Abstract
Insulin resistance and other features of the metabolic syndrome are increasingly recognized for their effects on cognitive health. To ascertain mechanisms by which this occurs, we fed mice a very high fat diet (60% kcal by fat) for 17 days or a moderate high fat diet (HFD, 45% kcal by fat) for 8 weeks and examined changes in brain insulin signaling responses, hippocampal synaptodendritic protein expression, and spatial working memory. Compared to normal control diet mice, cerebral cortex tissues of HFD mice were insulin-resistant as evidenced by failed activation of Akt, S6 and GSK3β with ex-vivo insulin stimulation. Importantly, we found that expression of brain IPMK, which is necessary for mTOR/Akt signaling, remained decreased in HFD mice upon activation of AMPK. HFD mouse hippocampus exhibited increased expression of serine-phosphorylated insulin receptor substrate 1 (IRS1-pS616), a marker of insulin resistance, as well as decreased expression of PSD-95, a scaffolding protein enriched in post-synaptic densities, and synaptopodin, an actin-associated protein enriched in spine apparatuses. Spatial working memory was impaired as assessed by decreased spontaneous alternation in a T-maze. These findings indicate that HFD is associated with telencephalic insulin resistance and deleterious effects on synaptic integrity and cognitive behaviors.
Introduction
“Metabolic syndrome” or “insulin resistance syndrome,” are terms for a still evolving disorder that clusters insulin resistance, impaired glucose regulation, dyslipidemia, abdominal obesity, and hypertension with risk for cardiovascular disease and type II diabetes (Kassi et al., 2011; Reaven, 2011) There is growing recognition that insulin resistance, type II diabetes and other features of the metabolic syndrome are also associated with brain disorders including Alzheimer’s disease and other neurodegenerative dementias (Baker et al.; Biessels et al., 2006; Correia et al., 2011; de la Monte, 2009; Haan, 2006; Neumann et al., 2008; Profenno et al.; Zhao and Townsend, 2009) depression (Amato et al., 1996; Knol et al., 2006; Okamura et al., 2000; Pan et al.; Pouwer et al., 2003; Weber-Hamann et al., 2006), and possibly schizophrenia, bipolar and anxiety disorders (Heppner et al., 2009; McEvoy et al., 2005; McIntyre et al., 2010).
The nature of these associations is complex. In Alzheimer’s disease (AD), research has described on the one hand that people with clinical AD, apolipoprotein E ε4 genotype and/or amyloid-β biomarkers exhibit abnormal insulin levels and insulin metabolism, while on the other hand, a history of type 2 diabetes and even normoglycemic insulin resistance increases risk for subsequent AD (Baker et al.; Biessels et al., 2006; Correia et al., 2011; de la Monte, 2009; Haan, 2006; Neumann et al., 2008; Profenno et al.; Schrijvers et al.; Zhao and Townsend, 2009). We recently reported direct evidence of brain insulin resistance in humans with mild cognitive impairment and AD as well as extensive abnormalities in the activation status of numerous constituents of the insulin – insulin receptor substrate-1 (IRS-1) – PI3k – Akt – mTOR and GSK3 pathways (Talbot et al., 2012). Such studies have stimulated interest in a role for insulin and other anti-diabetes medications for treatment of AD.
Reciprocal links between T2D and psychiatric disorders have also been described. For instance, depression increases risk for subsequent development of T2D (Knol et al., 2006) while the presence of T2D increases risk for incident depression. However, understanding of metabolic syndrome’s association with psychiatric disorders is more complicated than with AD, as these are frequently confounded by poor health status and medications, especially atypical antipsychotics which in themselves induce obesity and the metabolic syndrome (De Hert et al., 2012).
Insulin is known to have important effects on neurotransmission (Mielke and Wang, 2011). Insulin receptors are highly enriched in synaptosomes (Werther et al., 1989), have been co-localized with axon terminal markers synaptophysin and synapsin 1 (Mielke et al., 2006) and are found in post-synaptic density (PSD) fractions where they may interact with scaffolding proteins shank and PSD-95 via the insulin receptor tyrosine kinase substrate IRSp53 (Abbott et al., 1999; Bockmann et al., 2002). Insulin enhances neurite outgrowth, modulates catecholamine release and uptake, regulates trafficking of ligand-gated ion channels, regulates expression and localization of GABA, NMDA, and AMPA receptors, modulates activity dependent synaptic plasticity via NMDA and PI3K-Akt (van der Heide et al., 2005), and plays a critical role in the development and maintenance of excitatory synapses (Chiu et al., 2008).
There have been relatively few attempts to investigate how genetic or diet-induced insulin resistance affects cognition-related circuitry and neurotransmission in animal models. Furthermore, while alterations in neurotransmitter release and receptor expression, receptor trafficking and metabolism, long-term potentiation, hippocampal neurogenesis, and behavior have been described in animal models of diet-induced T2D, many findings are inconsistent or unreplicated (Boitard et al., 2012; Heyward et al., 2012; Hwang et al., 2010; Morrison et al., 2010; Pistell et al., 2010; Porter et al., 2012a; Porter et al., 2010; Tucker et al., 2012; Valladolid-Acebes et al., 2011; Yamada-Goto et al., 2012). The high fat diet (HFD) is a well-established approach to induce insulin resistance in peripheral organs and hypothalamus (Metlakunta et al., 2008; Obrosova et al., 2007; Winzell and Ahren, 2004). Many mechanisms and consequences of its effects are known in these systems. Here we demonstrate that HFD is associated with insulin resistance in the cerebral cortex of mice and we further elucidate HFD effects on critical insulin signaling pathway components with a particular focus on IPMK-mTOR/Akt, synaptodendritic molecular neuroanatomy and spatial working memory as potential mechanisms through which insulin resistance and the metabolic syndrome may lead to cognitive impairments.
Materials and methods
Animals and diets
Young adult C57BL/6J male mice (Jackson Laboratories, Bar Harbor, ME) were purchased at 8 weeks age and housed 5/cage under standard conditions in a temperature- and humidity-controlled facility with a 12-hour light-dark cycle (lights on at 07:00) at the University of Pennsylvania Translational Research Laboratories (Philadelphia, PA). They were acclimated to the environment for one week with standard laboratory pellet food and water freely available prior to assignment to HFD or control diet conditions. All experimental procedures were conducted in accordance with the guidelines published in the NIH Guide for Care and Use of Laboratory Animals and approved by the of Pennsylvania Institutional Animal Care and Use Committee.
Two cohorts were studied: 1) an extreme high fat diet (“HFD”, 60% kcal from fat, (D12492, Research Diets, Inc, New Brunswick, NJ) or the normal control diet (“NCD”), 10% kcal fat diet, D12450B,) for 17 days (n=5/group); and 2) a moderate HFD 45% kcal fat diet, D12451, Research Diets, Inc) or the NCD for 8 weeks (n=20/group).
In the very HFD cohort, mice were weighed and blood glucose levels were measured at 17 days, prior to sacrifice. In the moderate HFD cohort, weights were monitored weekly and glucose levels were monitored biweekly between 08:00 and 10:00 using a hand-held glucometer (One-Touch Ultra 2, Lifescan, Inc., Johnson & Johnson) with blood obtained by tail snip. At 8 weeks, 10 mice from each HFD and NCD condition underwent behavioral testing. Four days after these behavioral tests, all mice had a glucose tolerance test in which mice were administered glucose (1 g/kg, i.p.) after baseline measurement followed by blood glucose measurements at 20, 60, 90 and 120 minutes. Mice were killed two days later and brains removed and prepared for fresh and frozen tissue biochemistry and fixation and paraffinization for immunohistochemistry.
