Abstract
Insulin-like growth factors (IGF) 1 and 2 are known as potential mitogens for normal and neoplastic cells. IGF2 is a main fetal growth factor while IGF1 is activated through growth hormone action during postnatal growth and development. However, there is strong evidence that activation of IGF2 by its E2F transcription factor 3 (E2F3) is present in different types of cancer. Also high levels of IGF1 strongly correlate with cancer development due to anti-apoptotic properties and enhancement of cancer cell differentiation, which can be attenuated by IGFBP3. Head and neck cancer is known as one of the six most common human cancers. The main risk factor for head and neck cancer is consumption of tobacco and alcohol as well as viral and bacterial infection by stimulation of chronic local inflammation. There is also a genetic basis for this form of cancer; however, the genetic markers are not yet established. In this study we investigated the levels of the expression of IGF2, IGF1, E2F3 and IGFBP3 in human cancers and healthy tissues surrounding the tumor obtained from each of 41 patients. Our study indicated that there is no alteration of the level of expression of IGF2, E2F3 and IGF1 in Head and neck squamous cell carcinoma (HNSCC) cases studied in selected experimental population, but there was evidence for upregulation of pro-apoptotic IGFBP3 in cancer when comparing to healthy tissue. These important findings indicate that insulin-growth factors are not directly associated with HNSCC showing some variability between patients and location of tumor. However, elevated level of IGFBP3 suggests possible regulatory role of IGF signal by its binding protein in this type of tumors.
Keywords: oral cancer, IGF1, IGF2, survival
INTRODUCTION
Growth factors are strongly associated with cancer development. Insulin like growth factor 1 (IGF1) and IGF2 are major growth factors involved in development and growth. During fetal growth IGF2 plays the main role in development and mice with IGF2 disruption exhibit serious growth retardation at birth [1]. IGF2 is an imprinted gene expressed only by the parental allele and alterations of IGF2 imprinting are associated with childhood growth abnormalities [2, 3]. Biallelic expression of IGF2 observed in Beckwith-Wiedenmann syndrome leads to overgrowth and is believed to predispose cancer development [4]. Moreover, studies with mice showed high expression of IGF2 in different organs only during fetal life with dramatic down-regulation of its expression shortly after birth [5]. Importantly, the newest data indicate that different kinds of cancer are characterized by increased levels of IGF2 in adults. While IGF2 acts as a main growth factor during prenatal development IGF1, mainly regulated by growth hormone, takes over as a main growth factor during post-natal development [6]. As GH stimulates the growth and development by activation of hepatic production and release to circulation of IGF1 there is also tissue-specific local IGF1 production which is believed to be GH independent. The release of IGF1 during growth can also increase the risk of developing or accelerating the growth of cancer. There is strong evidence that animals with GH/IGF1 deficiency live longer than wild-type controls [6] and at the same time are protected from cancer [6]. It was shown that treatment of dwarf rats with GH that stimulate IGF1 production increased mammary cancer risk [7]. This suggests that growth factors and the very specific regulation of them during prenatal and postnatal development can predispose individuals to the development of cancer in adulthood.
One of the sixth most common human cancers are oral cancers, commonly referred to as head and neck cancers [8]. There are several types of head and neck cancers, but about 90% are squamous cell carcinomas [9]. About 35,000 Americans are diagnosed with oral or pharyngeal cancer per year [9], and the number would be even higher (54,000) if laryngeal cancer was included. Worldwide the problem is much greater, with over 640,000 new cases being found each year. Head and neck carcinogenesis is a multistep process as a result of several genetic alterations. The dominant important risk factors for the development of head and neck cancer are the consumption of tobacco [10] and alcohol [11] [12]. Other factors include genetics, Human papillomavirus (HPV) infection [13] [14], as well as inflammation [15]. Another aspect of this devastating disease is its high death rate is particularly high. Approximately 13,500 deaths are reported every year in USA. The high death rate of oral cancer is not because it is hard to discover or diagnose, but due to the cancer being neglected during patients’ daily life. Head and neck cancers can affect physical and mental health, strongly affects patients’ life quality and lifespan. Although improvement of early diagnosis of the cancer the best defense against this devastating disease[16] [17], regardless of early diagnosis and quick surgical intervention it is important to provide an appropriate strategy for treatment that includes chemotherapy and/or radiotherapy. Usually the therapy is adjusted individually based on the condition of the patients considering stage of tumor, over-all health and age of patients. However, depending on characteristics of each individual case the treatment can be successful or may fail to cure the cancer. To determine a potential role of growth factors in head and neck cancer we investigated the levels of the expression of IGF1, IGF2, E2F transcription factor 3 (E2F3) and IGF1 binding protein 3 (IGFBP-3) in tumor and healthy tissues from 41 cancer patients subjected to surgical treatment.
