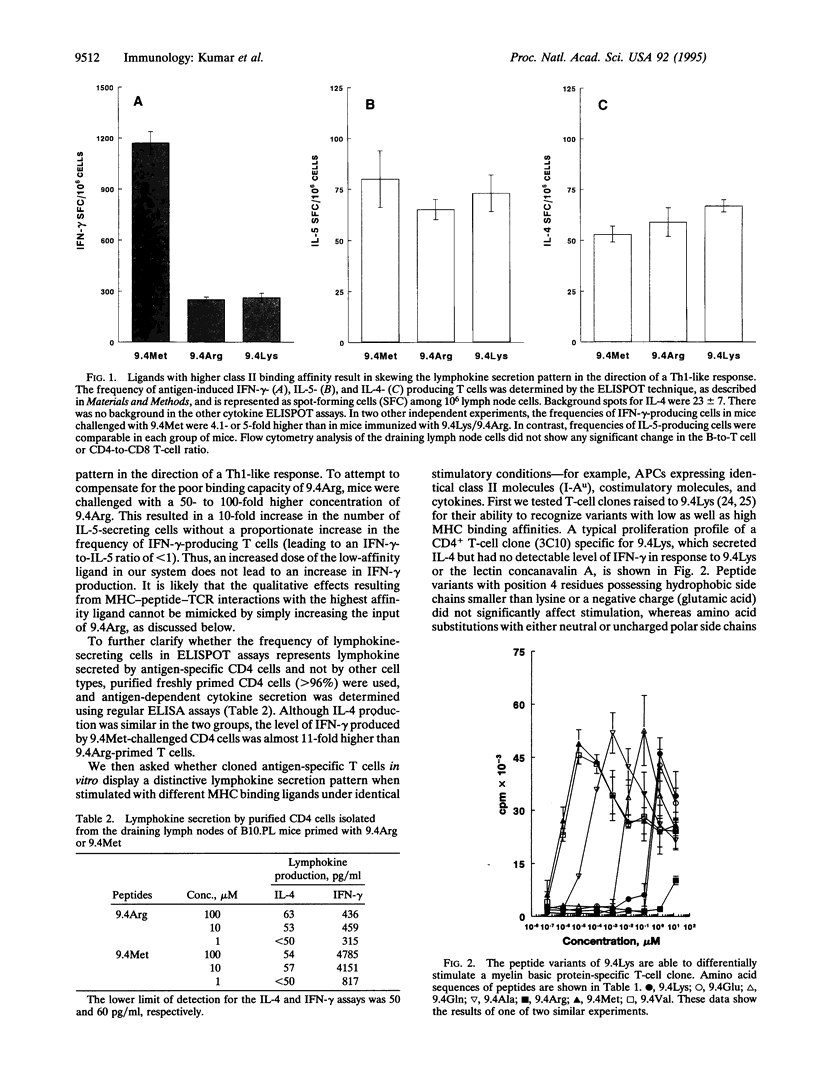
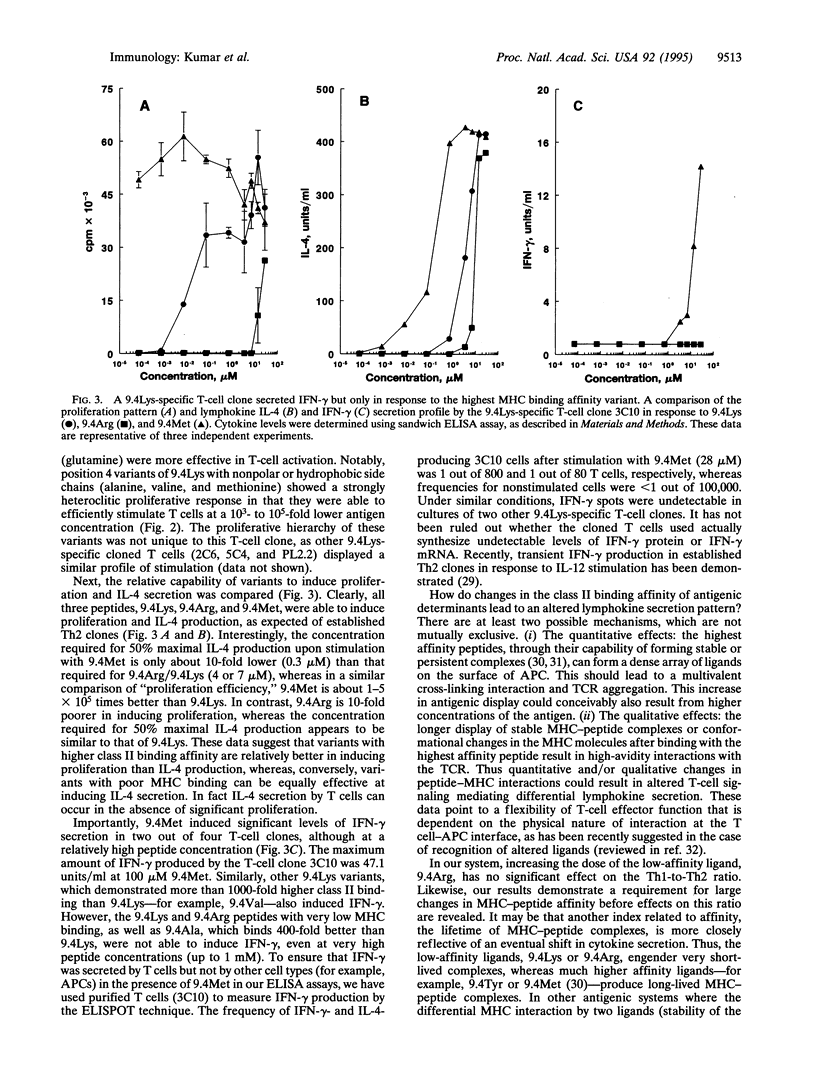
Abstract
Differential activation of CD4+ T-cell precursors in vivo leads to the development of effectors with unique patterns of lymphokine secretion. To investigate whether the differential pattern of lymphokine secretion is influenced by factors associated with either the display and/or recognition of the ligand, we have used a set of ligands with various class II binding affinities but unchanged T-cell specificity. The ligand that exhibited approximately 10,000-fold higher binding to I-Au considerably increased the frequency of interferon gamma-producing but not interleukin (IL) 4- or IL-5-secreting cells in vivo. Using an established ligand-specific, CD4+ T-cell clone secreting only IL-4, we also demonstrated that stimulation with the highest affinity ligand resulted in interferon gamma production in vitro. In contrast, ligands that demonstrated relatively lower class II binding induced only IL-4 secretion. These data suggest that the major histocompatibility complex binding affinity of antigenic determinants, leading to differential interactions at the T cell-antigen-presenting cell interface, can be crucial for the differential development of cytokine patterns in T cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhardwaj V., Kumar V., Geysen H. M., Sercarz E. E. Degenerate recognition of a dissimilar antigenic peptide by myelin basic protein-reactive T cells. Implications for thymic education and autoimmunity. J Immunol. 1993 Nov 1;151(9):5000–5010. [PubMed] [Google Scholar]
- Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1988 Sep;9(9):268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Miles C., Grey H. M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987 Mar 13;235(4794):1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I., Aebersold R., Ziltener H., Schrader J. W., Hood L. E., Kent S. B. Automated chemical synthesis of a protein growth factor for hemopoietic cells, interleukin-3. Science. 1986 Jan 10;231(4734):134–139. doi: 10.1126/science.3079915. [DOI] [PubMed] [Google Scholar]
- Curry R. C., Kiener P. A., Spitalny G. L. A sensitive immunochemical assay for biologically active MuIFN-gamma. J Immunol Methods. 1987 Nov 23;104(1-2):137–142. doi: 10.1016/0022-1759(87)90497-2. [DOI] [PubMed] [Google Scholar]
