Abstract
Resilience is an active process that involves a discrete set of neural substrates and cellular mechanisms and enables individuals to avoid some of the negative consequences of extreme stress. We have previously shown that dominant individuals show less stress-induced changes in behavior compared to subordinates using a conditioned defeat model in male Syrian hamsters (Mesocricetus auratus). To rule out pre-existing differences between dominants and subordinates, we examined whether 14 days of dominance experience is required to reduce the conditioned defeat response and whether the development of conditioned defeat resistance correlates with defeat-induced neural activation in select brain regions. We paired hamsters in daily 5-min aggressive encounters for 1, 7, or 14 days and then exposed animals to 3, 5-min social defeat episodes. The next day animals received conditioned defeat testing which involved a 5-min social interaction test with a non-aggressive intruder. In separate animals brains were collected after social defeat for c-Fos immunohistochemistry. We found that 14-day dominants showed a decreased conditioned defeat response compared to 14-day subordinates and controls, while 1-day and 7-day dominants did not differ from their subordinate counterparts. Also, the duration of dominance relationship was associated with distinct patterns of defeat-induced neural activation such that only 14-day dominants showed elevated c-Fos immunoreactivity in the ventral medial prefrontal cortex, medial amygdala, and lateral portions of the ventral medial hypothalamus. Our data suggest that resistance to social stress develops during the maintenance of dominance relationships and is associated with experience-dependent neural plasticity in select brain regions.
Keywords: dominance, stress, resilience, conditioned defeat, ventral medial prefrontal cortex, medial amygdala
1. Introduction
Social defeat is a robust stressor used to investigate behavioral and physiological responses to social stress and model the biological basis of stress-related mental illness [1, 2]. Conditioned defeat is a type of social stress model in male Syrian hamsters (Mesocricetus auratus) in which a brief social defeat results in a loss of species-typical territorial aggression and an increase in submissive and defensive behavior when animals are later tested with a small, nonaggressive intruder. Social defeat models, including conditioned defeat, allow for investigation of neurobiological mechanisms controlling vulnerability to the effects of social stress. For instance, we have previously shown that pairs of male Syrian hamsters with established dominance relationships respond differently to social defeat stress such that dominant animals show reduced conditioned defeat compared to subordinates [3, 4].
Exposure to chronic stress is a risk factor for the development of stress-related mental illness including major depression [5, 6], and exposure to an acute, traumatic stressor is an essential prerequisite for post-traumatic stress disorder [7–9]. However, in both cases not all individuals exposed to stressful events develop stress-related psychopathologies [10, 11]. Understanding the neurobiological mechanisms regulating vulnerability and resistance to the effects of stress is an important step toward advancing treatment options for stress-related psychopathology. Animal models have identified several brain regions critical for stress resistance including the ventral medial prefrontal cortex (vmPFC). The vmPFC, which includes the infralimbic cortex (IL) and prelimbic cortex (PL), controls affective processing and executive function [12, 13]. The vmPFC is also part of a neural circuit that inhibits neurons within the paraventricular nucleus of the hypothalamus that control the neuroendocrine stress response [14, 15]. Neural activation in the vmPFC is required for the stress resistance which develops following exposure to environmental enrichment [16], social dominance [4], and controllable stress [17]. For example, exposure to controllable stress has an immunizing effect on the development of learned helplessness such that uncontrollable stress fails to produce learned helplessness when rats are pre-exposed to controllable tail-shocks. Pharmacological inhibition of the vmPFC blocks the immunizing effect of controllable stress [18], and pharmacological activation of the vmPFC has an immunizing effect in the absence of controllable stress [19]. Also, stress-induced neural activation in the vmPFC is associated with individual differences in coping strategies and baseline trait anxiety in rodents [20, 21]. Overall, the vmPFC is a critical neural substrate that inhibits neuroendocrine and behavioral responses to stress.
Several other brain regions, outside of the vmPFC, have been implicated in resistance to the effects of stressful events. Six weeks of voluntary wheel running has been shown to prevent the shuttle box escape deficits that occur following uncontrollable stress [22]. A series of studies has shown that voluntary wheel running alters ΔFosB immunoreactivity in the nucleus accumbens [23], brain-derived neurotrophic factor mRNA expression in the hippocampus and amygdala [24], and 5-HT1A autoreceptor mRNA expression in the dorsal raphe nucleus [25]. Chronic social defeat stress in mice leads to heightened reactivity of the hypothalamic-pituitary-adrenal axis as well as increased anxiety and depression-like behavior [26]. However, about one-third of mice exhibit a resilient phenotype insofar as they do not exhibit social avoidance and anhedonia-like symptoms. Resilient mice show changes in the expression of K+ channels and AMPA receptor subunits which normalizes firing within a ventral tegmental-nucleus accumbens circuit [26, 27]. Dominant social status is also associated with coping style and neuroendocrine responses to stress. In Anolis lizards dominants have been described as adopting a proactive behavioral strategy during agonistic social encounters [28]. Dominant lizards also show elevated serotonin levels in the amygdala following restraint stress whereas subordinates show elevated dopamine levels [29]. In several species dominants show reduced basal or stress-induced glucocorticoid activity compared to subordinates [29–32]. Overall, resilience appears to be an active process that involves multiple brain regions and cellular mechanisms which facilitate coping with stress.
One limitation of studying the natural formation of dominance relationships is that subjects cannot be randomly assigned to dominant or subordinate status. Subjects may have pre-existing differences that correlate with both the probability of winning and responses to stress. The aim of this study was to examine the time course of the status-dependent changes in conditioned defeat and defeat-induced neural activation. We used the protein product of the immediate early gene c-fos as a marker of defeat-induced neural activation [33]. We hypothesized that dominants would show reduced conditioned defeat and elevated defeat-induced neural activation within brain regions such as the vmPFC compared to subordinates after 14 days, but not 1 or 7 days, of dominance experience. This approach allowed us to determine whether dominant and subordinate animals systemically differed in conditioned defeat and neural activation prior to dyadic dominance interactions or whether behavioral and physiological changes were experience-dependent.
2. Experimental Procedures
2.1 Subjects
Subjects were male Syrian hamsters (Mesocricetus auratus) obtained from our breeding colony that was originally derived from hamsters purchased from Charles River Laboratories (Wilmington, MA). Subjects were 3–4 months old (120–180 g) at the start of the study and were individually housed one week prior to the start of the study. Older hamsters (> 6 months, >190 g) were individually housed and used as resident aggressors for social defeat training. Younger hamsters (approx. 2 months, <120 g) were housed in groups of four and used as non-aggressive intruders for conditioned defeat testing. All animals were housed in polycarbonate cages (12 cm × 27 cm × 16 cm) with corncob bedding, cotton nesting materials, and wire mesh tops. Food and water were available ad libitum. Cages were not changed for one week prior to dominant-subordinate encounters to allow individuals to scent mark their territory. Subjects were handled daily for one week prior to dominant-subordinate encounters to habituate them to the stress of human handling. Animals were housed in a temperature controlled colony room (21 ± 2 °C) and kept on a 14:10 hr light:dark cycle to facilitate aggressive behavior. All behavioral protocols were performed during the first three hours of the dark phase of their cycle. All procedures were approved by the University of Tennessee Institutional Animal Care and Use Committee and are in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2 Behavioral Protocols
2.2.1 Dominant-Subordinate Encounters
To allow animals to establish social status, subjects within each cohort were weight-matched in resident-intruder dyads and paired in daily social encounters for 1 day, 7 days, or 14 days. Subjects were randomly assigned as a resident or intruder, and all social encounters occurred in the resident's home cage. The encounter on day 1 was 10 min in duration, while all subsequent encounters were 5 min. We have previously determined that a 10 min encounter on day 1 facilitates the formation of a dominance relationship, and that 5 min encounters on subsequent days maintain the dominance relationship and reduce the chance of wounding [3]. Dominant and subordinate animals were identified by the direction of agonistic behavior within each dyad. Subjects were pseudo-randomly assigned to the number of dominant-subordinate encounters. Dyads must have formed a dominant-subordinate relationship during their first encounter to be assigned to 1 day of social encounters. The remaining dyads, including some that formed a dominance relationship on the first day, were randomly assigned to 7 days or 14 days of social encounters. If a dyad did not form a dominance relationship after 5 daily encounters, that dyad was dropped from the study. Control subjects were individually housed one week prior to social defeat training and were handled daily during that time.
