Abstract
S-cysteinylated albumin and methionine-oxidized apolipoprotein A-I (apoA-I) have been posed as candidate markers of diseases associated with oxidative stress. Here, a dilute-and-shoot form of LC–electrospray ionization–MS requiring half a microliter of blood plasma was employed to simultaneously quantify the relative abundance of these oxidized proteoforms in samples stored at −80 °C, −20 °C, and room temperature and exposed to multiple freeze–thaw cycles and other adverse conditions in order to assess the possibility that protein oxidation may occur as a result of poor sample storage or handling. Samples from a healthy donor and a participant with poorly controlled type 2 diabetes started at the same low level of protein oxidation and behaved similarly; significant increases in albumin oxidation via S-cysteinylation were found to occur within hours at room temperature and days at −20 °C. Methionine oxidation of apoA-I took place on a longer time scale, setting in after albumin oxidation reached a plateau. Freeze–thaw cycles had a minimal effect on protein oxidation. In matched collections, protein oxidation in serum was the same as that in plasma. Albumin and apoA-I oxidation were not affected by sample headspace or the degree to which vials were sealed. ApoA-I, however, was unexpectedly found to oxidize faster in samples with lower surface-area-to-volume ratios. An initial survey of samples from patients with inflammatory conditions normally associated with elevated oxidative stress—including acute myocardial infarction and prostate cancer—demonstrated a lack of detectable apoA-I oxidation. Albumin S-cysteinylation in these samples was consistent with known but relatively brief exposures to temperatures above −30 °C (the freezing point of blood plasma). Given their properties and ease of analysis, these oxidized proteoforms, once fully validated, may represent the first markers of blood plasma specimen integrity based on direct measurement of oxidative molecular damage that can occur under suboptimal storage conditions.
Human serum albumin contains a single free cysteine residue (Cys34) that is susceptible to oxidation via disulfide-bond formation with free cysteine amino acids, resulting in S-cysteinylated (oxidized) albumin (1). Human apolipoprotein A-I (apoA-I)1 contains three methionine residues (Met86, Met112, and Met148) that can be oxidized to sulfoxides (2–4). The oxidized forms of both of these plasma/serum (P/S) proteins have been proposed as markers of conditions involving oxidative stress (5–9), including atherosclerosis (6–8). These proteins are readily analyzed intact via mass spectrometry in a single run using simple dilute-and-shoot techniques; thus if scientifically suitable, they are well positioned analytically to serve as clinical markers. At least some evidence exists, however, that both albumin (10) and apoA-I (6) are susceptible to artifactual oxidation ex vivo. Notably, the scientific literature in recent years has been relatively quiet with regard to both of these markers. We suspected that spontaneous artifactual oxidation of these proteins ex vivo led to their initial implication as markers of disease, but that the same phenomenon might have confounded efforts to clinically validate them (11, 12). Thus we undertook systematic studies of albumin and apoA-I oxidation ex vivo and found evidence indicating that rather than serving as markers of disease, oxidized albumin and apoA-I may serve as markers for improper handling and storage of blood P/S.
Improper biospecimen handling and storage can contribute to sample measurements that do not accurately reflect biological reality in vivo (13–16). This may introduce bias in analytical results, limiting the capacity for meaningful comparisons among patient groups (17–19). Thus careful pre-analytical sample handling is a vital component of both clinical investigation and biomarker research. For clinical assays, parameters that define proper sample handling and storage are generally determined during assay validation and are typically incorporated into laboratory standard operating procedures. In blood P/S-based biomarker development work, however, verification of sample integrity is sometimes overlooked or considered only as an afterthought. Contributing to this phenomenon is that fact that there are no universally accepted, globally applicable endogenous reference markers of P/S integrity. Indeed, there likely does not exist a single, individual marker capable of meeting this broad specification. Nonetheless, identification and standardization of quality control markers that cover this specific scope of application (i.e. proper storage conditions for blood P/S) represent an important goal of biobanking-related research (16, 20).
Betsou et al. (16) recently outlined and ranked some of the strongest candidates for use as quality control tools in biomarker research. Within the scope of tools for assessing proper handling and storage of P/S samples, nearly all markers are founded on the quantification of a nominal protein via a molecular-recognition-based assay. As a result, the indication of a loss of specimen integrity lies in an apparent loss of the target protein beyond the normal human reference range. Such loss is often ascribed to “degradation” and in many cases likely happens because of residual proteolytic activity that occurs at temperatures above the sample freezing point. In other cases, loss of the protein marker may be due to misfolding caused by repeated freeze–thaw cycles.
Though not frequently discussed, protein “degradation” ex vivo may also have roots in oxidative processes that are capable of disrupting protein–antibody interactions that serve as the basis for protein quantification. It is well known that in the absence of special precautions, disulfide bonds will form spontaneously between cysteine thiols. We have previously shown that this requires only the presence of atmospheric oxygen and trace metals and proceeds through a cysteine sulfenic acid intermediate (21). This mechanism also applies to S-cysteinylation of albumin (22–24), though disulfide exchange with cystine may be operative in P/S as well. Likewise, it is known that methionine-containing proteins and peptides will oxidize to sulfoxides spontaneously in the presence of atmospheric oxygen (25, 26); indeed, artifactual sulfoxidation of methionine residues in peptide-based proteomics work is well known. Oxidative modifications such as these have the potential to disrupt antibody interactions with the oxidized protein, resulting in low readings in molecular-recognition-based assays. Thus protein oxidation merits investigation as a protein “degradation” pathway.
As pointed out by Betsou et al. (16), markers that are highly sensitive to variations in specimen storage and handling conditions are likely to be the most useful. A considerable degree of change that occurs rapidly under undesirable conditions to which samples may be exposed, such as the state of being incompletely frozen (which for blood plasma occurs at temperatures above −30 °C (27–30)), is something to be sought after in a biospecimen-integrity marker. Herein we describe simple methods for the simultaneous relative quantification of oxidized albumin and apoA-I and present evidence that albumin and apoA-I can undergo major increases in oxidation ex vivo and thus may be useful markers of P/S specimen integrity.
EXPERIMENTAL PROCEDURES
Blood Plasma and Serum Sources
EDTA blood plasma specimens for time-course studies were collected via forearm venipuncture under institutional review board approval at the University of Southern California, processed within 30 min at room temperature, and immediately frozen at −80 °C. Samples were shipped on dry ice to Arizona State University, where they were received frozen and subsequently aliquoted for their respective time courses at various temperatures.
Matched EDTA plasma and serum samples from healthy volunteers were collected at Arizona State University under institutional review board approval. These samples were collected via forearm venipuncture according to NIH's Early Detection Research Network blood collection standard operating procedures (31, 32). Plasma samples were processed at room temperature, aliquoted, and placed in a −80 °C freezer within 35 min of collection; serum samples were placed at −80 °C within 95 min of collection.
