Abstract
This guideline contains the recommended vaccination schedules of dogs and cats from World Small Animal Veterinary Association (WSAVA) and American Animal Hospital Association (AAHA). In 2010, WSAVA published guidelines for the vaccination of dogs and cats. And, in 2011, AAHA also published guidelines for vaccination of dogs. In Korea, there is no published guideline for vaccination of dogs and cats yet. Therefore, the plane of vaccination also reports the present situation of vaccination schedule of dogs and cats in Korean animal hospitals.
Keywords: Vaccination, Dogs and cats, Korea
Guidelines for Vaccination of Dogs
Guidelines for vaccination of general veterinary practice by World Small Animal Veterinary Association (WSAVA) and American Animal Hospital Association (AAHA)
Table 1 summarizes vaccination schedule base on WSAVA vaccination guidelines in 2010 [1] and AAHA vaccination guideline in 2011 [2].
Table 1.
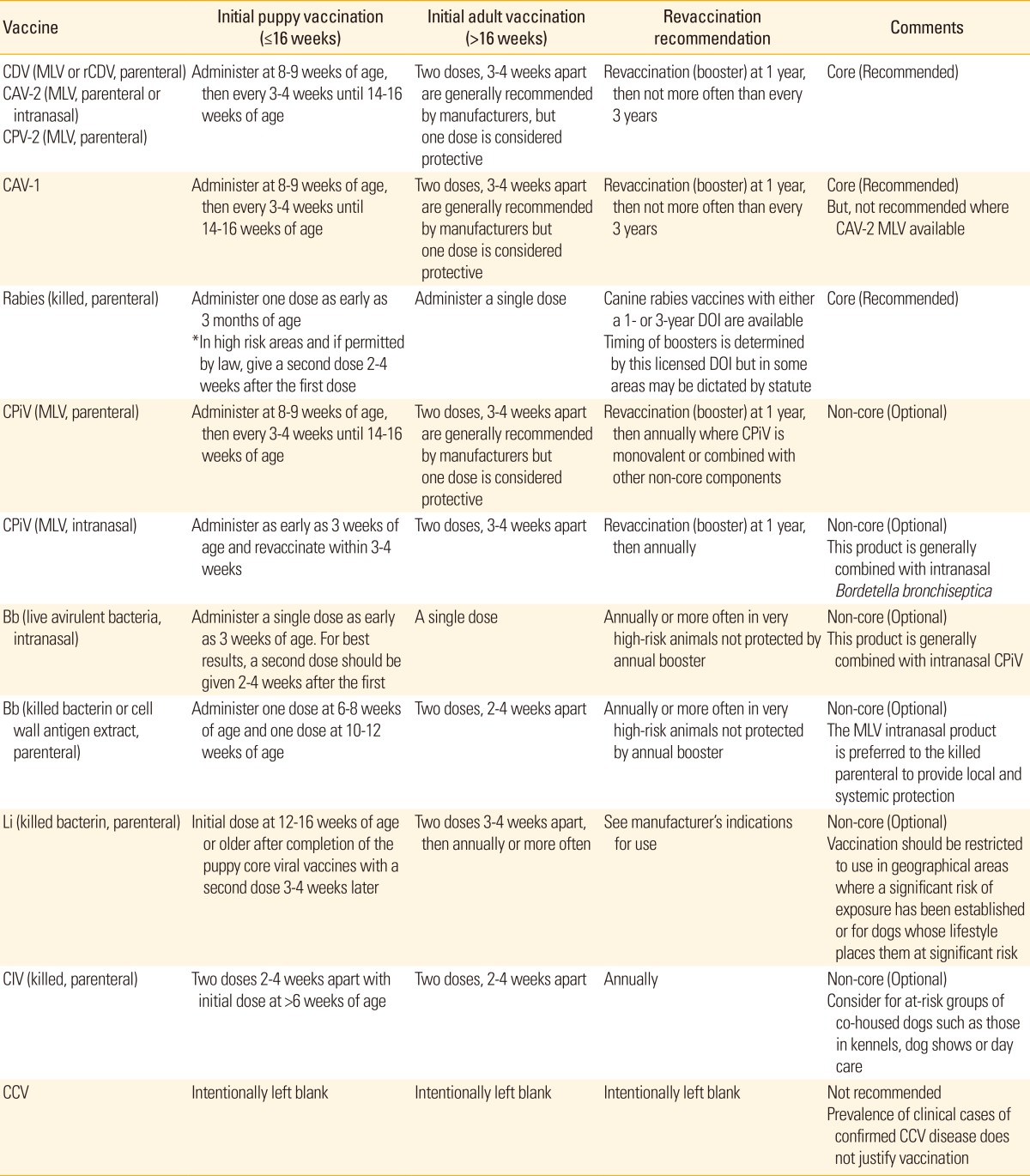
WSAVA, World Small Animal Veterinary Association; AAHA, American Animal Hospital Association; CDV, canine distemper virus; MLV, modified live vaccine; rCDV, recombinant canine distemper virus; CAV-2, canine adenovirus-2; CPV-2, canine parvovirus-2; CAV-1, canine adenovirus-1; DOI, duration of immunity; CPiV, canine parainfluenza virus; Bb, Bordetella bronchiseptica; Li, Leptosipria interrogans; CIV, canine influenza virus; CCV, canine corona virus.
