Abstract
Tuberculosis (TB) remains a worldwide health problem, causing around 2 million deaths per year. Despite the bacillus Calmette Guérin vaccine being available for more than 80 years, it has limited effectiveness in preventing TB, with inconsistent results in trials. This highlights the urgent need to develop an improved TB vaccine, based on a better understanding of host-pathogen interactions and immune responses during mycobacterial infection. Recent studies have revealed a potential role for autophagy, an intracellular homeostatic process, in vaccine development against TB, through enhanced immune activation. This review attempts to understand the host innate immune responses induced by a variety of protein antigens from Mycobacterium tuberculosis, and to identify future vaccine candidates against TB. We focus on recent advances in vaccine development strategies, through identification of new TB antigens using a variety of innovative tools. A new understanding of the host-pathogen relationship, and the usefulness of mycobacterial antigens as novel vaccine candidates, will contribute to the design of the next generation of vaccines, and to improving the host protective immune responses while limiting immunopathology during M. tuberculosis infection.
Keywords: Tuberculosis, BCG vaccine, Host-pathogen interactions, Innate immunity, Aadaptive immunity
Introduction
Tuberculosis (TB) remains a worldwide public health problem. In 2010, 75.7 of every 100,000 people were newly diagnosed in Korea, and among these newly diagnosed cases, around 12% were of drug-resistant TB [1]. There has been a global increase in multidrug- and drug-resistant TB cases [2]. The only licensed vaccine is the Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccine, a live attenuated strain of M. bovis, which has been used since 1921. Although the BCG vaccine gives consistent protection against TB infection in children, it is highly variable in protecting against adult pulmonary TB disease [3,4,5,6]. There is therefore an urgent need to develop a more effective vaccine to combat this notorious pathogen.
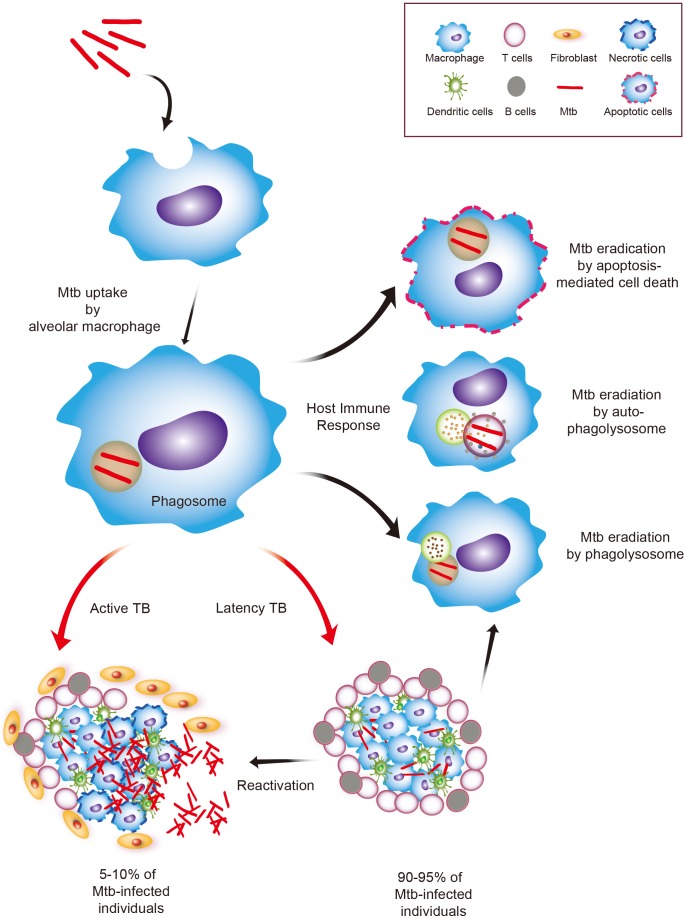
Mycobacterium tuberculosis (Mtb) is a successful human pathogen that can survive in the phagosomes and prevent normal phagosomal maturation [7]. Mtb can block the accumulation of ATPases and GTPases in the phagosomal vacuolar compartments, which interferes with phagosomal acidification [8]. This ability to subvert the host intracellular trafficking is a fundamental obstacle in the design and development of a novel vaccine [9]. To date, the main strategies for developing new vaccines to replace the BCG have focused on increasing the host protective immunity against TB. Although T cell-induced adaptive immune responses are thought to be essential for protective immunity to TB [10,11,12], emerging evidence suggests an important role for dendritic cells (DC) in initiating adaptive immunity in the development of a T cell-based protective vaccine [13]. Autophagy is a well-known intracellular degradation process of cytoplasmic constituents, including cellular organelles and protein aggregates [14,15]. It is now apparent that autophagy contributes to activation of the innate and adaptive immune responses, as well as antimicrobial responses against Mtb, through enhancing phagosomal maturation during Mtb infection [15,16,17]. More importantly, autophagy is essential for activation of immune responses via MHC class II presentation of vaccine candidate antigens [14,18]. Mtb is able to modulate the sentinel role of alveolar macrophages in alerting the surrounding cells in response to a pathogenic invasion, leading to a delay in antigen processing and priming of effector T cells [19]. This interplay between Mtb and the host cells, which is mediated through phagocyte receptor-mediated Mtb recognition, and the induction of appropriate innate immune responses, likely helps to trigger and enhance protective T cell immunity during mycobacterial infection [20].
Many Mtb immunodominant protein antigens have been identified and evaluated as potential candidates for vaccines against TB. Since the total nucleotide sequence of the Mtb genome was completed in 1998 [21] and re-annotated in 2002 [22], considerable effort has been devoted to understanding mycobacterial pathogenesis and protective immunity, by screening protein families that contain immunodominant antigens [23]. Recent discoveries of TB latency antigens have shed light on the possibility of developing post-exposure booster vaccines [24]. Additionally, new proteome-wide approaches in antigen discovery, from peptide libraries and protein microarrays, have opened up promising avenues in the search for new immunodominant antigens and potential vaccine candidates against TB [25]. In this review, we summarize the general host immune responses to Mtb and highlight the innate and adaptive protective immune responses, and autophagy activation against TB. We also discuss recent advances in the development of TB vaccines, especially in the discovery of antigens for protection against latent TB.
Overview of TB Pathogenesis
TB infection begins with mycobacterial access to the pulmonary alveoli, where the bacteria are internalized and replicate within alveolar macrophages and other phagocytes, including DC, that transport the bacteria to local, draining lymph nodes, where T cells and initiate adaptive immune responses are primed [10,26,27,28]. Mtb uses a variety of evasion strategies to resist attack from the host immune system, by blocking phagolysosome fusion and detoxifying reactive oxygen and nitrogen radicals [29]. In addition, virulent Mtb has multiple strategies to delay the early initial induction of T cell responses or to modulate antigen presentation to CD4+ T cells [27,29]. Recent studies have shown that the immune cells found in granuloma lesions have altered production capabilities of Th1 cytokines and bactericidal reactive nitrogen intermediates, with a high capacity to produce immunosuppressive interleukin (IL)-10 [29,30].
If the host immune system fails to clear Mtb, a unique pattern of immune responses is elicited, including formation of a fibrotic compartment granuloma, which is a hallmark of Mtb infection [7]. This is known as the Ghon complex [31], in which Mtb can persist in a non-metabolically active state during latent infection. However, recent studies in non-human primate models of latent TB have shown that Mtb is metabolically active and replicates in host tissues, without any clinical signs or symptoms of disease [32]. Indeed, there is a possibility that distinct Mtb subsets reside in different types of TB lesion, as seen in a monkey model of active TB [31]. Recent advances and detailed analysis of Mtb genes during latent TB infection have shed light on a potential use of dormancy antigens, including proteins encoded by the dormancy (dosR) regulon, to develop a vaccine against TB [24,33,34].
