Abstract
After more than 10 years of absence, in 2008 rabies re-emerged and spread in wild foxes in north-eastern Italy. In order to control the infection and to minimize the risk of human exposure, three oral foxes vaccination campaigns were first carried out by manual distribution of baits between January and September 2009, followed by four emergency oral rabies vaccination (ORV) campaigns by aerial distribution in the affected regions starting in December 2009. Ordinary aerial ORV campaigns followed in spring and fall 2011 and 2012, although no cases were reported after February 2011. In our paper, we describe the main characteristics of the rabies epidemic that occurred in north-eastern Italy in 2008–2011, with particular focus on the innovative systems that were implemented to manage and evaluate the efficacy of the aerial ORV. The Italian experience in containing and eliminating rabies in less than 3 years may provide information and suggestions for countries affected by rabies, and sharing a similar geomorphological conformation as Italy.
Keywords: Sylvatic rabies, Rabies vaccination, Aerial distribution, Red fox, GIS
Introduction
Urban and sylvatic rabies affected central and southern Italy until 1973 when urban rabies was definitely eradicated. Nevertheless, sporadic occurrences in wild animals were recorded in north-eastern Italy since late 1960s.1 After those cases, northern Italy experienced several sylvatic rabies epidemic waves between mid 1970s and mid 1990s.2 The emergence of rabies in the area was likely related to the epidemiological situation in the adjoining countries (France, Switzerland, Austria, and Yugoslavia). The first wave lasted about 10 years (1977–1986), and the whole Alps area was involved in the epidemic. The following waves were more spatially limited, affecting predominantly the north-eastern region of Friuli Venezia Giulia (FVG) (1988–1989 and 1991–1995) and the province of Bolzano (1993–1994). The 1977–1995 epidemic waves counted a total of 3333 cases; of these 98.2% (3273) occurred in wild animals. Red foxes (Vulpes vulpes) accounted for the greatest part of the cases (87.5%, 2916 cases), followed by mustelids (9.4%, 313 infected animals) and wild ruminants (2.9%, 97 cases). The last rabies case was identified in a red fox in 1995, and from 1997 Italy was declared rabies free.
After more than 10 years of absence, on 17 October 2008, the National Reference Centre identified a rabid fox in the municipality of Resia, located in FVG (Table 1), close to the border with Slovenia. Partial sequencing of the isolated rabies virus (RABV) strains showed 100% sequence identity with RABV isolates in Slovenia, Croatia, and other West Balkan countries.3,4 Following detection of the disease in foxes and its rapid spread in that region, four oral rabies vaccination (ORV) campaigns were organized and conducted in FVG by manual distribution of vaccine baits (SAG2). Although these efforts allowed to reduce the rabies incidence in the core area close to the Slovenian border, the disease continued spreading westward.
Table 1. Italian regions involved in the 2008–2011 sylvatic rabies epidemic.
| Area | Date of first rabies case | Date of last rabies case | Total number of cases |
| Friuli Venezia Giulia (FVG) (Udine, Pordenone, Trieste provinces) | 17 October 2008 | 4 May 2010 | 58 |
| Veneto (Belluno province) | 23 October 2009 | 14 February 2011 | 216 |
| Trento province | 12 February 2010 | 27 August 2010 | 8 |
| Bolzano province | 6 May 2010 | 17 June 2010 | 5 |
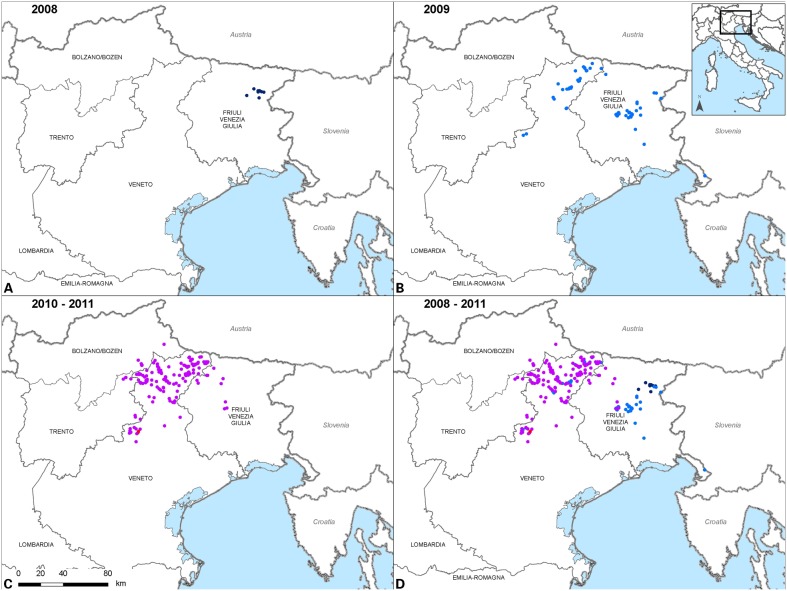
In November 2009, rabies was detected in the province of Belluno (Veneto region) (Table 1).5 As the disease was introduced in an unprotected fox population, within a month it rapidly spread to the Dolomiti Mountains (Fig. 1). The number of rabies cases dramatically increased in November–December 2009, reaching a peak in the first months of 2010, when the disease reached the autonomous provinces of Trento and Bolzano in February 2010 (Fig. 2). Rabies mostly circulated in the Veneto region, while only a limited number of rabies cases were observed in Trento and Bolzano provinces throughout the whole epidemic (five and eight in Bolzano and Trento provinces, respectively) (Table 1). The risk of further westward spreading of fox rabies not only to other previously rabies-free Italian regions but also to adjoining countries such as Austria and Switzerland forced competent authorities to take immediate actions. A first emergency ORV campaign was conducted in December 2009–January 2010 to address the problem. Successively emergency ORV campaigns were conducted in spring–summer and fall 2010. Spatial evaluation of both the ORV territorial coverage and the epidemiological situation was implemented through geographic information systems (GIS),6 and was integrated in the monitoring of the ORV campaigns, making it possible to promptly adapt the vaccination strategy.