Ex Vivo Insulin Stimulation and Western Blotting
Fresh frontal lobes from mice were cut at 0.4 mm intervals in sagittal and coronal planes using a McIlwain tissue chopper. The slices were dispersed in oxygenated artificial cerebrospinal fluid buffer (ACSF, 113mM NaCl, 4.5mM KCl, 1mM MgCl2, 25mM NaHCO3, 1mM NaH2PO4, 25mM Glucose, 2mM CaCl2) and then incubated with 10 nM insulin (Sigma, St. Louis, MO) for 10 min and washed with ice cold ACSF. Tissues were lysed in lysis buffer and subjected to SDS-PAGE and immunoblot, as previously described (Bang et al., 2012a; Chakraborty et al., 2010; Chen et al., 2013; Kim et al., 2011; Kim et al., 2007). Commercial phospho-specific antibodies for stimulated proteins of interest included Akt, GSK3β, S6 and Foxo1, with GAPDH as a loading control (all antibodies were from Cell Signaling, Danvers, MA). Immunoblotting was quantified using the ImageJ Software (Schneider et al., 2012) and data were expressed as a phospho-protein to total protein ratio.
Cells
Murine embryonic fibroblasts cells (MEFs) were prepared as described previously (Bang et al., 2012a) for use with AICAR (5-amino-1-β-D-ribofuranosyl-imidazole-4-carboxamide, Sigma) drug treatment as an AMPK activator. MEFs were grown in a humid atmosphere of 95% air and 5% CO2 at 37°C in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, L-glutamine (300ug/ml), penicillin (100 U/ml), and streptomycin (100 μg/ml). PC12 cells were obtained from the American Type Culture Collection (Manassas, VA) and used for insulin stimulation and transfection experiments. PC12 cells were maintained in in a humid atmosphere of 95% air and 5% CO2 at 37°C in Dulbecco’s modified Eagle’s medium supplemented with 5% horse serum, 5% calf serum, L-glutamine (300ug/ml), penicillin (100 U/ml), and streptomycin (100 μg/ml). One day after plating cells, PC12 cells were differentiated in Dulbecco’s modified Eagle’s medium supplemented with 1% horse serum, 1% Pen-Strep and 100ng / mL neuronal growth factor (NGF).
Overexpression of Plasmids into PC12 Cells
PC12 cells were plated at a density of 5 × 105 cells per well in six-well plates. Two or three days after plating, cells were transfected with Lipofectamine 2000 (Life Technologies, Grand Island, NY) according to the manufacture’s instruction using plasmids for AMPKα2, IPMK-myc, as previously described (Bang et al., 2012b)
Insulin Stimulation for Differentiated PC12 cells
To minimize the impact of differences in growth rates, cells were growth-arrested by overnight serum-starvation two days after transfection. Cells were then stimulated for various times with 100 nM insulin. Treatment was stopped by washing with ice-cold PBS and then flash-freezing the plates in liquid nitrogen.
Immunohistochemistry and Image Analysis
Fixed, paraffin-embedded tissues were cut in the coronal plane at 6μm on a rotary microtome that had been tested to assure invariant section thickness and then mounted on APES-coated slides. Dewaxed sections were immersed in 5% hydrogen peroxide dissolved in absolute methanol for 30 min to quench endogenous peroxidase activity. For antigen retrieval, the sections were boiled in 1 mmol/L ethylenediaminetetraacetic acid in 0.1 mol/L Tris buffer, pH 8.0, for 10 min. After cooling for 20 min and rinsing in water, followed by two changes of Tris-Triton (0.01% Triton X-100 in 0.1 mol/L Tris-HCl buffer, pH 7.6), sections were blocked for 45 min in 2% horse serum dissolved in Tris-Triton and incubated in the primary antibody. Antibodies for immunohistochemistry were directed at serine(S616)-phosphorylated IRS-1 (#44550G, 1:300; Invitrogen/Life Technologies, Inc, Grand Island, NY) and PSD-95 (#MAB1598, 1:200; Chemicon/Millipore, Billerica, MA), overnight at 4°C. After Tris-Triton rinses, sections were incubated in a biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) for an hour at room temperature. Sections were then treated for another hour at room temperature with an avidin-biotin-peroxidase complex made from a Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) and developed for 10 min in a solution containing 0.05% diaminobenzidine (Biogenex, San Ramon, CA) and 0.03% hydrogen peroxidase in Tris-Triton, supplemented with 0.25% NiSO4.H2O to amplify the immunohistochemical signal. After clearing in xylenes, tissue sections were coverslipped under Cytoseal 60 (Richard-Allan Scientific, Kalamazoo, MI). For double-immunofluorescence labeling, sections were co-incubated in antibodies against IRS1-pS616 (#44550G, 1:300, Invitrogen,) with secondary antibody Alexa Fluor 594 donkey anti-rabbit IgG (A21207, 1:500; Invitrogen, Grand Island, NY) and spinophilin (#14773, 1:500; Santa Cruz Biotechnology, Inc., Dallas, TX) with secondary antibody Alexa Fluor 488 donkey anti-mouse (A21202, 1:500, Invitrogen).
Immunolabeled slides were qualitatively examined and immunoreactivity was semi-quantitatively measured by net optical density (OD) in the moderate HFD cohort (n=10 each group) in the stratum lucidum of the CA3 region of the dorsal hippocampal formation at cytoarchitecturally similar rostral-caudal levels. Net OD was defined as the OD of the region of interest minus the OD of non-staining tissue background adjacent to the region of interest. OD analyses were performed on high resolution, gray-scale photoimages acquired under uniform light conditions and exposure times on an Olympus BX61 microscope (Olympus America, Inc, Center Valley, PA) with Retiga SRV deep-cooled digital CCD camera (QImaging, Surrey, BC, Canada) and Olympus DP software. OD was measured with ImageJ (Schneider et al., 2012). The operator was masked to identifying information throughout data accrual and analysis.
Behavioral Testing
Hippocampus-based short-term spatial memory was assessed using a spontaneous alternation paradigm in a T-maze (Dudchenko, 2004; Hughes, 2004). Spontaneous alternation measures an animal’s ability to remember which arm in a T-maze that it had previously entered and thus explore alternating arms of the maze in repeated trials. Moderate HFD mice and NCD control mice (n=10 per group) were habituated to the apparatus on the first day and tested on the subsequent day. Each mouse was placed in the start arm of the T-maze and allowed to select an arm to enter. Once the mouse chose an arm, it was confined to the arm for 30 seconds before it was returned to the start arm, and confined there for 5 seconds. The mouse was then allowed to choose an arm again. The mouse received a score of 0 if it chose the same arm, and a score of 1 if it alternated arms and the latency for making the choice was recorded. Data was accrued for 3 sets of 6 choice trials for a total of 18 trials per animal.
Data Analysis
Between group differences in the moderate HFD vs. NCD mouse cohort were assessed using repeated measures ANOVA for longitudinal weights, glucose levels and glucose tolerance tests. Student’s t-tests were used for Immunoblotting, immunohistochemical and behavioral data. All tests were two-tailed and statistical significance was defined as p<0.05. Statistical analyses were conducted with JMP9.0.0 (SAS, Cary, NC).
