Abstract
Dendritic cells (DCs) are professional antigen-presenting cells capable of initiating and regulating innate and adaptive immunity. The development of effective ways to produce a large number of DCs in laboratories made the use of DCs available in various vaccine approaches. Compared to conventional vaccines, focused on protective antibody responses, DC vaccines emphasize protective T cell immunity but might elicit strong antibody responses as well. In addition, the recent discoveries of functionally distinct DC subsets in various organs and tissues are likely to increase the potential of exploiting DCs in vaccines and immunotherapy. Vaccines composed of DCs generated ex vivo, pulsed with antigens, and matured prior to being re-infused to the body have been widely tried clinically but resulted in limited success due to various obstacles. In this review, new approaches that protein vaccines are selectively targeted to the endocytic C-type lectin receptors on surface of DCs in vivo are discussed.
Keywords: Antigen receptor, C-type lectins, Dendritic cells, Monoclonal antibody
Introduction
Vaccination is one of the great medical success stories, utilizing immune system. Vaccine requires the induction of antigen-specific, nontoxic, long-lived immune protection against diseases [1,2]. Since dendritic cells (DCs) were discovered and demonstrated to be the key inducers and regulators of immune responses, DCs became attractive tools for vaccine and immunotherapy [3,4,5]. Use of DCs for vaccination gave rise to the possibility of inducing strong T cell immunity to fight against cancer and infection, such as human immunodeficiency virus (HIV), and thus overcoming the limitation of conventional vaccines which rely largely on the induction of protective antibody responses [6].
Furthermore, the recent findings that classical DCs effectively control the tolerance of T cells expanded the potential of DC-based vaccines, beyond conventional immunogenic goals, to preventing or treating any disease related to immune system's abnormalities [2,5,6,7]. Especially, the late Ralph Steinman and his colleagues have pioneered the method of targeting antigen proteins to the endocytic receptors on DCs in vivo and thus efficiently exploiting the function of DCs as immunologically versatile regulators [2].
Dendritic Cells
DCs are specialized populations of leukocytes essential for controlling the immune system to tolerate or react properly against a vast number of different challenges it encounters. As professional antigen presenting cells (APCs) and sentinels of the immune system, DCs are located largely in the T-cell areas of lymphoid tissues and also in most tissues including body surfaces where they come across and seize antigens followed by migration to lymphoid organs.
The versatile roles of DCs in both immunogenic and tolerogenic functions can be explained by the transforming process of DCs known as maturation [2,3,7]. In homeostatic settings, immature DCs can actively induce T cell tolerance through induction of selection, anergy, or deletion of T cells including regulatory T (Treg) cells during development in thymus and periphery. Upon activation via signals from various receptors for antigens, cytokines, pathogen-associated molecular patterns, or damage-associated molecular patterns, DCs become mature by changing into an immunogenic phenotype and capable of inducing the activation of T cells [1,2,3].
Vaccination with Ex Vivo-Generated DCs
Provenge from Dendreon is the first Food and Drug Administration-approved autologous cellular immunotherapy for the treatment of advanced metastatic castrate resistant prostate cancer. Provenge is composed of not only DCs but also other autologous peripheral blood mononuclear cells, pulsed with granulocyte-macrophage colony-stimulating factor-fused prostatic acid phosphatase, a prostate self antigen, and incubated for 36 to 44 hours prior to being reinfused to the patient, which could help extend survival for a few months [8]. Clinical trials of various DC vaccines generated ex vivo and pulsed with cancer cells or antigens have proved safe and immunogenic against the cancers but only resulted in limited success [9]. There exist significant problems in current vaccines utilizing ex vivo-derived DCs, such as the practical difficulties of logistics and organization and the optimization of methods to overcome the limited efficacy likely due to their inefficiency in migration and antigen presentation following reinfusion to the body.
C-type Lectin Receptors on DCs
C-type lectin receptors (CLRs) have special importance for DCs to function as professional APCs. Compared to other pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs), NOD-like receptors, and RIG-I-like receptors, CLRs are composed of a large number of members which can recognize a broad range of their glycosylated ligand molecules and microbes. Upon recognition and binding of their ligand antigens, CLRs can take up and clear the antigens from environment and may generate signals for DCs to become mature. Especially, a variety of CLRs are highly expressed on DC surface where they perform as efficient endocytic receptors for antigens. This is important for DCs to function as principal sentinels and APCs of the immune system to capture and present antigens and thus to mount or tolerize adaptive immune responses to the antigens.