Blood from patients experiencing acute coronary syndrome symptoms (later confirmed as myocardial infarction) was collected upon patient arrival at the Maricopa Integrated Health Systems emergency room or cardiology clinic. Patients were consented and blood was collected via forearm venipuncture under institutional review board approval. Blood serum was processed at room temperature within 3 h of collection, and samples were stored for 0 to 6 weeks at −70 °C prior to transfer on dry ice to Arizona State University for analysis. Basic patient clinical information and laboratory data are provided in supplemental Tables S6 and S7.
Blood serum from prostate cancer patients was purchased from the Cooperative Human Tissue Network (an NIH-supported blood- and tissue-collection bank). Specimens were processed according to their standard protocols. The Cooperative Human Tissue Network stored the samples at −80 °C prior to distribution, when they were shipped on dry ice. Samples were subjected to two freeze–thaw and aliquoting cycles for other purposes prior to analysis for this study. Patient demographics and laboratory data are provided in supplemental Table S8.
Sample Preparation and Analysis
Plasma and serum samples were thawed at room temperature, mixed by vortexing, and then centrifuged at 13,000g for 1.5 min to sediment any particulates. 0.5 μl was removed and diluted into 500 μl of 0.1% (v/v) trifluoroacetic acid. 5 μl of this solution were injected without delay by a Spark Holland Endurance autosampler in microliter pick-up mode and loaded by an Eksigent nanoLC*1D at 10 μl/min using 80% water/20% acetonitrile with 0.1% formic acid onto a protein captrap (Michrom, city/country no longer commercially available) configured for unidirectional flow on a six-port diverter valve. The captrap was then washed for 3 min with this loading solvent. The flow rate over the protein captrap cartridge was then changed to 1 μl/min, and a linear gradient of increasing acetonitrile from 20% to 90% was employed to elute the proteins into the mass spectrometer. The captrap eluent was directed to a Bruker MicrOTOF-Q (Q-TOF) mass spectrometer operating in positive ion, TOF-only mode, acquiring spectra in the m/z range of 300 to 3000 with a resolving power of ∼20,000 m/Δm full width at half-maximum. Electrospray ionization settings for the Agilent G1385A capillary microflow nebulizer ion source were as follows: end plate offset -500 V, capillary -4500 V, nebulizer nitrogen 2 bar, and dry gas nitrogen 3.0 l/min at 225 °C. Notably, this electrospray ionization mass spectrometer has a source design in which the spray needle is kept at ground and the inlet of the instrument is brought to a high negative voltage (in positive ion mode). This design is important because it avoids the possibility of corona discharge and subsequent artifactual protein oxidation (33). Data were acquired in profile mode at a digitizer sampling rate of 2 GHz. Spectra rate control was by summation at 1 Hz.
Data Analysis
Approximately 1 min of recorded spectra were averaged across the chromatographic peak apex of each protein of interest. The electrospray ionization charge-state envelope was deconvoluted with Bruker DataAnalysis v3.4 software to a mass range of 1000 Da on either side of any identified peak. Deconvoluted spectra were baseline subtracted, and all peak heights were calculated. Peak heights were employed for quantification instead of area because of the incomplete resolution of some of the peaks involved. Tabulated mass spectral peak heights were exported to a spreadsheet for further calculation. The fractional abundance of S-cysteinylated (oxidized) albumin was determined by dividing the height of the mass spectral peak representing S-cysteinylated albumin by the sum of the peak heights for native and S-cysteinylated albumin.
ApoA1 contains three Met residues; thus each molecule of apoA-I may contain no, one, two, or three oxidized Met residues. The relative distribution of apoA-I in each of these four oxidation states can readily be determined via electrospray ionization MS. This degree of analytical clarity affords the expression of a unique measurement of apoA-I oxidation that we refer to as total weighted oxidation (TWO). This term refers to the weighted fractional abundance of maximum Met oxidation (i.e. the case in which all three Met residues of apoA-I are oxidized to sulfoxides). To calculate the TWO or percent total oxidation capacity (34), the mass spectral peak heights of native (unoxidized) apoA1 and of singly, doubly, and triply oxidized apoA1 are multiplied by 0, 0.33, 0.66, and 1, respectively, before normalizing for total apoA1 peak height.
Although the analytical methods employed in this study reproducibly measure the relative abundance of oxidized albumin and apoA-I proteoforms, they are not validated for absolute protein quantification. As a result, no conclusions can be drawn about protein degradation over time based on raw or charge deconvoluted signal intensity.
RESULTS
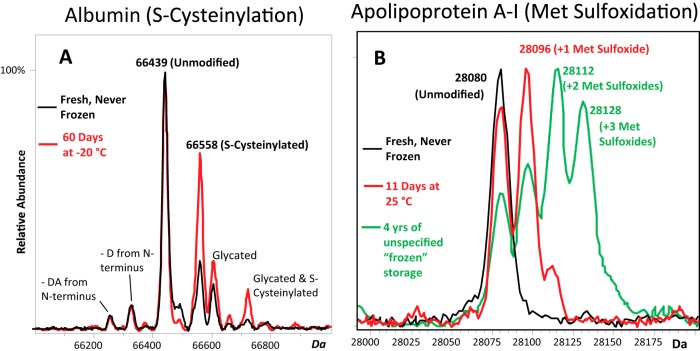
Dilution of blood P/S followed by direct analysis via mass spectrometry allows for the detection of both albumin and apoA-I. Bar-Or and colleagues have described dilute-and-shoot LC-MS methods for the analysis of intact albumin and its S-cysteinylated form (10, 35). We found that a similar approach could be used to analyze apoA-I simultaneously. This simple dilute-and-shoot method provided clean, easily interpreted mass spectra for the relative quantification of both albumin and apoA-I oxidation (Fig. 1). Raw spectra and peak tables corresponding to the charge deconvoluted spectra shown in Fig. 1 are provided in supplemental Figs. S1–S5 and supplemental Tables S1–S5. These supplemental figures and tables provide details on raw m/z values, charge states, MH+ protein masses from charge deconvoluted spectra, and typically observed peak heights. They represent data from across the entire qualitative and quantitative ranges of albumin and apoA-I proteoforms observed in these studies (i.e. no additional unique proteoforms of albumin or apoA-I were observed). Peak assignments in charge deconvoluted spectra were made on the basis of absolute mass. The calculated MH+ average mass of unmodified human albumin is 66,438.9 Da, and that of apoA-I is 28,079.6 Da. Based on analytical reproducibility data (described below), the unmodified form of albumin in charge deconvoluted spectra was measured at 66,439.45 ± 1.2 Da (S.E.). Unmodified apoA-I was measured at 28,079.6 ± 0.57 Da (S.E.). All modified forms of albumin (5, 10, 35) and apoA-I (6) have been described and published elsewhere and are in agreement with the modifications due to mild oxidation reported in these studies. Reproducibility and autosampler stability were validated to ensure the integrity of stability study results.