2.2.2 Social Defeat Training
Dominants and subordinates were exposed to social defeat training 24 hrs after their final dominance encounter such that defeats occurred 2, 8, or 15 days after the start of the experiment. Social experience controls were exposed to social defeat training after one week of individual housing, while the handled controls were not defeated (Fig. 1). Social defeat consisted of subjects being placed in the home cages of three different resident aggressors for three separate 5-min aggressive encounters. Resident aggressors are older, heavier male hamsters that have been singly housed for a prolonged period of time and display reliable aggression when confronted with intruders. Subjects received a 5-min rest period in their home cage between each aggressive encounter. Dominants and social experience controls often fought back against the resident aggressor during the first defeat but eventually lost and did not fight back during subsequent defeats. To correct for potential variation in the amount of aggression subjects received, we defined social defeat as starting at the resident aggressor's first attack that was accompanied by submissive behavior in a subject. Handled control subjects received 3, 5-min exposures to resident aggressor cages to control for olfactory cues that may impact behavior and neural activation.
Fig. 1.
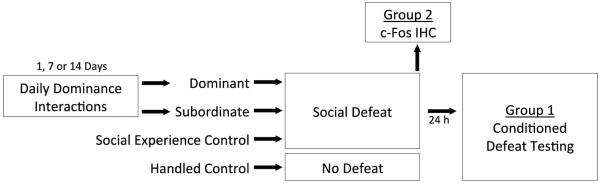
A schematic representation of the experimental design. Dominance interactions occurred daily for either 1, 7, or 14 days. Social experience controls and handled controls were singly housed one week prior to social defeat or no defeat, respectively. Group 2 animals were euthanized after social defeat training which occurred 24 hrs following the final dominance interaction for each pair. Group 1 animals were treated similarly and experienced conditioned defeat testing 24 hrs following social defeat.
Social defeats were digitally recorded for later behavioral analysis. We quantified the duration of aggression displayed by resident aggressors and the duration of submission displayed by subjects using the ethogram described below.
2.2.3 Conditioned Defeat Testing
To assess the effect of duration of dominance relationship on behavioral responses to social stress, animals were tested for conditioned defeat behavior 24 hours after social defeat training (Fig. 1, Group 1). Testing consisted of a 5-min social interaction test during which a non-aggressive intruder was placed in the subject's home cage. Non-aggressive intruders are younger, group-housed animals that display non-agonistic social and nonsocial behavior during conditioned defeat testing, and do not direct agonistic behavior toward the subject. We digitally recorded all conditioned defeat testing sessions and quantified the behavior of subjects using Noldus Observer software (Noldus Information Technology, Wageningen, Netherlands). We quantified the total duration of the following categories of behavior: submissive/defensive (flee, avoid, upright and side defensive postures, tail-up, stretch-attend, head flag); aggressive (chase, attack including bite, upright and side offensive postures); non-agonistic social (sniff, approach); and nonsocial (locomotion, grooming, nesting, feeding). We also recorded the frequency of attacks, flees, and stretch-attend postures. All behavioral scoring was performed by a researcher blind to the experimental treatment. To establish inter-rater reliability, we achieved 90% agreement on the duration of aggressive and submissive/defensive behavior in a subset of videos.
2.3 c-Fos Immunohistochemistry
Ninety minutes following the start of social defeat, animals were anesthetized with isoflurane and were transcardially perfused with 100ml of 0.1 M phosphate buffered saline (PBS) followed by 100ml of 4% paraformaldehyde solution (Fig. 1, Group 2). Brains were removed and soaked in 4% paraformaldehyde for 24 hours, followed by 0.1 M PBS/30% sucrose solution for 48 hours at 4°C, and then were stored in cryoprotectant. A consecutive series of 30 μm coronal sections were cut on a vibrating microtome reaction which involved a 15 min incubation in 3,3'-diaminobenzidine (DAB tablet, Sigma-Aldrich, St. Louis, MO) and nickel dissolved in PBS. The sections were washed five times with PBS and 5 times with distilled H2O prior to being mounted onto glass microscope slides. After air-drying, sections were dehydrated using a series of alcohols, cleared with citrisolv and coverslipped using DPX mountant (Sigma-Aldrich, St. Louis, MO). For each brain region, the tissue from all subjects was processed simultaneously.
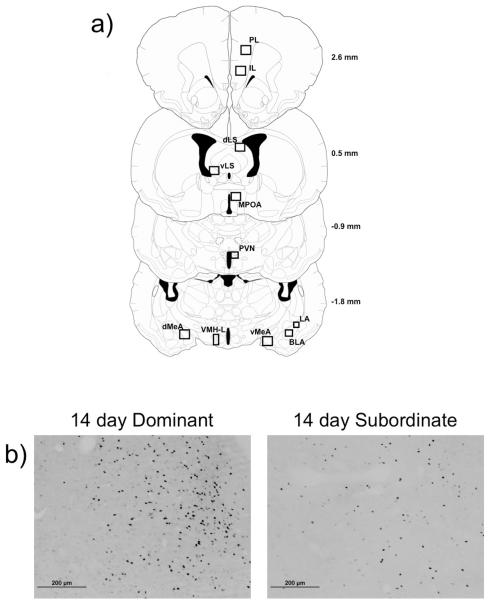
Images were captured at 10× magnification using an Olympus BX41 microscope. The number of c-Fos immunopositive cells was quantified in several brain regions including the basolateral amygdala (BLA), dorsal medial amygdala (dMeA), ventral medial amygdala (vMeA), paraventricular nucleus of the hypothalamus (PVN), lateral regions of the ventromedial hypothalamus (VMHL), ventral lateral septum (vLS), dorsal lateral septum (dLS), medial preoptic area (MPOA), prelimbic cortex (PL), and infralimbic cortex (IL). For each brain region, we recorded background immunoreactivity in unstained regions of each image. We defined immunopositive cells as those nuclei that showed staining 1.5x darker than the specific background immunoreactivity calculated for each image. MCID Core image analysis software (InterFocus Imaging, Cambridge, England) was used to quantify the number of c-Fos immunopositive cells and the analysis software reached 90% agreement with manual counting on a subset of images. Cell counts were limited to the area within defined boxes that were tailored to the size of each brain region (Fig. 2a). Sample images of c-Fos immunoreactivity in a 800 × 660 μm box within the vMeA of a defeated 14 day dominant and 14 day subordinate are shown in Figure 2b. For each brain region we quantified six nonconsecutive sections per individual along a rostral-caudal axis.