Results
Weight gain and hyperglycemia
Young adult mice were fed one of two HFDs: 1) an extreme HFD consisting of 60% kcal from fat for 17 days or a normal control diet (“NCD”) consisting of 10% kcal from fat (n=5 per group). This extreme HFD is known to induce systemic hyperglycemia and hyperinsulinemia within one week which persists indefinitely (Winzell and Ahren, 2004); and 2) a “moderate” HFD consisting of 45% kcal from fat vs. NCD for 8 weeks (n=20 per group). In the extreme HFD cohort, mean body weights prior to sacrifice at 17 days differed significantly (HFD 31.2 g [SD=1.8], NCD 26.3 [1.9], t=4.2, p=0.003) as did morning glucose levels (HFD 210.4 mg/dl [SD=9.3], NCD 167.2 [11.5], t=6.5, p=0.0002). In the moderate HFD cohort, weights and morning glucose levels also increased uniformly and significantly and glucose tolerance tests were markedly abnormal, indicating diabetes (see Supplement Figure S1).
High fat diet induces insulin resistance in frontal cortex and hippocampus
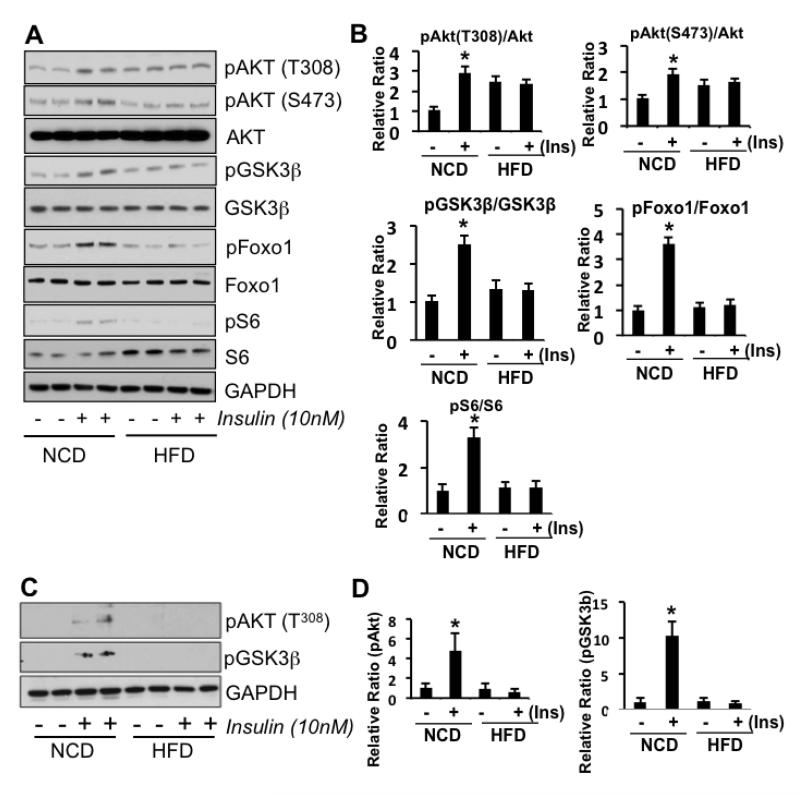
We used an ex vivo stimulation paradigm in fresh or frozen frontal cortex mouse tissue to test the baseline phosphorylation status and activation responses of key constituents of the insulin-PI3k-Akt-mTOR and GSK3 intracellular signaling pathways after stimulation of tissue with 10nM insulin. As portrayed in Figure 1A and B, we found that the extreme HFD induced a basal state of marginal hyperphosphorylation of Akt, but most remarkably, no activation of Akt with insulin stimulation nor its downstream targets FoxO1, GSK3β, and S6 compared to NCD mice. In the moderate HFD cohort tissues (Figure C and D), we found that basal phosphorylation states of Akt and GSK3β did not differ from NCD animals. However, as in tissues from the extreme HFD animals, these molecules failed to activate with insulin stimulation, while their NCD counterparts did. We also examined the effects of insulin with hippocampal slices and found the same patterns of responses; Akt activation in NCD mice but a lack of response in tissues from HFD mice (Supplement Figure S2). These data demonstrate frontal cortex and hippocampal insulin resistance from HFD.
Figure 1.
Representative Western blots of frontal brain tissue from HFD or NCD-fed mice with (+) or without (-) ex vivo insulin stimulation. Mice (n=5-10) were fed with either a 60% HFD for 17 days or 45% HFD for 8 weeks. A) Representative blots from pooled brain tissues of 60% HFD or NCD mice show that insulin stimulation in HFD mouse brain tissue fails to activate IRS-1, Akt, GSK3β, S6 (downstream of mTOR) and Foxo1 (a direct downstream target of Akt). GAPDH served as a loading control. B) Western blot quantitation in 60% HFD vs NCD mice. C) Representative blots from tissue samples from 45% HFD or NCD mice shows HFD mouse brain failure to activate AKT and GSK3 with insulin stimulation. D) Western blot quantitation in 45% HFD vs NCD mice. Student’s t-tests were performed (* p < 0.05).
High fat diet leads to decreased IPMK levels
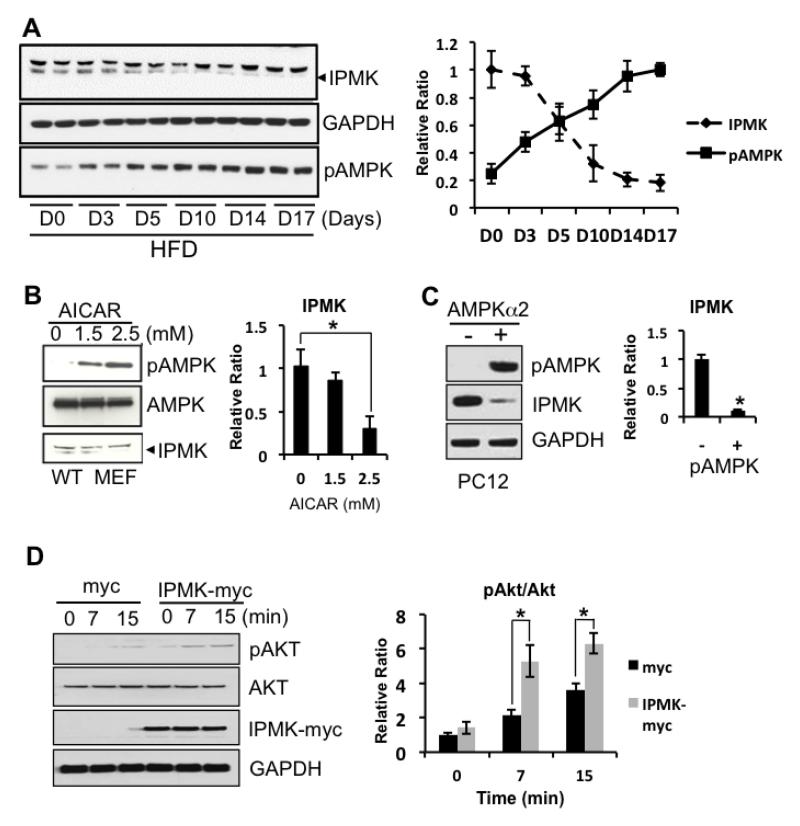
To investigate the mechanism by which insulin resistance may occur in the brain with HFD, we examined the expression levels of proteins that are involved in energy sensing as well as cofactors for Akt/mTOR pathways. In particular, we previously reported that IPMK is an essential protein for activation of the Akt/mTOR pathway as a genetic deletion of IPMK abolished either insulin or nutrients-mediated Akt/mTOR signaling (Bang et al., 2012a; Kim et al., 2011). Here, we found that IPMK expression levels in cortex decrease as phosphorylated AMPK (pAMPK) levels increase with HFD (Figure 2). Next, we used pharmacologic and genetic manipulation in cell-based systems to directly increase activity of AMPK and measure its effects on IPMK. First, we treated MEFs with AICAR, a pharmacological activator of AMPK for 3 hours under which AICAR is known to activate AMPK. We confirmed that IPMK expression levels decrease with AICAR treatment indicating AMPK activities and IPMK expression levels are inversely related (Fig. 2B). Next, we overexpressed AMPKα2, the catalytic subunit of AMPK in differentiated PC12 cells and examined the levels of IPMK expression. We observed that AMPK activity was increased by detecting increased pAMPKα2 while the level of IPMK level was decreased (Fig 2C). Finally, we overexpressed IPMK in differentiated PC12 cells and examined insulin responses. We found that increased IPMK expression enhanced insulin-mediated activation of Akt (Fig 2D). In sum, our data indicate that activation of AMPK in the brain leads to reduction in IPMK expression and this may, at least in part, explain how Akt/mTOR signaling is reduced in the ex vivo stimulation condition.
