Abstract
Post-translational modification by SUMO is a highly conserved pathway in eukaryotes that plays very important regulatory roles in many cellular processes. Deregulation of the SUMO pathway contributes to the development and progression of many diseases including cancer. Therefore, identifying additional SUMO substrates and studying how their cellular and biological functions are regulated by sumoylation should provide new insights. Our studies showed that sumoylation activity was significant in Xenopus egg extracts, and that a high level of sumoylation was associated with sperm chromatin when SUMO was incubated with Xenopus egg extracts. By isolating SUMO-conjugated substrates using His-tagged SUMO1 or SUMO2 proteins under denaturing conditions, we identified 346 proteins by mass spectrometry analysis that were not present in control pull-downs. Among them, 167 proteins were identified from interphase egg extracts, 86 proteins from mitotic phase egg extracts, and 93 proteins from both. Thirty-three proteins were pulled down by SUMO1, 85 proteins by SUMO2, and 228 proteins by both. We validated the sumoylation of five candidates, CKB, ATXN10, BTF3, HABP4, and BZW1, by co-transfecting them along with SUMO in HEK293T cells. Gene ontology analysis showed that SUMO substrates identified in this study were involved in diverse biological processes. Additionally, SUMO substrates identified from different cell cycle stages or pulled down by different SUMO homologs were enriched for distinct cellular components and functional categories. Our results comprehensively profile the sumoylation occurring in the Xenopus egg extract system.
Post-translational modification by small ubiquitin-like modifier (SUMO)1 is highly conserved from yeast to human. There are three well-characterized SUMO proteins in human: SUMO1, 2, and 3. SUMO2 and SUMO3 share about 92% identity and have no apparent functional differences. However, the identity between SUMO2/3 and SUMO1 is only 45%. SUMO1 has different dynamics and a distinct profile of target proteins from SUMO2/3, and some proteins with SUMO-interacting motifs (SIMs) distinguish SUMO1 from SUMO2/3. Therefore, protein substrates modified by different SUMO proteins may exert different functions (1).
Sumoylation is a multi-step enzymatic process. The SUMO precursor protein is initially processed at the C terminus by SUMO-specific proteases (SENPs) to generate mature SUMO with a C-terminal Gly-Gly motif. Then, an ATP-dependent activating E1 enzyme (a heterodimer of SAE1 and SAE2) and a conjugating enzyme E2 (UBC9) link sequentially to SUMO by forming a thioester bond between SUMO's C-terminal Gly and Cys residues in the active sites of these enzymes. In an E3 ligase dependent or independent manner, SUMO is conjugated through the C-terminal Gly-Gly motif onto the ε-amino group of specific Lys residues of substrates forming an isopeptide bond. The substrates can be modified by a single SUMO, multiple SUMOs at several Lys residues, or a chain of SUMOs. Conjugated SUMO can be recognized by SIMs in other proteins, allowing sumoylation to induce protein-protein interaction. Like ubiquitylation, sumoylation is also reversible because of the activity of desumoylating enzymes (2).
Sumoylation through altering protein stability, localization, interactions, or activity, is involved in diverse cellular processes, such as nuclear-cytosolic transport, transcriptional regulation (especially transcriptional repression), apoptosis, chromatin remodeling, metabolic regulation, response to stress, and progression through the cell cycle (3, 4). The sumoylation pathway contributes to the development and progression of many diseases including cancer (3, 5, 6). For example, a sumoylation-dependent transcriptional subprogram is required for Myc-driven tumorigenesis (7). Because the functional consequence of sumoylation is highly dependent on each individual substrate, identification of new SUMO modified proteins and further study of their functions will be important for understanding SUMO-dependent regulation and its role in disease.
Extracts prepared from the eggs of Xenopus laevis have proven to be extremely useful for studying cell cycle events in vitro (8). A female frog typically lays >1000 eggs overnight after hormone stimulation. Before fertilization, eggs are arrested by cytostatic factor (CSF) in Meiosis II, which resembles the mitotic phase in somatic cells. As the eggs contain highly concentrated proteins stockpiled for rapid cell divisions in early embryo development, extracts from these eggs are very useful for recapitulating the important events happening during mitosis, such as kinetochore assembly and spindle assembly (9). Moreover, CSF extracts can be induced to enter interphase by the addition of calcium to cause the degradation of CSF and mitotic cyclins, and can also be used to study the important events in interphase, such as nuclear envelope assembly, nuclear transport, and DNA replication (10). Reports from several labs have suggested the usefulness of the egg extract in studying sumoylation (11–14). However, unlike studies in yeast and mammalian cells (1, 15–18), a systematic large-scale isolation of SUMO substrates using this system has not been reported. Here, we studied sumoylation during different cell cycle stages using the Xenopus egg extract system and describe a method for identification of sumoylated proteins from Xenopus egg extracts by pulling down His-tagged SUMO mutant proteins under denaturing conditions. Mass spectrometry (MS) analysis and further study of the candidate proteins suggest the feasibility of using the Xenopus system for large-scale exploration of SUMO substrates and for investigating functions of sumoylation.
EXPERIMENTAL PROCEDURES
cDNAs and Expression Vectors
The cDNA clones of Xenopus laevis SUMO1b (NM_001096805) and SUMO2b (NM_001092126) were purchased from Open Biosystems. The human cDNAs of CKB (BC019281), ATXN10 (BC007508), BTF3 (NM_001037637), HABP4 (BC018788), and BZW1 (BC026303) were amplified from total RNA of HEK293T cells with RT-PCR. The human cDNA of RanGAP1 (accession, X82260) was gift from Dr. Martin Hetzer. The human cDNA of tumor suppressor p53 (accession, JQ694050) was gift from Dr. Clodagh O'Shea. The human cDNAs of SUMO E1 (SAE1 and SAE2), E2 (UBC9), SUMO1, and SUMO2 were gifts from Dr. Ronald Hay. PCR or PCR-based site-directed mutagenesis (QuikChange, Agilent Technology, Santa Clara, CA) was used to generate mutation in cDNAs. For all wild-type SUMO1 and SUMO2 constructs, the last four and two respectively amino acids after GG at the C terminus were deleted to encode mature processed SUMO. All the mutants used in this study were constructed based on their wild-type plasmid: SUMO1AA and SUMO2AA (mutating the last two Gly to Ala); SUMO1ΔGG and SUMO2ΔGG (deleting the last two Gly); SUMO1HFV and SUMO2QFI were described previously (19); SUMO1HFV-AA and SUMO2QFI-AA (mutating the last two Gly to Ala of SUMO1HFV and SUMO2QFI); SUMO1T96R and SUMO2T91R (mutating the Thr before GG to Arg); RanGAP1K15R; RanGAP1K524R; and RanGAP1K15RK524R. Mammalian cells expressing FLAG- or GFP-tagged constructs were made by cloning the cDNA into pFLAG-CMV-2 (Sigma) or pEGFP-C (Clontech, Mountain View, CA) vectors. For gene expression in bacteria, GFP-SUMO1 and SUMO2 mutant proteins were fusions of EGFP and SUMO1 or SUMO2 mutants, and were purified as 8His-tagged proteins from E. coli (20). The plasmid expressing His-FLAG-p53 protein was constructed by inserting the FLAG-tagged p53 into pET28a (Novagen, Madison, WI) vector. All the other GST or 6His-tagged constructs were made by cloning the cDNA into pGEX4T-1 (GE Healthcare) or pET28a (Novagen) vector.
Expression and Purification of Recombinant Proteins
All recombinant proteins were expressed in BL21Rosetta (DE3) competent cells (Novagen) with 0.1 mm IPTG induction at 25 °C for 5 h. To purify His-tagged proteins, the bacterial pellet from each liter of the culture was resuspended with 30 ml of cold NaP buffer (50 mm Na2HPO4, pH 7.0, and 300 mm NaCl) containing 1 mm imidazole, 0.25% TritonX100 and 1 mm PMSF, and broken by Emulsifier (EmulsiFlex-C3, AVESTIN Inc., Ottawa, Canada). The clarified supernatants were incubated with cobalt beads (Clontech #635502, 3 ml for 1 liter culture) at 4 °C for 2 h, and loaded onto an Econo-Pac Chromatography column (Bio-Rad, #732–1010EDU). The column was washed with 50 bead volume of NaP buffer containing 1 mm imidazole and 0.1 mm PMSF. The proteins were eluted using NaP buffer containing 200 mm imidazole. To purify GST-fusion proteins, HEPES buffer (50 mm HEPES, pH 8.0, and 300 mm NaCl) containing 0.5% TritonX100, 1 mm DTT, and 1 mm PMSF were used for lysis. GST beads (GenScript, #L00206) were used for binding proteins and washed with HEPES buffer containing 0.1% TrionX100 and 0.1 mm PMSF. Proteins were eluted by using HEPES buffer containing 20 mm reduced glutathione. All the purified proteins were changed into buffer using PD10 desalting columns (GE Healthcare, #17–0851-01) and concentrated using Millipore's Amicon Ultra centrifugal filter devices (#UFC900324) depending on the experiments.
Sumoylation Assays in Xenopus Egg Extracts
The methods for preparing interphase Xenopus egg extracts, mitotic Xenopus egg extracts, isolating demembranated Xenopus sperm chromatin, in vitro reconstitution, and fluorescence microscopy have been described previously (9–11, 21, 22). For conjugation assays, 1 μl of bacterially expressed His-GFP-SUMO proteins (5∼20 μg/μl) and 1 μl of demembranated Xenopus sperm (100,000 sperm/μl) were added into 100 μl of freshly prepared Xenopus egg extracts and incubated for specific times. All the fluorescent images were captured using a Zeiss LSM 710 or 780 Laser Scanning Confocal Microscope with a 63× oil objective. For His-SUMO pull-down assays, 30 μg of His-SUMO1T96R or His-SUMO2T91R proteins and 3 μl of demembranated Xenopus sperm were added into 250 μl of freshly prepared Xenopus egg extracts and incubated for 60 min. Xenopus egg extracts with sperm addition but no His-SUMO protein addition was used as a control. It is hard to determine the exact concentration of endogenous SUMO proteins in Xenopus egg extracts because of their conjugation, but we estimate the amount of added recombinant SUMO protein used here was approximately a four- to 10-fold excess over the level of endogenous SUMO based on the published literatures (9, 11, 12).