Fig. 2.
The diagrams indicate the location of brain regions selected for c-Fos quantification (a). The diagrams were modified from the hamster atlas of Morin & Wood and values indicate the distance from bregma [71]. The box sizes used for quantification are as follows (width × height): 440 μm × 400 μm (BLA); 325 μm × 650 μm (VMHL); 439 μm × 330 μm (PVN); 500 μm × 500 μm (dLS, vLS, BNST, MPOA); 870 μm × 660 μm (dMeA, vMeA, PL, IL). Representative photomicrograph of the vMeA from a defeated 14 day dominant and 14 day subordinate used for c-Fos quantification (b). Black dots represent c-Fos immunopositive nuclei.
2.4 Group 1: Conditioned Defeat Behavior
We tested the hypothesis that dominant individuals would show reduced submissive and defensive behavior during conditioned defeat testing compared to subordinates and social experience controls after 14 days of social encounters, but not after 1 or 7 days of social encounters. Following the establishment of dominance relationships, dominants, subordinates, and social experience controls received social defeat training (dominant 1 day, N = 13; subordinate 1 day, N = 14; dominant 7 day, N = 12; subordinate 7 day, N = 11; dominant 14 day, N = 13; subordinate 14 day, N = 12; social experience control, N = 14) and handled controls were not defeated (handled controls, N = 12). All subjects experienced conditioned defeat testing. Sample sizes were reduced slightly when nine individuals were removed from statistical analysis because of wounding that occurred during social defeat training or technical issues with conditioned defeat testing such as aggression by intruders.
2.5 Group 2: Defeat-induced Neural Activation
We tested the hypothesis that dominant individuals would show differences in defeat-induced c-Fos immunoreactivity in select brain regions compared to subordinates and social experience controls after 14 days of social encounters, but not after 1 or 7 days of social encounters. Following the establishment of dominance relationships, dominants, subordinates, and empty cage controls received social defeat (dominant 1 day, N = 14; subordinate 1 day, N = 14; dominant 7 day, N = 13; subordinate 7 day, N = 13; dominant 14 day, N = 14; subordinate 14 day, N = 14; social experience control, N = 13) and handled controls were not defeated (handled control, N = 13). Sample sizes were reduced slightly when three individuals were removed from statistical analysis because of wounding that occurred during social defeat training.
2.6 Data Analysis
Due to the unbalanced factorial design, we analyzed the data from social defeat training, conditioned defeat testing, and c-Fos immunohistochemistry with two separate statistical tests. A 2-way analysis of variance (ANOVA) test was used to investigate an interaction between social status (2 levels) and duration of dominance relationship (3 levels). A 1-way ANOVA with Tukey post-hoc tests was used to investigate planned comparisons between controls and dominant and subordinate groups. The occurrence of counter attacking during social defeat training was analyzed using a Chi-square test. Results were considered significant when the α level was P ≤ 0.05.
3. Results
3.1 Dominant-Subordinate Encounters
On average, dominance relationships were decided on day 1.4 ± 0.08. In pairs that did not form a dominance relationship during the first encounter, both animals were often aggressive until one animal submitted during the second or third encounter. In all cases, dominance relationships were stable once established. Subordinates displayed elevated and stable submissive/defensive behavior compared to dominants, and they never displayed aggressive behavior after the formation of dominance relationships.
3.2 Social Defeat Training
Dominants, subordinates, and controls did not significantly differ in either the amount of aggression received or the amount of submission displayed (data not shown). However, social status and duration of dominance relationships influenced whether individuals counterattacked the resident aggressor during the first social defeat episode. After 1 day of social encounters, dominant and subordinate individuals were equally likely to counterattack the resident aggressor (3 of 13 dominants 1 day; 0 of 14 subordinates 1 day; χ2 [1, n=27]=3.63, P>0.05). After 7 days of social encounters, dominant individuals were significantly more likely to counterattack the resident aggressor than subordinates (8 of 12 dominants 7 day; 0 of 11 subordinates 7 day; χ2 [1, n=23]=11.24, P<0.001) and social experience controls (3 of 14 social experience controls; χ2 [1, n=26]=6.58, P<0.025). After 14 days of social encounters, more dominants counterattacked than did subordinates (8 of 11 dominants 14 day; 0 of 12 subordinates 14 day; χ2 [1, n=23]=13.38, P<0.001) and social experience controls (3 of 14 social experience controls; χ2 [1, n=25]=5.42, P<0.025).
3.3 Conditioned Defeat Testing
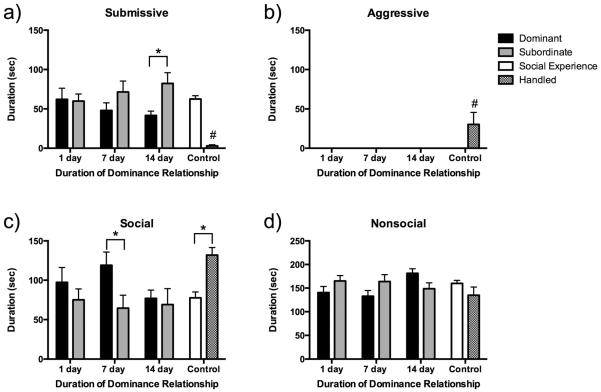
Subjects with 14 days of dominance experience showed reduced conditioned defeat behavior at testing, while neither 7 day dominants or 1 day dominants showed a reduction in conditioned defeat behavior (Fig. 3a). We found a significant main effect of social status on the duration of submissive/defensive behavior displayed at conditioned defeat testing, such that dominant individuals showed less submissive/defensive behavior than did subordinate individuals (F(2,69) = 5.009, P = 0.028). We found that the difference between dominant and subordinate individuals was not present following 1 day of dominant-subordinate encounters (P = 0.846) or 7 days of encounters (P = 0.109), while dominants displayed less submissive/defensive behavior after 14 days of encounters compared to 14 day subordinates (F(1,93) = 5.009, P < 0.0001; Tukey, P = 0.004). Social experience controls displayed an intermediate level of submissive/defensive behavior compared to 14 day dominants (P = 0.124) and 14 day subordinates (P = 0.153). All defeated groups showed significantly more submissive/defensive behavior at testing than did handled controls (P < 0.05). We found no effect of social status or duration of dominance relationship on the frequency of fleeing or stretch-attend postures in dominant and subordinate individuals or in comparison to controls during conditioned defeat testing (data not shown).
Fig. 3.
Durations (mean ± SE) of submissive/defensive (a), aggressive (b), social (c), and nonsocial (d) behaviors are shown during a 5 min test with a non-aggressive intruder. Dominant (black bars) and subordinate (gray bars) animals received social defeat 24 h prior to conditioned defeat testing. Social experience controls received social defeat 24 h prior to testing (white bars) whereas handled controls did not experience social defeat (hatched bars). An asterisk (*) indicates a significant difference between the bracketed bars and pound sign (#) indicates that handled controls are significantly different from all other groups (P < 0.05).