Figure 2.
A potential interaction between IPMK and pAMPK. A) Mice fed a 60% HFD for 17 days show a gradual decrease in IPMK while the levels of pAMPK increase (means + SEM). B-C) When pAMPK is induced either by overexpression of catalytic subunit of AMPK in differentiated PC12 cells or by a pharmacological reagent, IPMK levels are decreased. D) Differentiated PC12 cells overexpressed with either myc or IPMK-myc were treated with 100nM insulin for various time points. Overexpression of IPMK further sensitizes insulin-mediated-activation of pAkt. (* p < 0.05)
High fat diet is associated with increased neuritic serine-phosphorylated IRS1 and decreased expression of post-synaptic protein markers
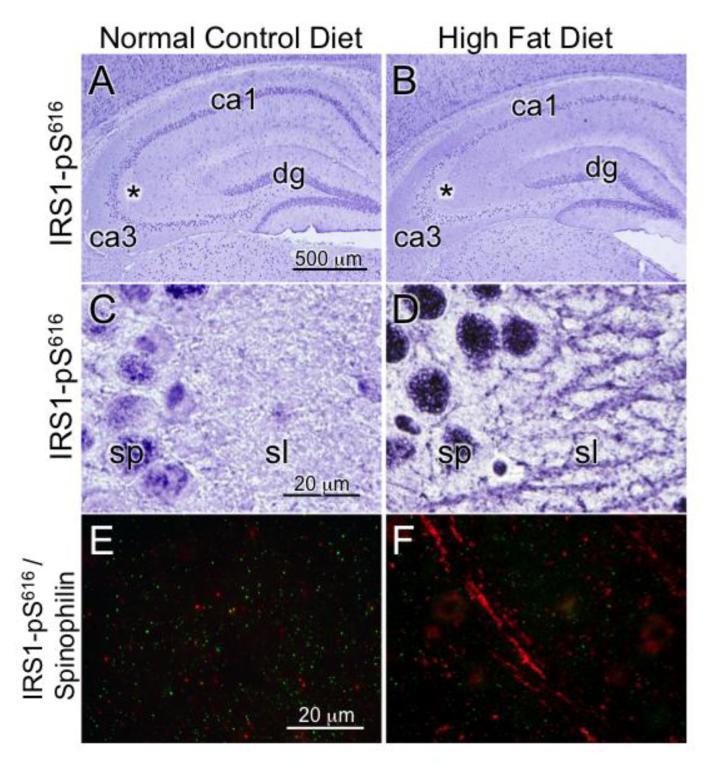
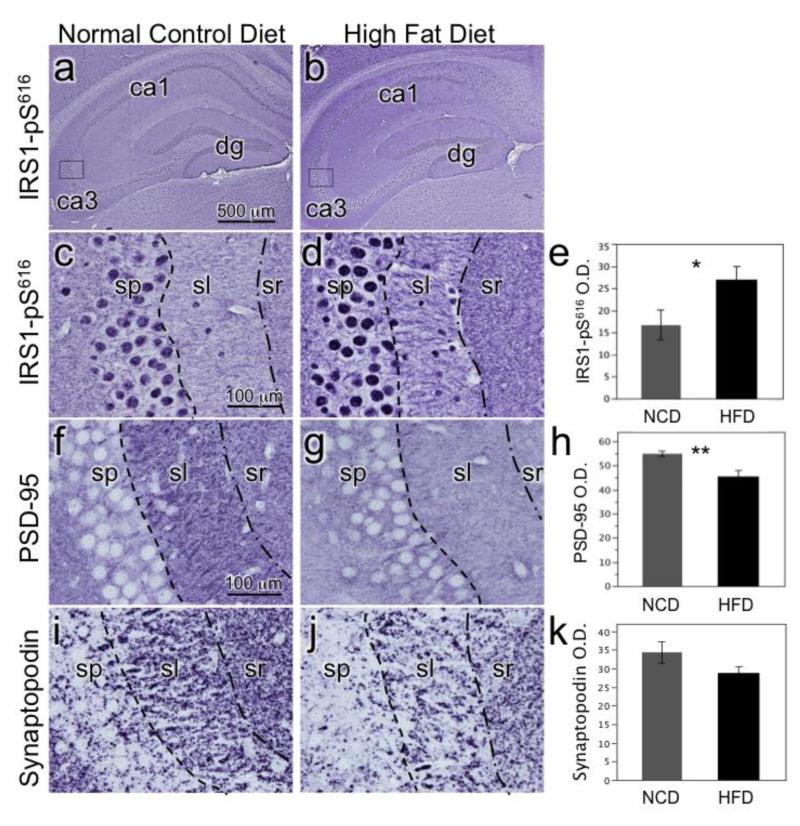
We previously reported marked increases in the expression of serine phosphorylated IRS1 (IRS1-pS312, pS616, pS636/9) in the insulin-resistant hippocampus of humans with mild cognitive impairment and AD (Talbot et al., 2012). In that and subsequent experiments, we found that IRS1-pS616 immunohistochemistry was a particularly robust indicator of abnormal neuronal cytoplasm and neurites in mild cognitive impairment and AD. Here, in both the extreme HFD (60%, Figure 3) and moderate HFD (45%, Figure 4) cohorts we found that IRS1-pS616 expression was increased in the hippocampus in HFD compared to NCD mice. Differences were especially evident in the CA3 subfield, with strong IRS1-pS616 decoration of dendrites in the stratum lucidum mossy fiber terminal zone in HFD mice. Optical density measurement of stratum lucidum in the moderate HFD cohort showed significantly increased IRS1-pS616 values in the HFD compared to NCD mice (t=2.22, p<0.04).
Figure 3.
HFD effects on hippocampal CA3 molecular neuroanatomy. Photomicorgraphs of hippocampus) from mice fed a 60% fat calorie HFD or NCD for 17 days. A) Low magnification image of hippocampus labeled for serine phosphorylated IRS1-pS616 with asterisk indicating location in CA3 where higher power image in C was captured. B) Low magnification image of HFD mouse with asterisk for image in D. C) CA3 stratum pyramidale (sp) immunolabeled IRS1-pS616 shows normal nuclear labeling and very little cytoplasmic membrane or neurite labeling in stratum lucidum (sl = mossy fiber terminal zone). D) HFD mouse CA3 shows increase in overall immunolabeling with abnormal neuritic labeling. E) NCD CA3 stratum lucidum with pseudocolored double immunofluorescence labeling for pS-IRS1 (red) and spinophilin (green) at high magnification. Spinophilin is a regulatory subunit of protein phosphatase-1 catalytic subunit that is highly enriched in dendritic spines. Note the virtual absence of pS-IRS1 labeling of this dendrite and spine-populated region. F) HFD mouse double immunolabeling shows increased pS-IRS1 dendritic labeling in red and decreased numbers of labeled spines in green.
Figure 4.