- Evavold B. D., Sloan-Lancaster J., Allen P. M. Tickling the TCR: selective T-cell functions stimulated by altered peptide ligands. Immunol Today. 1993 Dec;14(12):602–609. doi: 10.1016/0167-5699(93)90200-5. [DOI] [PubMed] [Google Scholar]
- Fairchild P. J., Wildgoose R., Atherton E., Webb S., Wraith D. C. An autoantigenic T cell epitope forms unstable complexes with class II MHC: a novel route for escape from tolerance induction. Int Immunol. 1993 Sep;5(9):1151–1158. doi: 10.1093/intimm/5.9.1151. [DOI] [PubMed] [Google Scholar]
- Fujihashi K., McGhee J. R., Beagley K. W., McPherson D. T., McPherson S. A., Huang C. M., Kiyono H. Cytokine-specific ELISPOT assay. Single cell analysis of IL-2, IL-4 and IL-6 producing cells. J Immunol Methods. 1993 Apr 2;160(2):181–189. doi: 10.1016/0022-1759(93)90176-8. [DOI] [PubMed] [Google Scholar]
- Hood L., Kumar V., Osman G., Beall S. S., Gomez C., Funkhouser W., Kono D. H., Nickerson D., Zaller D. M., Urban J. L. Autoimmune disease and T-cell immunologic recognition. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 2):859–874. doi: 10.1101/sqb.1989.054.01.100. [DOI] [PubMed] [Google Scholar]
- Hsieh C. S., Heimberger A. B., Gold J. S., O'Garra A., Murphy K. M. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., Tripp C. S., Wolf S. F., O'Garra A., Murphy K. M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993 Apr 23;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Kamogawa Y., Minasi L. A., Carding S. R., Bottomly K., Flavell R. A. The relationship of IL-4- and IFN gamma-producing T cells studied by lineage ablation of IL-4-producing cells. Cell. 1993 Dec 3;75(5):985–995. doi: 10.1016/0092-8674(93)90542-x. [DOI] [PubMed] [Google Scholar]
- Kumar V., Sercarz E. E. The involvement of T cell receptor peptide-specific regulatory CD4+ T cells in recovery from antigen-induced autoimmune disease. J Exp Med. 1993 Sep 1;178(3):909–916. doi: 10.1084/jem.178.