There was no effect of social status or duration of dominance relationship on the duration of aggressive behavior displayed at conditioned defeat testing, as all groups failed to display any aggressive behavior (Fig. 3b). The only subjects to display aggressive behavior during conditioned defeat testing were handled controls, which displayed significantly more aggressive behavior than all other groups (P < 0.05). Additionally, handled controls attacked nonaggressive intruders 1.25 (± 0.66) times during testing which was significantly greater than in any of the defeat groups (P < 0.05).
There was a significant main effect of social status on the duration of social behavior displayed during conditioned defeat testing, with dominant individuals displaying more social behavior than subordinate individuals (F(2,69) = 4.462, P = 0.038) (Fig.3c). Status-dependent differences in social behavior reached statistical significance only in animals with 7 days of dominance experience (F(1,93) = 5.384, P < 0.0001; Tukey, P = 0.014). Seven day dominant individuals showed significantly more social behavior during conditioned defeat testing than 1 day subordinates (P = 0.034), 14 day dominants (P = 0.046), 14 day subordinates (P = 0.021), and social experience controls (P = 0.045). Similarly, handled controls displayed significantly more social behavior during conditioned defeat testing than 1 day subordinates (P = 0.007), 7 day subordinates (P = 0.003), 14 day dominants (P = 0.010), 14 day subordinates (P = 0.004), and social experience controls (P = 0.009).
There was an interaction of social status and duration of dominance relationship on the duration of nonsocial behavior displayed at conditioned defeat testing (F(2,69) = 4.085, P = 0.021) (Fig. 3d). This result indicates that the maintenance of dominance relationships for 14 days increased the amount of nonsocial behavior in dominants but decreased it in subordinates. However, post-hoc tests revealed no significant differences between any groups (F(1,93) = 1.931, P = 0.073).
3.4 c-Fos Immunohistochemistry
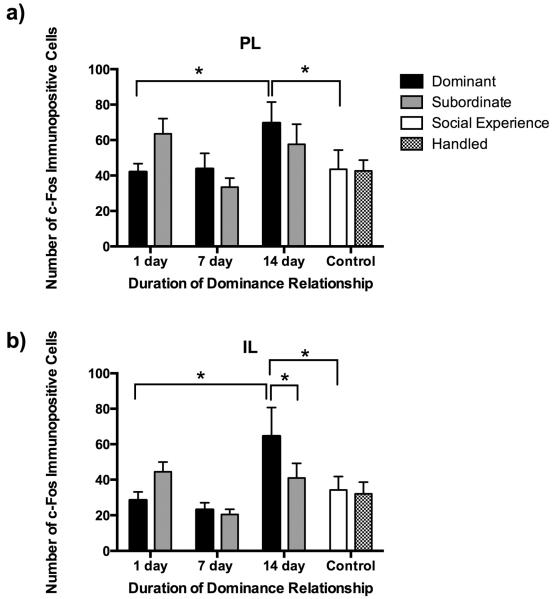
In the PL there was a main effect of duration of dominance relationship such that individuals with 14 days of social encounters had significantly more defeat-induced c-Fos immunopositive cells than did individuals with 7 days of social encounters (Fig 4a; F(2,58) = 4.214, P = 0.02; Tukey, P = 0.005). Also, 14 day dominants showed an increased number of c-Fos immunopositive cells compared to both 1 day dominants (F(7,76) = 2.763, P = 0.013; Tukey, P = 0.024) and social experience controls (P = 0.011).
Fig. 4.
Number (mean ± SE) of c-Fos immunopositive cells in subregions of the ventral medial prefrontal cortex measured following social defeat. Dominants (black bars), subordinates (gray bars), and social experience controls (white bars) were exposed to social defeat, whereas handled controls (hatched bars) were not. In the PL (a), there was a main effect of duration of dominance relationship. In the IL (b), there was an interaction between duration of dominance relationship and social status. Post-hoc tests revealed significant differences between bracketed bars represented by an asterisk (*, P < 0.05).
In the IL there was a significant social status x duration of dominance relationship interaction (Fig. 4b; F(2,53) = 3.194, P = 0.049). Specifically, 14 day dominants had more defeat-induced c-Fos immunopositive cells than did 14 day subordinates (F(7,70) = 3.206, P = 0.005; Tukey, P = 0.032), while there was no effect of social status at either 1 day or 7 days (P > 0.05). Also, 14 day dominants showed more c-Fos immunopositive cells than did 1 day dominants (P = 0.001) and social experience controls (P = 0.011).
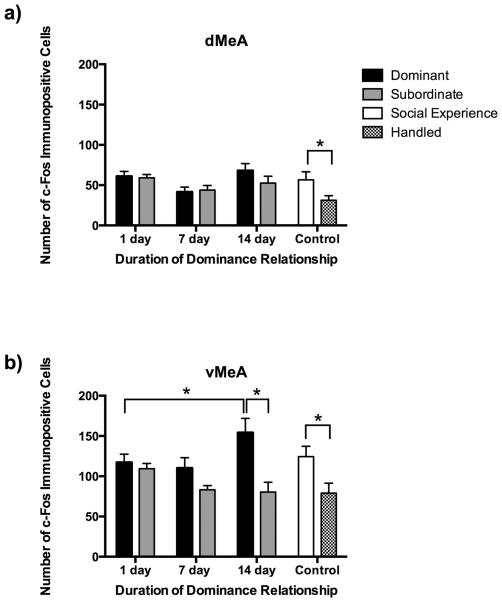
In the dMeA we found a main effect of duration of dominance relationship (Fig. 5a; F(2,67) = 4.685, P = 0.012). Subjects with 7 days of social encounters had significantly fewer c-Fos immunopositive cells than did both 1 day animals (P = 0.011) and 14 day animals (P = 0.012). Also, social defeat increased c-Fos immunoreactivity in control animals such that social experience controls had significantly more c-Fos immunopositive cells than did handled controls (F(7,86) = 2.824, P = 0.005; Tukey, P = 0.017).
Fig. 5.
Number (mean ± SE) of c-Fos immunopositive cells measured in subregions of the medial amygdala following social defeat. Dominants (black bars), subordinates (gray bars), and social experience controls (white bars) were exposed to social defeat, whereas handled controls (hatched bars) were not. In the dMeA (a), there was a main effect of duration of dominance relationship. In the vMeA (b), there was an interaction between duration of dominance relationship and social status. Post-hoc tests revealed significant differences between bracketed bars represented by an asterisk (*, P < 0.05).
In the vMeA there was a significant social status x duration of dominance relationship interaction (Fig. 5b; F(2,66) = 4.612, P = 0.013). Specifically, 14 day dominants showed increased c-Fos immunopositive cells compared to 14 day subordinates (F(7,85) = 4.963, P< 0.0001; Tukey, P < 0.0001), whereas 1 day and 7 day dominants did not significantly differ from corresponding subordinates (P > 0.05). In addition, 14 day dominants had more c-Fos immunopositive cells following social defeat than did 1 day dominants (P = 0.023). Finally, social defeat increased c-Fos immunoreactivity in the vMeA such that social experience controls showed more c-Fos immunopositive cells than did handled controls (P = 0.009).