In addition to their functional roles, CLRs are useful in distinguishing the different subsets of DCs in various tissues and organs. In immune cells, mainly 2 groups of CLRs are found: 1) type I transmembrane proteins with multi-lectins or multiple carbohydrate recognition domains (CRDs), such as CD205/DEC205, CD206/MMR, etc., and 2) type II transmembrane proteins with a single CRD, such as CD209/DC-SIGN, CD207/langerin, CD303/BDCA-2, Dectin, etc. Such CLRs as CD303, CD207, and CD205 are routinely used to identify and purify distinctive subsets of DCs in blood, skin, and lymphoid tissues.
Targeting vaccine proteins to CD205 on DCs in vivo
CD205, also known as DEC205 (a type I transmembrane protein containing deca lectin domains with molecular weight of 205 kDa), is the first identified DC-specific receptor. A monoclonal antibody (mAb) named NLDC145 was shown to specifically label DCs in lymphoid tissues [10], and the cDNA encoding the protein reactive to NLDC145 was cloned and named DEC205 [11]. Studies revealed that, when bound with surrogate ligands, CD205 could function as an efficient endocytic receptor that delivered the ligands to antigen processing pathways for the presentation on both class I and II MHC molecules [11,12,13]. Recently, a class of oligonucleotides with nonmethylated cytosine-guanosine (CpG) motifs were identified as ligands of CD205 [14], which implies that CD205 might be an endocytic receptor for both antigen and maturation stimulus in DCs.
When a peptide antigen was fused to a recombinant anti-CD205 mAb and delivered to CD205 on DCs in mouse, the responding T cells become eliminated or unresponsive to a further re-stimulation of the same peptide [15,16], resulting in tolerance of antigen-specific T cells. This proves that DCs in vivo actively establish T cell tolerance by presenting antigens from self and environment during the steady state. In contrast, antigen-conjugated anti-CD205 mAb co-injected with anti-CD40 antibody induced strong and lasting T cell immunity against the antigen [13,15,17]. Therefore, CD205 could also become a target on DCs in vivo exploited for antibody-based vaccine delivery.
Injected whether intravenously or subcutaneously, the anti-CD205 mAb fused with antigen was targeted to DCs in spleen and lymph nodes within 30 minutes [17]. With adjuvant, such as anti-CD40 and/or PRR agonist, anti-CD205 mAb-conjugated antigens could generate antigen-specific T cell responses with much higher efficiency, i.e., at least 100 to 1,000 fold more than unconjugated and control mAb-conjugated antigens [6,17]. Likewise, animals immunized with anti-CD205-conjugated antigens demonstrated that vaccines targeted to DCs in vivo produced the strong and long-lived memory responses of antigen-specific T cells [17,18]. In addition to the improved intensity and durable memory, targeting antigen to CD205 on DCs was able to generate the response of diverse T cell repertoires against various peptides from the antigen, efficiently presented by the MHC molecules of different haplotypes and individuals [18,19].
The protective immunity induced by the CD205-targeted vaccines was tested by various infection models. Mice challenged with either vaccinia virus or Yersinia pestis via airway route were effectively protected following vaccination with protective antigens conjugated to anti-CD205 mAb [18,20]. In those mice, the protective immunity generated by DC-targeted vaccine antigen was attributed to the efficient induction of antigen-specific helper T cells, accompanied by strong humoral immunity, i.e., high antibody titers against the antigen. Although the conventional, non-targeted vaccines immunized with alum adjuvant also induced high titers of antibodies and exhibited effective protection, only the DC-targeted vaccines were able to generate strong and durable T cell responses, implying that DC-targeted vaccines might be superior in the long run.