The reaction was terminated by adding 1 ml of NaP buffer (50 mm Na2HPO4, pH 7.0, and 300 mm NaCl) containing 25 mm N-ethylmaleimide (NEM) and 10 m urea at room temperature. 60 μl of cobalt beads (Clontech #635502) were incubated with the denaturing reaction at room temperature for 2 h and washed 8 times with 1 ml of NaP buffer containing 8 m urea. The proteins were eluted with 120 μl of NaP buffer containing 8 m urea and 200 mm imidazole. 10 μl of eluate was analyzed by SDS-PAGE and silver staining, and 90 μl was used for MS analysis. To detect if there were ubiquitin substrates contamination in the SUMO pull-down products, 4 μl of eluate was analyzed by Western blotting with anti-SUMO1 mouse monoclonal antibody (Invitrogen, #33–2400, 1:800), anti-SUMO2 mouse monoclonal antibody (Abcam, Cambridge, MA; ab81371, 1:1000), and anti-ubiquitin mouse monoclonal antibody (Santa Cruz, Santa Cruz, CA; sc-8017, 1:1000), respectively. One μl of Xenopus egg extract was loaded as control to test the antibodies. This His-SUMO pull-down assay was repeated three times using egg extracts from three different frogs.
In Vitro Sumoylation Assays
The method for in vitro sumoylation was based on the protocol published before (23). Briefly, a 20 μl-reaction was assembled by mixing all the components in the order of H2O, 2 μl of 10×Reaction buffer, 1 μl of substrate (1.5 μg/μl), 1 μl of GST-UBC9 (2 μg/μl), 1 μl of SUMO protein (2 μg/μl), 1 μl of GST-SAE1/SAE2 (0.5 μg/μl), and 2 μl of ATP (20 mm stock). After incubation at 30 °C for 2 h (or the indicated temperature and time), the reaction was terminated by adding 20 μl of 2×SDS sample buffer and boiled for 10 min at 95 °C. 5∼10 μl of final sample was loaded for immunoblotting.
Mass Spectrometry
Samples were first denatured in 8 m urea and subsequently reduced and alkylated with 10 mm Tris (2-carboxyethyl) phosphine hydrochloride (Sigma Aldrich, #C4706) and 55 mm chloroacetamide (Sigma-Aldrich, #22790) respectively. Overnight digestion with trypsin (Promega, Madison, WI; #V511A) was performed according to the manufacturer's specifications.
Protein digests were pressure-loaded onto 250 micron i.d. fused silica capillary (Polymicro Technologies, Phoenix, AZ; #160–2250-10) columns with a Kasil frit that were packed with 3 cm of 5 micron Partisphere strong cation exchange (SCX) resin (Whatman, #4621–1507) and 3 cm of 5 micron C18 resin (Phenomenex, Torrence, CA; #04A-4299). Following a desalting step, each bi-phasic column was connected to a 100 micron i.d. fused silica capillary (Polymicro Technologies, #1068150023) analytical column with a 5 micron pulled-tip that was packed with 10 cm of 5 micron C18 resin.
The MudPIT column was placed inline with an 1100 quaternary HPLC pump (Agilent Technologies). The eluted peptides were directly electrosprayed into an LTQ Orbitrap XL mass spectrometer (Thermo Scientific). The buffer solutions used were 5% acetonitrile/0.1% formic acid (buffer A), 80% acetonitrile/0.1% formic acid (buffer B), and 500 mm ammonium acetate/5% acetonitrile/0.1% formic acid (buffer C). An eleven-step MudPIT was run using salt pulses of 0%, 10%, 20%, 30%, 40%, 50%, 60%, 70%, and 100% buffer C and 90% buffer C/10% buffer B (twice). The 120 min elution gradient had the following profile: 10% buffer B beginning at 15 min to 40% buffer B at 105 min, continuing to 110 min.
One cycle consisted of one full scan mass spectrum (400–1600 m/z) in the Orbitrap at 60,000 resolution followed by five data-dependent collision induced dissociation (CID) MS/MS spectra in the LTQ. Charge state screening was enabled and unassigned charge states and charge state 1 were rejected. Dynamic exclusion was enabled with a repeat count of 1, a repeat duration of 30 s, an exclusion list size of 500 and an exclusion duration of 180 s. Dynamic exclusion early expiration was enabled with an expiration count of 3 and an expiration signal-to-noise ratio of 3. Application of mass spectrometer scan functions and HPLC solvent gradients were controlled by the Xcalibur data system (Thermo Scientific).
MS/MS spectra were extracted using RawXtract (version 1.9.9) with the default settings (24). MS/MS spectra were searched with the ProLuCID algorithm (version 1.3.5) (25) against a Xenopus laevis protein database (release date 02–08-2010) downloaded from Xenbase concatenated to a decoy database in which the sequence for each original database entry was reversed (26). A total of 68,260 protein entries were searched. Precursor mass tolerance was 50 ppm and fragment ion mass tolerance was 600 ppm. For protein identification, the ProLuCID search was performed using no enzyme specificity and static modification of cysteine caused by carboxyamidomethylation (57.02146). ProLuCID search results were assembled and filtered using the DTASelect (version 2.0.49) algorithm (27), requiring identified peptides to be fully tryptic and a minimum of one peptide per protein identification. The number of missed cleavages was not specified. The protein identification false positive rate was held below one percent and all peptide-spectra matches were required to have less than 10 ppm mass error. DTASelect assesses the validity of peptide-spectra matches using the cross-correlation score (XCorr) and normalized difference in cross-correlation scores (deltaCN). The search results are grouped by charge state and tryptic status and each sub-group is analyzed by discriminant analysis based on a non-parametric fit of the distribution of forward and reversed matches. For sumoylation site identification, a second ProLuCID search was performed using full enzyme specificity, static modification of cysteine caused by carboxyamidomethylation (57.02146) and differential modification of lysine caused by di-glycine remnant (114.042927). ProLuCID search results were assembled and filtered using the DTASelect (version 2.0.49) algorithm, requiring a minimum of one peptide per protein identification, a maximum of two missed cleavages per peptide and considering only modified peptides. The peptide identification false positive rate was held below one percent and all peptide-spectra matches were required to have less than 10 ppm mass error. Peptide count, spectral count, sequence coverage, NSAF value (28), and emPAI value (29) are reported for each protein identification as well as occurrences in different experiments. Candidate sumoylated proteins were those identified in either the SUMO1 or SUMO2 pull-down but not identified in the control pull-down.
Immunoprecipitation
Human embryonic kidney 293T (HEK293T) cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Plasmids expressing FLAG-tagged protein and GFP-SUMO were co-transfected with or without untagged UBC9 into each 10-cm dish of cells (the ratio is 8:8:4/0) using the calcium phosphate precipitation method. 48 h after transfection, each dish of cells was lysed with 1 ml lysis buffer (20 mm Tris-HCl, pH7.5, 150 mm NaCl, 0.5% Nonidet P-40, 10% glycerol, 1 mm EDTA, 20 mm NEM, 1 mm protease inhibitor mixture, and 1 mm PMSF) assisted with sonication bursts (3 × 10 s using a microtip on a Branson Sonifier 450 with output control set at 1.5). 20 μl of anti-FLAG M2 agarose beads (Sigma, #A2220) were incubated with the clarified cell lysates for 2 h at 4 °C, washed with lysis buffer for 6∼8 times and boiled with 60 μl of 2×SDS sample buffer at 95 °C for 10 min. 4∼10 μl of final sample was loaded for immunoblotting analysis. All the antibodies used here: anti-GFP rabbit polyclonal (homemade, 1:1000), anti-UBC9 rabbit polyclonal (affinity purified from rabbit antiserum raised against His-tagged human UBC9, 1:1000), anti-FLAG mouse monoclonal (Sigma, #F3165, 1:5000), and anti-α-tubulin (Sigma, #T5168, 1:5000).
Data Analysis
GenBank Protein Accessions and UniProt Accessions for Xenopus laevis (taxon ID 8355) were converted to Gene Symbols using bioDBnet (30) and DAVID (31). Human gene symbols were used for subsequent analyses because they are well-annotated relative to Xenopus. Gene list functional classification analysis was performed on sumoylated proteins using version 9.0 of the Protein ANalysis THrough Evolutionary Relationships (PANTHER) classification system (32). The Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) (33) was used to map interactions between the sumoylated proteins identified in this study. Only the highest confidence interactions (confidence score > 0.900) were retained. The Database for Annotation, Visualization and Integrated Discovery (DAVID) (31) was used to identify enriched terms among sumoylated proteins. Enriched terms were required to have a p value < 0.05 after Benjamini correction. SUMO substrates identified from different cell cycle stages (interphase and mitotic phase) or pulled down by different SUMO homologs (SUMO1 and SUMO2) were analyzed separately.
RESULTS
SUMO Proteins Bind to Sperm Chromatin during both Mitotic Phase and Interphase
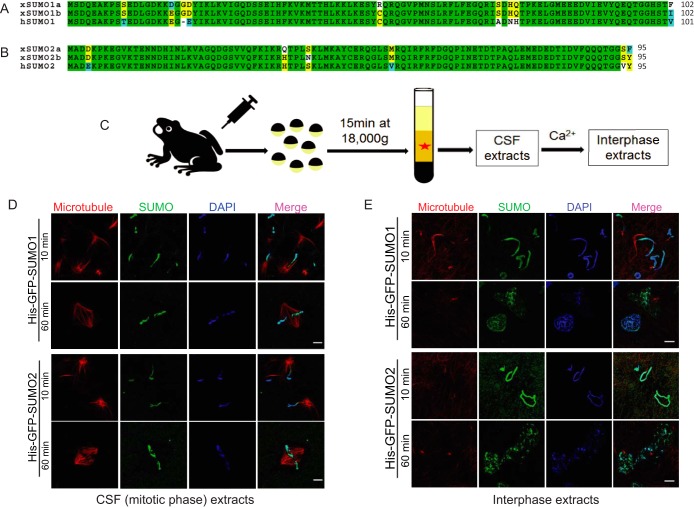
SUMO proteins in Xenopus laevis are very similar to their orthologs in human (34). There are several SUMO isoforms in Xenopus laevis: SUMO1a, SUMO1b, SUMO2a, SUMO2b, and SUMO3. Xenopus SUMO1a shares 97.1% similarity with SUMO1b, and SUMO1b is marginally more similar to human SUMO1 compared with SUMO1a (92.2% versus 91.2%) (Fig. 1A). Xenopus SUMO2a shares 96.8% similarity with SUMO2b, and SUMO2b is marginally more similar to human SUMO2 compared with SUMO2a (95.8% versus 94.7%) (Fig. 1B). Xenopus SUMO3 shares 98.9% similarity with human SUMO3 (supplemental Fig. S1A). Similar to the human SUMO proteins, Xenopus SUMO2 and SUMO3 are very similar (92.6% similarity). However, the similarity between Xenopus SUMO1 and SUMO2 is only 52.9% (supplemental Fig. S1B and S1C). Therefore, Xenopus SUMO1b and SUMO2b correspond to human SUMO1 and SUMO2 and can be used to carry out experiments in the Xenopus system. Functions of several well-characterized SUMO substrates, such as RanGAP1, PCNA, TOP2A, and PARP1, have already been studied in the Xenopus system (11–13, 35). CSF-arrested Xenopus egg extracts, which mimic the mitotic phase of somatic cells, and can be driven into interphase by adding calcium, have been extensively used for cell cycle studies (9, 21) (Fig. 1C). When demembranated Xenopus sperm chromatin was incubated with a mitotic Xenopus egg extract and purified His-GFP-tagged SUMO1 or SUMO2 proteins, a strong GFP signal appeared on the chromatin in less than 10 min and persisted for more than 60 min (Fig. 1D). We also found that a strong GFP signal appeared on the chromatin even after nuclear formation when the chromatin was incubated with His-GFP-tagged SUMO1 or SUMO2 proteins in interphase egg extracts (Fig. 1E). These observations suggested that His-GFP-tagged SUMO proteins could bind tightly to the chromatin in the presence of Xenopus egg extracts.