Host Protective Immune Responses during TB Infection
Despite efforts to clarify the host protective factors against TB, the complicated immune responses involved in determining disease outcomes are still poorly understood. Tissue destruction and pathogenesis during TB infection is not mediated by pathogens alone but is induced by an immunopathological inflammatory response of the host. Thus, the immunopathological inflammatory responses and protective immunity are a double edged sword in host-pathogen interactions during TB infection [35,36]. It is therefore plausible that the host-pathogen association determines the disease outcomes of TB infection, and a better understanding of host protective immunity is needed to design and develop new and improved vaccines. In this chapter, we discuss the general aspects of innate and adaptive immune responses in the context of protective immunity to TB, and review recent advances in autophagy pathway activation, in relation to a vaccine-development strategy.
Innate immune responses against TB
The mycobacterial cell wall consist of various biomolecular components, including hydrophobic mycolic acids, that are responsible for the acid-fast properties of Mtb, arabinogalactan, phosphatidyl-myo-inositol mannosides (PIMs), and peptidoglycans [37]. The other major components of the cell wall include mannose-containing biomolecules, including mannose-capped lipoarabinomannan (LAM), lipomannan (LM), and mannoglycoproteins [37]. Among the cell wall components, LM and LAM are major glycolipids and are thought to play an important role in TB pathogenesis through modulation of the host immune functions [38]. Mtb invasion starts with host recognition of Mtb outer surface molecules that can bind to the host pathogen-associated receptors, including toll-like receptor (TLR) and the c-type lectin family [35,39,40,41].
TLR is the best-characterized innate immune receptors. In mycobacterial infection, several TLRs are involved in recognition of Mtb components, and activation of the innate immune responses. For example, mycobacterial DNA exerts an immunostimulatory response through activation of TLR9 via its 5'-CG-3' CpG motif [42]. The mycobacterial glycolipids, including LM and PIM, are the main factors that activate TLR2 [43]. Several mycobacterial antigens, including a 19-kDa lipoprotein, activate innate immune responses through TLR2 [44]. In addition, several antigens are reported to activate TLR4 during a mycobacterial infection [45,46,47]. A potential role of TLR8 was suggested in TB-susceptibility in male TB patients, and in macrophage immune responses to BCG infection [48], although the detailed mechanism is not known. Previous studies have reported the role of TLRs in mycobacterial infection in the context of protective immunity and immunopathogenesis. Although this review does not go into a detailed discussion of TLR functions during TB, in vivo infections, and human genetic studies have demonstrated a partially redundant role of the TLRs in the host defense against TB infection. Indeed, multiple TLR mutations (TLR2 and TLR9 double knockout mice) led to greater susceptibility to Mtb infection [35,47,49,50].
The activation of the innate host defenses triggers intracellular signaling cascades, which results in the production of proinflammatory cytokines and chemokines, antimicrobial proteins, and antigen presentation [47]. Importantly, macrophages activated by Mtb recognition produce mainly proinflammatory cytokines, including tumor necrosis factor (TNF)-α, IL-1β, IL-18, and IL-12. An in vivo study demonstrated a critical requirement of TNF-α for protective immunity and survival after Mtb infection, as well as for granuloma formation [12]. Further genetic studies demonstrated an IL-12 receptor deficiency in patients with severe mycobacterial infection [51,52].
In the development of a vaccine, TLR ligand formulation has been suggested as having potential effects on immunostimulation as a vaccine adjuvant [53]. Recent studies have revealed the protective effects of glucopyranosyl lipid adjuvant, a TLR4 agonist, in combination with ID93, a TB vaccine antigen, to boost Th1 immune responses [54,55]. Furthermore, routes of vaccine delivery should also be considered in the design and development of vaccines. Thus, the adjuvant activity may require diverse TLR stimulation in the context of vaccine administration. For example, TLR5 signaling is potentially involved as a mucosal adjuvant in airway structural cells. Moreover, a recent study has shown that the adjuvant activity of a C-type lectin Mincle ligand, a synthetic analog of the mycobacterial glycolipid trehalose-6,6-dimycolate, requires MyD88-dependent mechanisms for induction of Th1 and Th17 immune responses [56].
Adaptive immune responses against TB
It is believed that the cellular immune response is an essential part of our protective immunity against TB. Although Th1-type CD4+ T cells producing interferon (IFN)-γ and TNF-α are necessary for protective immunity [10,11], recent studies of a cohort of BCG-vaccinated South African infants revealed that the protection against TB was not associated with the frequency of T cells producing IFN-γ, TNF-α, and IL-2 [57]. In addition, IL-17-producing CD4+ T cells are also protective and are required for the production of chemokines; the C-X-C motif chemokine ligand (CXCL) 9, CXCL10 and CXCL11, and for accumulation of IFN-γ-producing CD4+ T cells in the lung [58]. IL-17-mediated induction of CXCL13 in the lung is also crucial for recruiting CXCR5+ T cells within lymphoid structures, suggesting a significant role for the IL-17-CXCL13 pathway in improving the effects of a mucosal vaccine against TB [59]. Additionally, the cationic liposome adjuvant CAF01 (CAF01 is cationic adjuvant formulation 01), is beneficial for expansion of antigen-specific, long-term Th17 memory cells, and primes the Th1 and Th17 responses when challenged with an Mtb infection [60].
The BCG vaccination requires activation of the IL-23/IL-17 pathway for Th1 protective immunity [61]. Current vaccine development against TB is focused on induction of Th1 and Th17 memory cell proliferation, and immune responses for a protective outcome, while inhibiting immunosuppressive IL-10 responses [62,63]. However, in the absence of IL-10, the BCG-induced Th1-cell immune responses occur in an IL-17-independent manner [61]. The immunotherapeutic effects of BCG should be determined to prevent excessive inflammation caused by Th17 cells, whilst fine-tuning the total IFN-γ- and IL-17-mediated immune responses [64]. It is noted that modulation of the appropriate protective immune responses is mediated through enhanced CD8+ T cell activity, producing IFN-γ and an increase in the total γδ T cell population, in parallel with a reduction in Th17 cell numbers [64].
Although a Th1/Th2 balance is important and required for a vaccination response [65], earlier studies have shown that CD4+ Th2 cells are detrimental or do not participate in protective immunity during TB infection [66,67]. More recently, a significant role for B cells in shaping the host defense against Mtb infection has been suggested, mediated at least in part by decreasing the mycobacterial burden in the tissues, and by reducing the resultant inflammatory responses [68,69]. Notably, a recent pioneer study of a multiple antigen-presenting system to increase B-cell activation, as well as Th1 and Th17 responses, indicated its potential in the design of subunit vaccines with enhanced protective effects, mediated by multiple immune stimulation [70]. The knowledge obtained from studies of host immune responses during TB infection will contribute to the design of new TB vaccines and drugs.
Autophagy and its implication in vaccine development
Autophagy is an intracellular process for maintaining homeostasis during starvation or other stress conditions, including infectious stress [71]. It is now clear that the autophagy pathway is an essential component of the immune response against Mtb [15,16,72]. Since Mtb modulates phagosome maturation via various tactics in its attempts to evade the host immune systems, antibacterial autophagy activation through exogenous stimuli, including IFN-γ, vitamin D, rapamycin, as well as several TLRs, contribute to enhanced phagosomal acidification of Mtb within the host phagocytes [16,72,73,74].
