Abstract
Repeated exposure to noxious stimuli changes their painfulness, due to multiple adaptive processes in the peripheral and central nervous system. Somewhat paradoxically, repeated stimulation can produce an increase (sensitization) or a decrease (habituation) in pain. Adaptation processes may also be body-site-specific or operate across body sites, and considering this distinction may help explain the conditions under which habituation vs. sensitization occurs. To dissociate the effects of site-specific and site-nonspecific adaptation processes, we examined reported pain in 100 participants during counterbalanced sequences of noxious thermal stimulation on multiple skin sites. Analysis of pain ratings revealed two opposing sequential effects: repeated stimulations of the same skin site produced temperature-dependent habituation, whereas repeated stimulations across different sites produced sensitization. Stimulation trials were separated by ~20 seconds and sensitization was unrelated to the distance between successively stimulated sites, suggesting that neither temporal nor spatial summation occurred. To explain these effects, we propose a dynamic model with two adaptation processes, one site-specific and one site-nonspecific. The model explains 93% of the variance in the group-mean pain ratings after controlling for current stimulation temperature, with its estimated parameters showing evidence for habituation for the site-specific process and sensitization for the site-nonspecific process. The two pain-adaptation processes revealed in this study, and the ability to disentangle them, may hold keys to understanding multiple pain-regulatory mechanisms and their disturbance in chronic-pain syndromes.
Perspective
This article presents novel evidence for simultaneous site-specific habituation and site-nonspecific sensitization in thermal pain, which can be disentangled (and the direction and strength of each process estimated) by a dynamic model. The dissociation of site-specific and site-nonspecific adaptation processes may hold keys to understanding multiple pain-regulatory mechanisms in both healthy and patient populations.
Keywords: thermal pain, habituation, sensitization, dynamic model, somatotopic specificity
Introduction
Pain perception is strongly modulated by dynamic adaptive processes e.g., 6, 25, 40. Although the degree of pain is driven by the intensity of a noxious stimulus, there is also a substantial portion of variance arising from temporal adaptation processes that may or may not interact with stimulus intensity 21, 23. Many chronic-pain syndromes are characterized by disturbed pain-adaptation processes, such as a lack of habituation or abnormal sensitization e.g., 11, 15, 38, 49, 51, 58 which may reflect an increased excitability of central 62 and/or of peripheral 16 nociceptive neurons. The temporal dynamics of pain, and the ability to estimate them accurately, may hold keys to understanding multiple mechanisms of pain regulation, as well as the development of chronic pain 3, 9, 15, 49.
There are well-known dynamic effects in pain that occur during continuous or fast repetitive noxious stimulation, such as temporal summation13, 17, 24, 29, 33, 40, 41, 53 and offset analgesia (the disproportionately large decrease in thermal pain following a slight decrease in stimulus temperature)19, 63, 64. Temporal pain adaptation also occurs during sequences of more widely spaced noxious stimuli (e.g., separated by 10–80 seconds). Several studies have reported a rapid decrease in experienced pain over the course of such stimulus series 8, 14, 25, 34, although increases in pain over time have also been reported 5, 32. As is common in the pain literature, we will use the terms ‘habituation’ and ‘sensitization’ to refer to the general class of adaptive processes whereby current experienced pain is decreased or increased (respectively) by previous painful stimuli (note that some authors use habituation to refer only to non-sensorimotor mechanisms 20, 45, 55; we do not make that commitment here).
The variety of temporal pain-adaptation effects implies the existence of multiple different pain-adaptation processes. Because changes in pain ratings over the course of repeated noxious stimulation reflect the combined effects of these processes, dynamic effects can appear complex and their various components may be difficult to disentangle in standard statistical analyses. However, these effects may be well explained by dynamic models that capture the adaptation processes underlying these effects. For example, Cecchi et al. 6 recently developed a model of thermal-pain perception that can accurately predict the temporal evolution of continuous pain ratings during sustained heat stimuli, by modeling the various processes that underlie the transformation of thermal heat to pain perception. In the present study, we aimed to characterize the processes underlying sequential effects on pain ratings during series of repeated thermal stimuli.
One important factor that affects which pain-adaptation processes predominate during repeated exposure to noxious stimuli may be whether these stimuli are applied to the same or to different body sites. Although it has been argued that site-specific and site-nonspecific effects reveal peripheral versus central adaptation processes, respectively18, this is not necessarily true: although pain-adaptation effects that occur during successive stimulations of different body sites must indeed originate in the central nervous system, changes in pain produced by repeated stimulation of the same skin site can be either peripheral or central in origin. Nonetheless, different processes likely mediate changes in pain that occur during repeated stimulation of the same vs. different body sites: a somatotopically-specific adaptation process vs. a more general adaptation process that operates across body sites. However, previous studies on the temporal dynamics of pain have largely neglected this distinction; hence the respective directions of both types of adaptation effects (habituation or sensitization) remain to be explored. We dissociated site-specific and site-nonspecific pain-adaptation effects by analyzing variations in reported pain during carefully counterbalanced sequences of repeated thermal stimuli on the same and different skin sites. We first examine the respective effects of site-specific and site-nonspecific repetition, and their interactions with stimulus intensity, using a standard regression analysis. We next propose a dynamic model to characterize the underlying processes of these effects.
Materials and Methods
Participants
One hundred healthy participants completed the experiment (mean age = 23.5, range = 18–52 years; 47 males, 38 females, 15 sex not reported; 84 right-handed, 4 left-handed, 2 ambidextrous, 10 handedness not reported). Participants reported no history of psychiatric, neurological, or pain disorders, no current pain, and no intake of analgesics on the testing day. All participants gave informed consent and received $12 per hour for their participation. The experiment was approved by the institutional review board of the University of Colorado Boulder.
Procedure
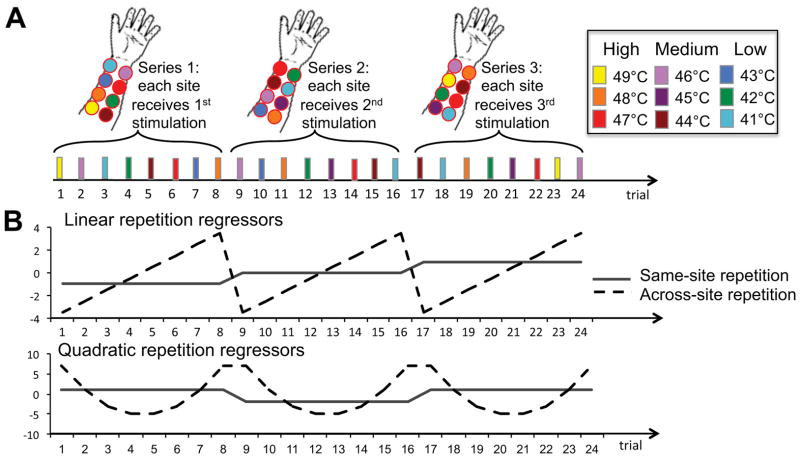
Testing took place while the participant was sitting in a comfortable chair designed to reduce spontaneous movement. We applied a sequence of 24 thermal stimuli of 11 seconds each (peak temperature = 41–49°C; 1.75 s ramp up, 7.5 s at peak temperature, 1.75 s ramp down) to eight sites on the volar surface of participants’ left inner forearms, using a 16×16 mm Peltier thermode (Medoc Ltd., Ramat Yishai, Israel). The sites were organized in a 4×2 layout, as illustrated in Figure 1A, for 62 participants, and in an 8×1 layout (i.e., 8 sites aligned in one line along the inner forearm) for 38 participants. Adjacent stimulation sites were separated by ~1 cm. The 24 stimuli were logically divided into three successive series of eight stimuli. During each series, each of the eight skin sites was stimulated once, in random order (Fig. 1A).
Figure 1.