Fig. 1.
Charge deconvoluted electrospray ionization mass spectra of (A) albumin and (B) apoA-I from healthy donors showing increasing S-cysteinylated albumin and methionine-oxidized apoA-I under less-than-ideal storage conditions. Red and black spectra are from the same individual sample, aged as indicated. As evidenced, albumin is subject to minor N-terminal truncations as well as glycation (i.e. covalent glucose attachment to protein amino groups akin to the diabetes marker HbA1c). There are three methionine residues in apoA-I, permitting up to three sulfoxidation events, each of which shifts the mass of the protein up by 16 Da. The heavily oxidized apoA-I sample from a healthy donor (green spectrum) was obtained from a for-profit biobank after four years of storage under unspecified “frozen” storage conditions. (A lot-paired sample from a different healthy individual was similarly oxidized; not shown.)
Analytical Reproducibility of the Method
Intra- and interday reproducibility of the analytical method were evaluated. For albumin, samples from three males and three females were analyzed in quadruplicate on three separate days. The average fractional abundance of S-cysteinylated albumin in these samples was 0.25. The average within-day precision (expressed as %cv) was 5.2%, and the total interday precision was 6.2%. ApoA-I oxidation was not detected in any of these samples. Data on the reproducibility of the apoA-I oxidation assay were thus collected from a separate sample that had been allowed to sit at room temperature for several days to build up a moderate degree of oxidation (about 40% TWO) and then placed back at −80 °C. Analysis of six replicates on three separate days revealed the ApoA-I TWO within-day precision as 1.7% and the interday precision as 1.8%. The fractional abundance of S-cysteinylated albumin in this sample was 0.44 with intraday reproducibility of 4.0% and interday precision at 6.7%.
Autosampler Stability
To assess the potential for preparing P/S for walk-away autosampler-based analysis, fresh plasma from a healthy donor was diluted in the usual manner (1000-fold in 0.1% trifluoroacetic acid), aliquoted into a 96-well plate, and set in the LC-MS autosampler at 10 °C for serial injections (as described above). Albumin S-cysteinylation was stable, but apoA-I oxidation began to develop within about 5 h (24 injections) (supplemental Fig. S6). In previous work (36) we found that the addition of 1 mm MetSer dipeptide (which serves as a competitive inhibitor of protein oxidation) can delay for hours the methionine oxidation of other proteins that have been pre-isolated from serum and are present at low concentrations in a similarly acidic solution. For apoA-I in diluted plasma, however, 5 mm MetSer was insufficient to prevent oxidation of methionine residues. In consideration of these results, all samples were diluted immediately before injection onto the LC-MS.
The Role of Storage Temperature
Albumin and apoA-I in blood plasma readily oxidized over time at temperatures of −20 °C or warmer. In albumin, the single free cysteine residue (Cys34) underwent disulfide bond formation and/or disulfide exchange with free cysteine amino acids and/or cystine, respectively, that were naturally present in the blood plasma (37), forming S-cysteinylated albumin (Fig. 1A). ApoA-I was susceptible to spontaneous methionine sulfoxidation (Fig. 1B). Because there are three Met residues in human apoA-I, up to three oxidation events may occur per molecule.
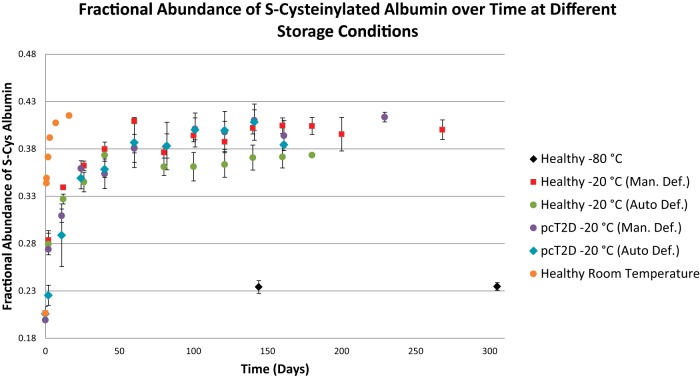
Albumin oxidation took place within hours at room temperature, reaching its peak within about a week. The process was slower at −20 °C, reaching a plateau in about six weeks (Fig. 2). Plasma sample storage in a manually defrosted freezer versus in an automatically defrosted freezer was irrelevant (Fig. 2). Notably, however, the automatically defrosted freezer caused sample dehydration, even when samples were stored in a test tube with inner threads and a sealing o-ring (as ice crystals could redeposit in the cap (supplemental Fig. S7)); based on this, plasma samples should never be stored in this type of freezer. At −80 °C, albumin S-cysteinylation was stable for over 300 days. Also shown in Fig. 2A are data from a 46-year-old donor with poorly controlled type 2 diabetes (HbA1c of 9.5; triglycerides > 1000 mg/dl) and a history of coronary artery disease including myocardial infarction. Relative to the healthy donor, there was no difference in either the starting fractional abundance of S-cysteinylated albumin or its plateau point at −20 °C.
Fig. 2.
Increasing abundance of S-cysteinylated albumin in plasma over time at −80 °C, −20 °C, and room temperature (25 °C). One sample was collected from a single healthy donor and a second sample was obtained from a single poorly controlled type 2 diabetic (pcT2D) with a history of coronary artery disease and myocardial infarction. Both samples were collected fresh and started on Day 0 at ∼0.20. Each sample was then aliquoted into two sets of three different vial types and stored in either an auto-defrost (n = 3) or a manual-defrost freezer (n = 3). An additional aliquot from the healthy donor was set aside for the room temperature time course. Auto-defrost freezers caused sublimation and/or evaporation of P/S water, resulting in sample dehydration (supplemental Fig. S7). Storage in such freezers is not recommended. Shown is the average ± S.E. of three aliquots per point (fewer for later auto-defrost freezer points as a result of sublimation-mediated sample loss), stored (to no effect; supplemental Fig. S8) in different types of vials with different headspaces and degrees of sealing. If no error bars are shown, only one aliquot was analyzed. The slight initial increase in the sample stored at −80 °C is likely due to the fact that the sample was measured and then aliquoted; during the aliquoting process the sample was at 4 - 25 °C for over an hour. The first time point for the room temperature sample was measured at 17.5 h.