Chronic moderate HFD effects on hippocampal CA3 molecular neuroanatomy. Photomicrographs are from mice fed a 45% fat calorie HFD or NCD for 8 weeks. A) Low magnification image of NCD mouse hippocampus labeled for IRS1-pS616 with box indicating location in CA3 where higher power images in C was taken. B) Low magnification image of 45% HFD mouse with box for image in D. C) NCD mouse CA3 labeled for IRS1-pS61 shows typical nuclear staining in stratum pyramidale (sp) and weak labeling of stratum lucidum (sl) and stratum radiatum (sr). D) 45% HFD mouse labeled for IRS1-pS616 shows increased overall labeling and distinctly labeled dendrites. E) Bar graph depicts mean optical density (O.D.) values (+SEM) of sl in NCD and 45% HFD mice (*p<0.05). F) NCD mouse CA3 labeled for PSD-95, an important scaffolding protein for post-synaptic densities. G) 45% HFD mouse CA3 shows decreased PSD-95 immunolabeling in sl. H) Graph depicting decreased PSD-95 expression in sl of HFD mice (**p<0.005). I) NCD mouse CA3 labeled for synaptopodin, an actin-associated protein essential for the formation of spine apparatuses in spines. Note its punctate appearance denoting spines. J) 45% HFD mouse CA3 shows decreased density of synaptopodin puncta in sl. K) Graph portrays decreased synaptopodin spine density in HFD mice (p<0.1).
Insulin has been shown to have important effects on synapses and HFD has been previously reported to impair hippocampal long-term potentiation (Mielke and Wang, 2011; Porter et al., 2012a). Based on the above IRS1-pS616 findings and reported work showing that insulin stimulates translation of the post-synaptic density scaffolding protein PSD-95 and promotes dendritic spine formation via insulin’s activation of PI3K-Akt-mTOR and Rac1 pathways (Lee et al., 2011), we hypothesized that HFD-induced insulin resistance would decrease expression of PSD-95 and dendritic spines. As depicted in Figure 4, we observed that both HFDs resulted in decreased PSD-95 expression and dendritic spine markers in CA3. In the extreme HFD mice, this was seen by decreased spinophilin-labeled spines in the presence of increased IRS1-pS616 (Figure 3). In the chronic, moderate HFD group, there was also a significant reduction in the expression of PSD-95 (t=3.36, p = 0.005) and a trend towards a reduction in synaptopodin-labeled spines (t=1.70, p<0.10)(Figure 4).
High fat diet mice show impaired spatial working memory
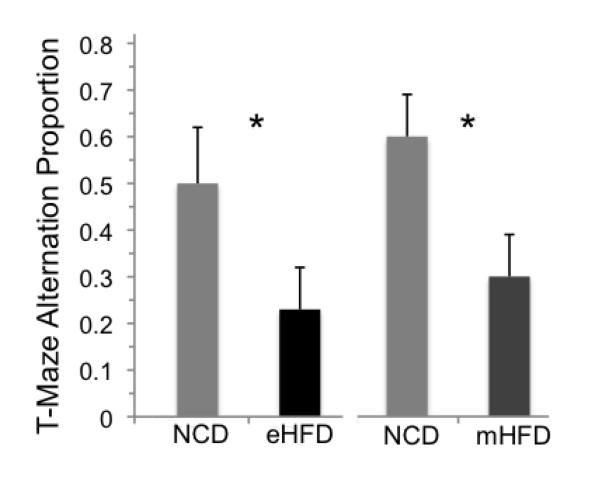
Spontaneous alternation measures an animal’s ability to explore alternating arms of the maze in repeated trials by remembering which arm in a T-maze that it had previously entered. Normal performance demonstrates that they retain a working memory of the arm they’ve just entered, reflecting short-term spatial memory that is dependent on hippocampal processing (Dudchenko, 2004). Mice ordinarily show a significant preference for alternation. T-maze spontaneous alternation testing was conducted in the moderate HFD cohort mice (n=10/group). As seen in Figure 5, NCD mice exhibited significantly greater preference for alternation than both their 60% 17-day HFD (t=1.96, p<0.04) and 45% 8-week HFD counterparts (t=2.22, p<0.04). No differences in motor activity were observed between groups as gauged by latencies in the T-maze (HFD average latency 16.7 seconds [5.9], NCD average 23.3 [5.9], t=0.8, p=0.43).
Figure 5.
Mice fed an extreme HFD (60%, eHFD) vs. normal control diet (NCD) for 17 days or moderate HFD (45%, mHFD) vs. NCD for 8 w show impaired working memory measured by spontaneous alternation. There were significant reductions in the normal preference for alternating arms in the T-maze in both experiments (mean proportion + SEM, *p < 0.05) without differing in response latency (both > p = 0.24), indicating that the mice differed in working memory but not in their physical ability to explore the T-maze.
Discussion
We provide direct evidence that relatively brief exposure to an extreme 60% HFD and more chronic exposure to a moderate 45% HFD induce telencephalic insulin resistance in the setting of decreased IPMK and increased pAMPK levels in the cortex and in association with systemic hyperglycemia. We further show that HFD is associated with abnormal neuroanatomic integrity of hippocampal CA3 dendrites and spines and reduced brain functioning as evidenced by impaired spatial working memory.
Cortical and hippocampal insulin resistance was demonstrated by a failure of key components of the PI3K-Akt-mTOR insulin signaling pathway to activate with insulin stimulation as well as the abnormal expression in neurons of serine-phosphorylated (inhibited) IRS1 which links insulin receptors to this major signaling pathway. It is likely that there are multiple causes of this neuronal insulin resistance due to HFD. One possibility is that chronic neuronal hyperactivation by HFD-associated hyperinsulinemia leads to a feedback down-regulation of insulin signaling. Such a phenomenon has been demonstrated in primary cortical neuron cultures exposed to excessive insulin in the media (Gupta et al., 2011). Other possibilities include HFD-induced alterations in incretin production and activity, inflammatory cascades, advanced glycation end-products, free fatty acids, reactive oxygen species, and nutrient-sensing homeostatic dysregulation that have been investigated in hypothalamic functioning in obesity (Williams, 2012). These may have similar effects in telencephalon too.
To further understand the mechanism by which systemic hyperglycemia induces insulin resistance in neurons, we examined various proteins involved in energy homeostasis and nutrient sensing. Our data showing reduced expression levels of IPMK in HFD mice indicate that this important component of nutrient sensing (Hardie et al., 2012b) may play a mechanistic role in regulating the insulin-PI3K-Akt-mTOR pathway in the telencephalon. We previously reported that IPMK, which possesses both inositol phosphate kinase and lipid kinase activities, regulates amino acid signaling to mTORC1 (Kim et al., 2011). This regulation is independent of IPMK’s catalytic function, instead reflecting its binding with mTOR and raptor, which maintains the mTOR-raptor association. Therefore IPMK is a physiologic mTOR cofactor, serving as a determinant of mTORC1 stability and signaling and a decrease in IPMK expression will likely impair mTOR signaling. In addition, another study demonstrated that IPMK is required for PI3K/Akt signaling and directly contributes to insulin- and other growth factor-mediated activation of the PI3K/Akt pathway (Maag et al., 2011). PIP3 especially, generated by IPMK, further augments growth factor-induced Akt signaling, indicating a critical role of IPMK in this pathway. Hence it is tempting to speculate that a specific loss of IPMK in the frontal lobe resulted in HFD-induced insulin resistance.