Repetition effects. A) Design (stimulation sites not drawn to scale). The experiment consisted of 3 successive series of 8 trials. Within each series, we applied 8 successive thermal stimuli (11 seconds each, separated by ~ 20 seconds) to 8 different skin sites. Thus, in the first 8-trial series each site is stimulated for the first time, whereas the second and third 8-trial series involve repeated stimulations of these same sites. B) Repetition regressors. We modeled the linear and quadratic effects of site-specific repetition and site-nonspecific repetition (and their interactions, as well as the effects of temperature, and temperature by repetition interactions; not shown).
Two seconds after each stimulus, participants used a computer mouse with their right hand to rate the overall amount of pain they experienced on that trial, on a 100-unit visual analog scale with anchors of no pain (0) and worst-imaginable pain (100) 42. Following the pain rating, the experimenter moved the thermode to another skin site and then after a variable interval of 1–4 seconds the next thermal stimulus started. The interval between successive stimuli was approximately 20 seconds (including the time needed for the participant to make the overall-pain rating and for the experimenter to move the thermode to a new site). Thus, each skin site was stimulated 3 times, separated by 8 trials or ~4 minutes on average.
Each skin site received one low-temperature (41, 42, or 43°C), one medium-temperature (44, 45, or 46°C), and one high-tempera ture stimulus (47, 48, or 49°C). In total, one low, one medium, and one high temperature were used twice and all other temperatures were used 3 times during the entire experiment. Between stimuli the thermode maintained a baseline temperature of 32°C.
Regression analysis
We conducted multi-level regression analyses on the pain ratings, using a customized version of Matlab’s glmfit function (T.D.W.; glmfit_multilevel which is part of the Multilevel Mediation Toolbox, available at http://wagerlab.colorado.edu/tools; see 1, 30, 60 for details on the implementation of our multi-level modeling procedures). We included regressors for the following effects of interest: “Temperature” (9 levels), “Site-specific repetition” (3 levels), and “Site-nonspecific repetition” (8 levels). Fig. 1B illustrates the repetition regressors. The Site-specific repetition regressor indicated whether the currently stimulated skin site was stimulated for first, second, or third time. We reset the Site-nonspecific repetition regressor at the beginning of each 8-trial series in order to orthogonalize the regressors coding for site-specific and -nonspecific repetition. We modeled these effects as continuous regressors, with linear and quadratic effects. To fully characterize the data, we also modeled the interactions between site-specific and site-nonspecific repetition. This resulted in a fully orthogonal set of regressors coding for site-specific repetition, site-nonspecific repetition, and their interactions. All regressors were centered, and all interaction regressors were calculated from centered variables.
To assess the dependence of temporal pain dynamics on the current stimulation temperature we modeled the interactions between each repetition effect and current stimulation temperature. To assess the effects of the previous stimulation temperature, we conducted additional analyses that included a regressor coding for the temperature of either the immediately preceding stimulus or the most recent stimulus applied to the same site as the current stimulation.
After running the regression model including all above-mentioned regressors, we excluded the regressors that did not predict pain rating (ps > 0.1) for our final regression model. We report the final model’s results, which are very similar to those from the initial full model. We further examined the nature of the significant interaction effects using repeated-measures ANOVAs.
Dynamic model
The above-described regression analyses test for the presence of site-specific and -nonspecific repetition effects on pain ratings, but do not inform about the underlying processes that give rise to these effects. To address this issue, we developed a dynamic model that characterizes the effects of past thermal stimuli on reported pain (Fig. 2). The model was implemented in Matlab (R2012a; Mathworks, Natick, MA). We will first provide a qualitative description of the model, followed by its algorithmic details.
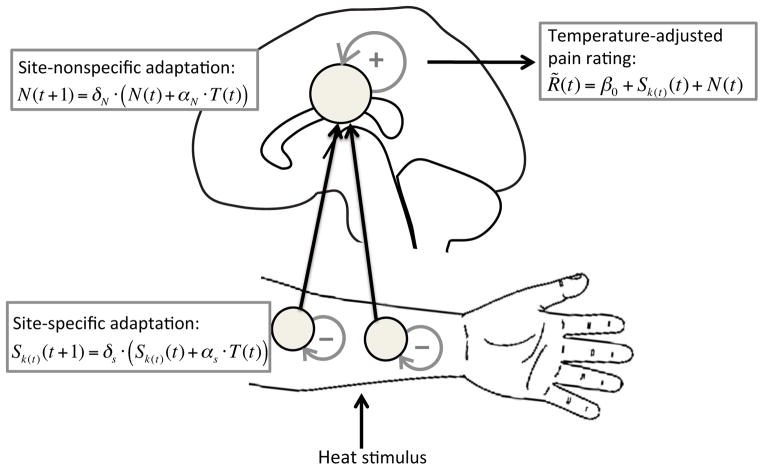
Figure 2.
Illustration of the dynamic model. The model predicts trial-by-trial dynamics of temperature-adjusted pain rating R̃(t), i.e., fluctuations in reported pain that are not due to variation in the current stimulus intensity. R̃(t) is modeled as a sum of site-nonspecific and site-specific adaptation processes, plus an intercept (β0). To this end, the model assumes both a site-nonspecific state variable (N) and a site-specific state variable (S), which are both updated as a function of noxious input. Note that although we displayed site-specific adaptation at a peripheral level (on the stimulated skin sites), this is illustrative only; site-specific habituation may also have a central contribution. Similarly, although we display site-nonspecific adaptation in the cortex, we do not know where in the central nervous system this effect arises (this could be in the spinal cord, brainstem or cortex).
Qualitative description of the model
Our model assumes that each time someone receives a thermal stimulus, that person’s pain sensitivity, and therefore the degree of pain s/he perceives in response to subsequent stimuli, is dynamically updated. Thus, the perceived pain induced by a thermal stimulus depends on the number and the intensity of previous stimuli. To allow different temporal dynamics for site-specific and site-nonspecific repetition, each thermal stimulus updates both the sensitivity level of the specific skin site it is applied to, and a general site-nonspecific sensitivity level. Both updating processes can cause either habituation (decreased sensitivity) or sensitization (increased sensitivity), depending on the direction of the updating process, and the overall effect of past stimuli on perceived pain is defined as the sum of both effects. Thus, the site-specific and site-nonspecific adaptation processes can (partially) cancel each other out if they are in opposite directions, and are additive if they are in the same direction.
The direction and strength of each updating process are determined by the model parameter α (αS for the site-specific adaptation, and αN for the site-nonspecific adaptation): negative values of α for a participant result in habituation (sensitivity decreases following each stimulus) and positive values of α result in sensitization (sensitivity increases following each stimulus), and both effects are stronger for larger absolute values of α. Because it has been reported that high-intensity heat stimuli result in stronger subsequent pain adaptation than low-intensity stimuli (e.g.,28), our model assumes that the degree of sensitivity updating following a thermal stimulus also depends on that stimulus’s intensity. To this end, the change in sensitivity following a heat stimulus is scaled by the intensity of that stimulus.
Because pain habituation and sensitization are nonlinear processes, which have been shown to asymptote after a certain number of stimuli (e.g.,25, 32), our model allows both updating processes to asymptote after a certain number of stimulus repetitions. This is implemented in the model through an exponential decay process, which rate is controlled by model parameter δ (δS for the site-specific process, and δN for the site-nonspecific process, allowing different decay rates for the two updating processes).
To summarize, our model assumes that past thermal stimuli affect stimulus-evoked pain through two dynamic processes: (i) a sensitivity-updating process, which causes someone’s sensitivity to thermal stimuli to increase or decrease with repeated stimulation (controlled by model parameter α), and (ii) a decay process, which allows this updating process to asymptote after a sufficient number of stimuli (controlled by model parameter δ). Two copies of these processes operate in parallel, on the sensitivity level of the currently stimulated skin site and on a general, site-nonspecific sensitivity level.
Finally, we would like to note the similarity and differences between our model and a recently proposed model of pain dynamics during individual periods of continuous thermal stimulation6. Cecchi et al. (2012) developed a dynamic model that can explain a variety of temporal effects on continuous pain ratings during sustained thermal stimuli, including offset analgesia. Their model includes a temperature-dependent “force” and a decay term, which are functionally similar to the α and δ parameters of our model, respectively. Cecchi et al.’s model also contains a “dynamic-restoring force” which captures the effects of fast changes in stimulus intensity; because we did not model moment-by-moment pain dynamics during individual stimuli, our model did not include this component. An important novel feature of our model is that it assumes that the same qualitative dynamics work in parallel on site-specific and -nonspecific adaptation processes.