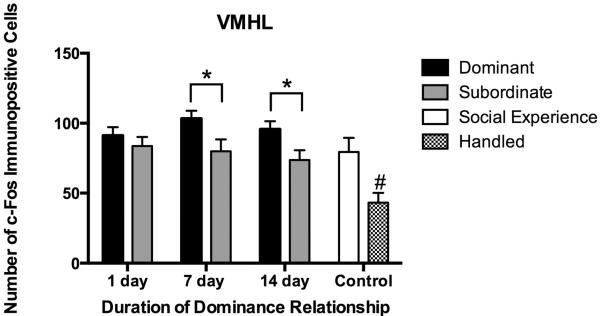
In the VMHL we found a main effect of social status in which dominants had more c-Fos immunopositive cells than subordinates (Fig. 6; F(1,55) = 9.72, P = 0.003). Significant increases in the number of c-Fos immunopositive cells were found in 7 day dominants compared to 7 day subordinates (F(7,72) = 5.470, P < 0.0001; Tukey, P = 0.031) and in 14 day dominants compared to 14 day subordinates (P = 0.039). Also, social defeat produced robust c-Fos immunoreactivity in the VMHL as all defeated groups showed significantly more c-Fos immunopositive cells compared to handled controls (P < 0.01).
Fig. 6.
Number (mean ± SE) of c-Fos immunopositive cells in the VHML measured following social defeat. Dominants (black bars), subordinates (gray bars), and social experience controls (white bars) were exposed to social defeat, whereas handled controls (hatched bars) were not. There was a significant main effect of social status and significant differences between bracketed bars are represented by an asterisk (*, P < 0.05). Also, handled controls were significantly different than all other groups (#, P < 0.05).
In the BLA there was a significant social status x duration of dominance relationship interaction on c-Fos immunoreactivity (Table 1; F(2,64) = 8.24, P = 0.01). We found that 1 day subordinates had more c-Fos immunopositive cells than 1 day dominants (F(7,83) = 5.485, P < 0.0001; Tukey, P = 0.01), while 14 day subordinates had fewer c-Fos immunopositive cells than did 14 day dominants (P = 0.016). Also, defeat-induced c-Fos immunoreactivity appeared to habituate in subordinates as 14 day subordinates had significantly fewer c-Fos immunopositive cells than did 1 day subordinates (P < 0.0001).
Table 1.
Number of c-Fos immunopositive cells (mean ± SE) following social defeat training.
| Brain Region | 1 Day | 7 Day | 14 Day | Controls | ||||
|---|---|---|---|---|---|---|---|---|
| Dominant (N = 8–13) | Subordinate (N = 8–12) | Dominant (N = 7–12) | Subordinate (N = 9–13) | Dominant (N = 7–13) | Subordinate (N = 7–11) | Social Experience (N = 7–11) | Handled (N = 8–11) | |
| BLA | 16.7 ± 1.3* | 23.2 ± 1.3 | 10.5 ± 1.2 | 11.6 ± 1.5 | 17.5 ± 2.4* | 10.8 ± 1.9 | 15.1 ± 2.1 | 17.9 ± 3.0 |
| dLS | 20.6 ± 5.2 | 22.1 ± 2.1 | 21.8 ± 4.6 | 16.7 ± 3.6 | 35.1 ± 4.8** | 27.7 ± 4.5** | 28.5 ± 7.6 | 20.6 ± 4.2 |
| vLS | 56.6 ± 10.5 | 80.2 ± 14.7 | 55.5 ± 7.7 | 52.9 ± 10.7 | 92.7 ± 16.9 | 71.3 ± 11.2 | 59.0 ± 10.5^ | 24.6 ± 4.0 |
| MPOA | 25.4 ± 6.2 | 40.3 ± 6.5 | 31.0 ± 6.4 | 28.1 ± 4.7 | 34.1 ± 4.7 | 32.5 ± 6.5 | 28.2 ± 8.8^ | 11.6 ± 1.9 |
| PVN | 56.9 ± 11.6 | 74.5 ± 12.2 | 68.7 ± 15.6 | 35.2 ± 7.4 | 85.3 ± 20.2 | 79.3 ± 26.9 | 59.9 ± 7.9^ | 25.8 ± 6.1 |
Note: Sample sizes vary because some individuals were excluded from data analysis due to poor tissue quality.
indicates that 1-day and 14-day dominants significantly differed from 1-day and 14-day subordinates, respectively (p < .05).
indicates that animals paired for 14 days significantly differed from animals paired for 1 or 7 days (p < .05).
indicates a significant difference compared to corresponding handled controls (p < .05). BLA – basolateral amygdala, dLS – dorsal lateral septum, vLS – ventral lateral septum, MPOA – medial preoptic area, PVN – paraventricular nucleus of the hypothalamus.
We found few changes in defeat-induced c-Fos immunoreactivity in the dLS, vLS, MPOA, and PVN (Table 1). In the dLS there was a main effect of duration of dominance relationship, such that 14 days of social encounters resulted in significantly more c-Fos immunopositive cells than either 1 day or 7 days of social encounters (F(2,63) = 4.334, P = 0.017; Tukey, P = 0.017, P = 0.004, respectively). In other brain regions we found an effect of social defeat only such that social experience controls displayed significantly more c-Fos immunopositive cells than did handled controls in the vLS (F(7,82) = 3.457, P = 0.006; Tukey, P = 0.018), MPOA (F(7,81) = 4.624, P = 0.008; Tukey, P = 0.011), and PVN (F(7,79) = 3.716, P = 0.004; Tukey, P = 0.009). Finally, we observed very low c-Fos immunoreactivity and did not quantify cells in the central amygdala, lateral amygdala, dorsal hippocampus, dentate gyrus, bed nucleus of the stria terminalis, and nucleus accumbens.
4. Discussion
4.1 Behavioral response
We found that resistance to the effects of social defeat in dominant male hamsters is an experience-dependent phenomenon resulting from the maintenance of dominance status. Dominant individuals only showed resistance to conditioned defeat, indicated by decreased submissive/defensive behavior compared to subordinates, following 14 days of dominance experience. Dominance status maintained for either 1 day or 7 days was not sufficient to produce resistance to conditioned defeat. In previous studies, it has been unclear whether dominants are resistant to the effects of social defeat or whether subordinates are susceptible, or both [4]. These data suggest that while neither dominants nor subordinates significantly differ in conditioned defeat response at day 14 compared to day 1, maintenance of dominance relationships produces both a resistant behavioral phenotype in dominants and a vulnerable behavioral phenotype in subordinates. This idea is consistent with the finding that social experience controls display an intermediate level of submissive and defensive behavior compared to 14 day dominants and subordinates. The effects if dominance social status are specific to submissive/defensive behavior at conditioned defeat testing, as 14 day dominants and subordinates displayed no significant differences on aggressive, social, or nonsocial behavior. We found that 7 day dominants show increased non-agonistic social behavior during testing compared to 7 day subordinates which suggests that some behavioral changes may precede resistance to conditioned defeat. Our findings are consistent with a growing body of literature that differential responses to stress can develop following specific experiences such as environmental enrichment [16], exercise [34], controllable stress exposure [35], and social dominance [29].