Fig. 1.
SUMO proteins associate with Xenopus sperm chromatin during both mitotic phase and interphase. A, Sequence comparison of Xenopus laevis SUMO1a (NP_001083717), SUMO1b (NP_001090274) and human SUMO1 (NP_003343). Completely conserved residues are shaded green, identical residues are shaded yellow, similar residues are shaded cyan, and different residues are shaded white. B, Sequence comparison of Xenopus laevis SUMO2a (NP_001080085), SUMO2b (NP_001085595) and human SUMO2 (NP_008868). Completely conserved residues are shaded green, identical residues are shaded yellow, similar residues are shaded cyan, and different residues are shaded white. C, CSF-arrested Xenopus egg extract preparation. CSF extracts can be induced to enter interphase by the addition of calcium. D, SUMO proteins associate with sperm chromatin during mitotic phase. Sperm chromatin isolated from male Xenopus was incubated with mitotic Xenopus egg extracts and purified His-GFP-tagged SUMO1 or SUMO2 protein. A strong GFP signal appeared on the chromatin in less than 10 min and persisted for more than 60 min. Microtubule structures formed by rhodamine-labeled tubulin indicate the cell cycle stage of Xenopus egg extracts. DAPI was used for DNA staining. Scale bar equals 10 μm. E, SUMO proteins also associate with sperm chromatin during interphase. The interphase Xenopus egg extract was induced from mitotic egg extract by adding calcium. Sperm chromatin isolated from male Xenopus was incubated with interphase Xenopus egg extract and purified His-GFP-tagged SUMO1 or SUMO2 protein. A strong GFP signal appeared on the chromatin in less than 10 min and persisted even after nuclear formation. Microtubule structures formed by rhodamine-labeled tubulin indicate the cell cycle stage of Xenopus egg extract. DAPI was used for DNA staining. Scale bar equals 10 μm.
Sumoylation Activity is High in Xenopus Egg Extracts
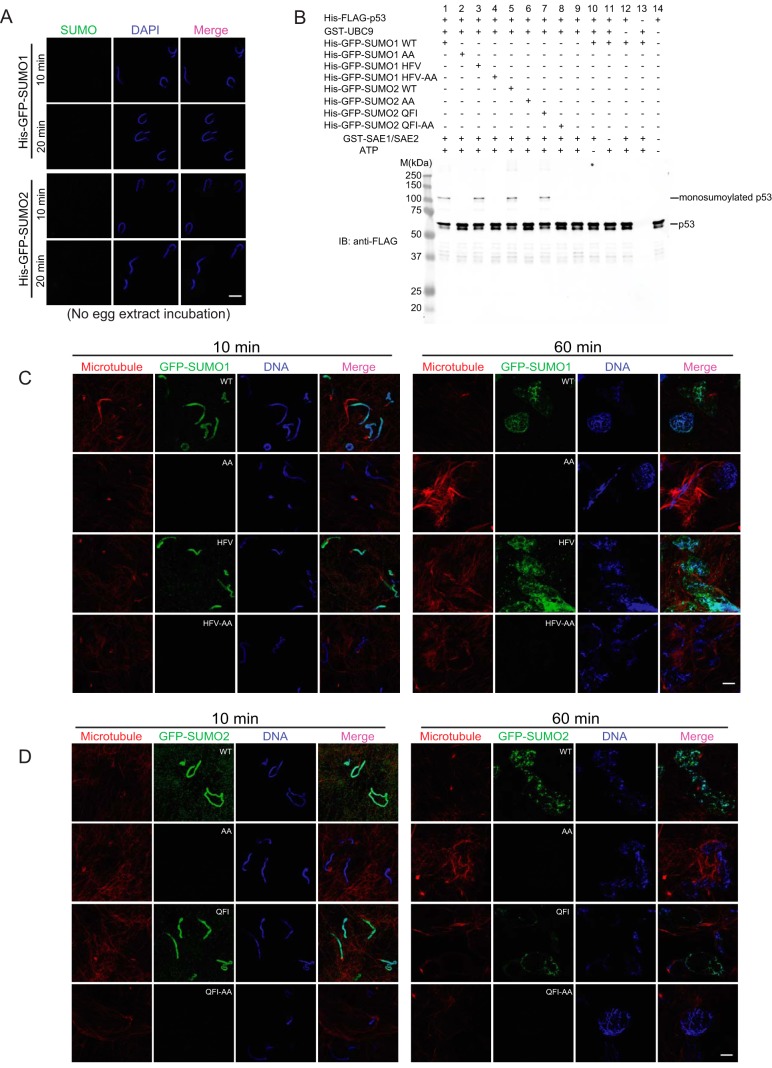
To test whether Xenopus egg extracts are necessary for SUMO binding to chromatin, we incubated sperm chromatin with His-GFP-SUMO proteins and buffer instead of egg extract. Regardless of the incubation length, no GFP signal was observed on chromatin (Fig. 2A), indicating that the Xenopus egg extract is essential for the association of SUMO with sperm chromatin. To determine if the chromatin association was caused by protein-protein interaction (SIM binding) or rather to authentic sumoylation (conjugation), we made several SUMO mutant constructs: SUMO1AA and SUMO2AA are conjugation-defective mutants; SUMO1HFV and SUMO2QFI are SIM binding mutants (19); and SUMO1HFV-AA and SUMO2QFI-AA are double mutants. We expressed and purified them as His-GFP-tagged fusion proteins and analyzed them by Coomassie blue staining (supplemental Fig. S2). To determine whether these SUMO mutants are functional, we tested their conjugation activity by in vitro sumoylation assay. The well-characterized SUMO substrate and tumor suppressor p53 was purified as a His-FLAG fusion protein, and used as a substrate in this assay. As expected, only wild-type and SIM-binding mutants of SUMO could be conjugated to p53. The conjugation mutants (SUMO1AA and SUMO2AA) and double mutants (SUMO1HFV-AA and SUMO2QFI-AA) could not be conjugated (Fig. 2B). Then we incubated sperm chromatin with the different His-GFP-SUMO mutant proteins and Xenopus egg extracts, and found GFP signal only appeared on chromatin incubated with wild-type and SIM-binding mutant SUMO proteins (Fig. 2C and 2D). This indicated that the observed SUMO association with chromatin was caused by sumoylation and not SIM binding. Interestingly, sumoylation not only took place on chromatin, many non-chromatin proteins in egg extracts were also sumoylated, as we observed the GFP signal outside chromatin (Figs. 1D, 1E, 2C, and 2D). Moreover, we also observed high levels of UBC9 protein in the egg extracts (data not shown), again reflecting the high sumoylation activity in Xenopus egg extracts. Here we concluded that SUMO proteins associate with chromatin through sumoylation, and that Xenopus egg extracts have significant sumoylation activity.
Fig. 2.
Sumoylation activity is high in Xenopus egg extracts. A, SUMO proteins do not associate with sperm chromatin in the absence of Xenopus egg extracts. No GFP signal was observed on sperm chromatin incubated with His-GFP-SUMO proteins and buffer instead of egg extract. DAPI was used for DNA staining. Scale bar equals 10 μm. B, SUMO mutant proteins were tested by in vitro sumoylation assay. Bacterially expressed and purified His-FLAG-tagged human p53 protein was used as a SUMO substrate. Monosumoylated His-FLAG-p53 is visualized at ∼105 kDa. Both wild-type and SIM binding mutants of SUMO proteins could be conjugated to p53, whereas the conjugation-defective mutants failed to be conjugated. C, SUMO1 could be conjugated to sperm chromatin. Sperm chromatin isolated from male Xenopus was incubated with interphase Xenopus egg extracts and purified His-GFP-tagged SUMO1 mutant proteins. A strong GFP signal appeared on the chromatin in Xenopus egg extracts in less than 10 min and persisted even after the nuclear formation in WT and HFV groups. No GFP signal was observed on sperm chromatin with the AA or HFV-AA SUMO mutants. Microtubule structures formed by rhodamine-labeled tubulin indicate the cell cycle stage of Xenopus egg extracts. DAPI was used for DNA staining. Scale bar equals 10 μm. D, SUMO2 could be conjugated on sperm chromatin. Sperm chromatin isolated from male Xenopus was incubated with interphase Xenopus egg extracts and purified His-GFP-tagged SUMO2 mutant proteins. A strong GFP signal appeared on the chromatin with WT and QFI SUMO. No GFP signal was observed with the AA or QFI-AA SUMO mutants. Microtubule structures formed by rhodamine-labeled tubulin indicate the cell cycle stage of Xenopus egg extracts. DAPI was used for DNA staining. Scale bar equals 10 μm.
