner M, Wagner P, Kruck S, Claussen CD, Stenzl A, Schlemmer HP, Schilling D. MRI-guided prostate biopsy detects clinically significant cancer: analysis of a cohort of 100 patients after previous negative TRUS biopsy. World journal of urology. 2012;30:213–218. doi: 10.1007/s00345-011-0675-2. [DOI] [PubMed] [Google Scholar]
- 22.Pinto PA, Chung PH, Rastinehad AR, Baccala AA, Jr, Kruecker J, Benjamin CJ, Xu S, Yan P, Kadoury S, Chua C, Locklin JK, Turkbey B, Shih JH, Gates SP, Buckner C, Bratslavsky G, Linehan WM, Glossop ND, Choyke PL, Wood BJ. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. The Journal of urology. 2011;186:1281–1285. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durmus T, Stephan C, Grigoryev M, Diederichs G, Saleh M, Slowinski T, Maxeiner A, Thomas A, Fischer T. Detection of prostate cancer by real-time MR/ultrasound fusion-guided biopsy: 3T MRI and state of the art sonography. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin. 2013;185:428–433. doi: 10.1055/s-0032-1330704. [DOI] [PubMed] [Google Scholar]
- 24.Krieger A, Susil RC, Menard C, Coleman JA, Fichtinger G, Atalar E, Whitcomb LL. Design of a novel MRI compatible manipulator for image guided prostate interventions. IEEE transactions on bio-medical engineering. 2005;52:306–313. doi: 10.1109/TBME.2004.840497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holm HH, Juul N, Pedersen JF, Hansen H, Stroyer I. Transperineal 125iodine seed implantation in prostatic cancer guided by transrectal ultrasonography. The Journal of urology. 1983;130:283–286. doi: 10.1016/s0022-5347(17)51108-8. [DOI] [PubMed] [Google Scholar]
- 26.Van Gellekom MP, Moerland MA, Battermann JJ, Lagendijk JJ. MRI-guided prostate brachytherapy with single needle method--a planning study. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2004;71:327–332. doi: 10.1016/j.radonc.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen PL, Chen MH, Zhang Y, Tempany CM, Cormack RA, Beard CJ, Hurwitz MD, Suh WW, D’Amico AV. Updated results of magnetic resonance imaging guided partial prostate brachytherapy for favorable risk prostate cancer: implications for focal therapy. The Journal of urology. 2012;188:1151–1156. doi: 10.1016/j.juro.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank SJ, Stafford RJ, Bankson JA, Li C, Swanson DA, Kudchadker RJ, Martirosyan KS. A novel MRI marker for prostate brachytherapy. International journal of radiation oncology, biology, physics. 2008;71:5–8. doi: 10.1016/j.ijrobp.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 29.Reuter HJ. Endoscopic cryosurgery of prostate and bladder tumors. The Journal of urology. 1972;107:389–393. doi: 10.1016/s0022-5347(17)61036-x. [DOI] [PubMed] [Google Scholar]
- 30.Maccini M, Sehrt D, Pompeo A, Chicoli FA, Molina WR, Kim FJ. Biophysiologic considerations in cryoablation: a practical mechanistic molecular review. International braz j urol: official journal of the Brazilian Society of Urology. 2011;37:693–696. doi: 10.1590/s1677-55382011000600002. [DOI] [PubMed] [Google Scholar]
- 31.Lian H, Guo H, Gan W, Li X, Yan X, Wang W, Yang R, Qu F, Ji C. Cryosurgery as primary treatment for localized prostate cancer. International urology and nephrology. 2011;43:1089–1094. doi: 10.1007/s11255-011-9952-7. [DOI] [PubMed] [Google Scholar]
- 32.Aus G. Current status of HIFU and cryotherapy in prostate cancer--a review. European urology. 2006;50:927–934. doi: 10.1016/j.eururo.2006.07.011. discussion 934. [DOI] [PubMed] [Google Scholar]
- 33.Williams AK, Martinez CH, Lu C, Ng CK, Pautler SE, Chin JL. Disease-free survival following salvage cryotherapy for biopsy-proven radio-recurrent prostate cancer. European urology. 2011;60:405–410. doi: 10.1016/j.eururo.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Lindner U, Lawrentschuk N, Trachtenberg J. Image guidance for focal therapy of prostate cancer. World journal of urology. 2010;28:727–734. doi: 10.1007/s00345-010-0604-9. [DOI] [PubMed] [Google Scholar]