As autophagy plays an important role in the delivery of intracellular materials to stimulate adaptive immune responses, it has been suggested that autophagy contributes to vaccine stimulation of protective immunity through enhancing antigen presentation to T cells [75,76,77,78]. Recent studies to discern the mechanisms of the yellow fever vaccine, YF-17D, have shown that YF-17D-induced GCN2 expression induces autophagy activation which enhances antigen presentation to T cells [76]. Since YF-17D is one of the most successful human vaccines [79], these findings are encouraging, and suggest that autophagy activation should be considered in the development of an improved TB vaccine. Previous studies on autophagy and the improvement of vaccines revealed that autophagy-activation in antigen-presenting cells led to enhancement of Mtb localization with autophagosomes and lysosomes, and increased Th1-mediated protection, in a mouse infection model [80].
Recent findings using DNA vaccines co-encapsulated in two Mtb plasmids, Ag85B and the kinase-defective mammalian target of rapamycin, suggested that autophagy activation during DNA vaccination elicited higher antibody responses as well as production of IFN-γ and IL-2 in the spleen [81]. These data strongly suggest an important role of autophagy in DNA vaccines that induce a protective immune response [81]. Additionally, the virulent Mtb strain (H37Rv) impaired autophagosome-lysosome fusion through the ESX-1 system, whereas avirulent Mtb (H37Ra) and BCG did not inhibit autophagic flux [82]. The inhibitory effect of virulent Mtb on autophagy activation was counteracted by treatment with rapamycin, which led to enhanced IL-12 production by DC and expansion of Th1 responses during Mtb infection [82]. To date, vaccine efficacy studies using autophagy activation have been performed in vitro and at the concept level. Future studies are urgently needed to clarify the role of autophagy in TB prevention, using multiple animal models and clinical trials.
Potential Vaccine Antigens for Protection against TB
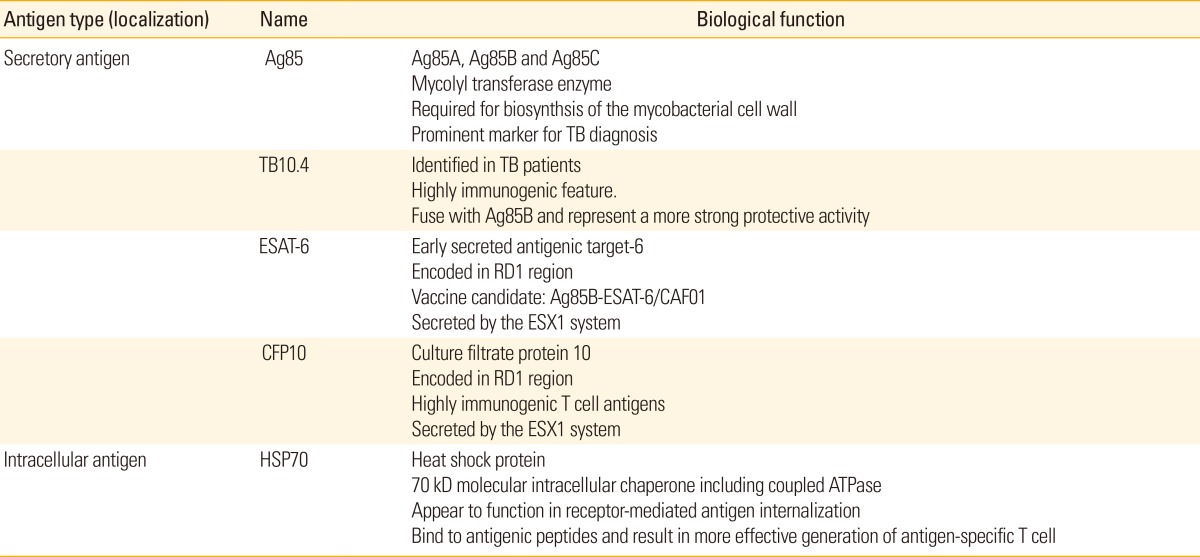
Numerous efforts have focused on identifying new mycobacterial antigens for the development of an effective TB vaccine. We discuss the principal mycobacterial protein antigens and their implications in the development of a TB vaccine (Table 1).
Table 1.
Potential candidates for TB vaccine in Mycobacterium tuberculosis protein antigens
TB, tuberculosis; RD1, regions of differences 1.
Antigen 85 complex
The antigen 85 (Ag85) complex comprises the mature secretory proteins of Mtb and BCG, and is encoded by three separate genes on the mycobacterial genome; it consists of three related antigens, Ag85A (32 kDa), Ag85B (30 kDa), and Ag85C (32.5 kDa) [83,84]. These proteins exhibit enzymatic mycolyl transferase activity and are pivotal constituents for the biosynthesis of the mycobacterial cell wall during the pathogenesis of TB [85,86]. The Ag85 complex can be detected in the blood and sputum of pulmonary TB patients, the cerebrospinal fluid of TB meningitis patients, as well as in mycobacterial liquid culture media, and thus is regarded as a prominent marker for TB diagnosis [87,88]. Ag85A and Ag85B are the major Mtb secretory proteins, and display key immunoprotective activities against TB in mouse and guinea pig infection models [89].
Clinical trials using viral vectors or protein adjuvants, including Ag85, have been undertaken to develop a replacement for the BCG vaccine. Modified vaccinia virus Ankara (MVA) 85A, is a recombinant strain, expressing Ag85A of Mtb that uses MVA as delivery system [90]. BCG-induced protection was enhanced by boosting with MVA85A in the mouse [91], guinea pig [92], and rhesus macaque models [93]. Additionally, MVA85A has been evaluated in human subjects, including healthy/BCG-vaccinated, BCG-naïve, latent Mtb, and human immunodeficiency virus-infected patients in phase I clinical trials at Oxford University (UK), since 2002 [94,95,96], and further in phase I and IIa clinical trials in the UK, South Africa, The Gambia and Senegal [97,98,99,100]. More recently, phase IIb clinical trials have evaluated the safety and efficacy of MVA85A in healthy/BCG vaccinated infants (Fig. 1) [101].
Fig. 1.
A schematic diagram of host immune system against infection of Mycobacterium tuberculosis (Mtb). Mtb is inhaled by aerosols and transmitted to the lungs, where it is phagocytized by alveolar macrophages and eliminated via various mechanisms including apoptosis and autophagy. If the bacteria growth is arrested, but not eradicated, in early stage of infection, then the disease is preserved in latent condition without symptoms of tuberculosis (TB) in 90-95% of individuals. The phase is successfully finished with appropriate induction of host innate immune responses. Otherwise, in 5-10% of cases, Mtb is replicated into macrophages and disseminated to other tissue and organ, where TB develops with typical clinical symptoms including weight loss, pain in the chest, frequent coughing, and fibrosis of lung.
Hybrid 1 is comprised of two fused, secreted antigens; early secreted antigenic target-6 (ESAT-6) and Ag85B [102], and was administered with an adjuvant, such as dimethyl dioctadecyl ammonium bromide-monophosphoryl lipid A [102], IC31 [103], and mucosal adjuvant LTK63 [104]. Preclinical studies demonstrated that hybrid 1 had a protective effect against an Mtb challenge in mouse [104,105] and guinea pig models [92,106,107]. This fusion protein was further investigated in a recent phase I clinical trial in which hybrid 1 was shown to be strongly immunogenic for both antigenic components and appeared safe in TB-naïve volunteers [108].