Quantitative description of the model
In our model, each skin site, k, is associated with a state variable, Sk, which characterizes that site’s level of sensitization or habituation at any given time and is dynamically updated as a function of noxious input. If Sk > 0 then stimuli at site k will be perceived as more intense than normal (somatotopic sensitization), and if Sk < 0 then stimuli will be perceived as less intense than normal (somatotopic habituation). The model also assumes a site-nonspecific state variable, N, which represents general habituation (N < 0) or sensitization (N > 0) across all sites. N is also dynamically updated as a function of noxious input. Thus the Sk and N state variables can separately capture site-specific and site-nonspecific pain dynamics.
The purpose of the model is to predict the trial-to-trial dynamics of pain as a function of a sequence of noxious stimuli, above and beyond effects of the stimulus intensity itself. Thus, we define the temperature-adjusted pain rating, R̃(t), as the residual on trial t obtained by regressing each participant’s pain ratings on the linear and quadratic Temperature predictors. This adjusted pain rating is then modeled as a sum of site-specific and site-nonspecific sequence effects, plus an intercept (β0):
| (1) |
Here k(t) is the site stimulated on trial t, and Sk(t)(t) and N(t) are the current levels of site-specific and site-nonspecific sensitization (if positive) or habituation (if negative).
The remainder of the model concerns the dynamics of S and N. The value of each of these variables reflects effects of past stimuli — stimuli applied to each separate site in the case of S, and all stimuli in the case of N — that are assumed to decay exponentially across time. S and N are each governed by two free parameters, αS and δS, and αN and δN, respectively. Following each trial t, the state of adaptation at the stimulated site, Sk(t), is incremented in proportion to the current temperature, with a constant of proportionality determined by αS. We defined current temperature as the difference between the stimulus temperature and the baseline temperature (i.e., T = stimulus temperature minus 32° C), so that the baseline temperature produces no adaptation. In the interval between trials t and t + 1, Sk is assumed to decay toward zero at a rate determined by δS, which is constrained to lie between 0 and 1 (a smaller value of δS indicates a faster decay rate). These assumptions lead to the following dynamics for S:
| (2) |
Thus, αS determines the direction and magnitude of the sensitization/habituation effect. If αS > 0, then stimulation of a skin site results in sensitization, whereas if αS < 0 then stimulation results in habituation. δS determines the rate of decay, or the effective timescale of site-specific sensitization/habituation.
The site-nonspecific adaptation state, N, follows the same dynamic principles. Because N is affected by stimulation at all skin sites, it is incremented by the stimulus on every trial, by an amount proportional to the current temperature and a magnitude parameter αN. Then it decays between trials with rate δN (constrained to lie between 0 and 1). Therefore, the dynamics for N are described by:
| (3) |
As with site-specific adaptation, δN determines the decay rate or effective timescale of site-nonspecific sensitization/habituation (a smaller value of δN indicates a faster decay rate), and αN determines its direction and magnitude, with αN > 0 producing site-nonspecific sensitization and αN < 0 producing site-nonspecific habituation.
Model estimation
We estimated the four parameters of the dynamic model by minimizing the sum of the squared error (SSE) between the observed trial-by-trial temperature-adjusted pain ratings and those predicted by the model. To optimize the parameter fits we used Matlab’s fmincon function 7, a constrained nonlinear optimization algorithm, with thirty randomized starting parameter estimates. We fitted the model separately to each participant’s data, to obtain estimates of each parameter per participant (α̂N, α̂S, δ̂N and δ̂S). We tested whether α̂N and α̂S (the signed magnitude parameters of the site-nonspecific and site-specific temporal adaptation, respectively) significantly differ from 0 by means of one-sample t-tests.
After fitting the model to each participant’s individual data, we computed the group-mean observed and model-predicted temperature-adjusted pain ratings on each of the 24 trials. The group-averaged trial-by-trial data contain much less noise than the single-trial data in individual participants, especially given our large number of participants (N=100). Therefore, a comparison of the observed and model-predicted group-mean data indicates how well the model explains the systematic pattern of trial-by-trial dynamics in pain ratings.
Model comparison
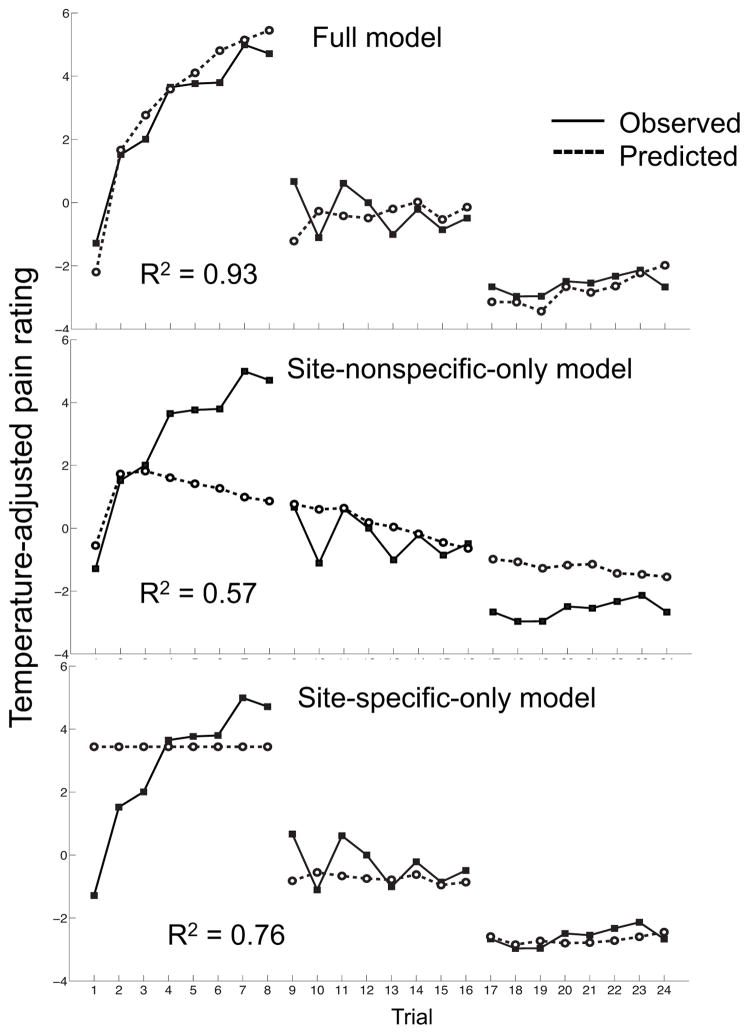
We tested the advantage of our model over simpler models that assume only site-specific (“Site-specific-only model”) or only site-nonspecific (“Site-nonspecific-only model”) dynamics. We created these simpler models by removing either the N(t) or the Sk(t)(t) term from the full model (Equation 1), i.e. by setting N(t)=0 in the Site-specific-only model or Sk(t)(t)=0 in the Site-nonspecific-only model. We then compared the proportion of variance explained by the full model with those explained by each of the two simpler models.