MATERIALS AND METHODS
Patients
Forty-one patients with Head and neck squamous cell carcinoma (HNSCC) were included in the study with 28 males and 13 females. The average age of the participants was 59 years with the youngest patient being 37 years old and the oldest patient 70 years old. Patients diagnosed with HNSCC were subjected to surgical treatment in The Greater Poland Cancer Center. Material collected during the surgery included cancer tissue and, as a control, normal epithelium tissue was collected within the range of 2 cm distal from tumor margins from the same patient. Tumor grade of differentiation was evaluated following WHO criteria and the TNM classification was with accordance of International Union Against Cancer (UICC). Patients’ clinical data is summarized in Table 1. All patients included in the study had not been treated with chemo- or radiotherapy prior to surgical intervention. The samples were divided into three groups according to the localization of tumor: larynx (n=25), oral cavity (n= 10) and pharynx (n= 5). The study was approved by the Institutional Review Board of University of Medical Sciences in Poznan and informed consent was obtained from all patients.
Table 1.
Summary of patients’ clinical data.
| Gender | female | 13 | |||
| male | 28 | ||||
| Median age at diagnosis (y) | 59 | ||||
| Anatomic site | larynx | oral cavity | pharynx | other | |
| Total number | 25 | 10 | 5 | 1 | |
| Tumor grade | Low-grade (I) | 0 | 2 | 1 | 0 |
| Intermediate-grade (II) | 20 | 6 | 4 | 0 | |
| High-grade (III) | 3 | 2 | 0 | 1 | |
| Stage | Low (I, II) | 5 | 3 | 1 | 0 |
| High (III, IV) | 18 | 7 | 4 | 1 | |
| M status | positive | 1 | 0 | 0 | 0 |
| negative | 24 | 10 | 5 | 1 | |
| N status | Low (0, I) | 25 | 10 | 5 | 1 |
| High (II, III) | 0 | 0 | 0 | 0 | |
Exclusion criteria
According to the study protocol, patients with local recurrences and second primary tumor were excluded from experimental groups. Also patients with a previous history of chemo- or radiotherapy were excluded.
Quantitative RT-PCR
Tumour and normal tissue were collected during surgery and did not cause any additional risk for patient. Tissues were frozen in −80°C immediately after surgery was completed. Total RNA was extracted using TRI Reagent (Sigma) according to the manufacture’s protocol. The concentration and purity of the eluted RNA samples were determined using the Take3 plate and the Epoch plate reader (BioTek). Complementary DNA was synthesized using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Quantitative PCR was performed on the ABI 7900HT fast real-time PCR system, using Fast SYBR Green master mix with Rox (Applied Biosystems, Foster City, CA). Primers for IGF1 (PPH00167C), IGF2 (PPH00168B), and E2F3 (PPH00917F) were purchased from SAbiosciences. Primer sequences for IGFBP-3 are: forward 5′-GCATGCTAAAGACAGCCAGC-3′, and reverse 5′-TCTCTACGGCAGGGACCATA-3′.
Statistical analysis
Analyses involved paired t-tests comparing gene expression values between tumor and healthy tissues. Given the exploratory nature of the analyses, we did not consider corrections for multiple comparisons. In order to determine if increases in expression levels of the genes in the tumors were associated with death or recurrence, we used Cox proportional hazards models [18] where death (or recurrence) was taken as the dependent variable with days until death (or recurrence) denoting survival times. 16 individuals died and 15 had recurrent tumors. Individuals who did not die (or who did not have recurrent tumors) were lost to follow-up after some period of time and were treated as censored observations. Gene expression levels in the normal and tumor tissue, as well as the differences between normal and tumor tissue, sex, age, and tumor grade were treated as predictors of death in the models. We fit models that considered gene expression levels for each gene independently as well as models that included all four genes as simultaneous predictors.