SUMO Substrates can be Isolated using the Xenopus Egg Extract System
The Xenopus egg extract is an open system and contains highly concentrated proteins for early embryo development, which make it ideal for biochemical study and screening (9, 21). We chose to use this system to identify additional SUMO substrates at different cell cycle stages through mass spectrometry (MS) for the following reasons: (1) it is easy to prepare both mitotic phase and interphase Xenopus egg extracts; (2) significant sumoylation activity is present in Xenopus egg extracts; and (3) sumoylation of several well-characterized proteins have been identified in this system (11, 12, 35). To facilitate subsequent MS analysis and increase the probability of identifying the sumoylation site(s) on substrate proteins directly, the SUMO remnants on the targeted Lys following tryptic digestion need to be shortened. For this purpose, we generated two additional Xenopus SUMO mutants, SUMO1T96R and SUMO2T91R, by mutating the Thr before Gly-Gly at the C terminus to Arg. This allows the use of a MS-based method similar to that used for ubiquitylation studies, in which the Arg before Gly-Gly motif at the C terminus is cleaved by trypsin. After trypsin digestion, peptides conjugated to SUMO will have only a short Gly-Gly tag on the targeted Lys (16, 17, 36) (Fig. 3A). The peptide mass search algorithm can also identify the fused peptides with sumoylation site by the presence of 114.042927 Da additional mass on Lys (37, 38). We purified these two mutants as 6His-tagged fusion proteins and analyzed them by Coomassie blue staining (Fig. 3B). To determine if these SUMO mutants have comparable conjugation efficiency as wild-type SUMO, we performed in vitro sumoylation assays using His-FLAG-tagged p53 as substrate described earlier. The SUMO1T96R and SUMO2T91R mutants were as efficiently conjugated to p53 as wild-type SUMO, whereas the conjugation-defective mutants, SUMO1ΔGG and SUMO2ΔGG, were not (Fig. 3C). Freshly prepared mitotic phase or interphase egg extracts from three different frogs and demembranated Xenopus sperm chromatin were incubated with His-SUMO1T96R or His-SUMO2T91R proteins for 60 min to allow substrate sumoylation. The reaction was terminated by adding denaturing buffer containing 8 m urea to fully denature proteins, and incubated with cobalt beads under denaturing condition at room temperature for 2 h to capture His-tagged sumoylated proteins. The beads were washed thoroughly and eluted with denaturing buffer containing imidazole, and the eluted proteins were digested by trypsin and analyzed by MS (Fig. 3D).
Fig. 3.
A method to identify SUMO substrates using the Xenopus egg extract system. A, The carboxyl termini of mature ubiquitin, Xenopus SUMO1, and SUMO2 are depicted. Two SUMO mutants, SUMO1T96R and SUMO2T91R were created by mutating the Thr before Gly-Gly to Arg. B, Bacterially expressed and purified His-tagged Xenopus SUMO1T96R and SUMO2T91R proteins were analyzed by SDS-PAGE followed by Coomassie blue staining. C, SUMO1T96R and SUMO2T91R proteins were tested by in vitro sumoylation assay. Both of them could be conjugated to p53 in an in vitro sumoylation assay as efficiently as wild-type SUMO1 and SUMO2. Monosumoylated His-FLAG-p53 is visualized at ∼77 kDa. The purification of His-FLAG-p53 protein contained a minor partially degraded species, which also resulted in a minor sumoylated band at ∼75 kDa. D, A flow chart showing how sumoylated proteins are identified using the Xenopus egg extract system followed by mass spectrometry analysis. After incubation of Xenopus egg extracts and sperm chromatin with or without His-tagged SUMO mutant proteins, the whole reaction product is denatured with 8 m urea. Sumoylated proteins are isolated by pulling down His-tagged SUMO using cobalt beads and digested by trypsin. Peptides with or without Gly-Gly-Lys feature are analyzed by MS.
Isolated SUMO Substrates were Analyzed by MS and Validated for their Sumoylation
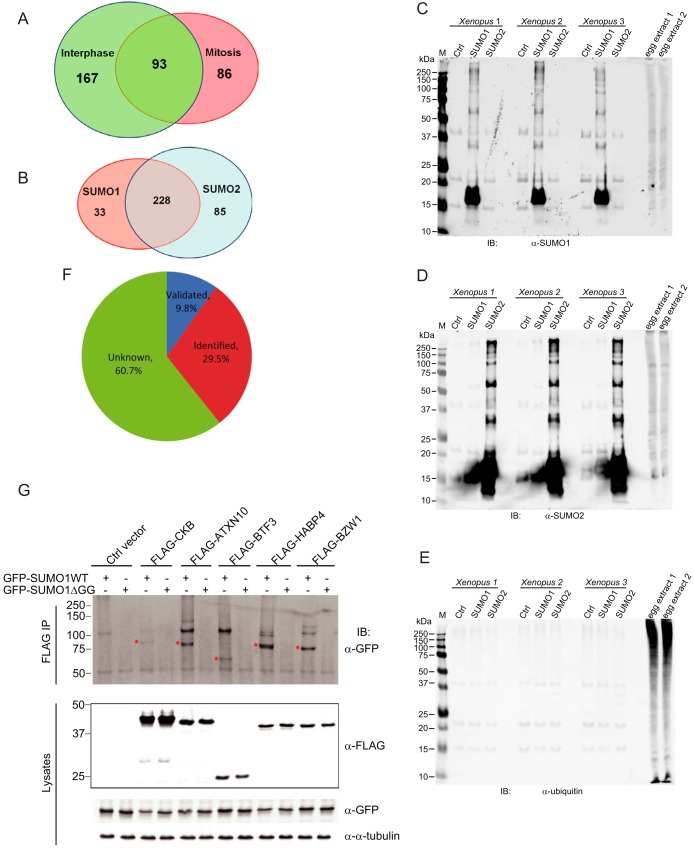
Analysis of three pull-down preparations by SDS-PAGE and silver staining, revealed different binding patterns for SUMO1, SUMO2, and control (without SUMO protein addition) samples (supplemental Fig. S3A and S3B). Proteins recognized from the peptides identified from mitotic phase or interphase egg extracts by MS were carefully annotated (supplemental Table S1 and S2). Proteins pulled down by either SUMO1 or SUMO2 or both, but absent in the control sample, were considered as high confidence proteins and subject to the subsequent analyses. A total of 179 and 260 nonredundant proteins were identified as SUMO conjugated proteins in mitotic phase and interphase samples, respectively (supplemental Table S1 and S2). When compared, 86 proteins are mitotic phase specific, 167 are interphase specific, and 93 proteins are overlapping (Fig. 4A and supplemental Table S3). Among the total 346 proteins, 33 are conjugated specifically to SUMO1 and 85 specifically to SUMO2, with an overlap of 228 proteins (Fig. 4B and supplemental Table S3). We note that in different cell cycle stage groups, the distribution of SUMO1 and SUMO2 substrates are very distinct (supplemental Fig. S3C). Among the 179 mitotic phase candidates, 72 are SUMO1 specific, 57 are SUMO2 specific, and 50 are shared by both (supplemental Fig. S3D and supplemental Table S3). However, among 260 interphase candidates, only 3 proteins are SUMO1 specific, and 189 are shared by both SUMO1 and SUMO2 (supplemental Fig. S3E and supplemental Table S3).
Fig. 4.
Analysis of SUMO substrates identified by MS. A, Venn diagram comparing the identified SUMO candidates from interphase sample and mitotic phase sample. B, Venn diagram comparing the different SUMO candidates in SUMO1 and SUMO2 pull-downs. C, SUMO1 pull-down proteins could be recognized by anti-SUMO1 antibody. SUMO pull-down products from three independent experiments were performed immunoblotting and 1 μl of Xenopus egg extracts were loaded as control to test the antibody. D, SUMO2 pull-down proteins could be recognized by anti-SUMO2 antibody. SUMO pull-down products from three independent experiments were performed immunoblotting and 1 μl of Xenopus egg extracts were loaded as control to test the antibody. E, SUMO pull-down proteins could not be recognized by anti-ubiquitin antibody. SUMO pull-down products from three independent experiments were performed immunoblotting and 1 μl of Xenopus egg extracts were loaded as control to test the antibody. F, Pie chart showing the percentage of total SUMO substrates that have been validated, identified or unknown prior to this study. G, Validation of the sumoylation of CKB, ATXN10, BTF3, HABP4, and BZW1. FLAG-tagged CKB, ATXN10, BTF3, HABP4, or BZW1 was co-transfected with GFP-SUMO1 in HEK293T cells followed by anti-FLAG immunoprecipitation and immunoblotting. The bands showing sumoylated protein are highlighted by red star. The additional GFP-positive bands above the highlighted bands in the SUMO1 WT lanes may be because of the multiple sumoylation of candidate proteins.
To determine if the isolated His-SUMO conjugated proteins contain ubiquitylated species, that might occur as a result of SUMO-targeted ubiquitin ligase activity, the same pull-downs were analyzed by performing immunoblotting assays with anti-SUMO1, -SUMO2, or -ubiquitin specific antibodies. In contrast to SUMO1 or SUMO2 antibodies that specifically recognized certain proteins on the blot, the ubiquitin antibody did not detect any protein, which suggests that the isolated sumoylated proteins are not appreciably ubiquitylated (Fig. 4C, 4D, and 4E). To try to identify sites of sumoylation we searched for the Gly-Gly-Lys feature as is done routinely in ubiquitylation studies (37, 38). However, Gly-Gly modified fused peptides were identified from only 14 and 16 proteins from mitotic phase and interphase samples, respectively (data not shown). The low efficiency of identifying this feature is likely because of the large amount of unmodified peptides in the protein digests that masked the peptides containing the conjugation site.
To see how reliable the Xenopus system is for SUMO substrate isolation, we analyzed all 346 proteins identified through searching PubMed (http://www.ncbi.nlm.nih.gov/pubmed), BioGRID (http://thebiogrid.org/), and PhosphoSitePlus (http://www.phosphosite.org). Thirty-four (9.8%) proteins have been previously validated for their sumoylation, and another 102 (29.5%) have been identified as SUMO substrates in other high throughput screens performed in mammalian cells or budding yeast (Fig. 4F and supplemental Table S3), indicating that our isolation method and MS analysis was in a good agreement with previous studies. Our identification of 210 proteins with previously undiscovered SUMO modification demonstrates the value of using Xenopus system for this large-scale screening.