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetti R., Gerosa F., Giudizi M. G., Biagiotti R., Parronchi P., Piccinni M. P., Sampognaro S., Maggi E., Romagnani S., Trinchieri G. Interleukin 12 induces stable priming for interferon gamma (IFN-gamma) production during differentiation of human T helper (Th) cells and transient IFN-gamma production in established Th2 cell clones. J Exp Med. 1994 Apr 1;179(4):1273–1283. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Schumacher J. H., Fiorentino D. F., Leverah J., Moore K. W., Bond M. W. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. J Immunol. 1990 Nov 1;145(9):2938–2945. [PubMed] [Google Scholar]
- Murray J. S., Pfeiffer C., Madri J., Bottomly K. Major histocompatibility complex (MHC) control of CD4 T cell subset activation. II. A single peptide induces either humoral or cell-mediated responses in mice of distinct MHC genotype. Eur J Immunol. 1992 Feb;22(2):559–565. doi: 10.1002/eji.1830220239. [DOI] [PubMed] [Google Scholar]
- Nelson C. A., Petzold S. J., Unanue E. R. Peptides determine the lifespan of MHC class II molecules in the antigen-presenting cell. Nature. 1994 Sep 15;371(6494):250–252. doi: 10.1038/371250a0. [DOI] [PubMed] [Google Scholar]
- Parish C. R. The relationship between humoral and cell-mediated immunity. Transplant Rev. 1972;13:35–66. doi: 10.1111/j.1600-065x.1972.tb00059.x. [DOI] [PubMed] [Google Scholar]
- Pfeiffer C., Stein J., Southwood S., Ketelaar H., Sette A., Bottomly K. Altered peptide ligands can control CD4 T lymphocyte differentiation in vivo. J Exp Med. 1995 Apr 1;181(4):1569–1574. doi: 10.1084/jem.181.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–257. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- Röcken M., Saurat J. H., Hauser C. A common precursor for CD4+ T cells producing IL-2 or IL-4. J Immunol. 1992 Feb 15;148(4):1031–1036. [PubMed] [Google Scholar]
- Scott P., Kaufmann S. H. The role of T-cell subsets and cytokines in the regulation of infection. Immunol Today. 1991 Oct;12(10):346–348. doi: 10.1016/0167-5699(91)90063-Y. [DOI] [PubMed] [Google Scholar]
- Seder R. A., Gazzinelli R., Sher A., Paul W. E. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder R. A., Paul W. E., Davis M. M., Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992 Oct 1;176(4):1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares L. R., Sercarz E. E., Miller A. Vaccination of the Leishmania major susceptible BALB/c mouse. I. The precise selection of peptide determinant influences CD4+ T cell subset expression. Int Immunol. 1994 May;6(5):785–794. doi: 10.1093/intimm/6.5.785. [DOI] [PubMed] [Google Scholar]