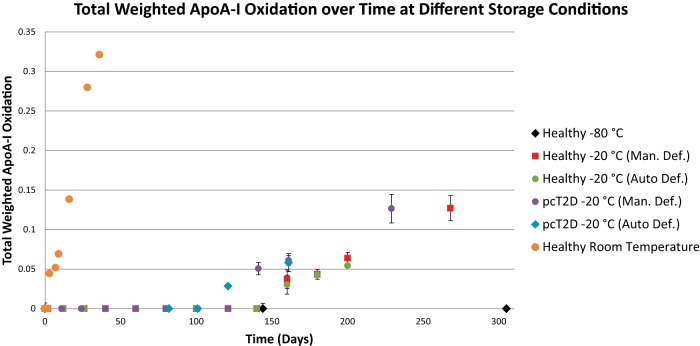
ApoA-I oxidation began to occur within a week at room temperature (Fig. 3) and continued to increase rapidly over the course of a month. Analysis ceased at 36 days due to the fact that storage at room temperature for 36 days is well outside the range of normal plasma sample handling. (Notably, no signs of mold, fungal, or bacterial growth were observed during this time or for a few months afterward.) Initially, no apoA-I oxidation was evident in any specimen, including that from the poorly controlled diabetic. ApoA-I appeared stable for three months at −20 °C, but after about four months of storage at −20 °C it began to show evidence of oxidation, which increased steadily thereafter in all samples (Fig. 3). ApoA-I was stable for over 300 days at −80 °C (Fig. 3).
Fig. 3.
Apolipoprotein A-I oxidation in plasma over time at −80 °C, −20 °C, and room temperature (25 °C). All samples were collected fresh and started on Day 0 with no oxidation. Up to three oxidation events may occur per apoA-I molecule, and total weighted oxidation was calculated as described in the text. Samples were the same as those described in Fig. 2.
Effect of Storage Vial Headspace and Degree of Sealing
For the time course experiments depicted in Figs. 2 and 3, each 50-μl plasma sample stored at −20 °C was aged in triplicate but in separate vials. Vial 1 consisted of a 1.5-ml Eppendorf snap-cap tube with a hole punched in the top. Vial 2 consisted of the same test tube but with no hole. Vial 3 consisted of a 2-ml conical bottom plastic tube with an inner threaded screw cap and an o-ring to facilitate sealing. Neither the type of test tube nor the degree of vial sealing made a significant difference in the oxidation rate of albumin or apoA-I (supplemental Figs. S8 and S9).
Freeze–Thaw Cycles
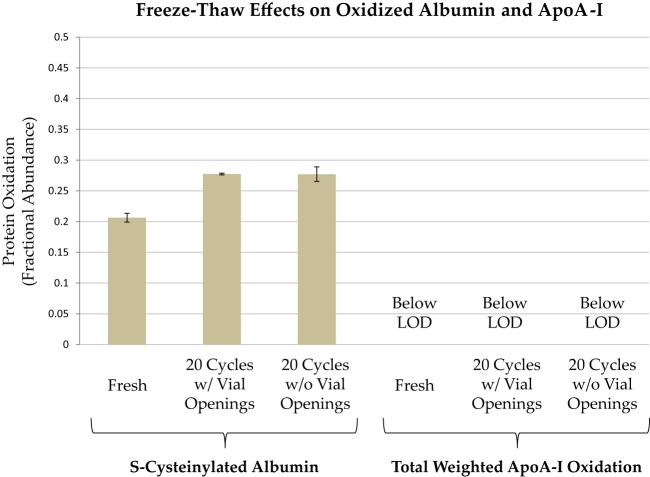
Freeze–thaw cycles are often suspected to contribute to sample instability. To assess the effect of freeze–thaw cycles on albumin and apoA-I oxidation, two 50-μl aliquots from a healthy donor were stored in screw-cap vials equipped with a sealing o-ring at −80 °C and subjected to 20 freeze–thaw cycles. The starting fractional abundance of S-cysteinylated albumin was 0.21 ± 0.0071 (n = 6 replicates), and there was no evidence of apoA-I oxidation. Each day the samples were thawed at room temperature, immediately mixed, very briefly centrifuged to remove plasma from the test tube walls, and placed back in storage at −80 °C. For one of the samples, the cap was briefly removed and then replaced each day prior to re-freezing—a procedure intended to simulate the minimum exposure needed to remove a specimen from the freezer, take an aliquot, and then return it to storage. To determine whether fresh air exposure in addition to freeze–thaw cycles affected albumin or apoA-I oxidation, the cap was never removed from the second sample until the final analysis. After 20 such freeze–thaw cycles (and an estimated total thawed time of 300 min), the fractional abundance of S-cysteinylated albumin in the repeatedly opened vial had reached 0.28 ± 0.0014 (n = 3 replicate analyses), and that in the once-opened vial had reached 0.28 ± 0.012 (n = 3 replicate analyses), indicating a small increase in albumin oxidation in accord with total thawed time, but no effect of vial opening and renewed air exposure during each thaw cycle (Fig. 4). No apoA-I oxidation was evident in either sample.
Fig. 4.
Freeze–thaw cycle effects on albumin and apoA-I oxidation. Following initial analysis (n = 6), a fresh sample was split into two vials, each of which was subjected to 20 freeze–thaw cycles prior to analysis in triplicate (average ± S.E. shown). One vial was opened at each thaw cycle, and the other vial was not opened until 20 freeze–thaw cycles were complete. For S-cysteinylated albumin, the differences between the fresh sample and the freeze–thaw cycled samples were significant (Mann–Whitney U test, p < 0.05) but in accord with a total thawed time of about 300 min (see Fig. 2).
Blood Collection Type
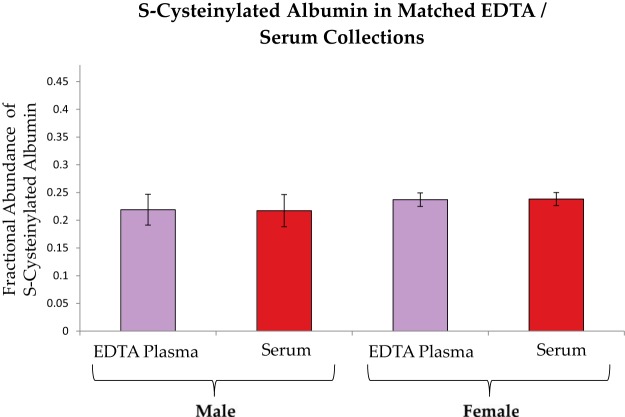
Matched EDTA plasma and serum sample sets from two healthy males and two healthy females were collected fresh to determine whether plasma differs from serum with regard to initial measurements of albumin and apoA-I oxidation. Plasma samples were processed, aliquoted, and placed in a −80 °C freezer within 35 min of collection; serum samples were placed at −80 °C within 95 min of collection. Aliquots were thawed and analyzed in duplicate within four months. Albumin S-cysteinylation was minimal, and no differences were evident in its fractional abundance between males and females or between EDTA plasma and serum (Fig. 5). No apoA-I oxidation was evident.