Consistent with our observation that sumoylated proteins are present on chromatin, 90 of the proteins we identified have been annotated as chromatin-associated proteins (supplemental Table S4). We grouped them according to their reported or predicted functions with reference to the DAVID database (http://david.abcc.ncifcrf.gov/) and GeneCards (http://www.genecards.org/). Seventeen of them play roles in chromatin remodeling, 57 of them are transcriptional regulators, and 16 of them are involved in DNA repair, stability, and replication. Among these 90 chromatin-associated proteins, 52 have been previously reported to be sumoylated and 54 have been reported to play roles in tumorigenesis and cancer progression (Table I and supplemental Table S4), which might indicate a potential connection between sumoylation of these proteins and their functions in cancer.
Table I. Chromatin-associated proteins identified by mass spectrometry. Proteins are highlighted in bold if their sumoylation has been reported. Proteins are underlined if they have functions in cancer.
| Function | Proteins identified by mass spectrometry |
|---|---|
| Chromatin remodeling | H1FOO, HDAC2, CHD4, NCL, SUPT7L, CHAF1A, HIST1H2AM, HMGB2, BAZ1B, HDAC1, NPM2, H3F3A, HIST1H2BA, HIST1H2AD, HABP4, HMGB1, MCM2 |
| Transcriptional regulation | GTF2IRD1, POLR3D, POLR3E, SUMO1, TOP2A, TRIM28, UBE2I, DPPA2, HIC2, DNMT1, GATAD2A, ILF3, PIAS4, RUVBL1, SNIP1, SOX3, USP10, BTF3L4, WDR77, ARID3A, DAXX, FOXK2, FUBP3, GZF1, HMCES, IRF2BPL, MAZ, MBD1, MNT, MYEF2, POU5F1, SOX11, SUB1, ZFR, ZNF208, ZNF521, ZNF652, ZNF653, SOX1, SUMO2, ZNF462, ZNF585A, PIAS1, BTF3, PDCD4, TLE4, ETV6, HMBOX1, KLF17, SND1, UBTF, ZBTB49, ZNF577, ZIC4, ZNF585B, MORC3, PRMT1 |
| DNA repair/stability/replication | LIG1, PARP1, PCNA, TSN, NASP, ORC4, POLA1, POLD3, WRN, RFC5, BRIP1, MCM4, ORC3, PRIM2, DDB1, PLK1 |
To assess how conserved sumoylation in the Xenopus egg extract is compared with human cells, we selected for validation five candidate proteins that were conjugated with SUMO1 in our original assays, and had not previously been reported to be sumoylated. The FLAG-tagged candidate proteins were transiently co-expressed with GFP-SUMO1 and with or without UBC9 in HEK293T cells, followed by immunoprecipitation (IP) using FLAG M2 antibody agarose beads and immunoblotting with anti-FLAG and anti-GFP antibodies to detect sumoylation. We used RanGAP1 as a positive control, analyzing wild-type RanGAP1 and three RanGAP1 mutants, including two sumoylation-defective mutants, K524R (39) and K15RK524R, and a sumoylation-competent mutant, K15R. Only wild-type and K15R mutant RanGAP1 were conjugated by SUMO1; no conjugation was observed with SUMO1ΔGG; and UBC9 co-transfection or not had no effect on RanGAP1 sumoylation (supplemental Fig. S3F). To test candidate proteins we co-transfected constructs expressing FLAG-tagged CKB, ATXN10, BTF3, HABP4 or BZW1, and SUMO1WT or SUMO1ΔGG mutant, with (supplemental Fig. S3G) or without UBC9 (Fig. 4G), and found that all of the five candidates could be sumoylated. Based on these results, we believe that the sumoylation of many substrates is conserved between Xenopus egg extracts and human cells. CKB (Creatine Kinase, Brain) is a cytoplasmic enzyme involved in energy homeostasis. It reversibly catalyzes the transfer of phosphate between ATP and various phosphogens such as creatine phosphate (40). ATXN10 is the protein product of the pathogenic gene for spinocerebellar ataxia type 10. Decreased expression of ATXN10 has been shown to contribute to the loss of cerebellar neurons, which suggests its important role in neuron survival (41). BTF3 (Basic Transcription Factor 3) can form a stable complex with RNA polymerase II and is required for the initiation of transcription (42). HABP4 (Hyaluronan Binding Protein 4) and BZW1 (Basic Leucine Zipper And W2 Domains 1) are two other transcriptional regulators with limited functional information available. Further studying their sumoylation may help us uncover their functions.
Functional Analysis of the SUMO Substrates
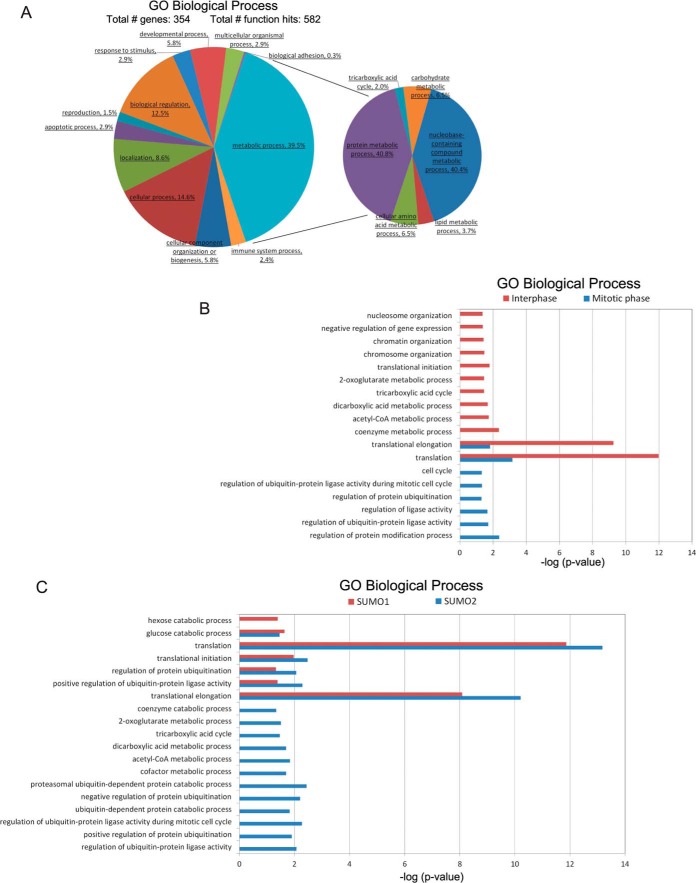
We analyzed our proteomic data using three bioinformatic analysis tools, the Protein Analysis Through Evolutionary Relationships (PANTHER) (http://www.pantherdb.org/) classification system, the Database for Annotation, Visualization and Integrated Discovery (DAVID) (http://david.abcc.ncifcrf.gov/), and the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) (http://string-db.org/). Using the PANTHER resource we performed gene ontology (GO) analysis of the total 346 SUMO substrates for terms associated with the biological process domain and have identified multiple processes in which these substrates are involved, such as metabolic process, cellular process, and response to stimulus (Fig. 5A and supplemental Table S5). For example, TOP2A (DNA topoisomerase II alpha) is an important player in several cellular processes, such as cell cycle. It is critical for chromosome segregation, which highly depends on its sumoylation. Decreased sumoylation of TOP2A impairs its accumulation at inner centromeres and proper separation of sister chromatids, blocking the cell cycle (43). The most represented biological process (with 39.5%) was linked to metabolic process (Fig. 5A and supplemental Table S5), in which proteins were found to play roles in protein, nucleotide, carbohydrate, lipid, or amino acid metabolic processes (Fig. 5A). Although it is known that sumoylation helps maintain overall metabolic homeostasis and facilitates cellular stress response through the regulation and/or adaptation of the most fundamental metabolic processes, the exact molecular mechanism is still unknown (44). Several SUMO substrates, such as PKM2, G6PD, LDHC, PGK1, and GBE1, identified in this study and others are well-known metabolic enzymes (15, 45–47). Investigating how sumoylation regulates their functions will undoubtedly improve our understanding of metabolic regulation.
Fig. 5.
Functional analysis of SUMO substrates. A, Pie chart showing gene ontology analysis of the SUMO candidate proteins. The genes were grouped into categories defined by GO Biological Processes by the PANTHER classification system. The largest category is that containing gene products that play roles in metabolic process. B, Bar chart showing enrichment p values of SUMO substrates identified from interphase or mitotic phase for GO Biological Process terms generated by DAVID database. C, Bar chart showing enrichment p values of SUMO1 and SUMO2 substrates for GO Biological Process terms generated by DAVID database.
We also performed GO analysis of all SUMO substrates for the molecular function domain and found two categories, binding and catalytic activity, were predominant (with 36.3% and 33.2%, respectively) (supplemental Fig. S4A and supplemental Table S5). A pie chart of binding sub-categories suggested that nucleic acid binding proteins were a majority (supplemental Fig. S4B) and play a very important role in transcriptional regulation (data not shown). The pie chart of catalytic activity sub-categories listed diverse enzymatic functions (supplemental Fig. S4C), suggesting many sumoylated proteins function in metabolism (data not shown). These analyses revealed that the SUMO substrates we identified from the Xenopus egg extract system were functionally diverse and the two major processes they involved in were transcription and metabolism.
The number and identity of isolated substrates from interphase samples are quite distinct from substrates isolated from mitotic phase samples. To explore the enrichment of these substrates in different cellular components, biological processes and molecular functions, we analyzed them by using the DAVID functional annotation tool. Interphase substrates are more involved in activities such as metabolism, DNA replication, transcription and translation, whereas mitotic substrates are more involved in cell cycle events and the ubiquitin proteasome pathway (Fig. 5B, S4D and supplemental Table S6).
SUMO1 has substantially different localization and dynamics compared with SUMO2 (48). SUMO2 prefers to form poly-SUMO chains compared with SUMO1 and is more responsive to environmental stress. SUMO1 and SUMO2 have distinct but also partially overlapping subsets of modified proteins (1, 49), which is consistent with our results. Among all the 346 protein substrates identified, 33 proteins are SUMO1 specific, 85 proteins are SUMO2 specific, and 228 proteins are shared by both (Fig. 4B and supplemental Table S3). By analyzing these proteins using the DAVID functional annotation tool to see for enrichment in different cellular components, biological processes and molecular functions, we found that both SUMO1 and SUMO2 substrates have many overlapping functions, especially translation. SUMO2 substrates are more involved in activities such as metabolism and the ubiquitin proteasome pathway (Fig. 5C, S4E and supplemental Table S6).