Guidelines for vaccination of dogs in Korean animal hospitals
Table 2 shows vaccination schedules of dogs currently carried out in Korean animal hospitals.
Table 2.
Guidelines for vaccination of dogs in Korean animal hospitals
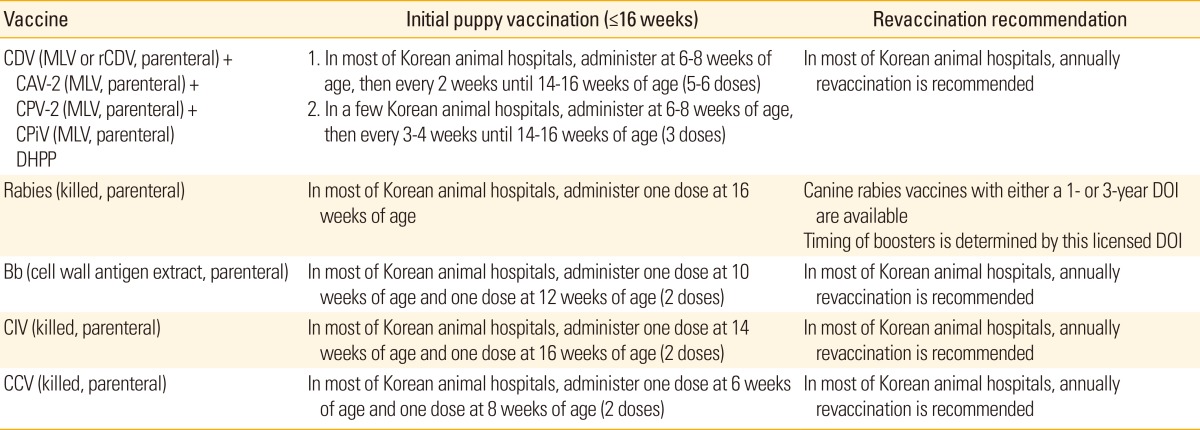
CDV, canine distemper virus; MLV, modified live vaccine; rCDV, recombinant canine distemper virus; CAV-2, canine adenovirus-2; CPV-2, canine parvovirus-2; CPiV, canine parainfluenza virus; DHPP, combination vaccines that include CDV (Distemper)+CAV-2 (Hepatitis)+CPV (Parvo)+CPiV (Parainfluenza); DOI, duration of immunity; Bb, Bordetella bronchiseptica; CIV, canine influenza virus; CCV, canine corona virus.
Guidelines for Vaccination of Cats
Vaccination guideline for general veterinary practice by WSAVA
Table 3 summarizes WSAVA vaccination guidelines in 2010 [1].
Table 3.
Guidelines for vaccination of cats by WSAVA [1]
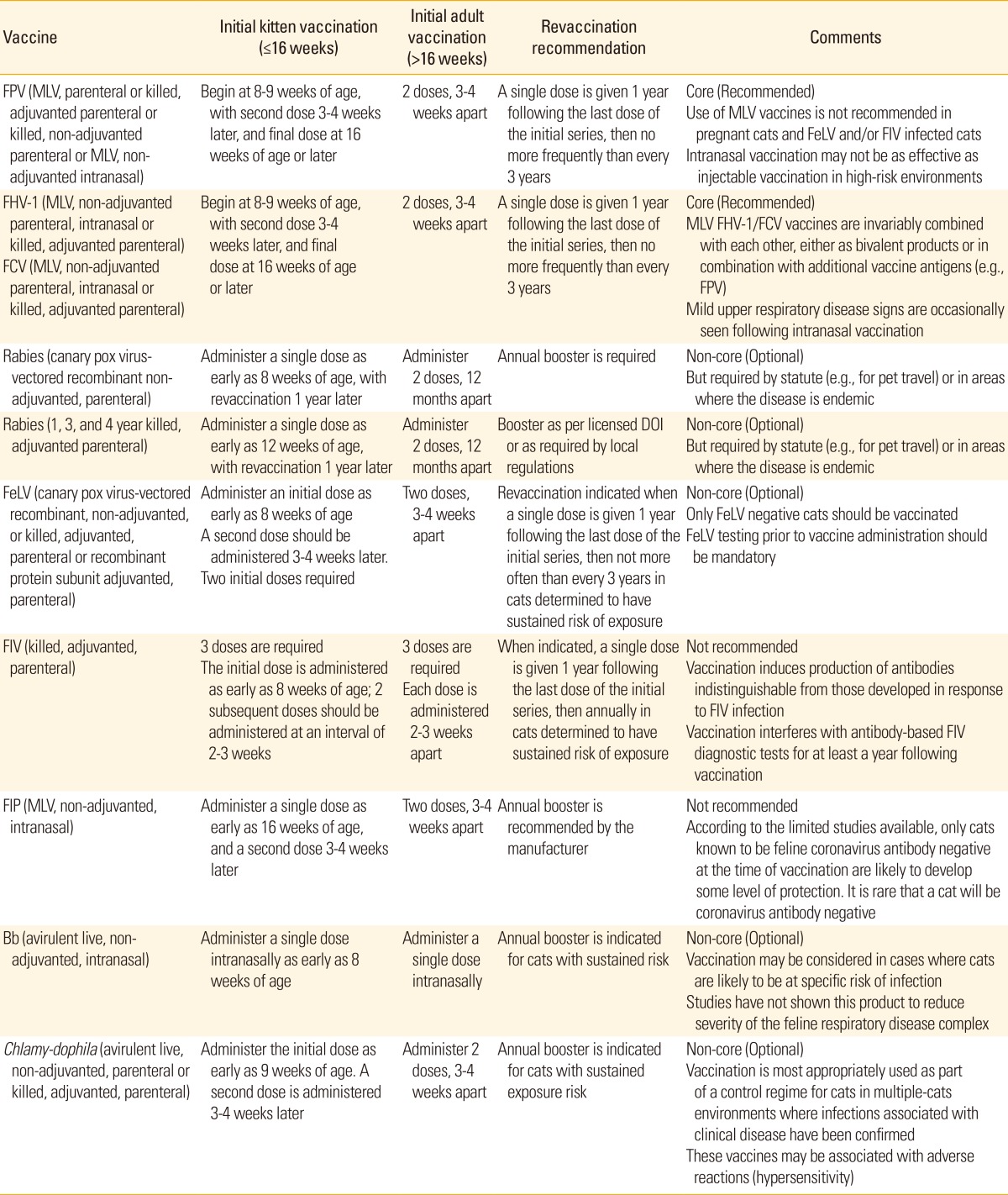
WSAVA, World Small Animal Veterinary Association; FPV, feline panleukopenia virus; MLV, modified live vaccine; FeLV, feline leukemia virus; FIV, feline immunodeficiency virus; FHV-1, feline herpes virus-1; FCV, feline calici virus; DOI, duration of immunity; FIP, feline infectious peritonitis; Bb, Bordetella bronchiseptica.