Fig. 5.
Albumin oxidation in matched EDTA plasma and serum collections from two healthy male and two healthy female donors. Each sample was analyzed in duplicate. No significant differences were detectable between plasma and serum or between males and females. Bars represent averages ± S.E.
Surface-area-to-volume Effects
Surface-area-to-volume (sa/vol) ratio effects on albumin and apoA-I oxidation were investigated at room temperature by dividing a fresh plasma sample from a healthy volunteer into 100-μl, 200-μl, and 400-μl aliquots in cylindrical, 8-mm internal diameter polypropylene screw-cap test tubes. Additional 10-μl aliquots were placed into a 1.5-ml conical-bottom polypropylene snap-cap test tube to represent an extreme case of high sa/vol.
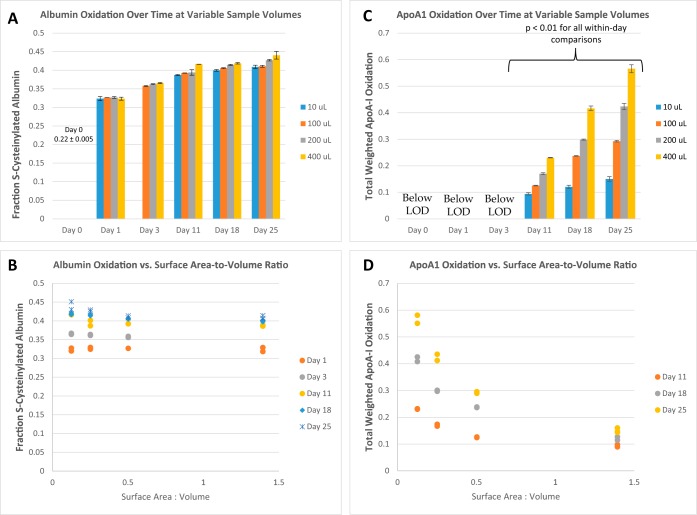
The fraction of S-cysteinylated albumin increased to a maximum of about 0.4 in all samples at a similar same rate, including the 10-μl sample (Figs. 6A and 6B). apoA-I oxidation varied systematically with sa/vol ratio, but in the direction opposite that expected (Figs. 6C and 6D): pairwise comparisons of apoA-I oxidation for all sa/vol ratios were statistically significant on days 11, 18, and 25 (analysis of variance; p < 0.01 for all Tukey pairwise comparisons). Within each day the Spearman coefficient of determination for apoA-I oxidation versus the sa/vol ratio was greater than 0.9 (p < 0.001).
Fig. 6.
Oxidation of albumin (A and B) at room temperature in samples of different volumes depicted over time (A) and, alternatively, at different surface-area-to-volume ratios (B). Analogous data are shown for apoA-I oxidation (C and D). Bar graphs in A and C represent the average of duplicate analyses with the error bars representing the actual points. Individual data points are shown in the scatter plots in B and D. As shown in C, pairwise comparisons of apoA-I oxidation for all sa/vol ratios were statistically significant on Days 11, 18, and 25 (analysis of variance; p < 0.01 for all Tukey pairwise comparisons). Within each day the Spearman coefficient of determination for apoA-I oxidation versus the sa/vol ratio was greater than 0.9 (p < 0.001).
Initial Survey of Reference Ranges for Oxidized ApoA-I in Heart Disease and Prostate Cancer
Samples from a healthy individual and a poorly controlled type 2 diabetic with a history of heart disease were collected for the temperature time course studies. As described above, albumin oxidation in these samples was minimal (∼20% in each sample), and apoA-I oxidation was not detectable. To further provide an initial assessment of baseline reference ranges in patients with diseases normally associated with increased oxidative stress, serum samples from two different patient cohorts that were originally collected for different purposes, but stored and handled under known conditions, were analyzed for apoA-I oxidation. Albumin oxidation was also assessed in these samples, but because the serum samples may have been kept at room temperature for variable amounts of time (up to 3 h) prior to processing, some degree of albumin oxidation above 0.2 was expected and observed.
In the first set, serum was collected from 24 patients presenting to the emergency room or cardiology clinic with acute coronary syndrome and subsequently determined to have had a myocardial infarction. Patient demographics and laboratory data are provided in supplemental Tables S6 and S7. Eight of these patients had type 2 diabetes. Samples were processed within 3 h of being drawn and were stored for 0 to 6 weeks at −70 °C and then transferred on dry ice to a −80 °C freezer for indefinite storage prior to analysis. No oxidation of apoA-I was detected in any of these samples. The average fraction of albumin in the S-cysteinylated form was 0.29 ± 0.097 S.E. (see Fig. 2 for comparison). Seven samples were measured with levels below 0.25.
The second set of samples from diseased individuals consisted of serum from 24 prostate cancer patients. Patient demographics and laboratory data are provided in supplemental Table S8. These samples were obtained from the Cooperative Human Tissue Network, an NIH-supported blood- and tissue-collection bank. They were collected, processed, and stored under Cooperative Human Tissue Network Standard Operating Procedures, which precluded any storage at −20 °C. These samples were stored at −80 °C before shipment on dry ice and storage at −80 °C prior to analysis. Two rounds of aliquoting took place in-house prior to sample analysis. No oxidation of apoA-I was detected in any of these samples. The average fraction of albumin in the S-cysteinylated form was 0.38 ± 0.047 S.E. Seven samples were measured with levels below 0.35.
DISCUSSION
The results presented here demonstrate the susceptibility of blood plasma albumin and apoA-I to spontaneous oxidation at temperatures of −20 °C and above. Measurements from dozens of patients ranging from healthy to very ill with conditions typically associated with high oxidative stress (e.g. myocardial infarction) suggest that most individuals probably lack a readily detectable degree of oxidized apoA-I in circulation (i.e. less than ∼ 3% TWO). Because the storage and handling histories of individual samples within the clinical sample cohorts employed here were not tracked in extreme detail, we cannot be sure that the elevated levels of albumin S-cysteinylation observed in patients with oxidative-stress-associated conditions were strictly due to handling. It is clear, however, that numerous patients experiencing myocardial infarction (with or without type 2 diabetes) have levels of albumin S-cysteinylation consistent with freshly collected samples from healthy individuals.