Results
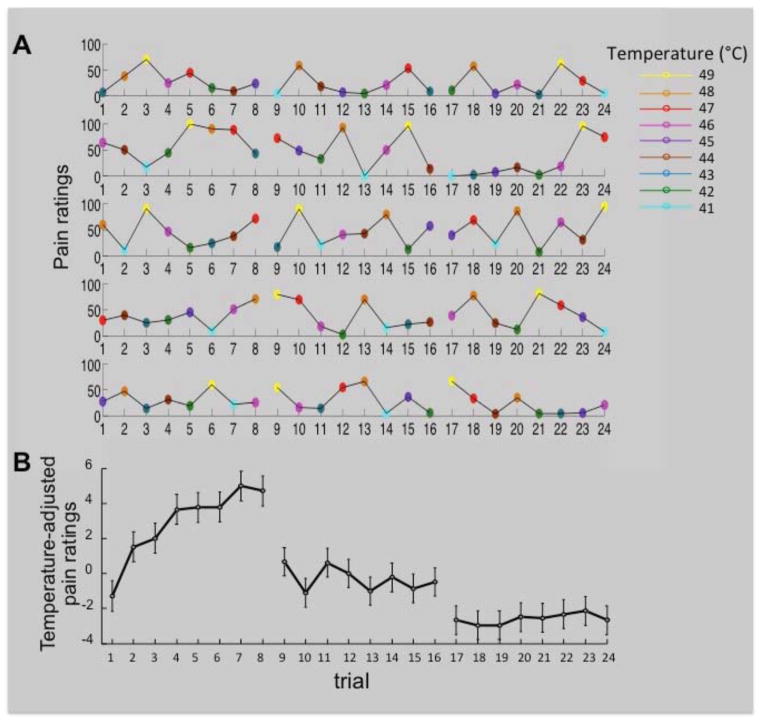
Fig. 3A shows five randomly selected participants’ pain ratings on every trial of the experiment. Note that each of 8 skin sites received their first stimulation during trials 1–8, and their second and third stimulation during trials 9–16 and 17–24, respectively. Whereas the effects of stimulus temperature are easily noticeable in these plots, the effects of temporal adaptation are more difficult to detect due to the trial-by trial variation in stimulus temperature and the noise inherent in single-trial/single-participant data. To examine the systematic changes in pain ratings over the course of the 24 stimulation trials, above and beyond effects of stimulus intensity, we regressed out the effects of temperature (i.e., we removed the variance in pain ratings that was accounted for by the linear and quadratic effects of temperature) and plotted the group-mean temperature-adjusted pain ratings on each trial of the experiment (Figure 3B). Figure 3B indicates that (i) there was an overall decrease in pain ratings across the three successive 8-trial series (site-specific habituation), and (ii) pain ratings gradually increased (site-nonspecific sensitization) during the first, but not during the second and third, stimulation series. We will formally test these observations in the next two subsections, using a multi-level regression analysis and our dynamic model, respectively.
Figure 3.
A) Pain ratings on each trial of the experiment, color-coded for stimulus temperature, for 5 randomly selected participants. Note that in the first 8-trial series each of eight skin sites are stimulated for the first time, whereas the second and third 8-trial series involve repeated stimulations of these same sites. B) The group-mean temperature-adjusted pain rating on each trial of the experiment. We obtained temperature-adjusted pain ratings by taking the residuals from a regression of pain rating on the linear and quadratic effects of temperature. Error bars are within-subject standard errors of the means (Loftus and Masson, 1994).
Regression results
Table 1 summarizes the effects of all significant predictors of pain ratings from the regression analysis.
Table 1.
Predictors of pain rating
| Coefficient | STE | Cohen’s d | t | p | |
|---|---|---|---|---|---|
| Intercept | 25.12 (13) | 1.29 | 1.9 | 19.4 | < 0.001 |
|
| |||||
| Temperature effects: | |||||
| Temperature L | 5.99 (2.6) | 0.25 | 2.3 | 23.57 | < 0.001 |
| Temperature Q | 0.68 (.46) | 0.04 | 1.5 | 15.59 | < 0.001 |
|
| |||||
| Repetition effects: | |||||
| Site-specific L | −2.56 (3.9) | 0.38 | 0.65 | −6.81 | < 0.001 |
| Site-nonspecific L | 0.24 (.97) | 0.09 | 0.25 | 2.59 | 0.01 |
| Specific L x Nonspecific L | −0.24 (1.2) | 0.11 | 0.21 | −2.17 | 0.03 |
| Specific Q x Nonspecific L | 0.21 (.77) | 0.07 | 0.27 | 3 | 0.004 |
| Specific L x Nonspecific Q | 0.11 (.48) | 0.05 | 0.20 | 2.27 | 0.03 |
|
| |||||
| Current temperature by repetition interactions: | |||||
| Temperature L x Specific L | 0.58 (1.1) | 0.11 | 0.53 | 5.38 | < 0.001 |
| Temperature L x Specific Q | −0.13 (.48) | 0.05 | 0.24 | −2.82 | 0.006 |
| Temperature Q x Specific L | 0.31 (.43) | 0.04 | 0.75 | 7.63 | < 0.001 |
Note. Standard deviations in parentheses; L = linear effect; Q = quadratic effect; STE = standard error of the mean
Effects of current stimulus intensity
As expected, pain ratings increased with increasing temperature, as reflected by the positive effects of temperature (Table 1, Figure 4). There were both linear and quadratic effects of temperature, suggesting a nonlinear relationship between pain rating and temperature that is consistent with previous studies 27, 56.
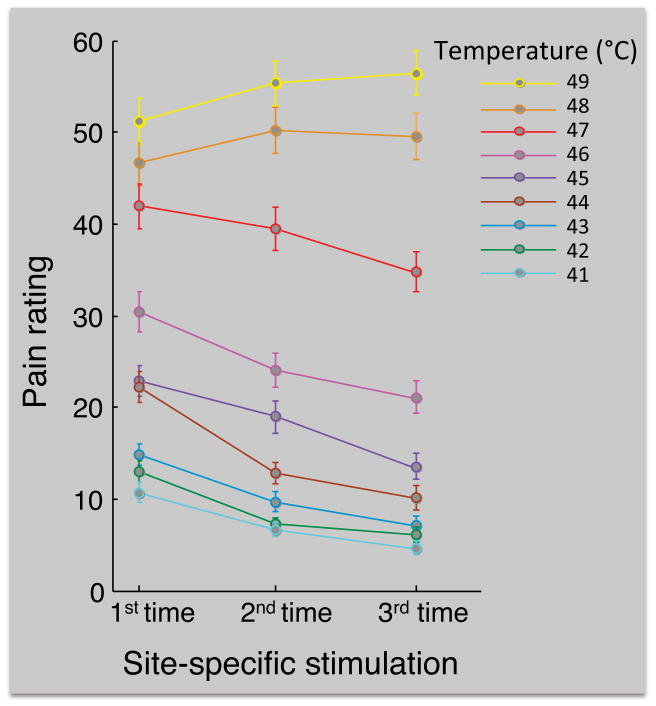
Figure 4.
Group-mean pain ratings as a function of current temperature and site-specific repetition. The first, second and third site-specific stimulation correspond to stimulation trials 1–8, 9–16, and 17–24, respectively. The average number of participants that contributed to each data point was 89 (range = 81–95, because for each participant three of the temperatures were used twice and all other temperatures were used 3 times; see Methods section). Error bars are between-subjects standard errors of the means.
Site-specific adaptation effects
Figure 4 shows the group-mean pain ratings for the first, second and third site-specific stimulation (i.e., the grand-average pain ratings for trials 1–8, 9–16, and 17–24, respectively), as a function of current stimulus temperature. The regression analysis revealed a negative linear effect of site-specific repetition (Table 1), reflecting the decrease in pain ratings across the three 8-trial series for most stimulus intensities. Because the site-specific repetition regressor was correlated with overall trial number, this effect could in principle be due to either site-specific habituation or a persistent site-nonspecific trial-by-trial habituation. However, the absence of evidence for a site-nonspecific habituation effect (but instead an increase in pain ratings during the first series, see next section) suggests that this effect was due to site-specific habituation.
There were also significant site-specific repetition × current temperature interactions (Table 1), reflecting that the site-specific habituation effect was strongest for low temperatures, and reversed for high temperatures (Figure 4). To further examine these interactions we tested the site-specific repetition effect separately for each level of current stimulation temperature, using repeated-measures ANOVAs. These analyses revealed that repeated stimulation of the same skin site resulted in a significant decrease in pain rating for 41 to 47°C stimuli (F(2,140) = 20.8, F(2,142) = 16.7, F(2,112) = 34.8, F(2,128) = 30.5, F(2,120) = 17.9, F(2,146) = 14.4, F(2,134) = 10.5, respectively, all ps < 0.001), no significant effect for 48°C stimuli (p = 0.43), and a significant increase in pain rating for 49°C stimuli (F(2,129) = 9.6, p < 0.001).