Regions of differences encoded proteins
Regions of differences (RD) encoded proteins, which are present in Mtb, M. africanum, and M. bovis genomes but absent from all BCG sub-strains and most environmental non-tuberculous mycobacteria [107,108], are promising candidate antigens for TB diagnosis and potential vaccines [109,110,111]. The 6-kDa ESAT-6 and culture filtrate protein 10, crucial components located in RD1 region, perform the dual roles of T cell activation and macrophage inhibition [112], and are present in the cell wall and culture supernatants of bacilli [113,114]. Previous studies have shown that the H56 subunit vaccine, a fusion-protein incorporating ESAT-6, Ag85B, combined with Rv2660c, conferred protective immunity, promoted CD4+ T cell responses, and controlled reactivation when administered post-exposure in mouse models of latent TB [115].
Several previous studies have investigated the role of Ag85-BESAT-6 (early secreted antigenic target of 6 kDa) as a highly efficient vaccine against TB [102,103,104]. The TB subunit vaccine containing Ag85B-ESAT-6/CAF01 induced sustained protective vaccine effects by inducing the proliferation of memory CD4+ T cells with TNF-α(+)IL-2(+) and IFN-γ(+) TNF-α(+)IL-2(+) multifunctional profiles [116]. Additionally, a novel TB protein, designated TB10.4, has been identified in infected TB patients and is considered a potential vaccine candidate because it induces considerable protection against TB [117]. Although Ag85B-ESAT-6 is an effective vaccine candidates, the new vaccine candidates as a substitute for ESAT-6 is required because ESAT-6 alone is also highly immunogenic. Previous study suggested that ESAT-6-related protein TB10.4, which was recognized by CD8+ T cells following infection with BCG and Mtb [118], is attractive vaccine candidate. Furthermore, a fusion protein elicits a greater response, for example, fusing Ag85B to TB10.4 produced induces a more effective immune response and was more highly protective against TB compared with vaccination with BCG or the individual antigens [116]. The fusion protein Ag85B-MPT64-Mtb8.4 had increased immunogenicity; i.e., induced greater cellular and humoral immune responses in mice, than Ag85B alone [117].
Other antigens for vaccine candidates
A novel approach has focused on the identification of vaccine candidates isolated from various body fluids from humans and animals infected with TB [119,120,121]. Reversed-phase high-performance liquid chromatography and mass spectrometric analysis identified several Mtb proteins (MT_1721, MT_1694, MT_2462, and MT_3444) from lung lesions and urine samples of pulmonary TB patients as candidate antigens for a vaccine and/or diagnostic assays for active TB [119]. Multiple efforts have focused on improving the immunogenicity of CD8+-and-CD4+ T cell activity by potent vaccine antigens [62]. A recent study of the MT1721-containing subunit and a DNA vaccine showed that use of MT1721 as the priming and boosting immunogen resulted in induction of both CD4+ Th1 and CD8+ T cell responses, which are required for an effective TB vaccine [122]. Moreover, urease-deficient BCG expressing both the Mtb-derived major membrane protein II and heat shock protein 70 efficiently activated human monocyte-derived DC, and induced differentiation of naïve T cells to antigen-specific CD4 and CD8 subsets [123].
Recent studies have revealed the role of several Mtb antigens in enhancing protective immunity through DC maturation and Th1 polarization [124,125]. For example, Mtb RpfB was found to be effective in DC activation, induced the production of high levels of proinflammatory cytokines by DC, and robustly activated adaptive immune responses through TLR4 activation [124]. Another DC-activating antigen, Rv0577, an Mtb complex-restricted protein involved in the methylglyoxal detoxification pathway, induced DC maturation and enhanced cytokine generation and polarization of Th1 responses [125].
Current Development of Live or Recombinant Mycobacterial Vaccines
There have been numerous efforts made to develop the live or recombinant mycobacterial vaccines to replace BCG vaccine though we have mainly described the subunit vaccines against TB in this review. Among them, several vaccine candidates including VPM 1002 (M. bovis BCG ∆ureC::hlyHM[R]) have been challenged to examine their immunogenicity and safety by clinical trials [126]. VPM 1002 was reported as one of the most safe and excellent vaccines to induce IFN-γ-producing T cells and antibody production activities in BCG-naïve and BCG-immunized healthy volunteers [127]. In addition, MTBVAC01, an attenuated Mtb vaccine candidate with an inactivated phoP gene encoding a key virulence protein for intracellular mycobacterial growth [128] is found to induce superior protective activity to BCG in preclinical studies [129].
Moreover, a double-blind phase 1 trial study showed that rBCG30 (recombinant BCG overexpressing Ag85b) had enhanced its ability to increase the number of Ag85b-specific CD4+ and CD8+ T cells as well as IFN-γ secretion in healthy human volunteers [130]. Previous studies showed that the Mtb72F, the stability-improved version of M72 construct candidate vaccine, exhibited a good clinical tolerance and a high immunogenicity in purified protein derivative-negative adults [131]. Recently, the phase I/II observer-blind controlled clinical trial study proposed that AS02 (adjuvant)-combined M72 vaccine induced strong and persistent cell-mediated and humoral immune responses in Mtb-naïve adults [132]. Further studies showed that AERAS-422 (research strain AFRO-1), a recombinant BCG strain co-expressing perfringolysin O, 85A, 85B, and Rv3407, had good safety profile and effects on mouse vaccine study by enhanced immune responses and increased survival to Mtb infection, compared to those vaccinated with the parental BCG strain [133]. Other studies revealed that MVA85 has a moral security in BCG-vaccinated adolescents and children, and a high immunogenicity with strong CD4+ T cell responses in both animals and humans [134]. However, recent clinical trials using MVA85A or AERAS-422 indicated an absence of efficacy and did not show any encouraging result [101]. So far, none of these recombinant or live vaccine candidates have been found to be more effective to be replaced with the BCG vaccine. The current and future efforts upon these trials with animal and human subjects will provide clearer insights into designing and development of effective vaccine formulation against Mtb infection.
Conclusion
The current BCG vaccine confers inconsistent protection against TB. The challenges in the development of new TB vaccines will be overcome by a better understanding of host-pathogen interactions during TB infection. Mtb is a successful pathogen that has developed multiple strategies to evade the host immune defenses. The innate immune system is a critical initial defense mechanism. Recognition of pathogens and induction of inflammatory cytokines and antimicrobial proteins contribute to the early clearance of Mtb during infection. Additionally, the appropriate induction of innate immune responses is required for establishment of efficient adaptive immune responses. If the immune activation is exacerbated, deleterious inflammatory responses may contribute to the immunopathogenesis of TB. Indeed, the Mtb-containing granuloma is a dynamic structure, which comprises immune cells in a localized site of infection, and is a hallmark of TB immunopathogenesis. Accumulating evidence has established a role for antibacterial autophagy in the defense against TB infection. Given the effects of autophagy in the induction of antigen presentation and T cell activation, information on its clinical applications will facilitate the development of new strategies to overcome the ability of Mtb to inhibit phagosomal acidification, and finally, in the design of new vaccines against TB.
To date, numerous approaches have been applied to identify candidate antigens that induce effective CD4+ and CD8+ T cell responses. The mycobacterial protein antigens described in this review are among the best-known immunodominant antigens, and are involved mainly in activation of the host immune responses in vitro and in vivo. Notably, several Mtb virulence factor components; i.e., ESAT-6 and other RD proteins, induce strong immune responses, making them excellent vaccine candidates. Despite the promise of some protein candidates, it is unlikely that any single protein antigen will confer protective effects against TB. Moreover, there is a lack of information on the effects of these antigens in preclinical and clinical trials. Although there is no live or recombinant candidate vaccine that is more effective than BCG vaccine in clinical trials, cumulative clinical studies will clarify to improve vaccine efficacy based on the safety, tolerance, and immunogenicity. To develop an effective TB vaccine that could replace the current BCG vaccine, the selection system must be able to identify candidates using a variety of in vivo models.