RESULTS
Gene expression analysis
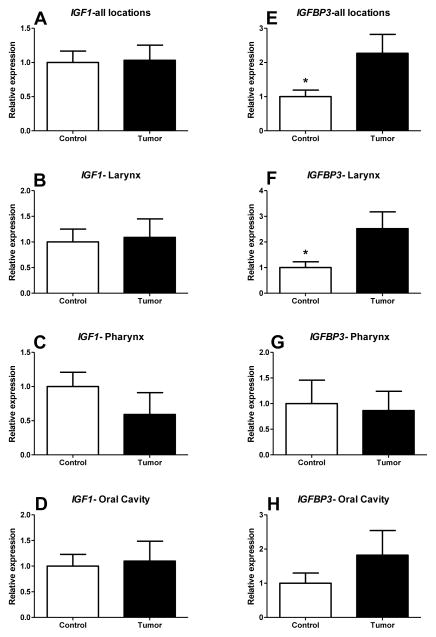
The analysis of all patients indicated no significant difference in IGF2 mRNA expression levels between healthy and tumor tissues (1.0±0.1909 and 1.365±0.3081 respectively; p=0.1585) (Figure 1A). Further, separation of patients according to the location of cancer also did not show any difference in the expression level of IGF2 when comparing healthy tissue with cancer tissue in larynx (1.0±0.2651 and 1.184±0.3525 respectively; p=0.3392), oral cavity (1.0±0.3578 and 1.937±0.6596 respectively; p=0.1152) and pharynx (1.0±0.2604 and 0.5220±0.2344 respectively; p=0.1056) (Figure 1A, B, C). E2F3 known as one of the stimulators of IGF2 expression activator also did not differ between healthy and tumor tissue in whole group (1.0±0.2209 and 1.133±0.2868 respectively; p=0.3577)(Figure 1E). There was also no alteration of E2F3 expression when analysing patients separately with cancer located in larynx (1.0±0.2867 and 1.465±0.5852 respectively; p=0.2396), oral cavity (1.0±0.3763 and 1.050±0.4959 respectively; p=0.4678) and pharynx (1.0±0.3183 and 1.6260±0.3603 respectively; p=0.2307) (Figure 1F, G, H). The expression level of IGF1 also did not differ between healthy and cancer tissues in analysis performed on all 41 patients (1.0±0.1671 and 1.032±0.2218 respectively; p=0.4536)(Figure 2A). There were also no differences detected in larynx (1.0±0.2514 and 1.090±0.3600 respectively; p=0.4196), oral cavity (1.0±0.2308 and 1.097±0.3890 respectively; p=0.4163) and pharynx (1.0±0.2086 and 0.5920±0.3195 respectively; p=0.1581)(Figure 2B,C,D). Interestingly, there was evidence for IGFBP3 mRNA level being upregulated in cancer when compared to healthy tissue (2.269±0.5522 and 1.0±0.1913 respectively; p=0.01131) (Figure 2E). Separate analysis of larynx cancer patients reflected the findings in whole experimental group indicating upregulation of IGFBP3 in cancer tissue (2.519±0.6573 and 1.0±0.2230 respectively; p=0.0168) (Figure 2F). The same binding protein did not differ in oral (1.0±0.2979 and 1.822±0.7213; p=0.1531) and pharynx cancer groups (1.0±0.4573 and 0.8640±0.3770; p=0.4121) (Figure 2G, H).
Figure 1.
The levels of relative expression of insulin-like growth factor 2 (IGF2) and E2F transcription factor 3 (E2F3) genes in Head and neck squamous cell carcinoma (HNSCC) and healthy tissues from the same patients. Means ± SEM.
Figure 2.