In previous studies, we have found that dominant individuals initially respond to social defeat by counter attacking their opponent [4, 36]. Similar to conditioned defeat behavior, the incidence of counter attacking was influenced by the duration of dominance relationship, with 7 day and 14 day dominants showing significantly more counter attacking of resident aggressors than corresponding subordinates and controls. Counter attacking the resident aggressor may represent a proactive coping style and is similar to how some rats respond to social defeat [20]. Coping style is typically considered a behavioral trait which remains stable [37], although in this case the experience of social dominance may generate a proactive coping style. If dominants use a proactive coping style in other situations their resistance to conditioned defeat may transfer to other domains. Interestingly, elevated offensive aggression has been associated with increased struggling during a forced swim test [38], increased shock probe burying [37], and increased active avoidance in a shuttle box [39]. An intriguing possibility for future studies is whether dominance status promotes resilience in other tests of anxiety-like behavior.
Importantly, our behavioral results suggest that even though subjects self-select into dominant and subordinate roles, resistance to conditioned defeat in dominants and susceptibility in subordinates is not related to pre-existing differences. Winning a single dominance interaction was not sufficient to alter how hamsters respond to social defeat in terms of counter aggression or the conditioned defeat response.
4.2 Neural activation
In several brain regions such as the PL, IL, and vMeA we found that defeat-induced c-Fos immunoreactivity increased in dominant animals only after they maintained dominant social status for two weeks. Importantly, these time-dependent changes in c-Fos immunoreactivity parallel the reduction of conditioned defeat in dominants. These results suggest that resistance to conditioned defeat in dominant individuals is associated with experience-dependent neural plasticity in select brain regions that develops during two weeks of social encounters.
We found that in both the IL and PL cortex 14 days of dominance experience leads to an increase in defeat-induced c-Fos immunoreactivity, compared to 1 day dominants and defeated controls. We also found that after 14 days of social encounters dominants display significantly more c-Fos immunoreactivity in the IL cortex compared to subordinates. These results are consistent with several animal models which indicate that neural activity within the vmPFC facilitates coping with stressful events. Optogenetic stimulation of vmPFC neurons reverses depression-like behavior in mice susceptible to chronic social defeat stress [40]. Brief maternal separation in squirrel monkeys promotes stress inoculation and increases cortical volume in the vmPFC [41]. Also, neural activity in the vmPFC is both necessary and sufficient for the resistance to learned helplessness conferred by prior experience with controllable stress [18, 19, 42]. Within the vmPFC, neural activity in both the IL and PL cortex can promote stress resistance. Pyramidal neurons in the IL cortex send projections to the BLA complex as well as intercalated amygdala cells to facilitate extinction of conditioned fear [43–45]. The IL cortex is also essential for the ability of enriched housing to promote resistance to chronic social defeat [16]. In contrast, pyramidal neurons in the PL cortex sends projections to DRN GABAergic cells which inhibit DRN serotonin activity [46, 47]. Controllable tail shock selectively activates PL neurons projecting to the DRN which can inhibit the DRN activation critical for the development of learned helplessness [48]. One possibility is that maintenance of dominance relationships generates experience-dependent neural plasticity in both IL-BLA and PL-DRN neural circuits to modulate responses to subsequent social stress.
Learned helplessness, environmental enrichment, and social dominance models also differ in the time course of events required for resilience. A single session of controllable tail shock increases intrinsic membrane excitability in PL pyramidal neurons and promotes resistance to learned helplessness [49]. In contrast, a single session of social dominance does not confer resistance to conditioned defeat. The neural plasticity associated with resistance to conditioned defeat in dominants requires two weeks of social encounters. In the environmental enrichment model neural plasticity also develops gradually as increased FosB/ΔFosB immunoreactivity requires three weeks of enriched housing [16]. IL lesions prior to environmental enrichment prevent resistance to chronic social defeat, while IL lesions prior to social defeat do not, suggesting that the IL is responsible for the development but not expression of stress resistance. In contrast, pharmacological inactivation of the vmPFC prior to social defeat or uncontrollable tail shock blocks the formation of conditioned defeat and learned helplessness, respectively [18, 36, 42]. Overall, these differences between models suggest that social and physical experiences generate stress resistance via separate cellular and molecular mechanisms in the PL and IL.
The MeA is a critical neural substrate for detecting biologically relevant odors and a key component of the brain's social behavior network [50, 51]. We have previously found that neural activation in the vMeA is associated with a reduction in conditioned defeat in dominant animals [4]. In the current study, defeat-induced neural activation in the vMeA increased only after two weeks of dominance experience. After 14 days of encounters, dominant individuals display significantly more defeat-induced c-Fos immunoreactivity compared to both 14 day subordinates and 1 day dominants. Because the time course of neural activation in the vMeA closely matches the time course of changes in conditioned defeat, the vMeA may be an important neural substrate controlling resistance to conditioned defeat in dominant individuals. Our findings are consistent with other research showing that the MeA regulates agonistic behavior [52–54]. In Syrian and long-tailed hamsters, aggressive encounters increase c-Fos expression in the MeA in both winners and losers [33, 55, 56]. In rats, social defeat increases c-Fos immunoreactivity in MeA cells that contain corticotropin-releasing factor type-2 receptor mRNA [57]. Also, pharmacological inactivation of the MeA during social defeat decreases the acquisition of the conditioned defeat response [58]. However, the MeA appears to modulate the acquisition of conditioned defeat via connections to other brain regions, such as the basolateral amygdala, because inhibition of protein synthesis in the MeA fails to disrupt the acquisition of conditioned defeat. One possible mechanism by which social dominance might modulate neural signaling in the MeA is via androgen receptors. The MeA contains an abundance of androgen receptors [59], and winning agonistic encounters can increase plasma testosterone levels and androgen receptor expression [60, 61]. Altogether, neural activation in the MeA is associated with both increased and decreased defensive behavior, which is likely modulated by neuropeptides and steroid feedback. Because the MeA contains medium-sized GABAergic principal neurons which colocalize a wide variety of neuropeptides and have a heterogeneous set of efferent projects [62, 63], it will be important to know the phenotype of the vMeA cells activated in dominant animals to better understand the development of conditioned defeat resistance.
Whereas the VMHL has not been associated with resistance to stress, it is a key brain region controlling aggressive behavior in hamsters [64, 65]. We found that dominant hamsters have greater defeat-induced c-Fos immunoreactivity in the VMHL compared to subordinates after both 7 days and 14 days of dominance experience. A proactive coping style during social defeat, such as displayed by dominants, has been associated with increased c-Fos expression in the VHML in studies of hamster aggression. While neural activation in the VHML may be associated with reduced conditioned defeat, it is also possible that neural activation in the VMHL is associated with counter attacking resident aggressors during social defeat training. This possibility is supported by the finding that dominants after 7 days and 14 days of experience are significantly more likely to counter attack resident aggressors than subordinates, a time course that matches VMHL c-Fos expression.
The BLA is a key brain region regulating stress-induced changes in behavior, including conditioned defeat. Several studies have indicated that the acquisition of conditioned defeat requires NMDA receptor activation, protein synthesis, and BDNF signaling [58, 66, 67]. Nevertheless, we found low c-Fos immunoreactivity in the BLA in all animals. Interestingly, we found that c-Fos immunoreactivity in the BLA decreased in subordinates during 14 days of social encounters such that on day 1 subordinates had greater defeat-induced neural activation than dominants, whereas on day 14 they had less. These findings suggest that defeat-induced c-Fos expression in the BLA habituates in subordinates and is inversely correlated with their conditioned defeat response after 14 days of social encounters. Similarly, other research indicates that neural activation in the BLA habituates after chronic social defeat [68], and interestingly behavioral and neuroendocrine habituation to repeated restraint stress depends on β-adrenergic receptor activity in the BLA [69]. Furthermore, social anxiety disorder is associated with increased neural habituation in the amygdala [70].