Footnotes
We are indebted to current and past members of our laboratory for discussions and investigations that contributed to this article. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2007-0054932). I apologize to colleagues whose work and publications could not be referenced owing to space constraints.
No potential conflict of interest relevant to this article was reported.
References
- 1.Kim HJ. Current status of tuberculosis in Korea. Korean J Med. 2012;82:257–262. [Google Scholar]
- 2.Abubakar I, Zignol M, Falzon D, et al. Drug-resistant tuberculosis: time for visionary political leadership. Lancet Infect Dis. 2013;13:529–539. doi: 10.1016/S1473-3099(13)70030-6. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann SH, Gengenbacher M. Recombinant live vaccine candidates against tuberculosis. Curr Opin Biotechnol. 2012;23:900–907. doi: 10.1016/j.copbio.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol. 1993;22:1154–1158. doi: 10.1093/ije/22.6.1154. [DOI] [PubMed] [Google Scholar]
- 5.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 6.Colditz GA, Brewer TF, Berkey CS, et al. Efficacy of BCG vaccine in the prevention of tuberculosis.: meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 7.O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 8.Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, et al. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich J, Doherty TM. Interaction of Mycobacterium tuberculosis with the host: consequences for vaccine development. APMIS. 2009;117:440–457. doi: 10.1111/j.1600-0463.2009.02458.x. [DOI] [PubMed] [Google Scholar]
- 10.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 11.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn JL, Goldstein MM, Chan J, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 13.Trumpfheller C, Longhi MP, Caskey M, et al. Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity. J Intern Med. 2012;271:183–192. doi: 10.1111/j.1365-2796.2011.02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gannage M, Munz C. Autophagy in MHC class II presentation of endogenous antigens. Curr Top Microbiol Immunol. 2009;335:123–140. doi: 10.1007/978-3-642-00302-8_6. [DOI] [PubMed] [Google Scholar]
- 15.Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5:527–549. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deretic V, Singh S, Master S, et al. Mycobacterium tuberculosis inhibition of phagolysosome biogenesis and autophagy as a host defence mechanism. Cell Microbiol. 2006;8:719–727. doi: 10.1111/j.1462-5822.2006.00705.x. [DOI] [PubMed] [Google Scholar]
- 17.Jo EK, Shin DM, Choi AM. Autophagy: cellular defense to excessive inflammation. Microbes Infect. 2012;14:119–125. doi: 10.1016/j.micinf.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Schmid D, Munz C. Immune surveillance via self digestion. Autophagy. 2007;3:133–135. doi: 10.4161/auto.3591. [DOI] [PubMed] [Google Scholar]
- 19.Shaler CR, Horvath C, Lai R, Xing Z. Understanding delayed T-cell priming, lung recruitment, and airway luminal T-cell responses in host defense against pulmonary tuberculosis. Clin Dev Immunol. 2012;2012:628293. doi: 10.1155/2012/628293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ottenhoff TH. New pathways of protective and pathological host defense to mycobacteria. Trends Microbiol. 2012;20:419–428. doi: 10.1016/j.tim.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Cole ST, Brosch R, Parkhill J, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 22.Camus JC, Pryor MJ, Medigue C, Cole ST. Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv. Microbiology. 2002;148(Pt 10):2967–2973. doi: 10.1099/00221287-148-10-2967. [DOI] [PubMed] [Google Scholar]
- 23.Louise R, Skjot V, Agger EM, Andersen P. Antigen discovery and tuberculosis vaccine development in the post-genomic era. Scand J Infect Dis. 2001;33:643–647. doi: 10.1080/00365540110026971. [DOI] [PubMed] [Google Scholar]
- 24.Singh S, Saraav I, Sharma S. Immunogenic potential of latency associated antigens against Mycobacterium tuberculosis. Vaccine. 2014;32:712–716. doi: 10.1016/j.vaccine.2013.11.065. [DOI] [PubMed] [Google Scholar]
- 25.Kunnath-Velayudhan S, Porcelli SA. Recent advances in defining the immunoproteome of Mycobacterium tuberculosis. Front Immunol. 2013;4:335. doi: 10.3389/fimmu.2013.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houben EN, Nguyen L, Pieters J. Interaction of pathogenic mycobacteria with the host immune system. Curr Opin Microbiol. 2006;9:76–85. doi: 10.1016/j.mib.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Urdahl KB, Shafiani S, Ernst JD. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol. 2011;4:288–293. doi: 10.1038/mi.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ernst JD. The immunological life cycle of tuberculosis. Nat Rev Immunol. 2012;12:581–591. doi: 10.1038/nri3259. [DOI] [PubMed] [Google Scholar]
- 29.Shaler CR, Kugathasan K, McCormick S, et al. Pulmonary mycobacterial granuloma increased IL-10 production contributes to establishing a symbiotic host-microbe microenvironment. Am J Pathol. 2011;178:1622–1634. doi: 10.1016/j.ajpath.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaler CR, Horvath CN, Jeyanathan M, Xing Z. Within the Enemy's Camp: contribution of the granuloma to the dissemination, persistence and transmission of Mycobacterium tuberculosis. Front Immunol. 2013;4:30. doi: 10.3389/fimmu.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barry CE, 3rd, Boshoff HI, Dartois V, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7:845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gideon HP, Flynn JL. Latent tuberculosis: what the host "sees"? Immunol Res. 2011;50:202–212. doi: 10.1007/s12026-011-8229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertholet S, Ireton GC, Ordway DJ, et al. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci Transl Med. 2010;2:53ra74. doi: 10.1126/scitranslmed.3001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin MY, Ottenhoff TH. Not to wake a sleeping giant: new insights into host-pathogen interactions identify new targets for vaccination against latent Mycobacterium tuberculosis infection. Biol Chem. 2008;389:497–511. doi: 10.1515/bc.2008.057. [DOI] [PubMed] [Google Scholar]
- 35.Jo EK. Mycobacterial interaction with innate receptors: TLRs, C-type lectins, and NLRs. Curr Opin Infect Dis. 2008;21:279–286. doi: 10.1097/QCO.0b013e3282f88b5d. [DOI] [PubMed] [Google Scholar]
- 36.Hossain MM, Norazmi MN. Pattern recognition receptors and cytokines in Mycobacterium tuberculosis infection: the double-edged sword? Biomed Res Int. 2013;2013:179174. doi: 10.1155/2013/179174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torrelles JB, Schlesinger LS. Diversity in Mycobacterium tuberculosis mannosylated cell wall determinants impacts adaptation to the host. Tuberculosis (Edinb) 2010;90:84–93. doi: 10.1016/j.tube.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Briken V, Porcelli SA, Besra GS, Kremer L. Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Mol Microbiol. 2004;53:391–403. doi: 10.1111/j.1365-2958.2004.04183.x. [DOI] [PubMed] [Google Scholar]
- 39.Korf J, Stoltz A, Verschoor J, De Baetselier P, Grooten J. The Mycobacterium tuberculosis cell wall component mycolic acid elicits pathogen-associated host innate immune responses. Eur J Immunol. 2005;35:890–900. doi: 10.1002/eji.200425332. [DOI] [PubMed] [Google Scholar]