The levels of relative expression of insulin-like growth factor 1 (IGF1) and IGF binding protein 3 (IGFBP3) genes in Head and neck squamous cell carcinoma (HNSCC) and healthy tissues from the same patients. (*) represent significant difference (P<0.05)
We want to emphasize that for some analyses, our small sample size may have led us to be underpowered to detect an effect. For example, a power analysis using the t-test module in the G*power program [19] for detecting a difference in IGF2 between cancerous and normal pharynx tissue suggested that we would need 10 samples (we had 5) for the observed effect size assuming a power level of 0.80 with an assumed type I error rate of 0.05.
Survival analysis
Patients’ post-treatment and survival
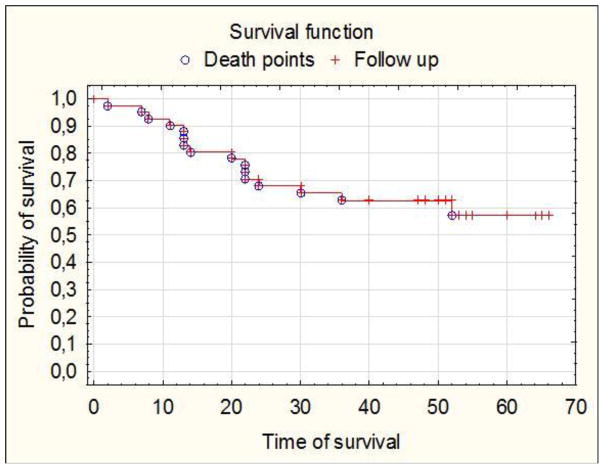
During patients follow-up period, there were 15 recurrences; the earliest recurrence was at 4 months after the operation and the latest at exactly 27 months after surgical intervention. During observation time there were 16 deaths, with the earliest at 2 months after operations the latest at 52 months after surgical treatment as presented by Kaplan-Meier (Figure 3).
Figure 3.
Kaplan-Meier survival curves representing patients’ post-surgical follow-up.
Cox proportional hazards model analysis of IGF1, IGF2, E2F3 and IGFBP3, when using the time until death from the time of the patients initial visit as the endpoint of interest (with age and sex as covariates), suggested that the difference in expression level between normal and tumor tissue for the E2F3 gene was the only gene to have expression differences associated with survival times in models that assessed the effects of all 4 genes simultaneously (p=0.038; Supplemental Table 1). Decreased expression of the E2F3 gene in the tumor relative to normal tissue was associated with recurrence time. Tumor-normal expression differences in the IGF2 gene showed an association with recurrence times when analyzed simultaneously with the other the genes, suggesting decreased expression in tumor relative to normal was associated with recurrence time (p=0.041; Supplementary Table 1). This was the case in models that only considered each gene’s effect in isolation of the others (p=0.044; Supplementary Table 1). We also found that elevated expression of the IGF1 gene in tumor tissue alone (i.e., not relative to normal tissue) was also associated with recurrence (p=0.0085; Supplementary Table 1). However, we must emphasize that our sample size was small with relatively few events (e.g., we had only 16 deaths and 15 recurrences among the 41 patients) and, as noted, given the exploratory nature of our analyses we did not control for multiple comparisons, so our results should be seen as highly preliminary.
DISCUSSION
There is a clear role of growth factors in cancer development and progression. IGF1 and IGF2 are known to decrease apoptosis and increase differentiation of cancer cells. Upon deregulation of growth factor expression, e.g. due to relaxation of IGF2 imprinting and overexpression during postnatal development there, is an increased risk of cancer development. Importantly the expression of IGF2 is regulated by E2F3. After birth E2F3 is strongly supressed which also downregulate the expression of IGF2 [20]. However, it was previously published that several types of cancer are characterized by elevated levels of IGF2 and E2F3 [20]. In contrast to observation in prostate, urinary bladder or Wilm’s tumor [20] our study of HNSCC cancer patients did not show any difference in IGF2 mRNA expression levels when cancer and healthy tissues from the same individuals were compared. This finding indicate that the expression of E2F3 and IGF2 in HNSCC does not play the same part in head and neck cancer biology as it has been indicated in other kinds of cancers characterized by elevated levels of E2F3 and IGF2 [20].