4.3 Conclusion
We have demonstrated that social status-associated resistance to conditioned defeat develops over a two-week period. Further, the time course for reduced conditioned defeat parallels the time course for defeat-induced neural activation in the IL, PL, and vMeA. These findings suggest that defeat-induced neural activity in these brain regions is experience-dependent and facilitates coping with social stress. Identifying the neural substrates that contribute to reduced conditioned defeat in dominant hamsters should help elucidate a neural circuitry controlling experience-dependent forms of resilience.
Highlights.
Hamsters show reduced conditioned defeat after 14 days of dominance experience.
14 days of dominance experience increases c-Fos expression in the IL, PL, and vMeA.
Development of reduced conditioned defeat parallels increased neural activation.
Resistance to social defeat stress requires experience-dependent neural plasticity.
Acknowledgements
We thank our team of undergraduates, most notably Jordan Lakin, Colleen McLaughlin, Ellen Ford, Cody Burleson and Anna Nelson. This work was supported by a National Institutes of Health grant R21 MH098190 and a University of Tennessee Professional Development Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Huhman KL. Social conflict models: can they inform us about human psychopathology? Horm Behav. 2006;50:640–6. doi: 10.1016/j.yhbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- [2].Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–9. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Morrison KE, Swallows CL, Cooper MA. Effects of dominance status on conditioned defeat and expression of 5-HT1A and 5-HT2A receptors. Physiology and Behavior. 2011;104:283–90. doi: 10.1016/j.physbeh.2011.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Morrison KE, Curry DW, Cooper MA. Social status alters defeat-induced neural activation in Syrian hamsters. Neuroscience. 2012;210:168–78. doi: 10.1016/j.neuroscience.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–39. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- [6].Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–41. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- [7].Risbrough VB, Stein MB. Role of corticotropin releasing factor in anxiety disorders: a translational research perspective. Horm Behav. 2006;50:550–61. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vermetten E, Bremner JD. Circuits and systems in stress. II. Applications to neurobiology and treatment in posttraumatic stress disorder. Depress Anxiety. 2002;16:14–38. doi: 10.1002/da.10017. [DOI] [PubMed] [Google Scholar]
- [9].Yehuda R. Post-traumatic stress disorder. N Engl J Med. 2002;346:108–14. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- [10].Agaibi CE, Wilson JP. Trauma, PTSD, and resilience: a review of the literature. Trauma Violence Abuse. 2005;6:195–216. doi: 10.1177/1524838005277438. [DOI] [PubMed] [Google Scholar]
- [11].Sarapas C, Cai G, Bierer LM, Golier JA, Galea S, Ising M, et al. Genetic markers for PTSD risk and resilience among survivors of the World Trade Center attacks. Dis Markers. 2011;30:101–10. doi: 10.3233/DMA-2011-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- [13].Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–84. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- [14].Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29:7330–40. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Radley JJ, Sawchenko PE. A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J Neurosci. 2011;31:9683–95. doi: 10.1523/JNEUROSCI.6040-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lehmann ML, Herkenham M. Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. J Neurosci. 2011;31:6159–73. doi: 10.1523/JNEUROSCI.0577-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–71. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- [18].Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26:13264–72. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Amat J, Paul E, Watkins LR, Maier SF. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience. 2008;154:1178–86. doi: 10.1016/j.neuroscience.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Walker FR, Masters LM, Dielenberg RA, Day TA. Coping with defeat: acute glucocorticoid and forebrain responses to social defeat vary with defeat episode behaviour. Neuroscience. 2009;162:244–53. doi: 10.1016/j.neuroscience.2009.04.041. [DOI] [PubMed] [Google Scholar]
- [21].Frank E, Salchner P, Aldag JM, Salome N, Singewald N, Landgraf R, et al. Genetic predisposition to anxiety-related behavior determines coping style, neuroendocrine responses, and neuronal activation during social defeat. Behav Neurosci. 2006;120:60–71. doi: 10.1037/0735-7044.120.1.60. [DOI] [PubMed] [Google Scholar]
- [22].Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, et al. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–98. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, et al. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res. 2011;217:354–62. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Greenwood BN, Strong PV, Foley TE, Fleshner M. A behavioral analysis of the impact of voluntary physical activity on hippocampus-dependent contextual conditioning. Hippocampus. 2009;19:988–1001. doi: 10.1002/hipo.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Greenwood BN, Foley TE, Day HE, Burhans D, Brooks L, Campeau S, et al. Wheel running alters serotonin (5-HT) transporter, 5-HT1A, 5-HT1B, and alpha 1b-adrenergic receptor mRNA in the rat raphe nuclei. Biol Psychiatry. 2005;57:559–68. doi: 10.1016/j.biopsych.2004.11.025. [DOI] [PubMed] [Google Scholar]
- [26].Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- [27].Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–52. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Korzan WJ, Summers CH. Behavioral diversity and neurochemical plasticity: selection of stress coping strategies that define social status. Brain Behav Evol. 2007;70:257–66. doi: 10.1159/000105489. [DOI] [PubMed] [Google Scholar]
- [29].Ling TJ, Forster GL, Watt MJ, Korzan WJ, Renner KJ, Summers CH. Social status differentiates rapid neuroendocrine responses to restraint stress. Physiol Behav. 2009;96:218–32. doi: 10.1016/j.physbeh.2008.10.004. [DOI] [PubMed] [Google Scholar]
- [30].McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Serotonin receptor binding in a colony model of chronic social stress. Biol Psychiatry. 1995;37:383–93. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- [31].Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, et al. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- [32].Honess PE, Marin CM. Behavioural and physiological aspects of stress and aggression in nonhuman primates. Neurosci Biobehav Rev. 2006;30:390–412. doi: 10.1016/j.neubiorev.2005.04.003. [DOI] [PubMed] [Google Scholar]
- [33].Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: defeat activates specific neurocircuits within the brain. J Neurosci. 1997;17:8842–55. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Greenwood BN, Foley TE, Burhans D, Maier SF, Fleshner M. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Res. 2005;1033:164–78. doi: 10.1016/j.brainres.2004.11.037. [DOI] [PubMed] [Google Scholar]
- [35].Maier SF, Watkins LR. Role of the medial prefrontal cortex in coping and resilience. Brain Res. 2010;1355:52–60. doi: 10.1016/j.brainres.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Morrison KE, Bader LR, McLaughlin CN, Cooper MA. Defeat-induced activation of the ventral medial prefrontal cortex is necessary for resistance to conditioned defeat. Behav Brain Res. 2013;243:158–64. doi: 10.1016/j.bbr.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Koolhaas JM, de Boer SF, Coppens CM, Buwalda B. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol. 2010;31:307–21. doi: 10.1016/j.yfrne.2010.04.001. [DOI] [PubMed] [Google Scholar]