SUMO pathway disruption commonly displays strong phenotypes. Budding yeast lacking SMT3 (the single SUMO homolog in Saccharomyces cerevisiae) fail to grow (50). Ubc9-depleted chicken cells undergo apoptosis (51). However, the function of individual SUMO modifications is often enigmatic, and interrupting the sumoylation of any individual SUMO substrate usually causes no notable phenotypes. A recent model discussing protein modification by SUMO may partially explain this (52). Psakhye and Jentsch, using DNA double-strand break repair as an example, showed that DNA damage triggered the sumoylation of several repair proteins in the same pathway. Only by eliminating sumoylation of several repair proteins in combination was the function of the homologous recombination pathway significantly affected (52). This suggests that linking sumoylation substrates through their protein interactions may help reveal the function of this modification.
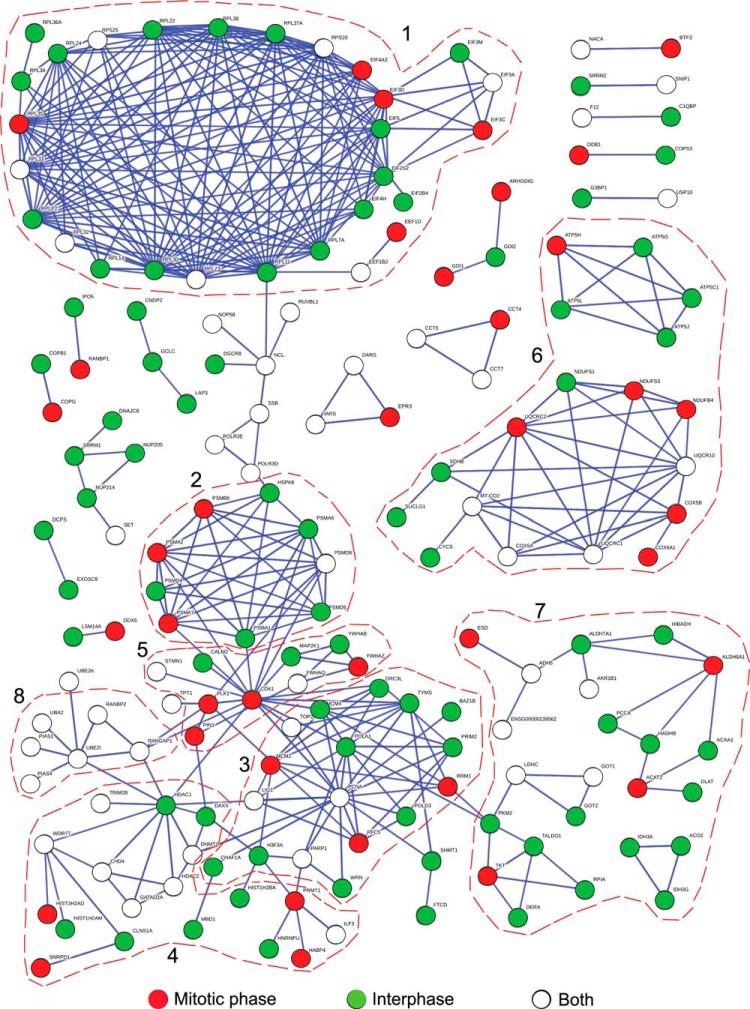
To explore if there are potential protein groups in our SUMO substrate pool, we analyzed all the SUMO candidate proteins using STRING (Table S7). For the convenience of comparing proteins identified from different cell cycle stages or different SUMO pull-downs, we made two STRING protein interaction networks by presenting sumoylated proteins in different colors; either denoting cell cycle phases (Fig. 6) or conjugation to SUMO1, SUMO2, or both (supplemental Fig. S5). In addition to the well-characterized UBE2I-UBA2-PIAS-RanGAP1-RanBP2 SUMO pathway complex, seven other protein complexes and pathways involved in specific cellular functions are highlighted (Fig. 6 and supplemental S5): (1) ribosome assembly and translation, including 17 ribosome subunits and 11 translation factors; (2) proteasomal degradation, including eight proteasome subunits; (3) DNA replication and repair, in which PCNA is a hub connected to several other proteins, such as RFC5, MCM2, and TOP2A; (4) chromatin remodeling and transcriptional regulation, containing several enzymes important for chromatin structure and gene expression, such as HDAC1, HDAC2, DNMT1, and CHD4; (5) cell cycle regulation, in which CDK1 plays important functions in cell cycle progression with other proteins; (6) the mitochondrial respiratory chain, composed of 18 proteins participating in oxidative phosphorylation and ATP synthesis; and (7) metabolic regulation, in which PKM2, ACAT2, ALDH5, etc., function as key players to coordinate other proteins functioning in glycolysis, lipid, or alcohol metabolism.
Fig. 6.
Functional protein interaction network analysis based on the STRING database. The image illustrates a network analysis of sumoylated proteins that was created with STRING. Proteins isolated from mitotic phase, interphase or both were highlighted in red, green or white, respectively. The protein complexes and pathways highlighted by red circle are involved in: (1) ribosome assembly and translation; (2) proteasomal degradation; (3) DNA replication and repair; (4) chromatin remodeling and transcriptional regulation; (5) cell cycle regulation; (6) the mitochondrial respiratory chain; (7) metabolic regulation; and (8) the sumoylation pathway.
SUMO substrates identified from the interphase egg extracts and the mitotic egg extracts both contributed to all eight of the protein complexes and pathways identified in the analysis with STRING (Fig. 6). Many of the protein complexes contained components identified in both the interphase and mitotic phase, indicating that their sumoylation may be ubiquitous throughout the cell cycle. In contrast, protein complexes involved in proteasomal degradation, cell cycle regulation and the mitochondrial respiratory chain contained mostly non-overlapping components, suggesting the presence of individual sub-networks that might be subject to differential regulation. Protein complexes involved in ribosome assembly and translation, metabolic regulation, and DNA replication and repair contained a majority of components identified only from the interphase egg extracts, which indicates that sumoylation may play a greater role in the regulation of these protein complexes outside the mitosis. Finally, we noted that protein complexes involved in proteasomal degradation, mitochondrial respiratory chain and metabolic regulation contained a number of components only identified from SUMO2 pull-downs (supplemental Fig. S5), indicating that SUMO2 may play a greater role in the regulation of these processes compared with SUMO1. Investigating whether and how sumoylation might facilitate the interaction among different members in the same complex or provide a means for the protein complex to interact with other SIM-containing proteins will further strengthen our understanding of these complexes and sumoylation study.
DISCUSSION
Xenopus SUMO proteins are highly similar to their orthologs in human (Figs. 1A, 1B, and supplemental Fig. S1) and the Xenopus egg extract can be flexibly cycled between mitotic phase and interphase (Fig. 1C), which makes the Xenopus system ideal for sumoylation studies in the cell cycle. We found GFP-tagged SUMO proteins conjugated to sperm chromatin and proteins in both mitotic and interphase Xenopus egg extracts (Figs. 1D, 1E, 2A, 2C, and 2D). Our analysis highlights the feasibility of using this system for large-scale explorations of SUMO substrates. By incubating His-tagged SUMO proteins with Xenopus sperm chromatin and CSF-arrested (mimicking the mitotic phase) or interphase Xenopus egg extracts, we isolated SUMO substrates under denaturing conditions, and identified 346 candidate proteins through MS analysis (Figs. 3, 4A, 4B, supplemental Figs. S3A, S3B, S3C, S3D, S3E, and supplemental Table S3), among which 210 proteins had not previously been reported to be modified by sumoylation (Fig. 4F and supplemental Table S3). Furthermore, we confirmed the sumoylation of five proteins (CKB, ATXN10, BTF3, HABP4, and BZW1) in HEK293T cells (Fig. 4G and supplemental Fig. S3G), demonstrating that the identification of sumoylated proteins using the Xenopus system is relevant to their human orthologs.
We had hoped to be able to identify the sumoylation sites of isolated candidate proteins using an MS-based Gly-Gly-Lys feature search method similar to that used for ubiquitylation studies (37, 38) (Fig. 3). However, this strategy was very inefficient as we only identified peptides with a Gly-Gly-Lys feature from 14 and 16 proteins from mitotic phase and interphase sample, respectively; of these, only a quarter lie in a sumoylation consensus motifs (ΨKxE/D, Ψ as a hydrophobic amino acid) (17) (data not shown). One major reason is likely to be the large amount of unmodified peptides in the digest that mask the peptides containing the conjugation site. To overcome this problem in future studies, enrichment of Gly-Gly-Lys featured peptides using anti-diGly-Lys MAb after trypsin digestion before MS analysis might be a better method (37, 38). Based on our experiments, contamination with ubiquitylated proteins, which will also generate Gly-Gly tagged peptides, seems unlikely to be a major problem (Figs. 4C, 4D, and 4E).
Sumoylation is essential for cell cycle regulation both in invertebrates and mammalian cells (53–55). Among the sumoylated proteins identified in this study, 167 proteins were specifically isolated from interphase sample, 86 proteins were from mitotic phase sample, and 93 proteins were from both (Fig. 4A and supplemental Table S3). Further Gene Ontology analysis suggests a distinct profile of functions and activities of substrates in interphase or mitotic phase. Whereas interphase substrates are more involved in activities such as metabolism, DNA replication, transcription, and translation, mitotic substrates are more involved in regulating the cell cycle and the ubiquitin proteasome pathway (Fig. 5B, S4D, and supplemental Table S6). Mitosis is a highly dynamic changing process. Different morphological transitions have to be coordinated in a temporal and spatial manner to allow for precise partitioning of the genetic material into two daughter cells. Protein post-translational modification of key mitotic factors is believed to be one of the major mechanisms ensuring faithful mitotic progression (56, 57). CDK1, one of the candidate sumoylated proteins identified in this study, is a highly conserved member of the serine/threonine kinase family, and plays an essential role in cell cycle regulation by phosphorylating a variety of substrates (58). However, whether sumoylation affects its kinase activity remains unknown.