Figure 1.
Geographical distribution of rabid foxes during the 2008–2011 rabies epidemic in Italy. Dots indicate the location where rabid foxes were found.
Figure 2.
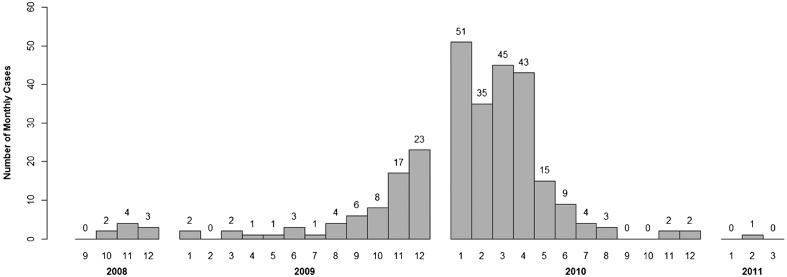
Number of rabid foxes per month during the 2008–2011 Italian rabies epidemic.
After April 2010, the number of observed cases started decreasing steeply, and only 15 cases were identified in May 2010; the last rabid fox was detected on 14 February 2011 (Fig. 2). A total of 287 cases were diagnosed in domestic and wild animals up to this date, out of which 243 (84.7%) were red foxes (V. vulpes), 10.1% of the cases occurred in other wild species (including 18 mustelids, 10 wild herbivores, and 1 rodent), and 5.2% were observed in domestic animals (three dogs, nine cats, two equines, and one bovine).No further rabies cases were diagnosed after 14 February 2011.
Emergency Orv of Foxes
Three ORV campaigns in foxes were conducted by manual distribution of vaccine baits exclusively in FVG between January and September 2009 (Table 2). Although the ORV campaigns allowed to reduce rabies incidence in the core area close to the Slovenian border, they did not prove to be sufficient to stop the westward spreading of the disease.
Table 2. Timetable of the vaccination activities carried out in 2009–2013. Dates of beginning and conclusion of the vaccination distribution and ORV efficacy monitoring are reported.
| Area | Nome | Activity | Beginning date | Conclusion date | Threshold altitude |
| FVG | First 2009 vacc. | Manual distr. | 1 January 2009 | 28 February 2009 | N/A |
| FVG | Second 2009 vacc. | Manual distr. | 1 May 2009 | 31 May 2009 | N/A |
| FVG | Third 2009 vacc. | Manual distr. | 1 September 2009 | 30 September 2009 | N/A |
| FVG | Fourth 2009 vacc. | Manual distr. | 1 December 2009 | 31 December 2009 | N/A |
| FVG, Veneto, Trento, Bolzano | First emerg. ORV | Aerial distr. | 28 December 2009 | 20 January 2010 | 1000 |
| Efficacy monit. | 19 February 2010 | 21 March 2010 | |||
| FVG, Veneto, Trento, Bolzano | Second emerg. ORV | Aerial distr. | 13 April 2010 | 28 June 2010 | 2300 |
| Efficacy monit. | 28 July 2010 | 27 August 2010 | |||
| FVG, Veneto, Trento, Bolzano | Third emerg. ORV | Aerial distr. | 23 August 2010 | 17 September 2010 | 2300 |
| Efficacy monit. | 17 October 2010 | 16 November 2010 | |||
| FVG, Veneto, Trento, Bolzano | Fourth emerg. ORV | Aerial distr. | 16 November 2010 | 16 December 2010 | 1500 |
| Efficacy monit. | 15 January 2011 | 14 February 2011 | |||
| FVG, Veneto, Trento, Bolzano | First ordinary ORV | Aerial distr. | 2 May 2011 | 25 May 2011 | 2300 |
| Efficacy monit. | 24 June 2011 | 24 July 2011 | |||
| FVG, Veneto, Trento, Bolzano | Second ordinary ORV | Aerial distr. | 14 November 2011 | 27 November 2011 | 1500 |
| Efficacy monit. | 27 December 2011 | 26 January 2012 | |||
| FVG, Veneto, Trento, Bolzano | Third ordinary ORV | Aerial distr. | 17 April 2012 | 19 May 2012 | 2300 |
| Efficacy monit. | 18 June 2012 | 18 July 2012 | |||
| FVG, Veneto, Trento, Bolzano | Fourth ordinary ORV | Aerial distr. | 13 November 2012 | 20 December 2012 | 1500 |
| Efficacy monit. | 19 January 2013 | 18 February 2013 | |||
| FVG | First preventive ORV | Aerial distr. | 11 May 2013 | 28 May 2013 | 2300 |
| Efficacy monit. | 27 June 2013 | 27 July 2013 |
FVG: Friuli Venezia Giulia; ORV: oral rabies vaccination.