The mechanism of albumin S-cysteinylation may involve disulfide exchange with cystine or direct reaction with cysteine through a cysteine sulfenic acid intermediate (21–24). As a result, the rate of formation of S-cysteinylated albumin is dependent on the P/S concentration of cystine and/or cysteine, O2, and redox active metals (21). Typical concentrations of cysteine, cystine, and total cysteine (present in the free form or as a mixed disulfide with other small molecules such as glutathione) in human P/S are ∼10 μm, 90 μm, and 200 μm, respectively (37, 38). At an average P/S albumin concentration of about 630 μm (39), it would take most of the total cysteine in P/S to increase the fraction of S-cysteinylated albumin from about 20% to the maximum values of over 40% observed in this study, suggesting a role for disulfide exchange in albumin S-cysteinylation. This analysis also suggests that specimens from different individuals may exhibit slightly variable ex vivo rates of albumin S-cysteinylation (though this was not apparent in the present study). Thus, besides endogenous levels of in vivo S-cysteinylated albumin, in future studies the populational variability in the rate of albumin S-cysteinylation and the maximum degree of S-cysteinylation will also need to be examined.
The ex vivo oxidative stability of albumin and apoA-I tracked strongly with the temperature and time at which samples were kept above −30 °C and not with the number of freeze–thaw cycles. This is consistent with the idea that multiple freeze–thaw cycles may lead to protein denaturation but thawed conditions are necessary to facilitate chemical reactions.
A limited amount of apoA-I oxidation may occur in vivo without being detected by the assay employed here. Our limit of detection for total weighted apoA-I oxidation was about 3%. Thus, at a typical P/S concentration of 1250 μg/ml (40), circulating concentrations of oxidized apoA-I may reach concentrations of 40 μg/ml without being detected by this assay. As a result, it is possible that some individuals may have oxidized apoA-I levels slightly below the detection threshold, whereas in others it might be well below this limit. Therefore, the possibility for varying degrees of P/S apoA-I oxidizability ex vivo must be considered. This may, in fact, be evidenced by the difference in the healthy and diabetic donors in the time to onset of detectable apoA-I oxidation at −20 °C (Fig. 3). As other P/S molecules are likely to be proportionally impacted in their susceptibility to oxidation alongside varying apoA-I oxidizability, this phenomenon is unlikely to affect the overall utility of oxidized apoA-I as a marker of P/S integrity.
As a lipoprotein, apoA-I is associated with HDL lipids and is uniquely susceptible to methionine-based sulfoxidation vis-à-vis oxidative transfer from lipid hydroperoxides (41, 42). Relative to other methionine-containing P/S proteins, this provides an additional pathway for oxidation and makes it likely that apoA-I methionine residues are more susceptible to oxidation than many, if not most, other P/S protein methionines. This is consistent with the observed inability of MetSer dipeptide to protect against apoA-I oxidation in the autosampler stability studies described above, whereas the dipeptide was able to protect against methionine oxidation of isolated Vitamin D binding protein (36). If proven, ex vivo sulfoxidation of apoA-I prior to that of other proteins would be highly useful in that it would permit the absence of apoA-I oxidation to serve as a molecular “all clear” sign that no other proteins have been damaged by methionine oxidation.
The rate dependence of apoA-I oxidation on the specimen sa/vol ratio requires careful consideration with regard to moving apoA-I methionine oxidation forward as a candidate marker of P/S integrity. Originally we anticipated that a higher sa/vol ratio might result in more rapid albumin and apoA-I oxidation. Although the sa/vol ratio was irrelevant to the rate of ex vivo albumin S-cysteinylation, it affected apoA-I oxidation in a manner opposite that expected. This suggests that P/S exposure to atmospheric oxygen is not a rate-limiting factor in albumin or apoA-I oxidation. In light of these findings and the fact that combined air and Cu2+ fails to oxidize isolated lipid-free apoA-I (41), we believe that the observed apoA-I oxidation was not simply due to the exposure of plasma to oxygen and the trace metals required to break the spin barrier for reaction of O2 with organic compounds. Peroxidation of HDL lipids in the presence of Cu2+ and oxygen (41) and the oxidative transfer from these lipid hydroperoxides of HDL to apoA-I methionine residues are well established (7, 41–45). Based on these phenomena, it appears that oxygen reacts with unsaturated lipids to form lipid hydroperoxides in a trace-metal catalyzed event. We theorize that in whole P/S the association of liquid-borne HDL particles with an air interface may render these particles dysfunctional with regard to the ability of lipid hydroperoxides to transfer their oxidative insults to apoA-I methionine residues. That is, at the surface, the unique phospholipid-bearing discoidal structure of HDL (in which apoA-I wraps around phospholipids like a belt (46, 47)) may functionally remove surface-associated HDL particles from solution and thereby make the methionine residues of apoA-I unavailable for oxidation by lipid hydroperoxides. Ultimately, the potential utility of apoA-I oxidation as a marker of P/S integrity depends on whether the stability of other methionine-containing clinical protein targets consistently track with apoA-I oxidation regardless of the sa/vol ratio. It might be necessary to define a maximum sa/vol ratio (minimum volume for a defined storage vessel) at which normal sample aging occurs and apoA-I serves as an effective surrogate marker of protein oxidation. Alternatively, if the relationship of apoA-I oxidation is tightly correlated with sa/vol ratio, it might be possible to normalize apoA-I oxidation results to the sa/vol ratio. It should be noted that the sa/vol ratio phenomenon observed for apoA-I might be applicable to other proteins that tend to associate with surfaces.
Oxidized albumin and apoA-I represent direct measurements of P/S protein oxidation, an important, often overlooked form of molecular damage that, as we have shown here, inevitably occurs at temperatures above −30 °C. Future studies will focus on (1) mapping the oxidation rates of albumin and apoA-I with the concurrent “loss” (as observed in ELISA) of clinical protein biomarkers with similarly oxidizable cysteine and methionine residues, (2) stabilizing apoA-I for autosampler based analysis, (3) establishing population reference ranges for in vivo oxidized levels of albumin and apoA-I, and (4) establishing population reference ranges for rates and maxima of ex vivo albumin and apoA-I oxidation.
Additional applications for this general approach of monitoring ex vivo protein oxidation may involve analysis of other, lower abundance proteins in specimens that have been depleted of the most abundant P/S proteins (e.g. via a MARS-14 depletion column). Suggested guidelines for target protein characteristics include the presence of a solvent-accessible free cysteine residue, low endogenous heterogeneity (e.g. minimal or highly consistent glycosylation), and relatively small protein size in order to maximize the ability to resolve proteoforms that differ by only one oxygen atom.
Conclusions
As candidate markers of blood P/S integrity that can be measured in a single assay, oxidized albumin and apoA-I have low sample volume requirements, useful dynamic ranges, and mechanisms and rates of oxidation that are likely to match or precede the oxidation of other proteins with similarly susceptible oxidation chemistries. If albumin is validated to have a low natural reference range in the population, its high sensitivity to ex vivo oxidation will make it a uniquely promising candidate for the general verification of biobanked P/S specimen integrity and a potential indicator of extremely well-handled samples. Likewise, the detection of any oxidized apoA-I appears to indicate exposure to suboptimal storage conditions.