- 35.Kitajima K, Kaji Y, Fukabori Y, Yoshida K, Suganuma N, Sugimura K. Prostate cancer detection with 3 T MRI: comparison of diffusion-weighted imaging and dynamic contrast-enhanced MRI in combination with T2-weighted imaging. Journal of magnetic resonance imaging: JMRI. 2010;31:625–631. doi: 10.1002/jmri.22075. [DOI] [PubMed] [Google Scholar]
- 36.Turkbey B, Pinto PA, Mani H, Bernardo M, Pang Y, McKinney YL, Khurana K, Ravizzini GC, Albert PS, Merino MJ, Choyke PL. Prostate cancer: value of multiparametric MR imaging at 3 T for detection--histopathologic correlation. Radiology. 2010;255:89–99. doi: 10.1148/radiol.09090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazaheri Y, Hricak H, Fine SW, Akin O, Shukla-Dave A, Ishill NM, Moskowitz CS, Grater JE, Reuter VE, Zakian KL, Touijer KA, Koutcher JA. Prostate tumor volume measurement with combined T2-weighted imaging and diffusion-weighted MR: correlation with pathologic tumor volume. Radiology. 2009;252:449–457. doi: 10.1148/radiol.2523081423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverman SG, Tuncali K, vanSonnenberg E, Morrison PR, Shankar S, Ramaiya N, Richie JP. Renal tumors: MR imaging-guided percutaneous cryotherapy--initial experience in 23 patients. Radiology. 2005;236:716–724. doi: 10.1148/radiol.2362041107. [DOI] [PubMed] [Google Scholar]
- 39.Gangi A, Tsoumakidou G, Abdelli O, Buy X, de Mathelin M, Jacqmin D, Lang H. Percutaneous MR-guided cryoablation of prostate cancer: initial experience. European radiology. 2012;22:1829–1835. doi: 10.1007/s00330-012-2411-8. [DOI] [PubMed] [Google Scholar]
- 40.Chopra R, Tang K, Burtnyk M, Boyes A, Sugar L, Appu S, Klotz L, Bronskill M. Analysis of the spatial and temporal accuracy of heating in the prostate gland using transurethral ultrasound therapy and active MR temperature feedback. Physics in medicine and biology. 2009;54:2615–2633. doi: 10.1088/0031-9155/54/9/002. [DOI] [PubMed] [Google Scholar]
- 41.Wust P, Cho CH, Hildebrandt B, Gellermann J. Thermal monitoring: invasive, minimal-invasive and non-invasive approaches. International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2006;22:255–262. doi: 10.1080/02656730600661149. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Barkauskas KJ, Nour SG, Duerk JL, Abdul-Karim FW, Saidel GM. Magnetic resonance imaging and model prediction for thermal ablation of tissue. Journal of magnetic resonance imaging: JMRI. 2007;26:123–132. doi: 10.1002/jmri.20956. [DOI] [PubMed] [Google Scholar]
- 43.Lindner U, Lawrentschuk N, Weersink RA, Davidson SR, Raz O, Hlasny E, Langer DL, Gertner MR, Van der Kwast T, Haider MA, Trachtenberg J. Focal laser ablation for prostate cancer followed by radical prostatectomy: validation of focal therapy and imaging accuracy. European urology. 2010;57:1111–1114. doi: 10.1016/j.eururo.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Ichikawa K, Hikita T, Maeda N, Takeuchi Y, Namba Y, Oku N. PEGylation of liposome decreases the susceptibility of liposomal drug in cancer photodynamic therapy. Biological & pharmaceutical bulletin. 2004;27:443–444. doi: 10.1248/bpb.27.443. [DOI] [PubMed] [Google Scholar]
- 45.McNichols RJ, Kangasniemi M, Gowda A, Bankson JA, Price RE, Hazle JD. Technical developments for cerebral thermal treatment: water-cooled diffusing laser fibre tips and temperature-sensitive MRI using intersecting image planes. International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2004;20:45–56. doi: 10.1080/02656730310001611035. [DOI] [PubMed] [Google Scholar]
- 46.Lindner U, Weersink RA, Haider MA, Gertner MR, Davidson SR, Atri M, Wilson BC, Fenster A, Trachtenberg J. Image guided photothermal focal therapy for localized prostate cancer: phase I trial. The Journal of urology. 2009;182:1371–1377. doi: 10.1016/j.juro.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 47.Woodrum DA, Gorny KR, Mynderse LA, Amrami KK, Felmlee JP, Bjarnason H, Garcia-Medina OI, McNichols RJ, Atwell TD, Callstrom MR. Feasibility of 3.0T magnetic resonance imaging-guided laser ablation of a cadaveric prostate. Urology. 2010;75:1514 e1511–1516. doi: 10.1016/j.urology.2010.01.059. [DOI] [PubMed] [Google Scholar]