Finally, we examined the interactions of site-specific repetition with previous stimulus temperature. To this end, we extended the regression model with a regressor coding for the temperature of the most recent stimulus applied to the same site (on average 8 trials ago). In this model we excluded trials 1–8, for which there were no previous stimulations on the same site. This analysis revealed that higher-intensity stimuli produced greater subsequent site-specific habituation when the same site was stimulated again (β̂ = −0.51, t = −4.14, p < 0.001). The effect of previous stimulus temperature did not interact with current stimulus temperature (β̂ = −0.09, t = −1.55, p = 0.12).
Site-nonspecific adaptation effects
In contrast to the (temperature-dependent) site-specific habituation, the regression analysis revealed a significant linear increase in pain rating during successive stimuli applied across different skin sites (site-nonspecific sensitization; Table 1). Site-nonspecific repetition did not interact with current stimulus temperature (ps > 0.1).
There were also several site-nonspecific repetition × site-specific repetition interactions (Table 1), reflecting that the site-nonspecific sensitization was restricted to the first 7–8 trials of the experiment (Fig. 3B). We conducted follow-up repeated measures ANOVAs on temperature-adjusted pain ratings to examine the site-nonspecific repetition effect separately for each of the 3 levels of site-specific repetition (i.e., separately for trials 1–8, 9–16, and 17–24). These analyses revealed a highly significant increase in pain during the first 8-trial series (F(7,693) = 5.76, p < .001), but no sequential effects during the second and third 8-trial series (ps > 0.6). Thus, successive stimuli applied to different skin sites only produced sensitization when the sites were stimulated for the first time. There are at least two possible explanations for this restriction of site-nonspecific sensitization to the first 8 stimulation trials: (I) repeated stimulation on a site may have abolished this effect; and (II) site-nonspecific sensitization may have reached asymptote after ~8 of the stimuli used in our experiment.
We also tested whether site-nonspecific sensitization depended on the intensity of the preceding stimulus. To this end, we extended the regression model with a regressor coding for the temperature of the immediately preceding stimulus, which was nearly always on a different skin site. In this model we excluded the first trial. This analysis revealed that higher-intensity stimuli produced greater sensitization on the following stimulation trial (β̂ = 0.26, t = 2.9, p = 0.005). Thus, whereas higher-intensity stimuli produce greater site-specific habituation (see previous section), they also produced greater site-nonspecific sensitization.
The site-nonspecific sensitization during the first series of stimuli might be explained by peripheral sensitization of the skin adjacent to the previously stimulated site. If so, we would expect observe greater sensitization when the current and previous stimulation sites are closer together, and when the preceding stimulus is more intense. We tested these predictions on the pain-rating data from the first 8 stimulation trials, using a multi-level regression model with regressors coding for the distance between the current and previous stimulation site, current stimulus temperature, previous stimulus temperature, and the distance by previous temperature interaction. Neither distance, nor the distance by previous temperature interaction significantly predicted pain rating (ps > 0.6), suggesting that the site-nonspecific sensitization could not be explained by a peripheral sensitization process. That the distance between successive stimulation sites did not affect pain ratings also suggests that the successive stimuli in our experiment did not result in a spatial-summation like effect (i.e., higher perceived pain for larger areas of noxious stimulation e.g.,43, which has been shown to be restricted to simultaneous inputs to nearby skin sites12).
Sex effects
Previous studies have shown stronger habituation effects in women than in men12, 22. To examine whether any of the revealed effects were driven by either the male or the female participants, we included sex as a between-subjects factor in the regression analysis (excluding the 15 participants whose sex was unknown). Controlling for sex did not change the significance of any of the predictors of pain rating reported in Table 1. However, this analysis did reveal a sex x site-specific repetition interaction, reflecting that the female participants showed stronger site-specific habituation (β̂ = 0.9, t = 2.37, p = .02), in line with previous findings 12, 22. This analysis also revealed a main effect of sex, reflecting higher pain ratings in the female participants (β̂ = −3.3, t = 2.38, p = .02), and a marginally significant sex x linear-temperature interaction, reflecting that the female participants’ pain ratings tended to be more strongly affected by stimulus temperature (β̂ = −0.52, t = 1.84, p = .069). None of the other regressors interacted with sex. We next conducted separate regression analyses for the male and female participants to test the presence of site-specific habituation in both groups. These analyses revealed highly significant effects site-specific repetition in both groups (β̂ = −1.63, t = 3.87, p < .001 and β̂ = −3.5, t = 5.31, p < .001 for the male and female participants, respectively). Thus, although site-specific habituation was stronger in the female participants, it was clearly present in the male participants as well.
Dynamic-model results
The linear and quadratic effects of stimulus temperature explained on average 79% of each individual participant’s trial-by-trial pain ratings. We examined how much of the residual variance (21%) could be accounted for by the temporal dynamics captured by our dynamic model. To this end, we fitted the model to the temperature-adjusted pain ratings of each individual participant. Fig. 5 (upper panel) shows the group-mean temperature-adjusted pain ratings on every trial of the experiment, as well as those predicted by the dynamic model. The model explained 93% of the variance in group-mean temperature-adjusted pain ratings across trials, suggesting that it accurately captured the pattern of systematic dynamic effects across trials, including the nonlinear, temperature-dependent effects captured in the standard regression analyses. The single-trial data from individual participants was, naturally, considerably noisier than the group-mean data; hence fitting the model to individual participants’ data resulted in less accurate predictions. The model still performed reasonably well, however, explaining on average 34% of the variance in individual, temperature-adjusted per-trial pain ratings (the remainder of the variance is presumably non-systematic noise in single-trial ratings or reflects other processes not captured by the model). Figure 6 shows the per-trial temperature-adjusted pain ratings in four individual participants. It can be seen that, through different values of the estimated model parameters, the model is able to capture a variety of different adaptation effects.
Figure 5.
The group-mean temperature-adjusted pain ratings on each trial (straight lines), and those predicted by the full dynamic model and two simpler models that capture only site-nonspecific or only site-specific dynamics (dotted lines).
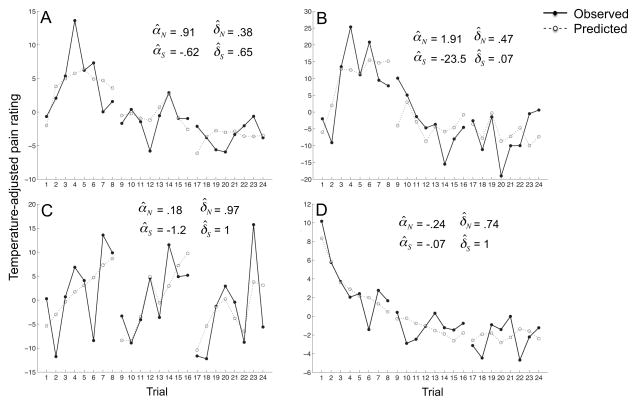
Figure 6.
Per-trial temperature-adjusted pain ratings of four individual participants. The estimated parameters from fits of our dynamic model to each participant’s data, and the pain ratings predicted by the dynamic model (dotted lines) are shown as well. Participants A and B are representative of the group-mean data: they show site-specific habituation, i.e., pain ratings decrease across the three 8-trial series when stimuli return to the same skin sites, and site-nonspecific sensitization during the first ~4 stimuli. Participant C also demonstrates site-specific habituation and site-nonspecific sensitization, but shows almost no decay of these processes, reflected in δ values of (almost) 1. This produces almost identical site-nonspecific sensitization effects during each 8-trial series, and a similar drop in pain ratings at the beginning of the second and third 8-trial series (when stimuli return to the same skin sites for the first and second times). Unlike most participants, participant D shows both site-specific and site-nonspecific habituation, reflected in a negative αS and αN, which produces a decrease in pain ratings both within and across the three 8-trial series.
