Abstract
Purpose
Protein cages are promising nanoplatform candidates for efficient delivery systems due to their homogenous size and structure with high biocompatibility and biodegradability. In this study, we investigate the potential of lumazine synthase protein cage as an antigen delivery system to dendritic cells (DCs), which induce antigen-specific T cell proliferation.
Materials and Methods
Ovalbumin (OVA) peptides OT-1 (SIINFEKL) and OT-2 (ISQAVHAAHAEINEAGR) were genetically inserted to lumazine synthase and each protein cage was over-expressed in Escherichia coli as a soluble protein. The efficiency of antigen delivery and the resulting antigen-specific T cell proliferation by DCs was examined in vitro as well as in vivo.
Results
We successfully generated and characterized OVA peptides carrying lumazine synthase protein cages. The OT-1 and OT-2 peptides carried by lumazine synthases were efficiently delivered and processed by DCs in vitro as well as in vivo, and induced proliferation of OT-1-specific CD8+T cells and OT-2-specific CD4+T cells.
Conclusion
Our data demonstrate the potential of lumazine synthase protein cage being used as a novel antigen delivery system for DC-based vaccine development in future clinical applications.
Keywords: Dendritic cells, Protein cage, Antigen presentation, Vaccines, Nanoparticles
Introduction
Protein cages, such as ferritins, small heat shock proteins, and viral capsids are promising nanoplatform candidates for efficient delivery systems because they have uniform size, structure, high biocompatibility and biodegradability [1, 2, 3, 4, 5, 6]. The protein cages are spontaneously self-assembled from multiple copies of one or a few types of protein subunits in a precisely controlled manner [1, 2, 3, 4, 5, 6]. Also, they can be genetically and chemically manipulated to have desired functionality by rational design on the basis of atomic resolution structural information.
The lumazine synthase, isolated from hyperthermophile Aquifex aeolicus (AaLS), consists of 60 identical subunits forming an icosahedral capsid architecture (T=1 state) with a 15.4 nm exterior and a 9 nm interior diameters, respectively [7]. The AaLS originally catalyzes the penultimate step in riboflavin biosynthesis within the cell. Outside of the cell, its hollow sphere architecture has been used as a template for encapsulation of cargo proteins such as green fluorescent proteins [8], human immunodeficiency virus proteases [9], crystallization of inorganic nanoparticles such as iron oxide [10], and fabrication of uniform layer-by-layer assemblies using non-covalent interaction between surface displayed hexahisitidine and Ni-NTA of the AaLS [11].
Dendritic cells (DCs) are the most potent professional antigen-presenting cells and key regulator of antigen-specific immune responses [12]. Immature DCs continously uptake and process antigens. Upon danger signal, the antigen-loaded DCs migrate into draining lymph nodes where DCs present antigens to naïve T cells leading to their proliferation and differentiation into effector cells; CD8+ cytotoxic T cells to kill infected target cells or CD4+ helper T cells to secrete cytokines and facilitate diverse forms of cellular and humoral immunity. Therefore, based on such seminal roles of DCs in the immune system, DC-based vaccine development has been a promising approach to direct antigen-specific adaptive immunity [13].
Efficient antigen delivery to DCs is the first step for DC-based vaccine development and simple and controllable antigen delivery system is required. Up to date, nano-sized materials, including polymers, inorganic nanoparticles, liposomes, and/or virus-like particle (VLP), have been extensively used as antigen delivery nanoplatforms for studying antigen-specific immune responses and further applied them to follow-up vaccine development. However, there is a limited amount of study utilizing protein cage nanoparticles to deliver antigens to DCs for immunization and vaccination. In our previous study, we utilized ferritin protein cage nanoparticles as versatile antigen delivery nanoplatforms for DC-based vaccine development and demonstrated DC-mediated antigen specific CD4+ and CD8+ T cell immune responses [14]. In the current study, we introduce lumazine synthase protein cage nanoparticles (AaLS) for carrying antigenic peptides to DCs and investigate DC-mediated antigen-specific T cell proliferation. Our data demonstrate the potential of AaLS as a novel antigen delivery system for DC-based vaccine development in future clinical applications.