Development of Clinical Vaccine Targeted to Human CD205
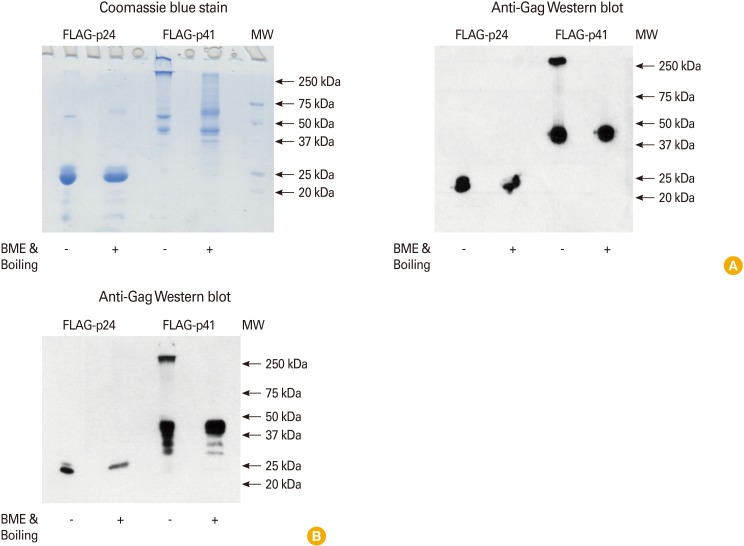
The first protein antigen of pathogens selected for CD205-targeted clinical vaccine was Gag protein of HIV-1, because the T cell immunity to Gag showed a protective potential [6]. The p41 fragment of Gag p55 was engineered to fuse with the C-terminus of heavy chain in anti-CD205 or control mAbs. The recombinant proteins of unconjugated and mAb-conjugated p41 were expressed in mammalian cell-lines such as HEK293T or CHO cells, secreted into culture media, and purified. Because the purified Gag p41 protein appeared to form aggregation, the p24 fragment of Gag p41 was also generated, expressed, purified, and then compared with p41. As shown in Fig. 1, the majority of p41 protein, even in cell culture media prior to purification, was aggregated and in complex with other molecules. However, the p24 protein was in a monomeric, soluble form when expressed from mammalian cells (Fig. 1). Therefore, to optimize the delivery of antigen to DCs and to rule out the artifact from aggregation, p24 was chosen as the Gag antigen for HIV vaccine.
Fig. 1.
Analyses of human immunodeficiency virus Gag proteins expressed from mammalian CHO cells. (A) Soluble, FLAG-tagged Gag p41 and p24 proteins were produced into culture media from the stably transfected CHO cells, followed by anti-FLAG affinity purification. Left panel: Five micrograms each of purified p41 and p24 was subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis with/without (+/-) treatment of β-mercaptoethanol (BME) and boiling at 100℃ for 5 minutes (Boiling). After electrophoresis, the gel was visualized with Coomassie blue stain. Aggregates on the border between stacking and resolving gels and on the bottom of loading wells are detected from the purified p41 without BME & Boiling. Right panel: Zero point five microgram each of purified p41 and p24 was subjected to SDS-polyacrylamide gel electrophoresis with/without BME & Boiling. After electrophoresis, the gel was transferred onto polyvinylidene difluoride (PVDF) membrane, blotted with anti-Gag antibody, and visualized with chemiluminescent reagent. Aggregates with high molecular weight are detected from the purified p41 without BME & Boiling. (B) Culture supernatants from stably transfected CHO cells expressing soluble, FLAG-tagged Gag p41 and p24 proteins were analyzed. Five microliters each of cell culture supernatants was subjected to SDS-polyacrylamide gel electrophoresis with/without BME & Boiling. After electrophoresis, the gel was transferred onto PVDF membrane, blotted with anti-Gag antibody, and visualized with chemiluminescent reagent. Aggregates with high molecular weight are detected from the cell culture supernatant of p41 without BME & Boiling.
To generate immunogenic DCs effectively in vivo, different types of stimulators were tested as adjuvant for the CD205-targeted p24 vaccination. In mice, amongst diverse agonists of different innate immune receptors, poly IC, a synthetic double-stranded RNA ligand to TLR3 and MDA-5, and its stabilized clinical version, poly ICLC or Hiltonol, were found most effective in stimulating the DCs targeted with p24 and thus inducing both T and B cell immunity via systemic interferon responses [21,22]. In humans, the treatment of poly ICLC induced the various pathways of innate immune responses broadly and efficiently, i.e., quite similarly to the highly effective yellow fever vaccine [23]. Therefore, poly ICLC was chosen for the adjuvant in clinical studies of the first vaccine targeted to DCs in vivo [6].