Table 2 shows the mean parameter estimates for fits of the model to each participant’s data. α̂N was positive for 74 of the 100 participants and mean α̂N was significantly greater than 0, t(99) = 2.2, p = 0.027, indicating site-nonspecific sensitization. By contrast, α̂S was negative for 86 of the 100 participants and mean α̂S was significantly less than 0, t(99) = −3.6, p < 0.001, indicating site-specific habituation. The decay rates δ̂N and δ̂S were not significantly different from each other, t(99) = 1.3, p = 0.21.
Table 2.
Mean parameter estimates (95% confidence interval on the mean in parentheses) for the fit of the full dynamic model and the two simpler control models to each participant’s data, and the proportion of variance explained by each model (R2).
| Parameter | Full model | Site-nonspecific-only model | Site-specific-only model |
|---|---|---|---|
| αN | 8.74 (1.0–16.5) | 5.87 (−4.8–16.6) | |
| αS | −18.0 (−28.0–−8.0) | −14.6 (−26.3–−3.0) | |
| δN | 0.55 (0.48–0.63) | 0.47 (0.39–0.55) | |
| δS | 0.49 (0.40–0.57) | 0.48 (.39–.56) | |
| R2 group-mean | .93 | .57 | .76 |
| R2 individual participants | .34 | .18 | .19 |
Finally, we compared the fit of our model with those of two simpler control models that capture only site-nonspecific or only site-specific dynamics (Fig. 5; Table 2). Whereas the full model predicted 93% of the variance of the group-mean data, the Site-nonspecific-only and Site-specific-only models predicted 57% and 76% respectively. At the level of individual participants, the full, Site-nonspecific-only and Site-specific-only models predicted on average 34%, 18%, and 19% of the variance, respectively. Thus, a combination of site-specific and -nonspecific dynamics explains the data considerably better than either one of these alone.
Discussion
Much of the variation in pain report is driven by variation in noxious stimulus intensity, but substantial adaptation effects—sequential effects of the stimulation history—can also strongly modulate pain. Adaptation effects include both habituation and sensitization across time, and may vary in their direction and magnitude across individuals. Predicting and explaining these dynamic effects may be clinically useful, and may also help prevent confounds between experimental manipulations and dynamic adaptation processes in research studies.
By dissociating site-specific and site-nonspecific adaptation processes, we found novel evidence for two opposing types of temporal dynamics in thermal pain. Repeated thermal stimulation on the same skin site produced habituation for all but the highest stimulation temperatures. In contrast, repeated stimulation across different skin sites produced sensitization. To parsimoniously explain these effects, we constructed a dynamic model that captures both types of adaptation processes. The model explained nearly all of the systematic trial-by-trial variance in pain ratings that remained after controlling for stimulus intensity. Because the model parameters were designed to reflect the underlying processes that give rise to temporal dynamics, they have a straightforward interpretation. In particular, αS and αN reflect the signed magnitudes of site-specific and site-nonspecific adaptation, respectively. These processes may manifest in multiple effects in standard statistical tests, which model the form of the data rather than its underlying processes. For example, the site-nonspecific sensitization effect that is only apparent in the first series of stimuli (the first 8 trials) produced complex site-specific by site-nonspecific interactions in our regression analysis, but can be explained relatively parsimoniously by the decay parameter of the model’s site-nonspecific adaptation process. That is, the model predicts that habituation/sensitization processes eventually saturate, which is why site-nonspecific sensitization was only observed during the first third of the stimulation trials. Thus, the model parameters complement standard statistical tests and provide additional insight into the temporal dynamics of thermal pain.
Site-specific habituation in this study depended on stimulus intensity in two ways: More-intense stimuli produced stronger habituation for subsequent stimulations on the same skin site, but more-intense (48–49°C) stimuli also reversed the habituation effect on the current trial, resulting in sensitization for the highest-intensity stimuli (more current pain for stimulations on previously stimulated sites). By suppressing mild repeated pain, while still allowing more biologically salient stimuli – which may, e.g., signal tissue damage – to get through, habituation for low- and sensitization for high-intensity current stimuli may serve an important adaptive role in optimizing survival behavior. This contrastive behavior is characteristic of systems under a balance of excitatory and inhibitory control, such as thalamic circuits that allow salient or attended visual percepts to get through while reducing background noise 47, 48.
If reported pain indeed reflects a mix of site-specific habituation and site-nonspecific sensitization processes, as our data suggest, this has important implications for experimental pain protocols. These opposing repetition effects may cancel each other out in some paradigms but not in others, depending on the timing, stimulus intensity, and the number of times the same vs. new skin sites are stimulated. When habituation and sensitization processes are equally strong, two opposing effects may produce an apparent lack of temporal pain modulation. When one of the effects predominates, temporal dynamics may confound experimental pain-modulation effects, especially those that develop over time (e.g., expectancy, learning and placebo effects), and those that systematically co-vary with presentation order or stimulus intensity. Such temporal confounds can be minimized by carefully matching the use of new and previously stimulated sites across experimental conditions.
Disturbed pain-adaptation processes play a key role in the pathophysiology of chronic pain. Patients with several chronic-pain conditions (e.g., fibromyalgia, migraine and chronic back pain) show reduced habituation, or abnormal sensitization instead of habituation, to repeated noxious stimuli, which is reflected in both their subjective pain and pain-related brain activation (e.g., 11, 15, 38, 49, 51, 58). Whether deficient pain habituation is a pre-dispositional factor that contributes to the development and/or persistence of chronic pain, or the result of an altered cortical state caused by the chronic pain is a matter of debate 10, 50. Different studies have attributed habituation deficits in chronic-pain patients to either cortical hyper-excitability, or a reduced baseline level of cortical activity leading to heightened stimulus-evoked responses 9. In addition to abnormal central adaptation, peripheral input to the central nervous system (e.g., nociceptor sensitization 16) also appears to play a crucial role in the initiation and maintenance of chronic pain 39, 44, 54, 59. Our dynamic model may be helpful in disentangling the underlying processes that give rise to pathological pain.
Although most of our participants showed site-specific habituation and site-nonspecific sensitization, this was not the case for everyone (see Figure 6). Thus, even within the healthy population there is considerable inter-individual variability in the temporal dynamics of pain. These individual differences may reflect inter-individual variability in the sensitivities and/or decay rates of the site-specific and site-nonspecific adaptation processes. A recent study examining the effects of repeated noxious thermal stimulation over the course of several days also found remarkable individual differences in pain adaptation: half of the participants showed habituation and the other half showed sensitization of their pain ratings52. Furthermore, those who sensitized, but not those who habituated, showed a reduction in grey matter density in several pain-processing brain regions on the last compared to the first stimulation day. Interestingly, similar reductions in grey matter density have been reported in chronic-pain patients31, suggesting that pain sensitization (in this case across several days of noxious stimulation) may indicate an increased risk for chronic-pain development. Indeed, initially acute pain following an injury can transform into chronic pain when nociceptor sensitization persists after resolution of the injury or when this triggers a prolonged increase in the excitability and synaptic efficacy of central nociceptive neurons (central sensitization; e.g., 61, 62). As our dynamic model parameters reflect individuals’ tendency to habituate/sensitize, another potential application of the dynamic model is the prediction of individuals’ risk for chronic pain development. Although the present study was not designed to explain individual differences, the dynamic model we developed can capture pain-adaptation effects at the group-mean level and provide estimates at the level of individual participants, thereby providing a foundation for assessment of individual differences. Future studies may measure person-level variables (e.g., pain history, psychopathology) that could serve as predictors of individual differences in pain adaptation, and relate these to individual participants’ estimated pain-adaptation rates.