Materials and Methods
Antigenic peptide addition and protein cage purification
The OT-1 (SIINFEKL) and OT-2 (ISQAVHAAHAEINEAGR) peptides were added at the N-terminal and the C-terminal ends of AaLS subunit, respectively, by an established polymerase chain reaction protocol using primers containing specific enzyme restriction sites and pETDuet based plasmids containing genes encoding AaLS protein as a template. The amplified DNAs were used to transform the competent Escherichia coli strain BL21 (DE), resulting in the over-expression in E. coli of the AaLS protein cages containing added antigenic peptides. The resultant protein was purified as described previously [11].
Mass spectrometry
The subunit masses of AaLS-OT-2, AaLS-OT-1, and wt AaLS protein cages were analyzed using an electrospray ionization time-of-flight mass (ESI-TOF) mass spectrometer (Xevo G2 TOF, Waters, Milford, MA, USA) interfaced to a Waters UPLC and an autosampler. Samples were loaded onto the MassPREP Micro desalting column (Waters) and eluted with a gradient of 5-95% (v/v) acetonitrile containing 0.1% formic acid with a flow rate of 300 µL/min [11]. Mass spectra were acquired in the range of m/z 500-3,000 and processed using MaxEnt 1 and MaxEnt 3 from MassLynx version 4.1 to obtain the average mass from multiple charge state distributions.
Mice
C57BL/6 mice were purchased from Taconic, and CD45.2+ OT-1 and CD45.2+ OT-2 mice were purchased from the Jackson Laboratory. All mice were maintained under specific pathogen-free conditions and used at 6-8 weeks with Institutional Animal Care and Use Committee guidelines.
Isolation of splenic CD11c+ DCs and ovalbumin-specific T cells
Single cell suspensions of splenocytes were prepared with 400 U/mL collagenase D (Roche, Basel, Switzerland) and CD11c+ cells were positively enriched with magnetic-activated cell sorting (MACS) sorting (Miltenyl Biotech, Bergisch Gladbach, Germany). Ovalbumin (OVA)-specific CD8+ or CD4+ T cells were prepared from OT-1 or OT-2 mice, respectively. Briefly, single cell suspensions from lymph nodes and spleens were prepared and CD8+ T cells or CD4+ T cells were positively enriched by MACS sorting. Sorted T cells showed >98% purity as detected by flow cytometry. All flow cytometry data were acquired by BD FACS Calibur and analyzed by FlowJo software (TreeStar, San Carlos, CA, USA).
Carboxyfluorescein succinimidyl ester-dilution assay to detect antigen-specific T cell proliferation in vitro and in vivo
For in vitro OVA-specific T cell proliferation assay, OT-1 or OT-2 T cells were stained with 0.5 µM of carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen, Carlsbad, CA, USA) at 1×107 cells/mL for 10 minutes at 37℃. CD11c+ DCs were pulsed with indicated proteins for 3 hours, washed and then co-cultured with the CFSE-labeled T cells at a ratio of 1:3 (DC, 1×105: T cell, 3×105) in a 96-well U-bottom plate at 37℃. After 4 days, cells were harvested and then stained for Vβ5.1/5.2 (Biolegend, San Diego, CA, USA), Vα2 and CD8 or CD4 (all from BD, San Jose, CA, USA). For in vivo OVA-specific T cell proliferation assay, naïve C57BL/6 mice were adoptively transferred with 5 µM CFSE-labeled OT-1 or OT-2 T cells intravenously at day -1. At day 0, mice were injected with indicated proteins in the footpads subcutaneously in the presence of 50 µg of poly (I:C) (Invivogen, San Diego, CA, USA) as an adjuvant. At day 3, single cell suspensions were prepared from lymph nodes. OVA-specific T cells (Vβ5.1/5.2+Vα2+CD8+ or Vβ5.1/5.2+Vα2+CD4+) were gated and progressive halving of CFSE fluorescence per cell was measured by flow cytometry.
Results
Surface and ribbon diagram representations of lumazine synthase (AaLS) were depicted (Fig. 1) and a schematic diagram showing DC-mediated antigen-specific T cell proliferation induced by AaLS carrying OT peptides was shown in Fig. 2.