To translate the preclinical studies of CD205-targeted DC vaccines into humans, a series of new anti-human CD205 mAbs were produced and tested. Due to the large size of CD205, early attempts to immunize animals with the limited sizes of CD205 protein fragments were able to generate only a few anti-human CD205 mAbs [24,25]. Therefore, a new effort was pursued to generate a large number of mAbs against the diverse epitopes in human CD205, for the selection of an anti-human CD205 mAb used in clinical studies. To this end, the extracellar domain of full-length CD205 protein was produced and used to immunize two different kinds of mice: mice carrying not mouse but human immunoglobulin genes [26] and mice deficient of CD205 gene, i.e., CD205 knockout mice [27]. Especially, from a single hybridoma fusion, the latter produced a large number, i.e., over 400 of anti-human CD205 mAbs, many of which were verified to bind not only human CD205 but also CD205 of mouse and other mammals [27]. This cross-species reactivity of anti-human CD205 mAbs has advantage that those mAbs can deliver the vaccines to DCs in humans as well as in mice and other animals. This will solve a need to generate animal models that express human CD205 in their DCs. However, fully human anti-human CD205 mAbs can be produced from the former mice [26], not requiring the further process to humanize mouse anti-human CD205 mAbs generated in the case of the latter mice.
From the mice with human immunoglobulin genes, 6 human anti-human CD205 mAbs were generated. Among those, mAbs cross-reactive to monkey CD205 were selected and compared for their vaccine-targeting capacities with an existing anti-human CD205 mAb [26]. For this job, a transgenic (Tg) mouse was generated to carry a full-length human CD205 cDNA under CD11c promoter, thus expressing the human CD205 endocytic receptor on mouse DCs. Then, anti-human CD205 mAb named 3G9 was determined most efficient to induce immune responses in the human CD205 Tg mice as well as in human peripheral blood cell cultures [26]. Therefore, mAb 3G9 was chosen to conjugate HIV Gag p24 and put forward to the first clinical trial of DC-targeting vaccines [6].
Targeting Vaccine Proteins to Other CLRs on DCs In Vivo
It had been shown that delivering an antigen to different subsets of DCs in vivo resulted in different immune responses. Antigen targeted to DCIR2-positive DCs stimulated CD4 T cells preferentially while CD205-positive DCs did CD8 T cells [28]. The difference of ability between those subsets of DCs was attributed to the differential expression of genes involved in the different pathways of antigen processing and presentation. When the protective immunity to an infection relied heavily on antibody response, the vaccine protein targeted to DCIR2, compared to CD205, on DCs in vivo induced higher antibody titers and thus achieved better protection [20].
Besides classical DCs in steady state, the role of a DC population(s) derived from monocytes in vivo had been suggested by animal models of various infections and by the monocyte-to-DC differentiation in vitro. DC-SIGN or CD209a was recently identified as a marker of monocyte-derived DCs in mouse lymph nodes during inflammation caused by Gram-negative bacteria and TLR4 agonists [29]. Upon administration of Gram-negative bacteria or TLR4 agonist like LPS, monocytes were mobilized to the T cell area of lymph nodes and fully differentiated to functional DCs expressing CD209a. Due to the newly generated mAbs with high affinities to CD209a [30], monocyte-derived inflammatory DCs could be detected in vivo [29]. With TLR4 agonist, targeting CD209a-positive DCs generated stronger antigen-specific T cell responses than targeting CD205-positive DCs, which implies that monocyte-derived inflammatory DCs might be better targets of vaccine delivery for the induction of protective immunity against microbial pathogens.
Future of Targeting Vaccines to DCs In Vivo
Now, beginning with targeting vaccines to CD205-positve DCs in vivo, various CLRs marking different subsets of DCs are being investigated for the delivery target of vaccine antigens in vivo. As seen in the cases of CD205, CD209a, and DCIR2, same antigens targeted to different subsets of DCs under distinct environments resulted in varied immune responses of T cells as well as B cells. Likely, there also exist more of unknown subsets of DCs with novel functions [31], yet to be found and targeted with vaccines in the future. Following characterization of the differences in the function of each vaccine targeted to a different subset of DCs in vivo, the differentially targeted vaccines could be combined to boost their activities or to generate a novel effect. In addition, the DC-targeted vaccine with combination of another vaccine in different platforms may intensify the immunogenicity of both vaccines, as shown in the combination of DC-targeting and attenuated viral vaccines [32].
Footnotes
I am grateful to Anthony Rodriguez and the Steinman Laboratory for their excellent support and Hye Young Na for her help with manuscript. This work was supported by a grant (AI093216) from the National Institutes of Health in the United States and by a grant (NRF-2013R1A1A2058427) from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education in the Republic of Korea.
No potential conflict of interest relevant to this article was reported.