- 40.Korbel DS, Schneider BE, Schaible UE. Innate immunity in tuberculosis: myths and truth. Microbes Infect. 2008;10:995–1004. doi: 10.1016/j.micinf.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 41.Torrelles JB, Azad AK, Schlesinger LS. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J Immunol. 2006;177:1805–1816. doi: 10.4049/jimmunol.177.3.1805. [DOI] [PubMed] [Google Scholar]
- 42.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 43.Ferwerda G, Girardin SE, Kullberg BJ, et al. NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog. 2005;1:279–285. doi: 10.1371/journal.ppat.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 45.Jo EK, Yang CS, Choi CH, Harding CV. Intracellular signalling cascades regulating innate immune responses to mycobacteria: branching out from Toll-like receptors. Cell Microbiol. 2007;9:1087–1098. doi: 10.1111/j.1462-5822.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- 46.Jung SB, Yang CS, Lee JS, et al. The mycobacterial 38-kilodalton glycolipoprotein antigen activates the mitogen-activated protein kinase pathway and release of proinflammatory cytokines through Toll-like receptors 2 and 4 in human monocytes. Infect Immun. 2006;74:2686–2696. doi: 10.1128/IAI.74.5.2686-2696.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kleinnijenhuis J, Oosting M, Joosten LA, Netea MG, Van Crevel R. Innate immune recognition of Mycobacterium tuberculosis. Clin Dev Immunol. 2011;2011:405310. doi: 10.1155/2011/405310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davila S, Hibberd ML, Hari Dass R, et al. Genetic association and expression studies indicate a role of toll-like receptor 8 in pulmonary tuberculosis. PLoS Genet. 2008;4:e1000218. doi: 10.1371/journal.pgen.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med. 2005;202:1715–1724. doi: 10.1084/jem.20051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Basu J, Shin DM, Jo EK. Mycobacterial signaling through toll-like receptors. Front Cell Infect Microbiol. 2012;2:145. doi: 10.3389/fcimb.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Altare F, Durandy A, Lammas D, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 52.de Jong R, Altare F, Haagen IA, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280:1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 53.Fox CB, Moutaftsi M, Vergara J, et al. TLR4 ligand formulation causes distinct effects on antigen-specific cell-mediated and humoral immune responses. Vaccine. 2013;31:5848–5855. doi: 10.1016/j.vaccine.2013.09.069. [DOI] [PubMed] [Google Scholar]
- 54.Orr MT, Fox CB, Baldwin SL, et al. Adjuvant formulation structure and composition are critical for the development of an effective vaccine against tuberculosis. J Control Release. 2013;172:190–200. doi: 10.1016/j.jconrel.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baldwin SL, Bertholet S, Reese VA, Ching LK, Reed SG, Coler RN. The importance of adjuvant formulation in the development of a tuberculosis vaccine. J Immunol. 2012;188:2189–2197. doi: 10.4049/jimmunol.1102696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Desel C, Werninghaus K, Ritter M, et al. The Mincle-activating adjuvant TDB induces MyD88-dependent Th1 and Th17 responses through IL-1R signaling. PLoS One. 2013;8:e53531. doi: 10.1371/journal.pone.0053531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kagina BM, Abel B, Scriba TJ, et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guerin vaccination of newborns. Am J Respir Crit Care Med. 2010;182:1073–1079. doi: 10.1164/rccm.201003-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khader SA, Bell GK, Pearl JE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 59.Gopal R, Rangel-Moreno J, Slight S, et al. Interleukin-17-dependent CXCL13 mediates mucosal vaccine-induced immunity against tuberculosis. Mucosal Immunol. 2013;6:972–984. doi: 10.1038/mi.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lindenstrom T, Woodworth J, Dietrich J, Aagaard C, Andersen P, Agger EM. Vaccine-induced th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset. Infect Immun. 2012;80:3533–3544. doi: 10.1128/IAI.00550-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gopal R, Lin Y, Obermajer N, et al. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur J Immunol. 2012;42:364–373. doi: 10.1002/eji.201141569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cayabyab MJ, Macovei L, Campos-Neto A. Current and novel approaches to vaccine development against tuberculosis. Front Cell Infect Microbiol. 2012;2:154. doi: 10.3389/fcimb.2012.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pitt JM, Stavropoulos E, Redford PS, et al. Blockade of IL-10 signaling during bacillus Calmette-Guerin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-gamma and IL-17 responses and increases protection to Mycobacterium tuberculosis infection. J Immunol. 2012;189:4079–4087. doi: 10.4049/jimmunol.1201061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zarate-Blades CR, Rodrigues RF, Souza PR, et al. Evaluation of the overall IFN-gamma and IL-17 pro-inflammatory responses after DNA therapy of tuberculosis. Hum Vaccin Immunother. 2013;9:1093–1103. doi: 10.4161/hv.23417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Agger EM, Cassidy JP, Brady J, Korsholm KS, Vingsbo-Lundberg C, Andersen P. Adjuvant modulation of the cytokine balance in Mycobacterium tuberculosis subunit vaccines; immunity, pathology and protection. Immunology. 2008;124:175–185. doi: 10.1111/j.1365-2567.2007.02751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lindblad EB, Elhay MJ, Silva R, Appelberg R, Andersen P. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect Immun. 1997;65:623–629. doi: 10.1128/iai.65.2.623-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ordway DJ, Costa L, Martins M, et al. Increased interleukin-4 production by CD8 and gammadelta T cells in health-care workers is associated with the subsequent development of active tuberculosis. J Infect Dis. 2004;190:756–766. doi: 10.1086/422532. [DOI] [PubMed] [Google Scholar]
- 68.Glatman-Freedman A. The role of antibody-mediated immunity in defense against Mycobacterium tuberculosis: advances toward a novel vaccine strategy. Tuberculosis (Edinb) 2006;86:191–197. doi: 10.1016/j.tube.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 69.Maglione PJ, Chan J. How B cells shape the immune response against Mycobacterium tuberculosis. Eur J Immunol. 2009;39:676–686. doi: 10.1002/eji.200839148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang F, Lu YJ, Malley R. Multiple antigen-presenting system (MAPS) to induce comprehensive B- and T-cell immunity. Proc Natl Acad Sci U S A. 2013;110:13564–13569. doi: 10.1073/pnas.1307228110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuma A, Hatano M, Matsui M, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 72.Jo EK, Yuk JM, Shin DM, Sasakawa C. Roles of autophagy in elimination of intracellular bacterial pathogens. Front Immunol. 2013;4:97. doi: 10.3389/fimmu.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 74.Yuk JM, Shin DM, Lee HM, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–243. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 75.Munz C. Enhancing immunity through autophagy. Annu Rev Immunol. 2009;27:423–449. doi: 10.1146/annurev.immunol.021908.132537. [DOI] [PubMed] [Google Scholar]