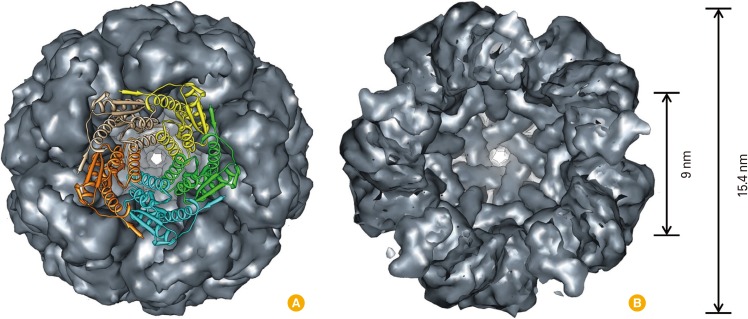
Fig. 1.
Surface and ribbon diagram representations of AaLS (PDB: 1HQK) looking down the five-fold symmetry axis (A) and clipped view showing the interior space (9 nm interior and 15.4 nm exterior diameters) of the protein cage (B). Five subunits were shown as ribbon diagram with individual colors.
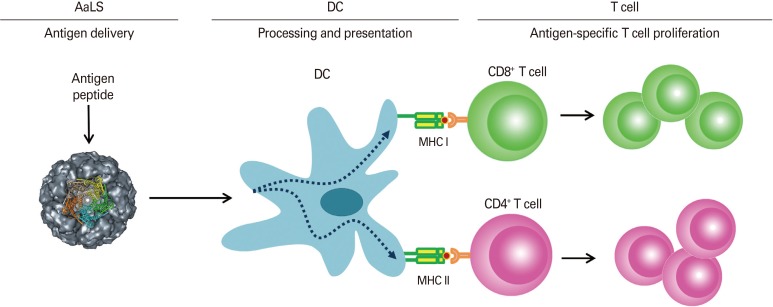
Fig. 2.
A schematic diagram showing dendritic cell (DC)-mediated antigen-specific T cell proliferation induced by lumazine synthase protein cage nanoparticles (AaLS) carrying OT peptides.
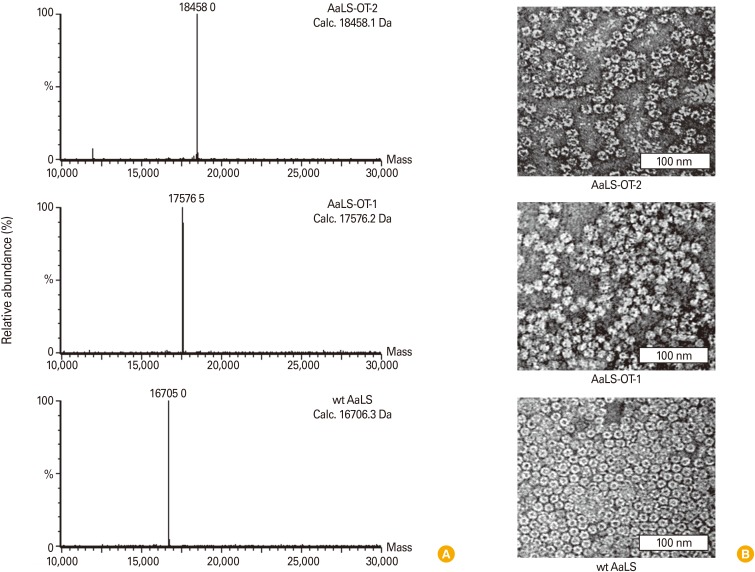
To adapt AaLS protein cage as antigen delivery nanoplatforms, we genetically introduced OT-1 (SIINFEKL) and OT-2 (ISQAVHAAHAEINEAGR) peptides to the N-terminal and the C-terminal ends of AaLS subunit, respectively. Antigenic peptide insertions were confirmed by DNA sequencing and molecular mass measurement of subunits of OT-1 or OT-2 peptide added AaLS protein cages (AaLS-OT-1 or AaLS-OT-2). AaLS-OT-1 and AaLS-OT-2 were individually over-expressed in E. coli as a soluble protein without noticeable amounts of precipitation. OT-peptide inserted AaLS protein cages were purified by the same method used to purify wild-type AaLS (wt AaLS), which do not have any OT peptide insertions. ESI-TOF mass spectrometry analysis indicated that the subunit masses of AaLS-OT-2, AaLS-OT-1, and wt AaLS protein cages were 18,458.0 Da, 17,576.5 Da, and 16,705.0 Da, respectively, which are well matched with their predicted masses (18,458.1 Da, 17,576.2 Da, and 16,706.3 Da, respectively) (Fig. 3A). Transmission electron microscopic images of stained AaLS-OT-2, AaLS-OT-1, and wt AaLS protein cages confirmed an intact cage architecture with a uniform size distribution (~16 nm) (Fig. 3B). These results indicate that AaLS-OT-2 and AaLS-OT-1 protein cages form an intact protein cage architecture without significant changes in size and composition.