References
- 1.Steinman RM. Dendritic cells in vivo: a key target for a new vaccine science. Immunity. 2008;29:319–324. doi: 10.1016/j.immuni.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Schuler-Thurner B, Palucka AK, Schuler G. Dendritic cells as vectors for therapy. Cell. 2001;106:271–274. doi: 10.1016/s0092-8674(01)00448-2. [DOI] [PubMed] [Google Scholar]
- 5.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 6.Trumpfheller C, Longhi MP, Caskey M, et al. Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity. J Intern Med. 2012;271:183–192. doi: 10.1111/j.1365-2796.2011.02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 8.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 9.Sabado RL, Bhardwaj N. Dendritic cell immunotherapy. Ann N Y Acad Sci. 2013;1284:31–45. doi: 10.1111/nyas.12125. [DOI] [PubMed] [Google Scholar]
- 10.Kraal G, Breel M, Janse M, Bruin G. Langerhans' cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med. 1986;163:981–997. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang W, Swiggard WJ, Heufler C, et al. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 12.Mahnke K, Guo M, Lee S, et al. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J Cell Biol. 2000;151:673–684. doi: 10.1083/jcb.151.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lahoud MH, Ahmet F, Zhang JG, et al. DEC-205 is a cell surface receptor for CpG oligonucleotides. Proc Natl Acad Sci U S A. 2012;109:16270–16275. doi: 10.1073/pnas.1208796109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawiger D, Masilamani RF, Bettelli E, Kuchroo VK, Nussenzweig MC. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. 2004;20:695–705. doi: 10.1016/j.immuni.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Bonifaz LC, Bonnyay DP, Charalambous A, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trumpfheller C, Finke JS, Lopez CB, et al. Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. J Exp Med. 2006;203:607–617. doi: 10.1084/jem.20052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozzacco L, Trumpfheller C, Siegal FP, et al. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc Natl Acad Sci U S A. 2007;104:1289–1294. doi: 10.1073/pnas.0610383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Do Y, Koh H, Park CG, et al. Targeting of LcrV virulence protein from Yersinia pestis to dendritic cells protects mice against pneumonic plague. Eur J Immunol. 2010;40:2791–2796. doi: 10.1002/eji.201040511. [DOI] [PubMed] [Google Scholar]
- 21.Trumpfheller C, Caskey M, Nchinda G, et al. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci U S A. 2008;105:2574–2579. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longhi MP, Trumpfheller C, Idoyaga J, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. 2009;206:1589–1602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caskey M, Lefebvre F, Filali-Mouhim A, et al. Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J Exp Med. 2011;208:2357–2366. doi: 10.1084/jem.20111171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo M, Gong S, Maric S, et al. A monoclonal antibody to the DEC-205 endocytosis receptor on human dendritic cells. Hum Immunol. 2000;61:729–738. doi: 10.1016/s0198-8859(00)00144-0. [DOI] [PubMed] [Google Scholar]
- 25.Kato M, McDonald KJ, Khan S, et al. Expression of human DEC-205 (CD205) multilectin receptor on leukocytes. Int Immunol. 2006;18:857–869. doi: 10.1093/intimm/dxl022. [DOI] [PubMed] [Google Scholar]
- 26.Cheong C, Choi JH, Vitale L, et al. Improved cellular and humoral immune responses in vivo following targeting of HIV Gag to dendritic cells within human anti-human DEC205 monoclonal antibody. Blood. 2010;116:3828–3838. doi: 10.1182/blood-2010-06-288068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park CG, Rodriguez A, Ueta H, et al. Generation of anti-human DEC205/CD205 monoclonal antibodies that recognize epitopes conserved in different mammals. J Immunol Methods. 2012;377:15–22. doi: 10.1016/j.jim.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dudziak D, Kamphorst AO, Heidkamp GF, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 29.Cheong C, Matos I, Choi JH, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143:416–429. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheong C, Matos I, Choi JH, et al. New monoclonal anti-mouse DC-SIGN antibodies reactive with acetone-fixed cells. J Immunol Methods. 2010;360:66–75. doi: 10.1016/j.jim.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gardner JM, Metzger TC, McMahon EJ, et al. Extrathymic Aire-expressing cells are a distinct bone marrow-derived population that induce functional inactivation of CD4(+) T cells. Immunity. 2013;39:560–572. doi: 10.1016/j.immuni.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flynn BJ, Kastenmuller K, Wille-Reece U, et al. Immunization with HIV Gag targeted to dendritic cells followed by recombinant New York vaccinia virus induces robust T-cell immunity in nonhuman primates. Proc Natl Acad Sci U S A. 2011;108:7131–7136. doi: 10.1073/pnas.1103869108. [DOI] [PMC free article] [PubMed] [Google Scholar]