Although our results provide strong evidence for the existence of two distinct and opposing pain-adaptation processes, the biological basis of these processes remains to be explored in future studies. The site-nonspecific sensitization effect most likely reflected central mechanisms, especially given its independence on the distance between successive stimulation sites. The site-specific habituation effect, on the other hand, could reflect peripheral and/or central processes. It is interesting to note that the characteristics of our observed site-specific habituation effect show a striking resemblance to the response dynamics of monkeys’ nociceptive afferent fibers during repeated heat stimulation 28, 37, 40, 57. The heat-evoked response of these nociceptive fibers rapidly decreases during the first ~5 stimuli, with the strongest decrease from the 1st to the 2nd stimulus. This suppressive effect of previous stimuli on nociceptive fibers’ responsivity increases with the intensity of the preceding heat stimulus, and takes more than 4 minutes to recover e.g.,28. These similarities between activity of peripheral nociceptive fibers and our site-specific habituation effect support the idea that site-specific habituation is, at least partly, peripheral in origin. However, our data does not provide conclusive evidence about this matter, and site-specific adaptation effects may also arise in the central nervous system. Site-specific habituation could, for example, originate from the suppression of pain-related activity in somatotopically organized spinal or cortical areas of the ascending pain pathway. Alternatively, if information about the stimulation sites is represented in the brain, site-specific habituation may be mediated by a descending pain-modulatory system that is somatotopically directed, perhaps similar to that underlying local placebo analgesia4, 36.
Neuroimaging studies could shine more light on the brain mechanisms underlying pain habituation and sensitization. A few studies have investigated the brain activation associated with pain adaptation during repeated stimulation 2, 5, 14, 35, as well as the role of the opioid system 14, 26, 46, but these studies did not dissociate site-specific and site-nonspecific effects. Pain-adaptation processes that are mediated at a peripheral level are expected to nonspecifically affect activation within all regions of the pain-processing network (similar to stimulus-intensity effects), whereas centrally mediated effects are likely associated with more specific activation, either within or outside the pain-processing network.
It remains to be explored whether the distinct effects of site-specific and -nonspecific repetition generalize to other types of pain—e.g., mechanical and electrical—and to other repetition rates. One caveat to the present experiment is that site-specific stimulations were separated by longer intervals than site-nonspecific stimulations, which may have influenced the results. However, we have preliminary data suggesting that site-specific habituation and site-nonspecific sensitization also occur during a stimulation protocol in which the same site is stimulated several times in a row before moving to the next site, which implies that our results were not due to the specific timings used in this experiment. Finally, we examined pain-modulation effects during a relatively limited number of trials; hence did not address adaptation effects that may occur during longer sequences of repeated stimulation. A recent study showed that experienced pain during longer series of repeated heat stimuli applied to the same skin site follows a bi-phasic time course, with initial habituation followed by sensitization25. Whether or not these two effects arise from the same underlying processes, and whether they can be predicted by our dynamic model, are interesting questions for future research.
To conclude, our results reveal complex, but systematic, temporal dynamics of pain, which can be well explained by a relatively simple dynamic model. The ability to disentangle site-specific and site-nonspecific dynamic effects may serve to uncover the mechanisms underlying both normal and pathological pain, and could eventually contribute to the diagnosis and treatment of pain disorders.
Acknowledgments
We would like to thank Luka Ruzic and Pa’Ticia Mac Moion for help with data collection, and Luke Chang and Marina López-Solà for helpful discussions.
Footnotes
The authors report no conflicts of interest.
Disclosures
This research was made possible with the support of National Institutes of Health grants NIMH 2R01MH076136 and R01DA027794-01 (to T.D.W.) and Air Force Office of Scientific Research grant FA9550-10-1-0177 (to M. Jones).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. J Neurosci. 2010;30:12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite AR, Gonzalez RG, Borsook D. Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magn Reson Med. 1999;41:1044–1057. doi: 10.1002/(sici)1522-2594(199905)41:5<1044::aid-mrm25>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 3.Becker S, Kleinbohl D, Baus D, Holzl R. Operant learning of perceptual sensitization and habituation is impaired in fibromyalgia patients with and without irritable bowel syndrome. Pain. 2011;152:1408–1417. doi: 10.1016/j.pain.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 4.Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Casey KL, Morrow TJ, Lorenz J, Minoshima S. Temporal and spatial dynamics of human forebrain activity during heat pain: analysis by positron emission tomography. J Neurophysiol. 2001;85:951–959. doi: 10.1152/jn.2001.85.2.951. [DOI] [PubMed] [Google Scholar]
- 6.Cecchi GA, Huang L, Hashmi JA, Baliki M, Centeno MV, Rish I, Apkarian AV. Predictive dynamics of human pain perception. PLoS Comput Biol. 2012;8:e1002719. doi: 10.1371/journal.pcbi.1002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman TF, Li YY. An interior trust region approach for nonlinear minimization subject to bounds. Siam J Optimiz. 1996;6:418–445. [Google Scholar]
- 8.Condes-Lara M, Calvo JM, Fernandez-Guardiola A. Habituation to bearable experimental pain elicited by tooth pulp electrical stimulation. Pain. 1981;11:185–200. doi: 10.1016/0304-3959(81)90004-X. [DOI] [PubMed] [Google Scholar]
- 9.Coppola G, Pierelli F, Schoenen J. Is the cerebral cortex hyperexcitable or hyperresponsive in migraine? Cephalalgia. 2007;27:1427–1439. doi: 10.1111/j.1468-2982.2007.01500.x. [DOI] [PubMed] [Google Scholar]
- 10.Coppola G, Pierelli F, Schoenen J. Reply to the topical review entitled “the phenomenon of changes in cortical excitability in migraine is not migraine-specific--a unifying thesis” by Anne Stankewitz and Arne May published in Pain 2009;145:14–7. Pain. 2010;149:407–408. doi: 10.1016/j.pain.2010.03.004. author reply 408–409. [DOI] [PubMed] [Google Scholar]
- 11.de Tommaso M, Libro G, Guido M, Losito L, Lamberti P, Livrea P. Habituation of single CO2 laser-evoked responses during interictal phase of migraine. J Headache Pain. 2005;6:195–198. doi: 10.1007/s10194-005-0183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Defrin R, Pope G, Davis KD. Interactions between spatial summation, 2-point discrimination and habituation of heat pain. Eur J Pain. 2008;12:900–909. doi: 10.1016/j.ejpain.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987;26:1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- 14.Ernst M, Lee MH, Dworkin B, Zaretsky HH. Pain perception decrement produced through repeated stimulation. Pain. 1986;26:221–231. doi: 10.1016/0304-3959(86)90077-1. [DOI] [PubMed] [Google Scholar]
- 15.Flor H, Diers M, Birbaumer N. Peripheral and electrocortical responses to painful and non-painful stimulation in chronic pain patients, tension headache patients and healthy controls. Neurosci Lett. 2004;361:147–150. doi: 10.1016/j.neulet.2003.12.064. [DOI] [PubMed] [Google Scholar]
- 16.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010;16:1248–1257. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granot M, Granovsky Y, Sprecher E, Nir RR, Yarnitsky D. Contact heat-evoked temporal summation: Tonic versus repetitive-phasic stimulation. Pain. 2006;122:295–305. doi: 10.1016/j.pain.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Greffrath W, Baumgartner U, Treede RD. Peripheral and central components of habituation of heat pain perception and evoked potentials in humans. Pain. 2007;132:301–311. doi: 10.1016/j.pain.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Grill JD, Coghill RC. Transient analgesia evoked by noxious stimulus offset. J Neurophysiol. 2002;87:2205–2208. doi: 10.1152/jn.00730.2001. [DOI] [PubMed] [Google Scholar]