Fig. 3.
Characterization of OT-peptide added AaLS protein cages. (A) Molecular masses of dissociated subunits of AaLS-OT-2 (top), AaLS-OT-1 (middle), and wt AaLS (bottom) protein cages. Calculated (Calc.) and measured molecular masses were indicated. (B) Transmission electron micrographic image of 2% uranyl acetate stained AaLS-OT-2 (top), AaLS-OT-1 (middle), and wt AaLS (bottom) protein cages confirmed an intact cage architecture with a uniform size distribution.
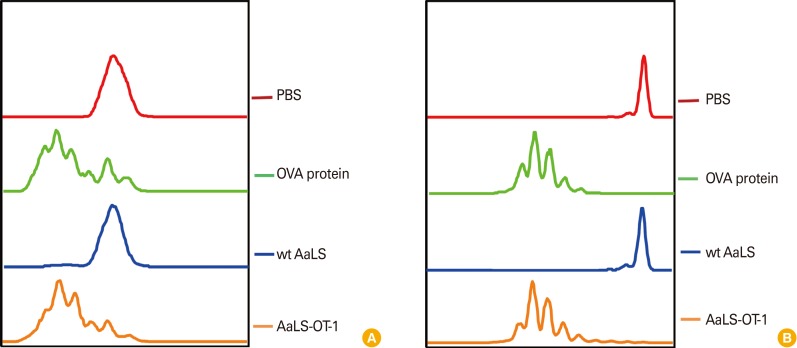
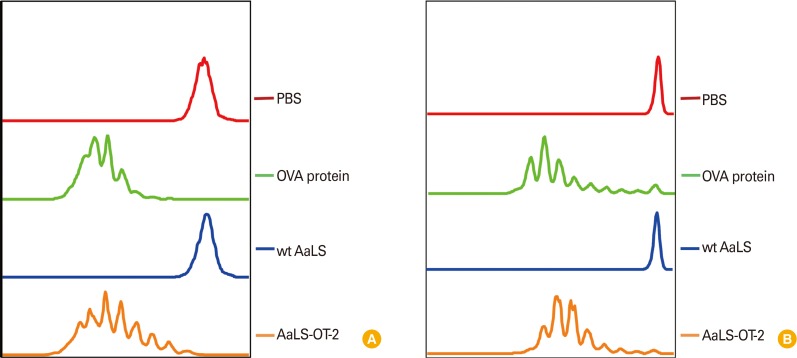
To study antigen-specific CD8+ T cell proliferation induced by DCs following antigen delivery by AaLS-OT-1 in vitro, DCs were pulsed with either wt AaLS or AaLS-OT-1 for three hours and were washed to ensure the presentation of only processed antigens. DCs pulsed with soluble OVA protein and wt AaLS were used as a positive and a negative control groups, respectively. Each group of DCs were then co-cultured with CFSE-labeled OT-1 T cells for four days and the proliferation of OT-1 peptide specific CD8+ T cells were measured by flow cytometry. While CFSE fluorescent signals of OT-1 T cells co-cultured with DCs pulsed with phosphate buffered saline or wt AaLS remain unchanged, those of OT-1 T cells co-cultured with DCs pulsed with soluble OVA protein or AaLS-OT-1 appeared as a series of peaks with lower CFSE fluorescence intensities indicating that OT-1 antigenic peptides delivered to DCs by AaLS effectively induced antigen-specific CD8+ T cell proliferation in vitro (Fig. 4A). Antigen-specific T cell proliferation induced by AaLS antigen delivery was also confirmed in vivo. Naïve mice were adoptively transferred intravenously with CFSE-labeled OT-1 T cells and were immunized with AaLS-OT-1, or with wt AaLS in the presence of poly (I:C) as an adjuvant. Soluble OVA protein (100 µg per mouse) was also used as a positive control in parallel. Similar to OT-1 peptide specific T cell proliferation data in vitro, CFSE signals of OT-1 T cells from mice immunized either with soluble OVA protein (a positive control) or AaLS-OT-1 showed serially diluted peaks with decreasing CFSE fluorescence intensity, suggesting that OT-1 antigenic peptides delivered to DCs by AaLS effectively induced antigen-specific CD8+ T cell proliferations in vivo as well (Fig. 4B).