Guidelines for vaccination of cats in Korean animal hospitals
Table 4 shows vaccination schedule of cats currently carried out in Korean animal hospitals.
Table 4.
Guidelines for vaccination of cats in Korean animal hospitals
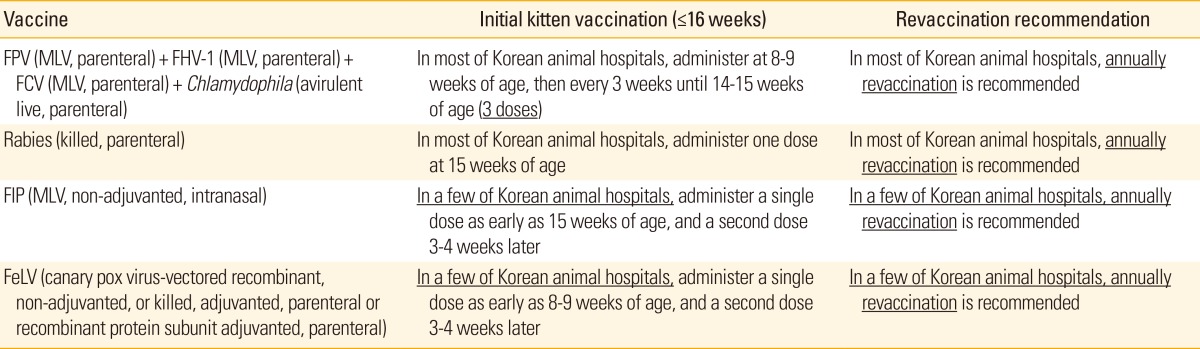
FPV, feline panleukopenia virus; MLV, modified live vaccine; FHV-1, feline herpes virus-1; FCV, feline calici virus; FIP, feline infectious peritonitis; FeLV, feline leukemia virus.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Vaccination Guidelines Group. Day MJ, Horzinek MC, Schultz RD. WSAVA guidelines for the vaccination of dogs and cats. J Small Anim Pract. 2010;51:1–32. doi: 10.1111/j.1748-5827.2010.00959a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Animal Hospital Association (AAHA) Canine Vaccination Task Force. Welborn LV, DeVries JG, et al. 2011 AAHA canine vaccination guidelines. J Am Anim Hosp Assoc. 2011;47:1–42. doi: 10.5326/jaaha-ms-4000. [DOI] [PubMed] [Google Scholar]