- 76.Ravindran R, Khan N, Nakaya HI, et al. Vaccine activation of the nutrient sensor GCN2 in dendritic cells enhances antigen presentation. Science. 2014;343:313–317. doi: 10.1126/science.1246829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ni Cheallaigh C, Keane J, Lavelle EC, Hope JC, Harris J. Autophagy in the immune response to tuberculosis: clinical perspectives. Clin Exp Immunol. 2011;164:291–300. doi: 10.1111/j.1365-2249.2011.04381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tey SK, Khanna R. Host immune system strikes back: autophagy-mediated antigen presentation bypasses viral blockade of the classic MHC class I processing pathway. Autophagy. 2012;8:1839–1841. doi: 10.4161/auto.21860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Monath TP. Review of the risks and benefits of yellow fever vaccination including some new analyses. Expert Rev Vaccines. 2012;11:427–448. doi: 10.1586/erv.12.6. [DOI] [PubMed] [Google Scholar]
- 80.Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Jr, Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–276. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- 81.Meerak J, Wanichwecharungruang SP, Palaga T. Enhancement of immune response to a DNA vaccine against Mycobacterium tuberculosis Ag85B by incorporation of an autophagy inducing system. Vaccine. 2013;31:784–790. doi: 10.1016/j.vaccine.2012.11.075. [DOI] [PubMed] [Google Scholar]
- 82.Romagnoli A, Etna MP, Giacomini E, et al. ESX-1 dependent impairment of autophagic flux by Mycobacterium tuberculosis in human dendritic cells. Autophagy. 2012;8:1357–1370. doi: 10.4161/auto.20881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Content J, de la Cuvellerie A, De Wit L, Vincent-Levy-Frebault V, Ooms J, De Bruyn J. The genes coding for the antigen 85 complexes of Mycobacterium tuberculosis and Mycobacterium bovis BCG are members of a gene family: cloning, sequence determination, and genomic organization of the gene coding for antigen 85-C of M. tuberculosis. Infect Immun. 1991;59:3205–3212. doi: 10.1128/iai.59.9.3205-3212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wiker HG, Sletten K, Nagai S, Harboe M. Evidence for three separate genes encoding the proteins of the mycobacterial antigen 85 complex. Infect Immun. 1990;58:272–274. doi: 10.1128/iai.58.1.272-274.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anderson DH, Harth G, Horwitz MA, Eisenberg D. An interfacial mechanism and a class of inhibitors inferred from two crystal structures of the Mycobacterium tuberculosis 30 kDa major secretory protein (Antigen 85B), a mycolyl transferase. J Mol Biol. 2001;307:671–681. doi: 10.1006/jmbi.2001.4461. [DOI] [PubMed] [Google Scholar]
- 86.Abou-Zeid C, Ratliff TL, Wiker HG, Harboe M, Bennedsen J, Rook GA. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect Immun. 1988;56:3046–3051. doi: 10.1128/iai.56.12.3046-3051.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malen H, Softeland T, Wiker HG. Antigen analysis of Mycobacterium tuberculosis H37Rv culture filtrate proteins. Scand J Immunol. 2008;67:245–252. doi: 10.1111/j.1365-3083.2007.02064.x. [DOI] [PubMed] [Google Scholar]
- 88.Kashyap RS, Ramteke SP, Deshpande PS, Purohit HJ, Taori GM, Daginawala HF. Comparison of an adenosine deaminase assay with ELISA for the diagnosis of tuberculous meningitis infection. Med Sci Monit. 2007;13:BR200–BR204. [PubMed] [Google Scholar]
- 89.Horwitz MA, Lee BW, Dillon BJ, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McShane H, Brookes R, Gilbert SC, Hill AV. Enhanced immunogenicity of CD4(+) t-cell responses and protective efficacy of a DNA-modified vaccinia virus Ankara prime-boost vaccination regimen for murine tuberculosis. Infect Immun. 2001;69:681–686. doi: 10.1128/IAI.69.2.681-686.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AV. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J Immunol. 2003;171:1602–1609. doi: 10.4049/jimmunol.171.3.1602. [DOI] [PubMed] [Google Scholar]
- 92.Williams A, Goonetilleke NP, McShane H, et al. Boosting with poxviruses enhances Mycobacterium bovis BCG efficacy against tuberculosis in guinea pigs. Infect Immun. 2005;73:3814–3816. doi: 10.1128/IAI.73.6.3814-3816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Verreck FA, Vervenne RA, Kondova I, et al. MVA.85A boosting of BCG and an attenuated, phoP deficient M. tuberculosis vaccine both show protective efficacy against tuberculosis in rhesus macaques. PLoS One. 2009;4:e5264. doi: 10.1371/journal.pone.0005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McShane H, Pathan AA, Sander CR, et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004;10:1240–1244. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 95.Sander CR, Pathan AA, Beveridge NE, et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in Mycobacterium tuberculosis-infected individuals. Am J Respir Crit Care Med. 2009;179:724–733. doi: 10.1164/rccm.200809-1486OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pathan AA, Sander CR, Fletcher HA, et al. Boosting BCG with recombinant modified vaccinia ankara expressing antigen 85A: different boosting intervals and implications for efficacy trials. PLoS One. 2007;2:e1052. doi: 10.1371/journal.pone.0001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Scriba TJ, Tameris M, Mansoor N, et al. Modified vaccinia Ankara-expressing Ag85A, a novel tuberculosis vaccine, is safe in adolescents and children, and induces polyfunctional CD4+ T cells. Eur J Immunol. 2010;40:279–290. doi: 10.1002/eji.200939754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brookes RH, Hill PC, Owiafe PK, et al. Safety and immunogenicity of the candidate tuberculosis vaccine MVA85A in West Africa. PLoS One. 2008;3:e2921. doi: 10.1371/journal.pone.0002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hawkridge T, Scriba TJ, Gelderbloem S, et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in healthy adults in South Africa. J Infect Dis. 2008;198:544–552. doi: 10.1086/590185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ibanga HB, Brookes RH, Hill PC, et al. Early clinical trials with a new tuberculosis vaccine, MVA85A, in tuberculosis-endemic countries: issues in study design. Lancet Infect Dis. 2006;6:522–528. doi: 10.1016/S1473-3099(06)70552-7. [DOI] [PubMed] [Google Scholar]
- 101.Tameris MD, Hatherill M, Landry BS, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381:1021–1028. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weinrich Olsen A, van Pinxteren LA, Meng Okkels L, Birk Rasmussen P, Andersen P. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85b and esat-6. Infect Immun. 2001;69:2773–2778. doi: 10.1128/IAI.69.5.2773-2778.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McShane H. Tuberculosis vaccines: beyond bacille Calmette-Guerin. Philos Trans R Soc Lond B Biol Sci. 2011;366:2782–2789. doi: 10.1098/rstb.2011.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dietrich J, Andersen C, Rappuoli R, Doherty TM, Jensen CG, Andersen P. Mucosal administration of Ag85B-ESAT-6 protects against infection with Mycobacterium tuberculosis and boosts prior bacillus Calmette-Guerin immunity. J Immunol. 2006;177:6353–6360. doi: 10.4049/jimmunol.177.9.6353. [DOI] [PubMed] [Google Scholar]
- 105.Kamath AT, Rochat AF, Valenti MP, et al. Adult-like anti-mycobacterial T cell and in vivo dendritic cell responses following neonatal immunization with Ag85B-ESAT-6 in the IC31 adjuvant. PLoS One. 2008;3:e3683. doi: 10.1371/journal.pone.0003683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Olsen AW, Williams A, Okkels LM, Hatch G, Andersen P. Protective effect of a tuberculosis subunit vaccine based on a fusion of antigen 85B and ESAT-6 in the aerosol guinea pig model. Infect Immun. 2004;72:6148–6150. doi: 10.1128/IAI.72.10.6148-6150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Agger EM, Rosenkrands I, Olsen AW, et al. Protective immunity to tuberculosis with Ag85B-ESAT-6 in a synthetic cationic adjuvant system IC31. Vaccine. 2006;24:5452–5460. doi: 10.1016/j.vaccine.2006.03.072. [DOI] [PubMed] [Google Scholar]