Similarly, the level of IGF1, the main postnatal growth factor, was also not altered in oral cancer tissues; however, there was a surprising increase of IGFBP3 mRNA in tumors. There is some evidence that IGFBP3 regulates the action of IGF1. Usually individuals with lower IGF1 and higher IGFBP3 would have lower risk of cancer [21], which would suggest that interaction of IGFBP3 with IGF1 would block the anti-apoptotic action if IGF1. However, at the same time binding of IGFBP3 to IGF1 can increase the half-life of IGF1 which could be rather negative for cancer patients. Moreover, the mRNA expression level in tumor and healthy tissue does not allow us to present clear conclusions without future studies of serum protein levels, binding assays and completing more mechanistic experiments involving the regulation of IGF1 by IGFBP3 in HNSCC. However, as serum levels of IGF1 and IGFBP3 can be associated with different types of cancer including prostate, colon/rectum, breast, lung, stomach [22–26] and even childhood leukaemias its role is still not well established in HNSCC. We would expect that high levels of serum IGF1 would be also associated with head and neck cancers, however, Brady et al. showed that circulating levels of both IGF1 and IGFBP3 protein is decreased in head and neck cancer patients [27]. On the other hand there is an evidence that IGF1 therepy for head and neck cancer undergoing radiation therapy may help with preservation and restoration of silvary gland function after radiation therapeutical approach [28]. Yet, the study of oral cancer cell line showed that there is positive association between autocrine production of IGF2 together with overexpression of IGF1-R and enhanced proliferation of oral cancer cells [29]. This could suggest that the lack of association between IGF1/IGF2 and HNSCC in selected population appears surprising; however, it could suggest that aside from local mRNA expression the circulating level of growth factors could be also important and have different role in HNSCC cancer development, etiology and during treatment which should be further investigated and correlated with protein levels of IGF1 and IGF2 in serum and tumor tissue. Additional complication for the study can also come from the cause of HNSCC such as genetic, environmental or viral infection as well as location. Our data indicated that there is a tendency towards upregulation of IGF2 while pulling all locations as well in larynx and oral cavity only. Interestingly, there was opposite trend indicating almost 50% down regulation of IGF2 in patients with tumor localized in pharynx. Symilar trend was observed for the expression of IGF1 and E2F3 mRNA in pharynx which could suggest differential biology and mechanism involved in HNSCC in this particular location.
In summary, in studied 41 patient affected by head and neck cancer we found that HNSCC are not associated with upregulated expression of IGF1 or IGF2 and observed elevation of IGFBP3 mRNA suggests that activation of IGF1 signal may be affected in these types of cancer cells. However, a suggestive relationship between E2F3 and survival suggests that a greater expression difference of E2F3 between normal and tumor tissue will possibly predict shorter survival of the patient affected by HNSCC.
Highlights.
Insulin growth factors are not directly associated with HNSCC
The expression of E3F3 suggest relationship with the prediction of survival of HNSCC patients
Acknowledgments
Research reported in this publication was supported by National Institute on Aging of the National Institutes of Health under award number R01AG032290, Polish National Science Centre N N403 186934 and NJS is funded in part by NIH grant 5 UL1 RR025774.