We also observed that AMPK, another key nutrient sensor protein, is activated in the brain with HFD exposure. AMPK is an evolutionarily conserved serine/threonine kinase that senses the energy status of cell and regulates fuel availability and is also activated by stressors that deplete cellular ATP, including hypoxia, ischemia, glucose deprivation, and uncouplers of oxidative phosphorylation. AMPK is ubiquitously expressed in the body and plays a major role in fatty acid synthesis and glucose homeostasis. In the periphery, AMPK activation is associated with decreased lipid formation, as AMPK phosphorylates acetyl-CoA carboxylase (ACC), inhibiting the generation of malonyl-CoA. Malonyl-CoA is a substrate for fatty acid synthase, so inhibition of ACC diminishes formation of fatty acids and lipids (Hardie et al., 2012a; Kahn et al., 2005). In the hypothalamus, AMPK acts in a seemingly reciprocal fashion to regulate food intake (Hardie et al., 2012a; Wolfgang and Lane, 2006). While it has been shown that hypothalamic AMPK responds to hormonal and nutrient signals to modulate food intake (Dagon et al., 2012; Xue and Kahn, 2006), it has not been examined whether or how AMPK in non-hypothalamic regions is regulated by energy status. Hence to the best of our knowledge, this is the first observation showing that the levels of non-hypothalamic AMPK are associated with a change in systemic energy balance created by the HFD. Importantly, our data showed that an increase in energy input also increased pAMPK levels in cortex. This is reminiscent of peripheral AMPK response but is an opposite response from that of the hypothalamus (decreased pAMPK) as previously described by us and others (Bang et al., 2012a; Minokoshi et al., 2004; Minokoshi et al., 2002). We speculate that this difference may contribute to neuronal insulin resistance. Moreover, it has been shown that AMPK can modulate the mTOR pathway in multiple ways. An increase of AMPK activity can directly disrupt mTORC1 by phosphorylating Raptor and inhibiting its activity via TSC1. In addition, we found that HFD leads to decreased expression levels of IPMK. Interestingly, it appears that activation of AMPK in cortex may make a direct contribution to a loss of IPMK, consequently leading to an insulin resistance in the brain. However, further studies are needed to understand the biochemical link between the two proteins.
Downstream abnormalities in synaptic anatomy and physiology that we observed in our HFD mice may be mechanistically related to HFD via PI3K-Akt-mTOR and GSK3 signaling as well. For instance, dysregulation or failure to activate mTOR and its associated downstream targets such as 4EBP1, particularly at the synapse, will lead to failed local RNA translation and protein synthesis in dendrites and spines (including PSD-95), axon terminals and growth cones (Gong et al., 2006; Kindler and Kreienkamp, 2012; Lee et al., 2011). mTOR also regulates synapses via S6K-mediated autophagy or Rho/Rac/PKC-mediate actin organization. mTOR inactivation by rapamycin triggers macroautophagy and this has been found to decrease axonal volumes, synaptic vesicle numbers and evoked neurotransmitter release (Hernandez et al., 2012). Concurrently, failure of Akt activation could decrease synaptic vesicle recycling and diminish synaptic strength (Smillie and Cousin, 2012). Failed activation of Akt also would disinhibit GSK3, thus promoting LTD and offsetting a balance between LTD and LTP (Peineau et al., 2007).
Behavioral and neuroplasticity deficits have been well established in streptozotocin-induced insulin deficiency rodent models of Type I diabetes (Artola et al., 2005; Gispen and Biessels, 2000; Ho et al., 2012), but there are relatively fewer reports on models of the much more prevalent metabolic syndrome/Type II diabetes associated with insulin resistance (Ho et al., 2013). Spatial memory deficits have been reported in genetic models employing the leptin-receptor mutant db/db mouse and Zucker rat (Li et al., 2002; Winocur and Greenwood, 2005), while leptin-deficient ob/ob mice have been reported to show normal memory (Finger et al., 2010; Porter et al., 2012b). HFDs have been reported to cause impairments in cognitive behaviors in rats (Greenwood and Winocur, 1990; Stranahan et al., 2008) and in many, but not all mouse model studies (Boitard et al., 2012; Heyward et al., 2012; Hwang et al., 2010; Morrison et al., 2010; Pistell et al., 2010; Porter et al., 2012a; Porter et al., 2010; Tucker et al., 2012; Valladolid-Acebes et al., 2011; Yamada-Goto et al., 2012). Reasons for the inconsistencies likely include the exact type of HFD (e.g. mild, moderate, or extreme HFD), duration of HFD, animal strain differences, and varying target behaviors or sensitivities of the specific tests used. Our behavioral data demonstrating impaired spontaneous alternation in a T-maze in HFD mice provides additional support for hippocampal-based memory dysfunction resulting from HFD, complementing other spatial and non-spatial memory tasks used in the aforementioned studies. Moreover, neurophysiological studies have found both LTP and LTD deficits in models of Type I and II diabetes, including streptozotocin, Zucker rat, db/db, ob/ob and HFD-induced models (Artola et al., 2005; Finger et al., 2010; Gispen and Biessels, 2000; Ho et al., 2012; Hwang et al., 2010; Li et al., 2002; Porter et al., 2012a; Porter et al., 2012b; Stranahan et al., 2008; Winocur and Greenwood, 2005) further corroborating our findings.
The behavioral and neuroplasticity abnormalities with HFD in rodents have been attributed to a variety of proximal neurobiological abnormalities. These include reduced hippocampal neurogenesis (Boitard et al., 2012; Can et al., 2012; Lindqvist et al., 2006), abnormalities of glutamate metabolism, NMDA and AMPA expression and activity (Valladolid-Acebes et al., 2012), and abnormalities in BDNF expression (Yamada-Goto et al., 2012). In addition, there has been one previous study to our knowledge that examined synaptic markers in mice fed a high-fat + high sugar diet. Stranahan et al. (Stranahan et al., 2008) reported decreased numbers of Golgi-stained dendritic spines in CA1 in the setting of normal dendrite lengths and arborization, as well as reduced expression of the presynaptic vesicle marker synaptophysin in hippocampal homogenate immunoblots and CA1 immunofluorescence-stained sections. Our immunohistochemical experiments measuring specific proteins enriched in postsynaptic densities (PSD-95) and dendritic spines (spinophilin and synaptopodin) found analogous reductions in these important synaptodendritic markers in CA3’s mossy fiber terminal zone. Notably, this region is particularly vulnerable to various environmental and pharmacological stressors, including psychosocial or restraint stress and corticosterone, that may also be exacerbated by brief exposure to streptozotocin-induced diabetes (Cohen et al., 2011; Magarinos and McEwen, 2000; Magarinos et al., 1997).
We recognize far more complexity and interactions involving HFD-induced alterations in insulin signaling pathways than we investigated here. We have demonstrated that HFD leads to synaptic loss, impaired memory functioning and brain insulin resistance within the Akt-mTOR & GSK3 pathway. This pathway plays a critical role in diabetes-related dysfunction in various organ systems including the brain, as exemplified by our recent investigation of insulin signaling in Alzheimer’s disease (Talbot et al., 2012). Importantly, our data suggest that a loss of the novel IPMK caused by an activation of cortical AMPK may be a potential mechanism by which cells develop insulin resistance leading to a decline in cognitive function.
Our findings lead to an interesting question of whether drugs used in Type II diabetes might improve cognitive disorders as some of these drugs (especially metformin) act in part through AMPK. Metformin is thought to act through activation of AMPK, but recent studies have shown that metformin can improve glucose homeostasis through non-AMPK mechanisms too (Foretz et al., 2010; Kalender et al., 2010; Miller et al., 2013). If so, is modulation of neuronal AMPK necessary to improve insulin resistance in the brain or is it possible even in the absence of neuronal AMPK? Better understanding of the cellular mechanisms by which this and other pathways link Type II diabetes and cognitive disorders will also identify novel entry points for prevention and potential therapeutics.