Fig. 4.
OT-1 antigenic peptides delivered by AaLS-OT-1 induce OT-1-specific CD8+ T cell proliferation in vitro and in vivo. (A) CD11c+ dendritic cells (DCs) were pulsed with phosphate buffered saline (PBS), soluble ovalbumin (OVA) protein, wt AaLS, or AaLS-OT-1 at 2 mg/mL for 3 hours. The DCs were washed and then co-cultured with carboxyfluorescein succinimidyl ester (CFSE)-labeled OT-1 T cells at a ratio of 1:3. Four days later, the proliferation of OT-1-specific CD8+ T cells was measured by flow cytometry. (B) Mice were adoptively transferred with CFSE-labeled OT-1 T cells and on the next day, they were immunized subcutaneously with PBS, 100 µg of OVA protein, 50 µg of wt AaLS, AaLS-OT-1 in the presence of an adjuvant. Three days later, the proliferation of OT-1-specific CD8+ T cells was measured by flow cytometry.
Similar approaches were applied to examine antigen-specific CD4+ T cell proliferation following antigen delivery via AaLS-OT-2 in vitro and in vivo. The data showed that only those of T cells co-cultured with DCs pulsed with soluble OVA protein (a positive control) or AaLS-OT-2 proliferated, showing that OT-2 antigenic peptides delivered to DCs by AaLS effectively induce antigen-specific CD4+ T cell proliferation in vitro (Fig. 5A). CFSE-labeled OT-2 T cells were adoptively transferred and proliferated when immunized either with soluble OVA protein or AaLS-OT-2, demonstrating that AaLS deliver OT-2 antigenic peptides to DCs efficiently in vivo as well (Fig. 5B). Taken together, these data demonstrate that antigenic peptides carried by AaLSs are successfully delivered, processed, and presented by DCs leading to antigen-specific T cell proliferation, which demonstrated the value of AaLS as a novel antigen delivery system.
Fig. 5.
OT-2 antigenic peptides delivered by AaLS-OT-2 induce OT-2-specific CD4+ T cell proliferation in vitro and in vivo. (A) CD11c+ dendritic cells (DCs) were pulsed with phosphate buffered saline (PBS), soluble ovalbumin (OVA) protein, wt AaLS, or AaLS-OT-2 at 2 mg/mL for 3 hours. The DCs were washed and then co-cultured with carboxyfluorescein succinimidyl ester (CFSE)-labeled OT-2 T cells at a ratio of 1:3. Four days later, the proliferation of OT-2-specific CD4+ T cells was measured by flow cytometry. (B) Mice were adoptively transferred with CFSE-labeled OT-2 T cells and on the next day, they were immunized subcutaneously with PBS, 100 µg of OVA protein, 50 µg of wt AaLS, AaLS-OT-2 in the presence of an adjuvant. Three days later, the proliferation of OT-2-specific CD4+ T cells was measured by flow cytometry.
Discussion
In this study, we propose a lumazine synthase protein cage as an efficient antigen delivery system to DCs and a potential candidate for a new DC-based vaccine carrier in the future. In comparison to other nano-sized materials such as polymers, inorganic nanoparticles, liposomes, and/or VLP, protein cage nanoparticles have the virtue of simplicity and reproducibility; protein cages are modified and produced by genetic engineering and thus, not only the ratios of antigen to carrier can be controlled consistently but also the original structure and functions of inserted antigens, which are mainly proteins, can be maintained intact. Besides, protein cages are biocompatible and degrade safely demonstrating high practical feature in future various clinical applications.