Footnotes
The Authors declare that there is no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.DeChiara TM, Efstratiadis A, Robertson EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990;345(6270):78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson-Smith AC, et al. Embryological and molecular investigations of parental imprinting on mouse chromosome 7. Nature. 1991;351(6328):667–70. doi: 10.1038/351667a0. [DOI] [PubMed] [Google Scholar]
- 3.Murphy R, et al. IGF2/H19 hypomethylation in a patient with very low birthweight, preocious pubarche and insulin resistance. BMC Med Genet. 2012;13:42. doi: 10.1186/1471-2350-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morison IM, et al. Somatic overgrowth associated with overexpression of insulin-like growth factor II. Nat Med. 1996;2(3):311–6. doi: 10.1038/nm0396-311. [DOI] [PubMed] [Google Scholar]
- 5.Lui JC, et al. An imprinted gene network that controls mammalian somatic growth is down-regulated during postnatal growth deceleration in multiple organs. Am J Physiol Regul Integr Comp Physiol. 2008;295(1):R189–96. doi: 10.1152/ajpregu.00182.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anisimov VN, Bartke A. The key role of growth hormone-insulin-IGF-1 signaling in aging and cancer. Crit Rev Oncol Hematol. 2013;87(3):201–23. doi: 10.1016/j.critrevonc.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsey MM, et al. Growth hormone-deficient dwarf animals are resistant to dimethylbenzanthracine (DMBA)-induced mammary carcinogenesis. Endocrinology. 2002;143(10):4139–42. doi: 10.1210/en.2002-220717. [DOI] [PubMed] [Google Scholar]
- 8.Williams HK. Molecular pathogenesis of oral squamous carcinoma. Mol Pathol. 2000;53(4):165–72. doi: 10.1136/mp.53.4.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jemal A, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 10.Warnakulasuriya S, Sutherland G, Scully C. Tobacco, oral cancer, and treatment of dependence. Oral Oncol. 2005;41(3):244–60. doi: 10.1016/j.oraloncology.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Hashibe M, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99(10):777–89. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 12.Ogden GR. Alcohol and oral cancer. Alcohol. 2005;35(3):169–73. doi: 10.1016/j.alcohol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Mao L, Hong WK. How does human papillomavirus contribute to head and neck cancer development? J Natl Cancer Inst. 2004;96(13):978–80. doi: 10.1093/jnci/djh209. [DOI] [PubMed] [Google Scholar]
- 14.Ang KK, Sturgis EM. Human papillomavirus as a marker of the natural history and response to therapy of head and neck squamous cell carcinoma. Semin Radiat Oncol. 22(2):128–42. doi: 10.1016/j.semradonc.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T, Tanaka M, Tanaka T. Oral carcinogenesis and oral cancer chemoprevention: a review. Patholog Res Int. 2011:431246. doi: 10.4061/2011/431246. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Petersen PE. Oral cancer prevention and control--the approach of the World Health Organization. Oral Oncol. 2009;45(4–5):454–60. doi: 10.1016/j.oraloncology.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 17.Gillenwater A, Papadimitrakopoulou V, Richards-Kortum R. Oral premalignancy: new methods of detection and treatment. Curr Oncol Rep. 2006;8(2):146–54. doi: 10.1007/s11912-006-0050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox DR, Oakes D. Monographs on statistics and applied probability. London ; New York: Chapman and Hall; 1984. Analysis of survival data; p. viii.p. 201. [Google Scholar]
- 19.Faul F, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 20.Lui JC, Baron J. Evidence that Igf2 down-regulation in postnatal tissues and up-regulation in malignancies is driven by transcription factor E2f3. Proc Natl Acad Sci U S A. 2013;110(15):6181–6. doi: 10.1073/pnas.1219079110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma J, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91(7):620–5. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 22.Chan JM, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279(5350):563–6. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 23.Giovannucci E, et al. A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiol Biomarkers Prev. 2000;9(4):345–9. [PubMed] [Google Scholar]
- 24.Hankinson SE, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351(9113):1393–6. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 25.Oh Y. IGFBPs and neoplastic models. New concepts for roles of IGFBPs in regulation of cancer cell growth. Endocrine. 1997;7(1):111–3. doi: 10.1007/BF02778076. [DOI] [PubMed] [Google Scholar]
- 26.Pollak M. Insulin-like growth factors and prostate cancer. Epidemiol Rev. 2001;23(1):59–66. doi: 10.1093/oxfordjournals.epirev.a000796. [DOI] [PubMed] [Google Scholar]
- 27.Brady G, et al. Serum levels of insulin-like growth factors (IGFs) and their binding proteins (IGFBPs), -1, -2, -3, in oral cancer. Int J Oral Maxillofac Surg. 2007;36(3):259–62. doi: 10.1016/j.ijom.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Victory K, et al. Head and neck tumor cell radiation response occurs in the presence of IGF1. J Dent Res. 2011;90(3):347–52. doi: 10.1177/0022034510388037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brady G, et al. Upregulation of IGF-2 and IGF-1 receptor expression in oral cancer cell lines. Int J Oncol. 2007;31(4):875–81. [PubMed] [Google Scholar]