Supplementary Material
Highlights.
HFD leads to failed activation of Akt/mTOR/GSK3β pathways by insulin in cortex
HFD leads to increased AMPK and correlated decreased IPMK levels
HFD is associated with increased IRS1-pS616 pathological neurites
HFD is associated with decreased PSD-95 and dendritic spine density
HFD impairs spatial working memory in mice
Acknowledgments
This work was supported by grants from the NIH DK084336, R01 MH086599 and T32 MH14654, the Allen H and Selma W. Berkman Charitable Trust and pilot grants from the University of Pennsylvania Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott MA, et al. The insulin receptor tyrosine kinase substrate p58/53 and the insulin receptor are components of CNS synapses. J Neurosci. 1999;19:7300–8. doi: 10.1523/JNEUROSCI.19-17-07300.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato L, et al. The Osservatorio Geriatrico of Campania Region Group Non-insulin-dependent diabetes mellitus is associated with a greater prevalence of depression in the elderly. Diabetes Metab. 1996;22:314–8. [PubMed] [Google Scholar]
- Artola A, et al. Diabetes mellitus concomitantly facilitates the induction of long-term depression and inhibits that of long-term potentiation in hippocampus. Eur J Neurosci. 2005;22:169–78. doi: 10.1111/j.1460-9568.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- Baker LD, et al. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol. 2010;68:51–7. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang S, et al. AMP-activated protein kinase is physiologically regulated by inositol polyphosphate multikinase. Proc Natl Acad Sci U S A. 2012a;109:616–20. doi: 10.1073/pnas.1119751109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang S, et al. Striatum specific protein, Rhes regulates AKT pathway. Neurosci Lett. 2012b;521:142–7. doi: 10.1016/j.neulet.2012.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels GJ, et al. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- Bockmann J, et al. ProSAP/Shank postsynaptic density proteins interact with insulin receptor tyrosine kinase substrate IRSp53. J Neurochem. 2002;83:1013–7. doi: 10.1046/j.1471-4159.2002.01204.x. [DOI] [PubMed] [Google Scholar]
- Boitard C, et al. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus. 2012;22:2095–100. doi: 10.1002/hipo.22032. [DOI] [PubMed] [Google Scholar]
- Can OD, et al. The effect of simvastatin treatment on behavioral parameters, cognitive performance, and hippocampal morphology in rats fed a standard or a high-fat diet. Behav Pharmacol. 2012;23:582–92. doi: 10.1097/FBP.0b013e328356c3f2. [DOI] [PubMed] [Google Scholar]
- Chakraborty A, et al. Inositol pyrophosphates inhibit Akt signaling, thereby regulating insulin sensitivity and weight gain. Cell. 2010;143:897–910. doi: 10.1016/j.cell.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, et al. Dexras1, a Small GTPase, Is Required for Glutamate-NMDA Neurotoxicity. J Neurosci. 2013;33:3582–7. doi: 10.1523/JNEUROSCI.1497-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu SL, et al. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron. 2008;58:708–19. doi: 10.1016/j.neuron.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JW, et al. Chronic corticosterone exposure alters postsynaptic protein levels of PSD-95, NR1, and synaptopodin in the mouse brain. Synapse. 2011;65:763–70. doi: 10.1002/syn.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia SC, et al. Insulin-resistant brain state: the culprit in sporadic Alzheimer’s disease? Ageing Res Rev. 2011;10:264–73. doi: 10.1016/j.arr.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagon Y, et al. p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin’s effect on food intake. Cell Metab. 2012;16:104–12. doi: 10.1016/j.cmet.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert M, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2012;8:114–26. doi: 10.1038/nrendo.2011.156. [DOI] [PubMed] [Google Scholar]
- de la Monte SM. Insulin resistance and Alzheimer’s disease. BMB Reports. 2009;42:475–481. doi: 10.5483/bmbrep.2009.42.8.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudchenko PA. An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev. 2004;28:699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Finger BC, et al. Leptin-deficient mice retain normal appetitive spatial learning yet exhibit marked increases in anxiety-related behaviours. Psychopharmacology (Berl) 2010;210:559–68. doi: 10.1007/s00213-010-1858-z. [DOI] [PubMed] [Google Scholar]
- Foretz M, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. The Journal of clinical investigation. 2010;120:2355–69. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gispen WH, Biessels GJ. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 2000;23:542–9. doi: 10.1016/s0166-2236(00)01656-8. [DOI] [PubMed] [Google Scholar]
- Gong R, et al. Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J Biol Chem. 2006;281:18802–15. doi: 10.1074/jbc.M512524200. [DOI] [PubMed] [Google Scholar]
- Greenwood CE, Winocur G. Learning and memory impairment in rats fed a high saturated fat diet. Behav Neural Biol. 1990;53:74–87. doi: 10.1016/0163-1047(90)90831-p. [DOI] [PubMed] [Google Scholar]
- Gupta A, et al. Peripheral insulin-sensitizer drug metformin ameliorates neuronal insulin resistance and Alzheimer’s-like changes. Neuropharmacology. 2011;60:910–20. doi: 10.1016/j.neuropharm.2011.01.033. [DOI] [PubMed] [Google Scholar]
- Haan MN. Therapy Insight: type 2 diabetes mellitus and the risk of late-onset Alzheimer’s disease. Nat Clin Pract Neurol. 2006;2:159–66. doi: 10.1038/ncpneuro0124. [DOI] [PubMed] [Google Scholar]
- Hardie DG, et al. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature reviews. Molecular cell biology. 2012a;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, et al. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012b;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner PS, et al. The association of posttraumatic stress disorder and metabolic syndrome: a study of increased health risk in veterans. BMC Med. 2009;7:1. doi: 10.1186/1741-7015-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez D, et al. Regulation of presynaptic neurotransmission by macroautophagy. Neuron. 2012;74:277–84. doi: 10.1016/j.neuron.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyward FD, et al. Adult mice maintained on a high-fat diet exhibit object location memory deficits and reduced hippocampal SIRT1 gene expression. Neurobiol Learn Mem. 2012;98:25–32. doi: 10.1016/j.nlm.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho N, et al. Depressive phenotypes evoked by experimental diabetes are reversed by insulin. Physiol Behav. 2012;105:702–8. doi: 10.1016/j.physbeh.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho N, et al. Effects of diabetes on hippocampal neurogenesis: Links to cognition and depression. Neurosci Biobehav Rev. 2013;37:1346–1362. doi: 10.1016/j.neubiorev.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev. 2004;28:497–505. doi: 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Hwang LL, et al. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity (Silver Spring) 2010;18:463–9. doi: 10.1038/oby.2009.273. [DOI] [PubMed] [Google Scholar]
- Kahn BB, et al. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell metabolism. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kalender A, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell metabolism. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassi E, et al. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, et al. Amino acid signaling to mTOR mediated by inositol polyphosphate multikinase. Cell Metab. 2011;13:215–21. doi: 10.1016/j.cmet.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SF, et al. From the Cover: Antipsychotic drug-induced weight gain mediated by histamine H1 receptor-linked activation of hypothalamic AMP-kinase. Proc Natl Acad Sci U S A. 2007;104:3456–9. doi: 10.1073/pnas.0611417104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler S, Kreienkamp HJ. Dendritic mRNA targeting and translation. Adv Exp Med Biol. 2012;970:285–305. doi: 10.1007/978-3-7091-0932-8_13. [DOI] [PubMed] [Google Scholar]