Future validation of the low natural reference ranges for the oxidized forms of these proteins in the population will be necessary before they can serve as endogenous reference markers for inappropriate sample handling and storage. In such a case they would represent the first markers of P/S specimen integrity based on direct measurement of oxidative molecular damage that occurs under less-than-ideal P/S storage conditions.
Supplementary Material
Footnotes
Author contributions: C.R.B., B.N., and C.B. designed research; C.R.B., D.S.R., S.J., M.R.S., and N.D.S. performed research; C.R.B. and S.J. analyzed data; C.R.B. wrote the paper; H.Y., B.N. and C.B. collected specimens.
* This work was supported by Awards R01DK082542 and R24DK090958 from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Yassine was supported by National Institutes of Health Grant No. K23HL107389 and American Heart Association Grant No. AHA12CRP11750017.
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- apoA-I
- apolipoprotein A-I
- P/S
- plasma/serum
- sa/vol
- surface area to volume
- TWO
- total weighted oxidation.
REFERENCES
- 1. Peters T. (1996) All About Albumin: Biochemistry, Genetics, and Medical Applications, Academic Press, San Diego [Google Scholar]
- 2. Anantharamaiah G. M., Hughes T. A., Iqbal M., Gawish A., Neame P. J., Medley M. F., Segrest J. P. (1988) Effect of oxidation on the properties of apolipoproteins A-I and A-II. J. Lipid Res. 29, 309–318 [PubMed] [Google Scholar]
- 3. von Eckardstein A., Walter M., Holz H., Benninghoven A., Assmann G. (1991) Site-specific methionine sulfoxide formation is the structural basis of chromatographic heterogeneity of apolipoproteins A-I, C-II, and C-III. J. Lipid Res. 32, 1465–1476 [PubMed] [Google Scholar]
- 4. Panzenbock U., Kritharides L., Raftery M., Rye K. A., Stocker R. (2000) Oxidation of methionine residues to methionine sulfoxides does not decrease potential antiatherogenic properties of apolipoprotein A-I. J. Biol. Chem. 275, 19536–19544 [DOI] [PubMed] [Google Scholar]
- 5. Bar-Or D., Heyborne K. D., Bar-Or R., Rael L. T., Winkler J. V., Navot D. (2005) Cysteinylation of maternal plasma albumin and its association with intrauterine growth restriction. Prenat. Diagn. 25, 245–249 [DOI] [PubMed] [Google Scholar]
- 6. Pankhurst G., Wang X. L., Wilcken D. E., Baernthaler G., Panzenbock U., Raftery M., Stocker R. (2003) Characterization of specifically oxidized apolipoproteins in mildly oxidized high density lipoprotein. J. Lipid Res. 44, 349–355 [DOI] [PubMed] [Google Scholar]
- 7. Panzenbock U., Stocker R. (2005) Formation of methionine sulfoxide-containing specific forms of oxidized high-density lipoproteins. Biochim. Biophys. Acta 1703, 171–181 [DOI] [PubMed] [Google Scholar]
- 8. Wang X. S., Shao B., Oda M. N., Heinecke J. W., Mahler S., Stocker R. (2009) A sensitive and specific ELISA detects methionine sulfoxide-containing apolipoprotein A-I in HDL. J. Lipid Res. 50, 586–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Borges C. R., Oran P. E., Buddi S., Jarvis J. W., Schaab M. R., Rehder D. S., Rogers S. P., Taylor T., Nelson R. W. (2011) Building multidimensional biomarker views of type 2 diabetes on the basis of protein microheterogeneity. Clin. Chem. 57, 719–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rael L. T., Bar-Or R., Ambruso D. R., Mains C. W., Slone D. S., Craun M. L., Bar-Or D. (2009) The effect of storage on the accumulation of oxidative biomarkers in donated packed red blood cells. J. Trauma 66, 76–81 [DOI] [PubMed] [Google Scholar]
- 11. Woodward M., Croft K. D., Mori T. A., Headlam H., Wang X. S., Suarna C., Raftery M. J., MacMahon S. W., Stocker R. (2009) Association between both lipid and protein oxidation and the risk of fatal or non-fatal coronary heart disease in a human population. Clin. Sci. 116, 53–60 [DOI] [PubMed] [Google Scholar]
- 12. Mullan A., Sattar N. (2009) More knocks to the oxidation hypothesis for vascular disease? Clin. Sci. 116, 41–43 [DOI] [PubMed] [Google Scholar]
- 13. Lippi G., Guidi G. C., Mattiuzzi C., Plebani M. (2006) Preanalytical variability: the dark side of the moon in laboratory testing. Clin. Chem. Lab. Med. 44, 358–365 [DOI] [PubMed] [Google Scholar]
- 14. Betsou F., Barnes R., Burke T., Coppola D., Desouza Y., Eliason J., Glazer B., Horsfall D., Kleeberger C., Lehmann S., Prasad A., Skubitz A., Somiari S., Gunter E. (2009) Human biospecimen research: experimental protocol and quality control tools. Cancer Epidemiol. Biomarkers Prev. 18, 1017–1025 [DOI] [PubMed] [Google Scholar]
- 15. Moore H. M., Compton C. C., Lim M. D., Vaught J., Christiansen K. N., Alper J. (2009) 2009 Biospecimen research network symposium: advancing cancer research through biospecimen science. Cancer Res. 69, 6770–6772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Betsou F., Gunter E., Clements J., DeSouza Y., Goddard K. A., Guadagni F., Yan W., Skubitz A., Somiari S., Yeadon T., Chuaqui R. (2013) Identification of evidence-based biospecimen quality-control tools: a report of the International Society for Biological and Environmental Repositories (ISBER) Biospecimen Science Working Group. J. Mol. Diagn. 15, 3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rai A. J., Gelfand C. A., Haywood B. C., Warunek D. J., Yi J. Z., Schuchard M. D., Mehigh R. J., Cockrill S. L., Scott G. B. I., Tammen H., Schulz-Knappe P., Speicher D. W., Vitzthum F., Haab B. B., Siest G., Chan D. W. (2005) HUPO Plasma Proteome Project specimen collection and handling: towards the standardization of parameters for plasma proteome samples. Proteomics 5, 3262–3277 [DOI] [PubMed] [Google Scholar]
- 18. Lim M. D., Dickherber A., Compton C. C. (2011) Before you analyze a human specimen, think quality, variability, and bias. Anal. Chem. 83, 8–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tuck M. K., Chan D. W., Chia D., Godwin A. K., Grizzle W. E., Krueger K. E., Rom W., Sanda M., Sorbara L., Stass S., Wang W., Brenner D. E. (2009) Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J. Proteome Res. 8, 113–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. LaBaer J. (2012) Improving international research with clinical specimens: 5 achievable objectives. J. Proteome Res. 11, 5592–5601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rehder D. S., Borges C. R. (2010) Cysteine sulfenic acid as an intermediate in disulfide bond formation and nonenzymatic protein folding. Biochemistry 49, 7748–7755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Turell L., Botti H., Carballal S., Ferrer-Sueta G., Souza J. M., Duran R., Freeman B. A., Radi R., Alvarez B. (2008) Reactivity of sulfenic acid in human serum albumin. Biochemistry 47, 358–367 [DOI] [PubMed] [Google Scholar]