SUMO1 and SUMO2 have been shown to have different conjugation dynamics and target profiles (1). SUMO1 or SUMO2 modification of different proteins could regulate distinct cell processes (54). Our results also suggest the possibility of differentiating SUMO1 or SUMO2 specific substrates using Xenopus system. We found 33 proteins could be specifically isolated with SUMO1 pull-down, 85 proteins with SUMO2 pull-down, and 228 proteins were isolated with both homologs (Fig. 4B and supplemental Table S3). In particular, during interphase, SUMO2 has more specific substrates than SUMO1 (supplemental Fig. S3D and supplemental Table S2). Analysis of proteins identified by SUMO1 and SUMO2 pull-downs suggests they may have overlapping functions and involved in similar protein interaction complexes (Fig. 5C, supplemental Figs. S4E, and S5). SUMO2 substrates were more involved in activities such as metabolism and the ubiquitin proteasome pathway. Further study of the sumoylation of non-overlapping candidates should be helpful in understanding the different functions of SUMO1 and SUMO2.
We found high sumoylation activity on sperm chromatin when it was incubated with Xenopus egg extracts (Fig. 1D, 1E, 2C, and 2D). Ninety proteins out of 346 total proteins were chromatin-associated proteins (Table I and supplemental Table S4). Chromatin-associated proteins play important roles in chromatin remodeling, transcriptional regulation, and DNA repair/stability/replication, and perturbation of these activities can contribute directly to tumorigenesis. Many chromatin-associated proteins, such as histones, p53, PCNA, and TOP2A, are targets of SUMO-modification and are regulated by sumoylation (11, 12, 59, 60). To explore SUMO substrates that specifically associate with chromatin using the Xenopus egg extract system, chromatin can be isolated by centrifugation through a glycerol cushion after prior incubation with His-tagged SUMO proteins and egg extracts for further analysis.
Because sumoylation has been shown to regulate transcription, DNA repair, nucleocytoplasmic transport and other nuclear functions numerously (4), this has led to the idea that sumoylation is restricted to the nuclear compartment and only nuclear proteins could be potentially sumoylated. However, as more SUMO substrates are identified, it is clear that sumoylation is not only a nuclear affair. Proteins from the cytoplasm, the plasma membrane, the endoplasmic reticulum, mitochondria, and ribosomes, have been shown to be SUMO substrates, and play many roles when they are sumoylated (16, 61). Therefore, the traditional method of isolating SUMO substrates by fractionating nuclei will underestimate the pool of SUMO substrates and the functions of sumoylation. Here we used Xenopus egg extract to isolate SUMO substrates, which allows us to study both nuclear and cytoplasmic proteins. As an open system, Xenopus egg extract is easy to operate and provides the complexity of total cytoplasm and most endo-membranes (21). The high concentration of stored proteins needed for rapid early embryo development will amplify the absolute number of sumoylated proteins and the dramatic effects of sumoylation in such a dynamic changing context. Our PANTHER analyses and STRING networks showed SUMO substrates were found in different cellular compartments with diverse functions besides transcriptional regulation, suggesting many other promising directions for sumoylation study (Fig. 5A, 6, supplemental Figs. S4A, S4B, S4C, and S5, supplemental Table S5 and S7).
Many proteins in our candidate list have been reported to be involved in cancer development (Table I). However, exactly how SUMO regulates the function of proteins in cancer is still elusive mainly because of the limited knowledge of the role of sumoylation in substrate proteins identified thus far. Investigation of novel SUMO targets throughout the cell cycle will be critical to better understand their roles in cancer development and progression.
Supplementary Material
Acknowledgments
We thank Yixian Zheng and Ona Martin for generous helps and support with the Xenopus system, Martin Hetzer and Robbie Schulte for RanGAP1 constructs and microscope access, Suzy Simon, Jill Meisenhelder, and Justin Zimmermann for ordering and general technical support, the Waitt Advanced Biophotonics Center Core facility for imaging technical support, and members of Hunter lab for critical comments.
Footnotes
Author contributions: L.M., A.A., H.S., and T.H. designed research; L.M. and A.A. performed research; L.M., H.S., M.J., and Y.S. contributed new reagents or analytic tools; L.M. and A.A. analyzed data; L.M., A.A., and T.H. wrote the paper; J.R.Y. provided lab space, instrumentation and funding; T.H. provided lab space and funding.
* This research was supported by NIH grants (5R01CA080100, 5R01CA82683, and P30CA14915, T.H.), NCRR grant (5P41RR011823-17, J.R.Y.), NIGMS grant (8P41GM103533-17, J.R.Y.) and a Genentech Foundation Fellowship for Cancer Research (L.M.). T.H. is a Frank and Else Schilling American Cancer Society Professor and holds the Renato Dulbecco Chair in Cancer Research.
 This article contains supplemental Figs. S1 to S5 and Tables S1 to S7.
This article contains supplemental Figs. S1 to S5 and Tables S1 to S7.
1 The abbreviations used are:
- SUMO
- small ubiquitin-like modifier
- SIM
- SUMO-interacting motif
- SENP
- SUMO-specific protease
- CSF
- cytostatic factor
- IPTG
- isopropyl β-D-1-thiogalactopyranoside
- NEM
- N-ethylmaleimide
- SCX
- strong cation exchange
- MudPIT
- Multidimensional Protein Identification Technology
- CID
- collision induced dissociation
- NSAF
- normalized spectral abundance factor
- emPAI
- exponentially modified Protein Abundance Index
- PANTHER
- Protein ANalysis THrough Evolutionary Relationships
- DAVID
- Database for Annotation, Visualization, and Integrated Discovery
- STRING
- Search Tool for the Retrieval of Interacting Genes/Proteins
- IP
- immunoprecipitation
- GO
- Gene Ontology
- RanGAP1
- Ran GTPase Activating Protein 1
- TOP2A
- DNA topoisomerase II alpha
- CKB
- creatine kinase, brain
- ATXN10
- spinocerebellar ataxia 10
- BTF3
- basic transcription factor 3
- HABP4
- hyaluronan binding protein 4
- BZW1
- basic leucine zipper and W2 domains 1
- CDK1
- cyclin-dependent kinase 1
- PCNA
- proliferating cell nuclear antigen
- PARP1
- poly (ADP-Ribose) polymerase 1
- PKM2
- pyruvate kinase, muscle.
REFERENCES
- 1. Vertegaal A. C., Andersen J. S., Ogg S. C., Hay R. T., Mann M., Lamond A. I. (2006) Distinct and overlapping sets of SUMO-1 and SUMO-2 target proteins revealed by quantitative proteomics. Mol. Cell. Proteomics 5, 2298–2310 [DOI] [PubMed] [Google Scholar]
- 2. Creton S., Jentsch S. (2010) SnapShot: The SUMO system. Cell 143, 848–848 e841 [DOI] [PubMed] [Google Scholar]
- 3. Flotho A., Melchior F. (2013) Sumoylation: a regulatory protein modification in health and disease. Annu. Rev. Biochem. 82, 357–385 [DOI] [PubMed] [Google Scholar]
- 4. Zhao J. (2007) Sumoylation regulates diverse biological processes. Cell. Mol. Life Sci. 64, 3017–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alarcon-Vargas D., Ronai Z. (2002) SUMO in cancer–wrestlers wanted. Cancer Biol. Ther. 1, 237–242 [DOI] [PubMed] [Google Scholar]
- 6. Lee* J. S., Choi* H. J., Baek S. H. (2009) Sumoylation and its contribution to cancer. SUMO regulation of cellular processes. In: Wilson V. G., ed., pp. 253–272, Springer; Netherlands [Google Scholar]
- 7. Kessler J. D., Kahle K. T., Sun T., Meerbrey K. L., Schlabach M. R., Schmitt E. M., Skinner S. O., Xu Q., Li M. Z., Hartman Z. C., Rao M., Yu P., Dominguez-Vidana R., Liang A. C., Solimini N. L., Bernardi R. J., Yu B., Hsu T., Golding I., Luo J., Osborne C. K., Creighton C. J., Hilsenbeck S. G., Schiff R., Shaw C. A., Elledge S. J., Westbrook T. F. (2012) A SUMOylation-dependent transcriptional subprogram is required for Myc-driven tumorigenesis. Science 335, 348–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lohka M. J., Maller J. L. (1985) Induction of nuclear envelope breakdown, chromosome condensation, and spindle formation in cell-free extracts. J. Cell Biol. 101, 518–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ma L., Tsai M. Y., Wang S., Lu B., Chen R., Iii J. R., Zhu X., Zheng Y. (2009) Requirement for Nudel and dynein for assembly of the lamin B spindle matrix. Nat. Cell Biol. 11, 247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Powers M., Evans E. K., Yang J., Kornbluth S. (2001) Preparation and use of interphase Xenopus egg extracts. Current protocols in cell biology/editorial board, Juan S. Bonifacino. [et al.] Chapter 11, Unit 11 10 [DOI] [PubMed] [Google Scholar]
- 11. Azuma Y., Arnaoutov A., Dasso M. (2003) SUMO-2/3 regulates topoisomerase II in mitosis. J. Cell Biol. 163, 477–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leach C. A., Michael W. M. (2005) Ubiquitin/SUMO modification of PCNA promotes replication fork progression in Xenopus laevis egg extracts. J. Cell Biol. 171, 947–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ryu H., Al-Ani G., Deckert K., Kirkpatrick D., Gygi S. P., Dasso M., Azuma Y. (2010) PIASy mediates SUMO-2/3 conjugation of poly(ADP-ribose) polymerase 1 (PARP1) on mitotic chromosomes. J. Biol. Chem. 285, 14415–14423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonne-Andrea C., Kahli M., Mechali F., Lemaitre J. M., Bossis G., Coux O. (2013) SUMO2/3 modification of cyclin E contributes to the control of replication origin firing. Nat. Commun. 4, 1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou W., Ryan J. J., Zhou H. (2004) Global analyses of sumoylated proteins in Saccharomyces cerevisiae. Induction of protein sumoylation by cellular stresses. J. Biol. Chem. 279, 32262–32268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galisson F., Mahrouche L., Courcelles M., Bonneil E., Meloche S., Chelbi-Alix M. K., Thibault P. (2011) A novel proteomics approach to identify SUMOylated proteins and their modification sites in human cells. Mol. Cell. Proteomics 10, M110 004796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matic I., Schimmel J., Hendriks I. A., van Santen M. A., van de Rijke F., van Dam H., Gnad F., Mann M., Vertegaal A. C. (2010) Site-specific identification of SUMO-2 targets in cells reveals an inverted SUMOylation motif and a hydrophobic cluster SUMOylation motif. Mol. Cell 39, 641–652 [DOI] [PubMed] [Google Scholar]