- Knol MJ, et al. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49:837–45. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- Lee CC, et al. Insulin promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR and Rac1 signaling pathways. Neuropharmacology. 2011;61:867–79. doi: 10.1016/j.neuropharm.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Li XL, et al. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113:607–15. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, et al. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol. 2006;13:1385–8. doi: 10.1111/j.1468-1331.2006.01500.x. [DOI] [PubMed] [Google Scholar]
- Maag D, et al. Inositol polyphosphate multikinase is a physiologic PI3-kinase that activates Akt/PKB. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:1391–6. doi: 10.1073/pnas.1017831108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Experimental diabetes in rats causes hippocampal dendritic and synaptic reorganization and increased glucocorticoid reactivity to stress. Proc Natl Acad Sci U S A. 2000;97:11056–61. doi: 10.1073/pnas.97.20.11056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, et al. Chronic stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci U S A. 1997;94:14002–8. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy JP, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80:19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, et al. Bipolar disorder and metabolic syndrome: an international perspective. J Affect Disord. 2010;126:366–87. doi: 10.1016/j.jad.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Metlakunta AS, et al. Hypothalamic phosphatidylinositol 3-kinase pathway of leptin signaling is impaired during the development of diet-induced obesity in FVB/N mice. Endocrinology. 2008;149:1121–8. doi: 10.1210/en.2007-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke JG, et al. Endogenous insulin signaling protects cultured neurons from oxygen-glucose deprivation-induced cell death. Neuroscience. 2006;143:165–73. doi: 10.1016/j.neuroscience.2006.07.055. [DOI] [PubMed] [Google Scholar]
- Mielke JG, Wang YT. Insulin, synaptic function, and opportunities for neuroprotection. Prog Mol Biol Transl Sci. 2011;98:133–86. doi: 10.1016/B978-0-12-385506-0.00004-1. [DOI] [PubMed] [Google Scholar]
- Miller RA, et al. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494:256–60. doi: 10.1038/nature11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–74. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–43. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Morrison CD, et al. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem. 2010;114:1581–9. doi: 10.1111/j.1471-4159.2010.06865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann KF, et al. Insulin resistance and Alzheimer’s disease: molecular links & clinical implications. Curr Alzheimer Res. 2008;5:438–47. doi: 10.2174/156720508785908919. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, et al. High-fat diet induced neuropathy of pre-diabetes and obesity: effects of “healthy” diet and aldose reductase inhibition. Diabetes. 2007;56:2598–608. doi: 10.2337/db06-1176. [DOI] [PubMed] [Google Scholar]
- Okamura F, et al. Insulin resistance in patients with depression and its changes during the clinical course of depression: minimal model analysis. Metabolism. 2000;49:1255–60. doi: 10.1053/meta.2000.9515. [DOI] [PubMed] [Google Scholar]
- Pan A, et al. Bidirectional association between depression and type 2 diabetes mellitus in women. Arch Intern Med. 170:1884–91. doi: 10.1001/archinternmed.2010.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peineau S, et al. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 2007;53:703–17. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Pistell PJ, et al. Cognitive impairment following high fat diet consumption is associated with brain inflammation. Journal of neuroimmunology. 2010;219:25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D, et al. Actions of incretin metabolites on locomotor activity, cognitive function and in vivo hippocampal synaptic plasticity in high fat fed mice. Peptides. 2012a;35:1–8. doi: 10.1016/j.peptides.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Porter DW, et al. Four weeks administration of Liraglutide improves memory and learning as well as glycaemic control in mice with high fat dietary-induced obesity and insulin resistance. Diabetes Obes Metab. 2010;12:891–9. doi: 10.1111/j.1463-1326.2010.01259.x. [DOI] [PubMed] [Google Scholar]
- Porter WD, et al. Liraglutide improves hippocampal synaptic plasticity associated with increased expression of Mash1 in ob/ob mice. Int J Obes (Lond) 2012b doi: 10.1038/ijo.2012.91. [DOI] [PubMed] [Google Scholar]
- Pouwer F, et al. Rates and risks for co-morbid depression in patients with Type 2 diabetes mellitus: results from a community-based study. Diabetologia. 2003;46:892–8. doi: 10.1007/s00125-003-1124-6. [DOI] [PubMed] [Google Scholar]
- Profenno LA, et al. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 67:505–12. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Reaven GM. The metabolic syndrome: time to get off the merry-go-round? J Intern Med. 2011;269:127–36. doi: 10.1111/j.1365-2796.2010.02325.x. [DOI] [PubMed] [Google Scholar]
- Schneider CA, et al. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijvers EM, et al. Insulin metabolism and the risk of Alzheimer disease: the Rotterdam Study. Neurology. 75:1982–7. doi: 10.1212/WNL.0b013e3181ffe4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie KJ, Cousin MA. Akt/PKB Controls the Activity-Dependent Bulk Endocytosis of Synaptic Vesicles. Traffic. 2012 doi: 10.1111/j.1600-0854.2012.01365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, et al. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–8. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–38. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KR, et al. Olfactory ability and object memory in three mouse models of varying body weight, metabolic hormones, and adiposity. Physiol Behav. 2012;107:424–32. doi: 10.1016/j.physbeh.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladolid-Acebes I, et al. High-fat diets induce changes in hippocampal glutamate metabolism and neurotransmission. Am J Physiol Endocrinol Metab. 2012;302:E396–402. doi: 10.1152/ajpendo.00343.2011. [DOI] [PubMed] [Google Scholar]
- Valladolid-Acebes I, et al. High-fat diets impair spatial learning in the radial-arm maze in mice. Neurobiol Learn Mem. 2011;95:80–5. doi: 10.1016/j.nlm.2010.11.007. [DOI] [PubMed] [Google Scholar]
- van der Heide LP, et al. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J Neurochem. 2005;94:1158–66. doi: 10.1111/j.1471-4159.2005.03269.x. [DOI] [PubMed] [Google Scholar]
- Weber-Hamann B, et al. Improved insulin sensitivity in 80 nondiabetic patients with MDD after clinical remission in a double-blind, randomized trial of amitriptyline and paroxetine. J Clin Psychiatry. 2006;67 doi: 10.4088/jcp.v67n1204. [DOI] [PubMed] [Google Scholar]
- Werther GA, et al. Localization and Characterization of Insulin-Like Growth Factor-I Receptors in Rat Brain and Pituitary Gland Using in vitro Autoradiography and Computerized Densitometry* A Distinct Distribution from Insulin Receptors. J Neuroendocrinol. 1989;1:369–77. doi: 10.1111/j.1365-2826.1989.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Williams LM. Hypothalamic dysfunction in obesity. Proc Nutr Soc. 2012;71:521–33. doi: 10.1017/S002966511200078X. [DOI] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol Aging. 2005;26(Suppl 1):46–9. doi: 10.1016/j.neurobiolaging.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl 3):S215–9. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- Wolfgang MJ, Lane MD. Control of energy homeostasis: role of enzymes and intermediates of fatty acid metabolism in the central nervous system. Annual review of nutrition. 2006;26:23–44. doi: 10.1146/annurev.nutr.25.050304.092532. [DOI] [PubMed] [Google Scholar]
- Xue B, Kahn BB. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J Physiol. 2006;574:73–83. doi: 10.1113/jphysiol.2006.113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada-Goto N, et al. Impairment of fear-conditioning responses and changes of brain neurotrophic factors in diet-induced obese mice. J Neuroendocrinol. 2012;24:1120–5. doi: 10.1111/j.1365-2826.2012.02327.x. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Townsend M. Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer’s disease. Biochim Biophys Acta. 2009;1792:482–96. doi: 10.1016/j.bbadis.2008.10.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.