- Soloway P., Fish S., Passmore H., Gefter M., Coffee R., Manser T. Regulation of the immune response to peptide antigens: differential induction of immediate-type hypersensitivity and T cell proliferation due to changes in either peptide structure or major histocompatibility complex haplotype. J Exp Med. 1991 Oct 1;174(4):847–858. doi: 10.1084/jem.174.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., Bradley L. M., Croft M., Tonkonogy S., Atkins G., Weinberg A. D., Duncan D. D., Hedrick S. M., Dutton R. W., Huston G. Helper T-cell subsets: phenotype, function and the role of lymphokines in regulating their development. Immunol Rev. 1991 Oct;123:115–144. doi: 10.1111/j.1600-065x.1991.tb00608.x. [DOI] [PubMed] [Google Scholar]
- Taguchi T., McGhee J. R., Coffman R. L., Beagley K. W., Eldridge J. H., Takatsu K., Kiyono H. Detection of individual mouse splenic T cells producing IFN-gamma and IL-5 using the enzyme-linked immunospot (ELISPOT) assay. J Immunol Methods. 1990 Mar 27;128(1):65–73. doi: 10.1016/0022-1759(90)90464-7. [DOI] [PubMed] [Google Scholar]
- Urban J. L., Kumar V., Kono D. H., Gomez C., Horvath S. J., Clayton J., Ando D. G., Sercarz E. E., Hood L. Restricted use of T cell receptor V genes in murine autoimmune encephalomyelitis raises possibilities for antibody therapy. Cell. 1988 Aug 12;54(4):577–592. doi: 10.1016/0092-8674(88)90079-7. [DOI] [PubMed] [Google Scholar]
- Wall M., Southwood S., Sidney J., Oseroff C., del Guericio M. F., Lamont A. G., Colón S. M., Arrhenius T., Gaeta F. C., Sette A. High affinity for class II molecules as a necessary but not sufficient characteristic of encephalitogenic determinants. Int Immunol. 1992 Jul;4(7):773–777. doi: 10.1093/intimm/4.7.773. [DOI] [PubMed] [Google Scholar]
- Wraith D. C., Smilek D. E., Mitchell D. J., Steinman L., McDevitt H. O. Antigen recognition in autoimmune encephalomyelitis and the potential for peptide-mediated immunotherapy. Cell. 1989 Oct 20;59(2):247–255. doi: 10.1016/0092-8674(89)90287-0. [DOI] [PubMed] [Google Scholar]