- 20.Groves PM, Thompson RF. Habituation: a dual-process theory. Psychol Rev. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- 21.Hardy JD, Stolwijk JA, Hammel HT, Murgatroyd D. Skin temperature and cutaneous pain during warm water immersion. J Appl Physiol. 1965;20:1014–1021. doi: 10.1152/jappl.1965.20.5.1014. [DOI] [PubMed] [Google Scholar]
- 22.Hashmi JA, Davis KD. Women experience greater heat pain adaptation and habituation than men. Pain. 2009;145:350–357. doi: 10.1016/j.pain.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Hashmi JA, Davis KD. Effects of temperature on heat pain adaptation and habituation in men and women. Pain. 2010;151:737–743. doi: 10.1016/j.pain.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 24.Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 25.Hollins M, Harper D, Maixner W. Changes in pain from a repetitive thermal stimulus: the roles of adaptation and sensitization. Pain. 2011;152:1583–1590. doi: 10.1016/j.pain.2011.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janicki P, Libich J, Gumulka W. Lack of habituation of pain evoked potentials after naloxone. Pol J Pharmacol Pharm. 1979;31:201–205. [PubMed] [Google Scholar]
- 27.Johnstone T, Salomons TV, Backonja MM, Davidson RJ. Turning on the alarm: the neural mechanisms of the transition from innocuous to painful sensation. Neuroimage. 2012;59:1594–1601. doi: 10.1016/j.neuroimage.2011.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaMotte RH, Campbell JN. Comparison of responses of warm and nociceptive C-fiber afferents in monkey with human judgments of thermal pain. J Neurophysiol. 1978;41:509–528. doi: 10.1152/jn.1978.41.2.509. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Simone DA, Larson AA. Windup leads to characteristics of central sensitization. Pain. 1999;79:75–82. doi: 10.1016/S0304-3959(98)00154-7. [DOI] [PubMed] [Google Scholar]
- 30.Lindquist MA, Spicer J, Asllani I, Wager TD. Estimating and testing variance components in a multi-level GLM. Neuroimage. 2012;59:490–501. doi: 10.1016/j.neuroimage.2011.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 32.May A, Rodriguez-Raecke R, Schulte A, Ihle K, Breimhorst M, Birklein F, Jurgens TP. Within-session sensitization and between-session habituation: A robust physiological response to repetitive painful heat stimulation. Eur J Pain. 2012;16:401–409. doi: 10.1002/j.1532-2149.2011.00023.x. [DOI] [PubMed] [Google Scholar]
- 33.Mendell LM. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966;16:316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- 34.Milne RJ, Kay NE, Irwin RJ. Habituation to repeated painful and non-painful cutaneous stimuli: a quantitative psychophysical study. Exp Brain Res. 1991;87:438–444. doi: 10.1007/BF00231861. [DOI] [PubMed] [Google Scholar]
- 35.Mobascher A, Brinkmeyer J, Warbrick T, Musso F, Schlemper V, Wittsack HJ, Saleh A, Schnitzler A, Winterer G. Brain activation patterns underlying fast habituation to painful laser stimuli. Int J Psychophysiol. 2010;75:16–24. doi: 10.1016/j.ijpsycho.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Montgomery G, Kirsch I. Mechanisms of placebo pain reduction: an empirical investigation. Psychological science. 1996;7:174–176. [Google Scholar]
- 37.Peng YB, Ringkamp M, Meyer RA, Campbell JN. Fatigue and paradoxical enhancement of heat response in C-fiber nociceptors from cross-modal excitation. J Neurosci. 2003;23:4766–4774. doi: 10.1523/JNEUROSCI.23-11-04766.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters ML, Schmidt AJM, Vandenhout MA. Chronic Low-Back Pain and the Reaction to Repeated Acute Pain Stimulation. Pain. 1989;39:69–76. doi: 10.1016/0304-3959(89)90176-0. [DOI] [PubMed] [Google Scholar]
- 39.Price DD, Craggs JG, Zhou Q, Verne GN, Perlstein WM, Robinson ME. Widespread hyperalgesia in irritable bowel syndrome is dynamically maintained by tonic visceral impulse input and placebo/nocebo factors: evidence from human psychophysics, animal models, and neuroimaging. Neuroimage. 2009;47:995–1001. doi: 10.1016/j.neuroimage.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 41.Price DD, Mao J, Frenk H, Mayer DJ. The N-methyl-D-aspartate receptor antagonist dextromethorphan selectively reduces temporal summation of second pain in man. Pain. 1994;59:165–174. doi: 10.1016/0304-3959(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 42.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 43.Price DD, McHaffie JG, Larson MA. Spatial summation of heat-induced pain: influence of stimulus area and spatial separation of stimuli on perceived pain sensation intensity and unpleasantness. J Neurophysiol. 1989;62:1270–1279. doi: 10.1152/jn.1989.62.6.1270. [DOI] [PubMed] [Google Scholar]
- 44.Price DD, Zhou Q, Moshiree B, Robinson ME, Verne GN. Peripheral and central contributions to hyperalgesia in irritable bowel syndrome. J Pain. 2006;7:529–535. doi: 10.1016/j.jpain.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, Coppola G, Geyer MA, Glanzman DL, Marsland S, McSweeney FK, Wilson DA, Wu CF, Thompson RF. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem. 2009;92:135–138. doi: 10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rennefeld C, Wiech K, Schoell ED, Lorenz J, Bingel U. Habituation to pain: further support for a central component. Pain. 2010;148:503–508. doi: 10.1016/j.pain.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 47.Robinson DL. Functional contributions of the primate pulvinar. Prog Brain Res. 1993;95:371–380. doi: 10.1016/s0079-6123(08)60382-9. [DOI] [PubMed] [Google Scholar]
- 48.Saalmann YB, Kastner S. Gain control in the visual thalamus during perception and cognition. Curr Opin Neurobiol. 2009;19:408–414. doi: 10.1016/j.conb.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith BW, Tooley EM, Montague EQ, Robinson AE, Cosper CJ, Mullins PG. Habituation and sensitization to heat and cold pain in women with fibromyalgia and healthy controls. Pain. 2008;140:420–428. doi: 10.1016/j.pain.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 50.Stankewitz A, May A. The phenomenon of changes in cortical excitability in migraine is not migraine-specific--a unifying thesis. Pain. 2009;145:14–17. doi: 10.1016/j.pain.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Stankewitz A, Schulz E, May A. Neuronal correlates of impaired habituation in response to repeated trigemino-nociceptive but not to olfactory input in migraineurs: An fMRI study. Cephalalgia. 2013;33:256–265. doi: 10.1177/0333102412470215. [DOI] [PubMed] [Google Scholar]
- 52.Stankewitz A, Valet M, Schulz E, Woller A, Sprenger T, Vogel D, Zimmer C, Muhlau M, Tolle TR. Pain sensitisers exhibit grey matter changes after repetitive pain exposure: A longitudinal voxel-based morphometry study. Pain. 2013;154:1732–1737. doi: 10.1016/j.pain.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 53.Staud R, Craggs JG, Robinson ME, Perlstein WM, Price DD. Brain activity related to temporal summation of C-fiber evoked pain. Pain. 2007;129:130–142. doi: 10.1016/j.pain.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Staud R, Nagel S, Robinson ME, Price DD. Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebo-controlled study. Pain. 2009;145:96–104. doi: 10.1016/j.pain.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- 56.Timmermann L, Ploner M, Haucke K, Schmitz F, Baltissen R, Schnitzler A. Differential coding of pain intensity in the human primary and secondary somatosensory cortex. J Neurophysiol. 2001;86:1499–1503. doi: 10.1152/jn.2001.86.3.1499. [DOI] [PubMed] [Google Scholar]
- 57.Treede RD, Meyer RA, Raja SN, Campbell JN. Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. J Physiol. 1995;483 (Pt 3):747–758. doi: 10.1113/jphysiol.1995.sp020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valeriani M, de Tommaso M, Restuccia D, Le Pera D, Guido M, Iannetti GD, Libro G, Truini A, Di Trapani G, Puca F, Tonali P, Cruccu G. Reduced habituation to experimental pain in migraine patients: a CO(2) laser evoked potential study. Pain. 2003;105:57–64. doi: 10.1016/s0304-3959(03)00137-4. [DOI] [PubMed] [Google Scholar]
- 59.Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain. 2003;105:223–230. doi: 10.1016/s0304-3959(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 60.Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage. 2009;47:821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 62.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yelle MD, Oshiro Y, Kraft RA, Coghill RC. Temporal filtering of nociceptive information by dynamic activation of endogenous pain modulatory systems. J Neurosci. 2009;29:10264–10271. doi: 10.1523/JNEUROSCI.4648-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yelle MD, Rogers JM, Coghill RC. Offset analgesia: a temporal contrast mechanism for nociceptive information. Pain. 2008;134:174–186. doi: 10.1016/j.pain.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]