- 48.Woodrum DA, Mynderse LA, Gorny KR, Amrami KK, McNichols RJ, Callstrom MR. 3.0T MR-guided laser ablation of a prostate cancer recurrence in the postsurgical prostate bed. Journal of vascular and interventional radiology: JVIR. 2011;22:929–934. doi: 10.1016/j.jvir.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 49.Johannsen M, Thiesen B, Wust P, Jordan A. Magnetic nanoparticle hyperthermia for prostate cancer. International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2010;26:790–795. doi: 10.3109/02656731003745740. [DOI] [PubMed] [Google Scholar]
- 50.Johannsen M, Gneveckow U, Taymoorian K, Thiesen B, Waldofner N, Scholz R, Jung K, Jordan A, Wust P, Loening SA. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: results of a prospective phase I trial. International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2007;23:315–323. doi: 10.1080/02656730601175479. [DOI] [PubMed] [Google Scholar]
- 51.Johannsen M, Gneveckow U, Thiesen B, Taymoorian K, Cho CH, Waldofner N, Scholz R, Jordan A, Loening SA, Wust P. Thermotherapy of prostate cancer using magnetic nanoparticles: feasibility, imaging, and three-dimensional temperature distribution. European urology. 2007;52:1653–1661. doi: 10.1016/j.eururo.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 52.Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyperthermia. Critical reviews in oncology/hematology. 2002;43:33–56. doi: 10.1016/s1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 53.Wust P, Gneveckow U, Johannsen M, Bohmer D, Henkel T, Kahmann F, Sehouli J, Felix R, Ricke J, Jordan A. Magnetic nanoparticles for interstitial thermotherapy--feasibility, tolerance and achieved temperatures. International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2006;22:673–685. doi: 10.1080/02656730601106037. [DOI] [PubMed] [Google Scholar]
- 54.Borle F, Radu A, Monnier P, van den Bergh H, Wagnieres G. Evaluation of the photosensitizer Tookad for photodynamic therapy on the Syrian golden hamster cheek pouch model: light dose, drug dose and drug-light interval effects. Photochemistry and photobiology. 2003;78:377–383. doi: 10.1562/0031-8655(2003)078<0377:eotptf>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 55.Koudinova NV, Pinthus JH, Brandis A, Brenner O, Bendel P, Ramon J, Eshhar Z, Scherz A, Salomon Y. Photodynamic therapy with Pd-Bacteriopheophorbide (TOOKAD): successful in vivo treatment of human prostatic small cell carcinoma xenografts. International journal of cancer. Journal international du cancer. 2003;104:782–789. doi: 10.1002/ijc.11002. [DOI] [PubMed] [Google Scholar]
- 56.Trachtenberg J, Bogaards A, Weersink RA, Haider MA, Evans A, McCluskey SA, Scherz A, Gertner MR, Yue C, Appu S, Aprikian A, Savard J, Wilson BC, Elhilali M. Vascular targeted photodynamic therapy with palladium-bacteriopheophorbide photosensitizer for recurrent prostate cancer following definitive radiation therapy: assessment of safety and treatment response. The Journal of urology. 2007;178:1974–1979. doi: 10.1016/j.juro.2007.07.036. discussion 1979. [DOI] [PubMed] [Google Scholar]
- 57.Lepor H. Vascular targeted photodynamic therapy for localized prostate cancer. Reviews in urology. 2008;10:254–261. [PMC free article] [PubMed] [Google Scholar]
- 58.Windahl T, Andersson SO, Lofgren L. Photodynamic therapy of localised prostatic cancer. Lancet. 1990;336:1139. doi: 10.1016/0140-6736(90)92626-s. [DOI] [PubMed] [Google Scholar]
- 59.Trachtenberg J, Weersink RA, Davidson SR, Haider MA, Bogaards A, Gertner MR, Evans A, Scherz A, Savard J, Chin JL, Wilson BC, Elhilali M. Vascular-targeted photodynamic therapy (padoporfin, WST09) for recurrent prostate cancer after failure of external beam radiotherapy: a study of escalating light doses. BJU international. 2008;102:556–562. doi: 10.1111/j.1464-410X.2008.07753.x. [DOI] [PubMed] [Google Scholar]