- [38].Veenema AH, Koolhaas JM, de Kloet ER. Basal and stress-induced differences in HPA axis, 5-HT responsiveness, and hippocampal cell proliferation in two mouse lines. Ann N Y Acad Sci. 2004;1018:255–65. doi: 10.1196/annals.1296.030. [DOI] [PubMed] [Google Scholar]
- [39].Benus RF, Bohus B, Koolhaas JM, Vanoortmerssen GA. Behavioral Strategies of Aggressive and Nonaggressive Male-Mice in Active Shock Avoidance. Behavioural Processes. 1989;20:1–12. doi: 10.1016/0376-6357(89)90008-9. [DOI] [PubMed] [Google Scholar]
- [40].Covington HE, 3rd, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 2010;30:16082–90. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Katz M, Liu C, Schaer M, Parker KJ, Ottet MC, Epps A, et al. Prefrontal plasticity and stress inoculation-induced resilience. Dev Neurosci. 2009;31:293–9. doi: 10.1159/000216540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Christianson JP, Thompson BM, Watkins LR, Maier SF. Medial prefrontal cortical activation modulates the impact of controllable and uncontrollable stressor exposure on a social exploration test of anxiety in the rat. Stress. 2009;12:445–50. doi: 10.1080/10253890802510302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–64. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Amir A, Amano T, Pare D. Physiological identification and infralimbic responsiveness of rat intercalated amygdala neurons. J Neurophysiol. 2011;105:3054–66. doi: 10.1152/jn.00136.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–38. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Varga V, Szekely AD, Csillag A, Sharp T, Hajos M. Evidence for a role of GABA interneurones in the cortical modulation of midbrain 5-hydroxytryptamine neurones. Neuroscience. 2001;106:783–92. doi: 10.1016/s0306-4522(01)00294-9. [DOI] [PubMed] [Google Scholar]
- [47].Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- [48].Baratta MV, Zarza CM, Gomez DM, Campeau S, Watkins LR, Maier SF. Selective activation of dorsal raphe nucleus-projecting neurons in the ventral medial prefrontal cortex by controllable stress. European Journal of Neuroscience. 2009;30:1111–6. doi: 10.1111/j.1460-9568.2009.06867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Varela JA, Wang J, Christianson JP, Maier SF, Cooper DC. Control over stress, but not stress per se increases prefrontal cortical pyramidal neuron excitability. J Neurosci. 2012;32:12848–53. doi: 10.1523/JNEUROSCI.2669-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–57. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- [51].Goodson JL, Kabelik D. Dynamic limbic networks and social diversity in vertebrates: from neural context to neuromodulatory patterning. Frontiers in Neuroendocrinology. 2009;30:429–41. doi: 10.1016/j.yfrne.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wang Y, He Z, Zhao C, Li L. Medial amygdala lesions modify aggressive behavior and immediate early gene expression in oxytocin and vasopressin neurons during intermale exposure. Behav Brain Res. 2013;245:42–9. doi: 10.1016/j.bbr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- [53].Rosvall KA, Bergeon Burns CM, Barske J, Goodson JL, Schlinger BA, Sengelaub DR, et al. Neural sensitivity to sex steroids predicts individual differences in aggression: implications for behavioural evolution. Proc Biol Sci. 2012;279:3547–55. doi: 10.1098/rspb.2012.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cheng SY, Taravosh-Lahn K, Delville Y. Neural circuitry of play fighting in golden hamsters. Neuroscience. 2008;156:247–56. doi: 10.1016/j.neuroscience.2008.07.048. [DOI] [PubMed] [Google Scholar]
- [55].Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66:721–36. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- [56].Pan Y, Xu L, Young KA, Wang Z, Zhang Z. Agonistic encounters and brain activation in dominant and subordinate male greater long-tailed hamsters. Horm Behav. 2010;58:478–84. doi: 10.1016/j.yhbeh.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Fekete EM, Zhao Y, Li C, Sabino V, Vale WW, Zorrilla EP. Social defeat stress activates medial amygdala cells that express type 2 corticotropin-releasing factor receptor mRNA. Neuroscience. 2009;162:5–13. doi: 10.1016/j.neuroscience.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Markham CM, Huhman KL. Is the medial amygdala part of the neural circuit modulating conditioned defeat in Syrian hamsters? Learn Mem. 2008;15:6–12. doi: 10.1101/lm.768208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Blake CB, Meredith M. Change in number and activation of androgen receptor-immunoreactive cells in the medial amygdala in response to chemosensory input. Neuroscience. 2011;190:228–38. doi: 10.1016/j.neuroscience.2011.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Fuxjager MJ, Forbes-Lorman RM, Coss DJ, Auger CJ, Auger AP, Marler CA. Winning territorial disputes selectively enhances androgen sensitivity in neural pathways related to motivation and social aggression. Proc Natl Acad Sci USA. 2010;107:12393–8. doi: 10.1073/pnas.1001394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Fuxjager MJ, Oyegbile TO, Marler CA. Independent and additive contributions of postvictory testosterone and social experience to the development of the winner effect. Endocrinology. 2011;152:3422–9. doi: 10.1210/en.2011-1099. [DOI] [PubMed] [Google Scholar]
- [62].McDonald AJ. Is there an amygdala and how far does it extend? An anatomical perspective. Ann N Y Acad Sci. 2003;985:1–21. doi: 10.1111/j.1749-6632.2003.tb07067.x. [DOI] [PubMed] [Google Scholar]
- [63].Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1995;360:213–45. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- [64].Delville Y, Mansour KM, Ferris CF. Serotonin blocks vasopressin-facilitated offensive aggression: interactions within the ventrolateral hypothalamus of golden hamsters. Physiol Behav. 1996;59:813–6. doi: 10.1016/0031-9384(95)02166-3. [DOI] [PubMed] [Google Scholar]
- [65].Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamster. Brain Behav Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- [66].Day DE, Cooper MA, Markham CM, Huhman KL. NR2B subunit of the NMDA receptor in the basolateral amygdala is necessary for the acquisition of conditioned defeat in Syrian hamsters. Behavioral Brain Research. 2011;217:55–9. doi: 10.1016/j.bbr.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Taylor SL, Stanek LM, Ressler KJ, Huhman KL. Differential brain-derived neurotrophic factor expression in limbic brain regions following social defeat or territorial aggression. Behav Neurosci. 2011;125:911–20. doi: 10.1037/a0026172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kollack-Walker S, Don C, Watson SJ, Akil H. Differential expression of c-fos mRNA within neurocircuits of male hamsters exposed to acute or chronic defeat. J Neuroendocrinol. 1999;11:547–59. doi: 10.1046/j.1365-2826.1999.00354.x. [DOI] [PubMed] [Google Scholar]
- [69].Grissom NM, Bhatnagar S. The basolateral amygdala regulates adaptation to stress via beta-adrenergic receptor-mediated reductions in phosphorylated extracellular signal-regulated kinase. Neuroscience. 2011;178:108–22. doi: 10.1016/j.neuroscience.2010.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sladky R, Hoflich A, Atanelov J, Kraus C, Baldinger P, Moser E, et al. Increased neural habituation in the amygdala and orbitofrontal cortex in social anxiety disorder revealed by FMRI. PLoS One. 2012;7:e50050. doi: 10.1371/journal.pone.0050050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Morin LP, Wood RI. A stereotaxic atlas of the golden hamster brain. Academic Press; New York: 2001. [Google Scholar]