Antigenic peptide insertion did not cause any significant conformational changes in AaLS. Moreover, such inserted antigenic peptides into AaLS were functional so that DCs could process and present them leading to the induction of antigen-specific T cell proliferation. The efficacy of antigen delivery to DCs via AaLSs was comparable to a conventional way of pulsing with soluble proteins to DCs, based on our data showing similar level of T cell proliferative responses between 100 µg of soluble OVA protein and 50 µg of AaLS-OTs per mouse (AaLS-OT-1 or AaLS-OT-2) where the contained amounts of OT-peptide epitopes were similar to each other. Further dosage experiments are necessary to examine the efficacy of antigen delivery via AaLSs.
To improve the efficacy as well as the specificity of antigen-delivery to DCs has been one of the main subjects for optimal DC-based vaccine development. Among various approaches, DC-targeting strategy might be the latest technology, which is composed of a fusion antibody against DC-specific antigen uptake receptors of which heavy chains are conjugated with an antigen of interest and a systemic administration of adjuvants for proper DC maturation [15]. To date, the concept of DC-targeting strategy and efficacy as a DC-based vaccine has been validated in various disease models [16, 17, 18]. Nonetheless, still following aspects of the DC-targeting strategy are needed to be considered for improved future clinical applications; limitations of size and solubility of the conjugated antigens to the DC-targeting antibodies [19], low cost effective strategy due to the requirement of mammalian cell transfection for functional antibody production [20], and any adverse hyperactivations caused by a systemic administration of adjuvants [21, 22]. Exploring nanotechnology of protein cage might provide useful solutions for these challenges and could advance the DC-based vaccine development since protein cage nanoplatforms feature with broader choices of size and solubility of antigens, more cost effective due to production in E. coli, as well as with co-insertion of selective adjuvants and antigens together. Furthermore, various protein cages containing selective antigens and adjuvants can be mixed and matched with known DC-targeting antibodies, and such interdisciplinary synergy will open a new avenue of DC-based vaccine design and development.
In conclusion, this study sets the stage for the analysis of functional roles of lumazine synthase protein cages as efficient DC-based vaccine carrier in various disease models.
Footnotes
This work was supported by Advanced Research Center (No. 2012-0008996) and Basic Science Research Program (No. 2013R1A1A2005545) of MEST through NRF of Korea, and the year of 2012 research fund (No. 1.120020.01) of UNIST.
No potential conflict of interest relevant to this article was reported.
References
- 1.Kang HJ, Kang YJ, Lee YM, Shin HH, Chung SJ, Kang S. Developing an antibody-binding protein cage as a molecular recognition drug modular nanoplatform. Biomaterials. 2012;33:5423–5430. doi: 10.1016/j.biomaterials.2012.03.055. [DOI] [PubMed] [Google Scholar]
- 2.Uchida M, Klem MT, Allen M, et al. Biological containers: protein cages as multifunctional nanoplatforms. Adv Mater. 2007;19:1025–1042. [Google Scholar]
- 3.Kang S, Uchida M, O'Neil A, Li R, Prevelige PE, Douglas T. Implementation of p22 viral capsids as nanoplatforms. Biomacromolecules. 2010;11:2804–2809. doi: 10.1021/bm100877q. [DOI] [PubMed] [Google Scholar]
- 4.Min J, Jung H, Shin HH, Cho G, Cho H, Kang S. Implementation of p22 viral capsids as intravascular magnetic resonance T1 contrast conjugates via site-selective attachment of Gd(III)-chelating agents. Biomacromolecules. 2013;14:2332–2339. doi: 10.1021/bm400461j. [DOI] [PubMed] [Google Scholar]
- 5.Kang YJ, Park DC, Shin HH, Park J, Kang S. Incorporation of thrombin cleavage peptide into a protein cage for constructing a protease-responsive multifunctional delivery nanoplatform. Biomacromolecules. 2012;13:4057–4064. doi: 10.1021/bm301339s. [DOI] [PubMed] [Google Scholar]