- 108.van Dissel JT, Arend SM, Prins C, et al. Ag85B-ESAT-6 adjuvanted with IC31 promotes strong and long-lived Mycobacterium tuberculosis specific T cell responses in naive human volunteers. Vaccine. 2010;28:3571–3581. doi: 10.1016/j.vaccine.2010.02.094. [DOI] [PubMed] [Google Scholar]
- 109.Wang S, Chen J, Zhang Y, et al. Mycobacterium tuberculosis region of difference (RD) 2 antigen Rv1985c and RD11 antigen Rv3425 have the promising potential to distinguish patients with active tuberculosis from M. bovis BCG-vaccinated individuals. Clin Vaccine Immunol. 2013;20:69–76. doi: 10.1128/CVI.00481-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ansari MA, Zubair S, Mahmood A, et al. RD antigen based nanovaccine imparts long term protection by inducing memory response against experimental murine tuberculosis. PLoS One. 2011;6:e22889. doi: 10.1371/journal.pone.0022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lindenstrom T, Agger EM, Korsholm KS, et al. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J Immunol. 2009;182:8047–8055. doi: 10.4049/jimmunol.0801592. [DOI] [PubMed] [Google Scholar]
- 112.Ganguly N, Siddiqui I, Sharma P. Role of M. tuberculosis RD-1 region encoded secretory proteins in protective response and virulence. Tuberculosis (Edinb) 2008;88:510–517. doi: 10.1016/j.tube.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 113.Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol. 2002;46:709–717. doi: 10.1046/j.1365-2958.2002.03237.x. [DOI] [PubMed] [Google Scholar]
- 114.Lewis KN, Liao R, Guinn KM, et al. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guerin attenuation. J Infect Dis. 2003;187:117–123. doi: 10.1086/345862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aagaard C, Hoang T, Dietrich J, et al. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med. 2011;17:189–194. doi: 10.1038/nm.2285. [DOI] [PubMed] [Google Scholar]
- 116.Dietrich J, Aagaard C, Leah R, et al. Exchanging ESAT6 with TB10.4 in an Ag85B fusion molecule-based tuberculosis subunit vaccine: efficient protection and ESAT6-based sensitive monitoring of vaccine efficacy. J Immunol. 2005;174:6332–6339. doi: 10.4049/jimmunol.174.10.6332. [DOI] [PubMed] [Google Scholar]
- 117.Luo Y, Wang B, Hu L, et al. Fusion protein Ag85B-MPT64 (190-198)-Mtb8.4 has higher immunogenicity than Ag85B with capacity to boost BCG-primed immunity against Mycobacterium tuberculosis in mice. Vaccine. 2009;27:6179–6185. doi: 10.1016/j.vaccine.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 118.Majlessi L, Rojas MJ, Brodin P, Leclerc C. CD8+-T-cell responses of Mycobacterium-infected mice to a newly identified major histocompatibility complex class I-restricted epitope shared by proteins of the ESAT-6 family. Infect Immun. 2003;71:7173–7177. doi: 10.1128/IAI.71.12.7173-7177.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kashino SS, Pollock N, Napolitano DR, Rodrigues V, Jr, Campos-Neto A. Identification and characterization of Mycobacterium tuberculosis antigens in urine of patients with active pulmonary tuberculosis: an innovative and alternative approach of antigen discovery of useful microbial molecules. Clin Exp Immunol. 2008;153:56–62. doi: 10.1111/j.1365-2249.2008.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mukherjee S, Kashino SS, Zhang Y, et al. Cloning of the gene encoding a protective Mycobacterium tuberculosis secreted protein detected in vivo during the initial phases of the infectious process. J Immunol. 2005;175:5298–5305. doi: 10.4049/jimmunol.175.8.5298. [DOI] [PubMed] [Google Scholar]
- 121.Napolitano DR, Pollock N, Kashino SS, Rodrigues V, Jr, Campos-Neto A. Identification of Mycobacterium tuberculosis ornithine carboamyltransferase in urine as a possible molecular marker of active pulmonary tuberculosis. Clin Vaccine Immunol. 2008;15:638–643. doi: 10.1128/CVI.00010-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cayabyab MJ, Kashino SS, Campos-Neto A. Robust immune response elicited by a novel and unique Mycobacterium tuberculosis protein using an optimized DNA/protein heterologous prime/boost protocol. Immunology. 2012;135:216–225. doi: 10.1111/j.1365-2567.2011.03525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mukai T, Tsukamoto Y, Maeda Y, Tamura T, Makino M. Efficient activation of human T cells of both CD4 and CD8 subsets by urease-deficient recombinant Mycobacterium bovis BCG that produced a heat shock protein 70-M. tuberculosis-derived major membrane protein II fusion protein. Clin Vaccine Immunol. 2014;21:1–11. doi: 10.1128/CVI.00564-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim JS, Kim WS, Choi HG, et al. Mycobacterium tuberculosis RpfB drives Th1-type T cell immunity via a TLR4-dependent activation of dendritic cells. J Leukoc Biol. 2013;94:733–749. doi: 10.1189/jlb.0912435. [DOI] [PubMed] [Google Scholar]
- 125.Byun EH, Kim WS, Kim JS, et al. Mycobacterium tuberculosis Rv0577, a novel TLR2 agonist, induces maturation of dendritic cells and drives Th1 immune response. FASEB J. 2012;26:2695–2711. doi: 10.1096/fj.11-199588. [DOI] [PubMed] [Google Scholar]
- 126.Rowland R, McShane H. Tuberculosis vaccines in clinical trials. Expert Rev Vaccines. 2011;10:645–658. doi: 10.1586/erv.11.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Grode L, Ganoza CA, Brohm C, Weiner J, 3rd, Eisele B, Kaufmann SH. Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine. 2013;31:1340–1348. doi: 10.1016/j.vaccine.2012.12.053. [DOI] [PubMed] [Google Scholar]
- 128.Perez E, Samper S, Bordas Y, Guilhot C, Gicquel B, Martin C. An essential role for phoP in Mycobacterium tuberculosis virulence. Mol Microbiol. 2001;41:179–187. doi: 10.1046/j.1365-2958.2001.02500.x. [DOI] [PubMed] [Google Scholar]
- 129.Arbues A, Aguilo JI, Gonzalo-Asensio J, et al. Construction, characterization and preclinical evaluation of MTBVAC, the first live-attenuated M. tuberculosis-based vaccine to enter clinical trials. Vaccine. 2013;31:4867–4873. doi: 10.1016/j.vaccine.2013.07.051. [DOI] [PubMed] [Google Scholar]
- 130.Hoft DF, Blazevic A, Abate G, et al. A new recombinant bacille Calmette-Guerin vaccine safely induces significantly enhanced tuberculosis-specific immunity in human volunteers. J Infect Dis. 2008;198:1491–1501. doi: 10.1086/592450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Leroux-Roels I, Leroux-Roels G, Ofori-Anyinam O, et al. Evaluation of the safety and immunogenicity of two antigen concentrations of the Mtb72F/AS02(A) candidate tuberculosis vaccine in purified protein derivative-negative adults. Clin Vaccine Immunol. 2010;17:1763–1771. doi: 10.1128/CVI.00133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leroux-Roels I, Forgus S, De Boever F, et al. Improved CD4(+) T cell responses to Mycobacterium tuberculosis in PPD-negative adults by M72/AS01 as compared to the M72/AS02 and Mtb72F/AS02 tuberculosis candidate vaccine formulations: a randomized trial. Vaccine. 2013;31:2196–2206. doi: 10.1016/j.vaccine.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 133.Sun R, Skeiky YA, Izzo A, et al. Novel recombinant BCG expressing perfringolysin O and the over-expression of key immunodominant antigens; pre-clinical characterization, safety and protection against challenge with Mycobacterium tuberculosis. Vaccine. 2009;27:4412–4423. doi: 10.1016/j.vaccine.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 134.Nicol MP, Grobler LA. MVA-85A, a novel candidate booster vaccine for the prevention of tuberculosis in children and adults. Curr Opin Mol Ther. 2010;12:124–134. [PubMed] [Google Scholar]