- 23. Turell L., Botti H., Carballal S., Radi R., Alvarez B. (2009) Sulfenic acid—a key intermediate in albumin thiol oxidation. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877, 3384–3392 [DOI] [PubMed] [Google Scholar]
- 24. Turell L., Carballal S., Botti H., Radi R., Alvarez B. (2009) Oxidation of the albumin thiol to sulfenic acid and its implications in the intravascular compartment. Braz. J. Med. Biol. Res. 42, 305–311 [DOI] [PubMed] [Google Scholar]
- 25. Linde S., Nielsen J. H., Hansen B., Welinder B. S. (1990) High-performance liquid chromatography of rat and mouse islet polypeptides: potential risk of oxidation of methionine residues during sample preparation. J. Chromatogr. 530, 29–37 [DOI] [PubMed] [Google Scholar]
- 26. Brot N., Weissbach H. (1991) Biochemistry of methionine sulfoxide residues in proteins. BioFactors 3, 91–96 [PubMed] [Google Scholar]
- 27. Farrugia A., Hill R., Douglas S., Karabagias K., Kleinig A. (1992) Factor VIII/von Willebrand factor levels in plasma frozen to -30 degrees C in air or halogenated hydrocarbons. Thromb. Res. 68, 97–102 [DOI] [PubMed] [Google Scholar]
- 28. MacKenzie A. P. (1980) First and second order transitions during the freezing and thawing of source plasma (human). American Institute of Chemical Engineers (New York) Symposium on Processing and Fractionation of Blood Plasma, Philadelphia, June 8–12 [Google Scholar]
- 29. Bravo M. I., Grancha S., Jorquera J. I. (2006) Effect of temperature on plasma freezing under industrial conditions. Pharmeur. Sci. Notes 2006, 31–35 [PubMed] [Google Scholar]
- 30. (2005) Human Plasma for Fractionation, Monograph 0853, Council of Europe, Strasbourg, France [Google Scholar]
- 31.The Early Detection Research Network (EDRN) Standard Operating Procedure (SOP) for Collection of EDTA Plasma.
- 32.The Early Detection Research Network (EDRN) Standard Operating Procedure (SOP) for Collection of Serum.
- 33. Boys B. L., Kuprowski M. C., Noel J. J., Konermann L. (2009) Protein oxidative modifications during electrospray ionization: solution phase electrochemistry or corona discharge-induced radical attack? Anal. Chem. 81, 4027–4034 [DOI] [PubMed] [Google Scholar]
- 34. Yassine H., Borges C. R., Schaab M. R., Billheimer D., Stump C., Reaven P., Lau S. S., Nelson R. (2013) Mass spectrometric immunoassay and MRM as targeted MS-based quantitative approaches in biomarker development: potential applications to cardiovascular disease and diabetes. Proteomics Clin. Applicat. 7, 528–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bar-Or D., Bar-Or R., Rael L. T., Gardner D. K., Slone D. S., Craun M. L. (2005) Heterogeneity and oxidation status of commercial human albumin preparations in clinical use. Crit. Care Med. 33, 1638–1641 [DOI] [PubMed] [Google Scholar]
- 36. Rehder D., Schaab M., Borges C. (2014) Buffers for stabilizing biological specimens and their use. U.S. Patent 8,669,111
- 37. Park Y., Ziegler T. R., Gletsu-Miller N., Liang Y., Yu T., Accardi C. J., Jones D. P. (2010) Postprandial cysteine/cystine redox potential in human plasma varies with meal content of sulfur amino acids. J. Nutr. 140, 760–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moriarty S. E., Shah J. H., Lynn M., Jiang S., Openo K., Jones D. P., Sternberg P. (2003) Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic. Biol. Med. 35, 1582–1588 [DOI] [PubMed] [Google Scholar]
- 39. Centers for Disease Control and Prevention and National Center for Health Statistics. (2006) National Health and Nutrition Examination Survey Data. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Hyattsville, MD [Google Scholar]
- 40. Maciejko J. J., Holmes D. R., Kottke B. A., Zinsmeister A. R., Dinh D. M., Mao S. J. T. (1983) Apolipoprotein-a-I as a marker of angiographically assessed coronary-artery disease. N. Engl. J. Med. 309, 385–389 [DOI] [PubMed] [Google Scholar]
- 41. Garner B., Witting P. K., Waldeck A. R., Christison J. K., Raftery M., Stocker R. (1998) Oxidation of high density lipoproteins. I. Formation of methionine sulfoxide in apolipoproteins AI and AII is an early event that accompanies lipid peroxidation and can be enhanced by alpha-tocopherol. J. Biol. Chem. 273, 6080–6087 [DOI] [PubMed] [Google Scholar]
- 42. Garner B., Waldeck A. R., Witting P. K., Rye K. A., Stocker R. (1998) Oxidation of high density lipoproteins. II. Evidence for direct reduction of lipid hydroperoxides by methionine residues of apolipoproteins AI and AII. J. Biol. Chem. 273, 6088–6095 [DOI] [PubMed] [Google Scholar]
- 43. Bowry V. W., Stanley K. K., Stocker R. (1992) High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc. Natl. Acad. Sci. U.S.A. 89, 10316–10320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sattler W., Christison J., Stocker R. (1995) Cholesterylester hydroperoxide reducing activity associated with isolated high- and low-density lipoproteins. Free Radic. Biol. Med. 18, 421–429 [DOI] [PubMed] [Google Scholar]
- 45. Mashima R., Yamamoto Y., Yoshimura S. (1998) Reduction of phosphatidylcholine hydroperoxide by apolipoprotein A-I: purification of the hydroperoxide-reducing proteins from human blood plasma. J. Lipid Res. 39, 1133–1140 [PubMed] [Google Scholar]
- 46. Wu Z., Wagner M. A., Zheng L., Parks J. S., Shy J. M., 3rd, Smith J. D., Gogonea V., Hazen S. L. (2007) The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction. Nat. Struct. Mol. Biol. 14, 861–868 [DOI] [PubMed] [Google Scholar]
- 47. Segrest J. P., Jones M. K., Klon A. E., Sheldahl C. J., Hellinger M., De Loof H., Harvey S. C. (1999) A detailed molecular belt model for apolipoprotein A-I in discoidal high density lipoprotein. J. Biol. Chem. 274, 31755–31758 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.