- 18. Panse V. G., Hardeland U., Werner T., Kuster B., Hurt E. (2004) A proteome-wide approach identifies sumoylated substrate proteins in yeast. J. Biol. Chem. 279, 41346–41351 [DOI] [PubMed] [Google Scholar]
- 19. Sun H., Leverson J. D., Hunter T. (2007) Conserved function of RNF4 family proteins in eukaryotes: targeting a ubiquitin ligase to SUMOylated proteins. EMBO J. 26, 4102–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun H., Hunter T. (2012) Poly-small ubiquitin-like modifier (PolySUMO)-binding proteins identified through a string search. J. Biol. Chem. 287, 42071–42083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hannak E., Heald R. (2006) Investigating mitotic spindle assembly and function in vitro using Xenopus laevis egg extracts. Nat. Protoc. 1, 2305–2314 [DOI] [PubMed] [Google Scholar]
- 22. Tsai M. Y., Wang S., Heidinger J. M., Shumaker D. K., Adam S. A., Goldman R. D., Zheng Y. (2006) A mitotic lamin B matrix induced by RanGTP required for spindle assembly. Science 311, 1887–1893 [DOI] [PubMed] [Google Scholar]
- 23. Vethantham V., Manley J. L. (2009) In vitro sumoylation of recombinant proteins and subsequent purification for use in enzymatic assays. Cold Spring Harb. Protoc. 2009, pdb prot5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McDonald W. H., Tabb D. L., Sadygov R. G., MacCoss M. J., Venable J., Graumann J., Johnson J. R., Cociorva D., Yates J. R., 3rd (2004) MS1, MS2, and SQT-three unified, compact, and easily parsed file formats for the storage of shotgun proteomic spectra and identifications. Rapid Commun. Mass Spectrom. 18, 2162–2168 [DOI] [PubMed] [Google Scholar]
- 25. Eng Jimmy K., McCormack Ashley L., III, Y. J. R. (1994) An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectr. 5, 976–989 [DOI] [PubMed] [Google Scholar]
- 26. Peng J., Elias J. E., Thoreen C. C., Licklider L. J., Gygi S. P. (2003) Evaluation of multidimensional chromatography coupled with tandem mass spectrometry (LC/LC-MS/MS) for large-scale protein analysis: the yeast proteome. J. Proteome Res. 2, 43–50 [DOI] [PubMed] [Google Scholar]
- 27. Tabb D. L., McDonald W. H., Yates J. R., 3rd (2002) DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J. Proteome Res. 1, 21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zybailov B., Mosley A. L., Sardiu M. E., Coleman M. K., Florens L., Washburn M. P. (2006) Statistical analysis of membrane proteome expression changes in Saccharomyces cerevisiae. J. Proteome Res. 5, 2339–2347 [DOI] [PubMed] [Google Scholar]
- 29. Ishihama Y., Oda Y., Tabata T., Sato T., Nagasu T., Rappsilber J., Mann M. (2005) Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell. Proteomics 4, 1265–1272 [DOI] [PubMed] [Google Scholar]
- 30. Mudunuri U., Che A., Yi M., Stephens R. M. (2009) bioDBnet: the biological database network. Bioinformatics 25, 555–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang da W., Sherman B. T., Lempicki R. A. (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 [DOI] [PubMed] [Google Scholar]
- 32. Mi H., Muruganujan A., Thomas P. D. (2013) PANTHER in 2013: modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Res. 41, D377–D386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Franceschini A., Szklarczyk D., Frankild S., Kuhn M., Simonovic M., Roth A., Lin J., Minguez P., Bork P., von Mering C., Jensen L. J. (2013) STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 41, D808–D815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saitoh H., Sparrow D. B., Shiomi T., Pu R. T., Nishimoto T., Mohun T. J., Dasso M. (1998) Ubc9p and the conjugation of SUMO-1 to RanGAP1 and RanBP2. Curr. Biol.: CB 8, 121–124 [DOI] [PubMed] [Google Scholar]
- 35. Joseph J., Tan S. H., Karpova T. S., McNally J. G., Dasso M. (2002) SUMO-1 targets RanGAP1 to kinetochores and mitotic spindles. J. Cell Biol. 156, 595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Knuesel M., Cheung H. T., Hamady M., Barthel K. K., Liu X. (2005) A method of mapping protein sumoylation sites by mass spectrometry using a modified small ubiquitin-like modifier 1 (SUMO-1) and a computational program. Mol. Cell. Proteomics : MCP 4, 1626–1636 [DOI] [PubMed] [Google Scholar]
- 37. Xu G., Paige J. S., Jaffrey S. R. (2010) Global analysis of lysine ubiquitination by ubiquitin remnant immunoaffinity profiling. Nat. Biotechnol. 28, 868–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim W., Bennett E. J., Huttlin E. L., Guo A., Li J., Possemato A., Sowa M. E., Rad R., Rush J., Comb M. J., Harper J. W., Gygi S. P. (2011) Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol. Cell 44, 325–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mahajan R., Gerace L., Melchior F. (1998) Molecular characterization of the SUMO-1 modification of RanGAP1 and its role in nuclear envelope association. J. Cell Biol. 140, 259–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bessman S. P., Carpenter C. L. (1985) The creatine-creatine phosphate energy shuttle. Annu. Rev. Biochem. 54, 831–862 [DOI] [PubMed] [Google Scholar]
- 41. Marz P., Probst A., Lang S., Schwager M., Rose-John S., Otten U., Ozbek S. (2004) Ataxin-10, the spinocerebellar ataxia type 10 neurodegenerative disorder protein, is essential for survival of cerebellar neurons. J. Biol. Chem. 279, 35542–35550 [DOI] [PubMed] [Google Scholar]
- 42. Zheng X. M., Black D., Chambon P., Egly J. M. (1990) Sequencing and expression of complementary DNA for the general transcription factor BTF3. Nature 344, 556–559 [DOI] [PubMed] [Google Scholar]
- 43. Dawlaty M. M., Malureanu L., Jeganathan K. B., Kao E., Sustmann C., Tahk S., Shuai K., Grosschedl R., van Deursen J. M. (2008) Resolution of sister centromeres requires RanBP2-mediated SUMOylation of topoisomerase IIalpha. Cell 133, 103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Anderson D. D., Stover P. J. (2009) The Roles of SUMO in Metabolic Regulation. SUMO Regulation of cellular processes. In: Wilson V. G., ed., pp. 137–149, Springer; Netherlands [Google Scholar]
- 45. Guo D., Han J., Adam B. L., Colburn N. H., Wang M. H., Dong Z., Eizirik D. L., She J. X., Wang C. Y. (2005) Proteomic analysis of SUMO4 substrates in HEK293 cells under serum starvation-induced stress. Biochem. Biophys. Res. Commun. 337, 1308–1318 [DOI] [PubMed] [Google Scholar]
- 46. Bruderer R., Tatham M. H., Plechanovova A., Matic I., Garg A. K., Hay R. T. (2011) Purification and identification of endogenous polySUMO conjugates. EMBO Rep. 12, 142–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vigodner M., Shrivastava V., Gutstein L. E., Schneider J., Nieves E., Goldstein M., Feliciano M., Callaway M. (2013) Localization and identification of sumoylated proteins in human sperm: excessive sumoylation is a marker of defective spermatozoa. Hum. Reprod. 28, 210–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ayaydin F., Dasso M. (2004) Distinct in vivo dynamics of vertebrate SUMO paralogues. Mol. Biol. Cell 15, 5208–5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Saitoh H., Hinchey J. (2000) Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. J. Biol. Chem. 275, 6252–6258 [DOI] [PubMed] [Google Scholar]
- 50. Johnson E. S., Schwienhorst I., Dohmen R. J., Blobel G. (1997) The ubiquitin-like protein Smt3p is activated for conjugation to other proteins by an Aos1p/Uba2p heterodimer. EMBO J. 16, 5509–5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hayashi T., Seki M., Maeda D., Wang W., Kawabe Y., Seki T., Saitoh H., Fukagawa T., Yagi H., Enomoto T. (2002) Ubc9 is essential for viability of higher eukaryotic cells. Exp. Cell Res. 280, 212–221 [DOI] [PubMed] [Google Scholar]
- 52. Psakhye I., Jentsch S. (2012) Protein group modification and synergy in the SUMO pathway as exemplified in DNA repair. Cell 151, 807–820 [DOI] [PubMed] [Google Scholar]
- 53. Watts F. Z. (2007) The role of SUMO in chromosome segregation. Chromosoma 116, 15–20 [DOI] [PubMed] [Google Scholar]
- 54. Zhang X. D., Goeres J., Zhang H., Yen T. J., Porter A. C., Matunis M. J. (2008) SUMO-2/3 modification and binding regulate the association of CENP-E with kinetochores and progression through mitosis. Mol. Cell 29, 729–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schimmel J., Eifler K., Sigurethsson J. O., Cuijpers S. A., Hendriks I. A., Verlaan-de Vries M., Kelstrup C. D., Francavilla C., Medema R. H., Olsen J. V., Vertegaal A. C. (2014) Uncovering SUMOylation dynamics during cell-cycle progression reveals FoxM1 as a key mitotic SUMO target protein. Mol. Cell 53, 1053–1066 [DOI] [PubMed] [Google Scholar]
- 56. Sumara I., Maerki S., Peter M. (2008) E3 ubiquitin ligases and mitosis: embracing the complexity. Trends Cell Biol. 18, 84–94 [DOI] [PubMed] [Google Scholar]
- 57. Medema R. H., Lindqvist A. (2011) Boosting and suppressing mitotic phosphorylation. Trends Biochem. Sci. 36, 578–584 [DOI] [PubMed] [Google Scholar]
- 58. Ubersax J. A., Woodbury E. L., Quang P. N., Paraz M., Blethrow J. D., Shah K., Shokat K. M., Morgan D. O. (2003) Targets of the cyclin-dependent kinase Cdk1. Nature 425, 859–864 [DOI] [PubMed] [Google Scholar]
- 59. Shiio Y., Eisenman R. N. (2003) Histone sumoylation is associated with transcriptional repression. Proc. Natl. Acad. Sci. 100, 13225–13230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Melchior F., Hengst L. (2002) SUMO-1 and p53. Cell Cycle 1, 245–249 [PubMed] [Google Scholar]
- 61. Geiss-Friedlander R., Melchior F. (2007) Concepts in sumoylation: a decade on. Nat. Rev. Mol. Cell Biol. 8, 947–956 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.