- 6.Uchida M, Kang S, Reichhardt C, Harlen K, Douglas T. The ferritin superfamily: supramolecular templates for materials synthesis. Biochim Biophys Acta. 2010;1800:834–845. doi: 10.1016/j.bbagen.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Meining W, Fischer M, Bacher A, Ladenstein R. X-ray structure analysis and crystallographic refinement of lumazine synthase from the hyperthermophile Aquifex aeolicus at 1.6 A resolution: determinants of thermostability revealed from structural comparisons. J Mol Biol. 2001;306:1099–1114. doi: 10.1006/jmbi.2000.4435. [DOI] [PubMed] [Google Scholar]
- 8.Worsdorfer B, Pianowski Z, Hilvert D. Efficient in vitro encapsulation of protein cargo by an engineered protein container. J Am Chem Soc. 2012;134:909–911. doi: 10.1021/ja211011k. [DOI] [PubMed] [Google Scholar]
- 9.Worsdorfer B, Woycechowsky KJ, Hilvert D. Directed evolution of a protein container. Science. 2011;331:589–592. doi: 10.1126/science.1199081. [DOI] [PubMed] [Google Scholar]
- 10.Shenton W, Mann S, Colfen H, Bacher A, Fischer M. Synthesis of Nanophase Iron Oxide in Lumazine Synthase Capsids This work was supported by the BBSRC (W.S.). We thank A. M. Seddon for help with transmission electron microscopy and analytical ultracentrifufation studies and G. D. Ruggiero for the generation of computer images. Angew Chem Int Ed Engl. 2001;40:442–445. [PubMed] [Google Scholar]
- 11.Moon H, Kim WG, Lim S, et al. Fabrication of uniform layer-by-layer assemblies with complementary protein cage nanobuilding blocks via simple His-tag/metal recognition. J Mater Chem B. 2013;1:4504–4510. doi: 10.1039/c3tb20554a. [DOI] [PubMed] [Google Scholar]
- 12.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 13.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 14.Han JA, Kang YJ, Shin C, et al. Ferritin protein cage nanoparticles as versatile antigen delivery nanoplatforms for dendritic cell (DC)-based vaccine development. Nanomedicine. 2014;10:561–569. doi: 10.1016/j.nano.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Trumpfheller C, Longhi MP, Caskey M, et al. Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity. J Intern Med. 2012;271:183–192. doi: 10.1111/j.1365-2796.2011.02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Do Y, Koh H, Park CG, et al. Targeting of LcrV virulence protein from Yersinia pestis to dendritic cells protects mice against pneumonic plague. Eur J Immunol. 2010;40:2791–2796. doi: 10.1002/eji.201040511. [DOI] [PubMed] [Google Scholar]
- 17.Flynn BJ, Kastenmuller K, Wille-Reece U, et al. Immunization with HIV Gag targeted to dendritic cells followed by recombinant New York vaccinia virus induces robust T-cell immunity in nonhuman primates. Proc Natl Acad Sci U S A. 2011;108:7131–7136. doi: 10.1073/pnas.1103869108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang B, Zaidi N, He LZ, et al. Targeting of the non-mutated tumor antigen HER2/neu to mature dendritic cells induces an integrated immune response that protects against breast cancer in mice. Breast Cancer Res. 2012;14:R39. doi: 10.1186/bcr3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trumpfheller C, Finke JS, Lopez CB, et al. Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. J Exp Med. 2006;203:607–617. doi: 10.1084/jem.20052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright A, Morrison SL. Effect of glycosylation on antibody function: implications for genetic engineering. Trends Biotechnol. 1997;15:26–32. doi: 10.1016/S0167-7799(96)10062-7. [DOI] [PubMed] [Google Scholar]
- 21.Lampkin BC, Levine AS, Levy H, Krivit W, Hammond D. Phase II trial of a complex polyriboinosinic-polyribocytidylic acid with poly-L-lysine and carboxymethyl cellulose in the treatment of children with acute leukemia and neuroblastoma: a report from the Children's Cancer Study Group. Cancer Res. 1985;45(11 Pt 2):5904–5909. [PubMed] [Google Scholar]
- 22.Batista-Duharte A, Lindblad EB, Oviedo-Orta E. Progress in understanding adjuvant immunotoxicity mechanisms. Toxicol Lett. 2011;203:97–105. doi: 10.1016/j.toxlet.2011.03.001. [DOI] [PubMed] [Google Scholar]