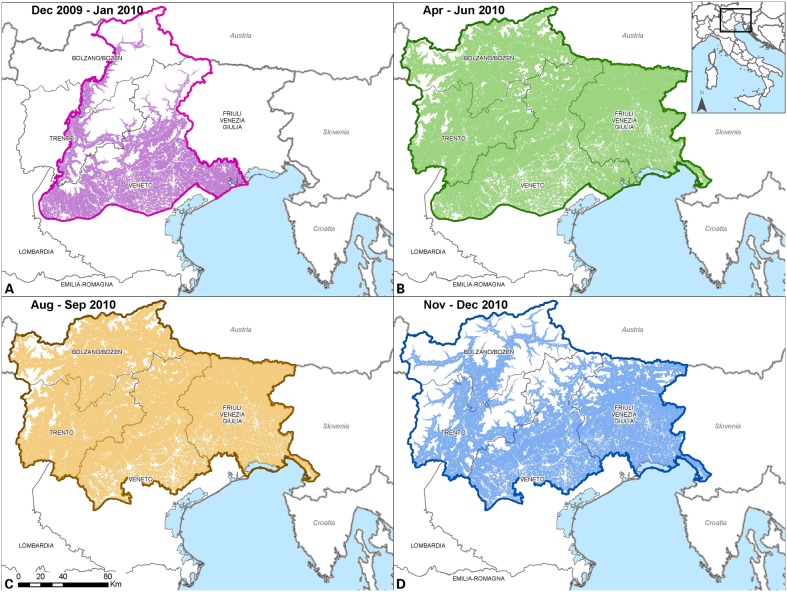
The emergence of rabies in Veneto region in 2009 prompted for more effective control measures to limit the further uncontrolled diffusion of the disease, and eventually eradicate it from the affected areas. Therefore between December 2009 and December 2010, four emergency ORV campaigns were planned and carried out by aerial distribution, according to the EU guidelines (Table 2, Fig. 3).7 Owing to the general geography of the Italian regions involved, helicopters were used for their superior manoeuvrability and better territorial coverage in hilly and mountainous areas. Further additional measures included (i) ban on hunting with dogs, (ii) ban on bringing dogs in woodland and in the countryside, (iii) increased passive surveillance on wildlife, and (iv) compulsory vaccination of dogs and domestic herbivores at pasture.8 Thirty days after the completion of each vaccination campaign, foxes were actively collected on a sample basis in order to carry out tests for the efficiency (level of bait uptake) and efficacy (antibody titration) of the ORV (Table 3).7 The deposition of tetracycline (the biomarker) in the jawbone of the tested foxes was considered as proof of bait uptake, and levels of antibody titre≧0.5 IU/ml, as determined in the blood clot collected from the heart, were considered when defining the population immunization.9
Figure 3.
Geographical extension and suitable areas of the emergency aerial vaccination. In the suitable vaccination areas are considered orography, hydrography, and presence of urbanized areas.
Table 3. Efficiency and efficacy of the aerial oral rabies vaccination (ORV) campaigns in foxes.
| ORV campaign | Type | Bait uptake(1) (%) | 95% C.I. | Herd immunity(2) (%) | 95% C.I. |
| December 2009–January 2010 | Emergency | 71.1 | 64.6–77.6 | 75.9 | 69.4–81.6 |
| May–June 2010 | Emergency | 63.2 | 59.7–66.9 | 69.1 | 65.1–73.0 |
| August–September 2010 | Emergency | 82.5 | 79.3–85.6 | 46.2 | 40.8–51.5 |
| November–December 2010 | Emergency | 91.8 | 88.3–95.3 | 77.8 | 73.8–81.4 |
| April–May 2011 | Ordinary | 67.7 | 61.7–73.6 | 74.6 | 70.7–78.3 |
| November–December 2011 | Ordinary | 86.5 | 82.3–90.6 | 46.3 | 42.3–49.3 |
| April–May 2012 | Ordinary | 92.7 | 89.5–96.0 | 60.1 | 56.1–64.1 |
| November–December 2012 | Ordinary | 87.4 | 83.8–90.4 | 49.5 | 44.5–54.5 |
| May 2013 | Preventive | 65.2 | 49.8–78.6 | 32.2 | 19.6–51.4 |
ORV: oral rabies vaccination.
Detection of biomarker in bones.
Percentage of tested animals with antibody titre > 0.5 UI/ml.
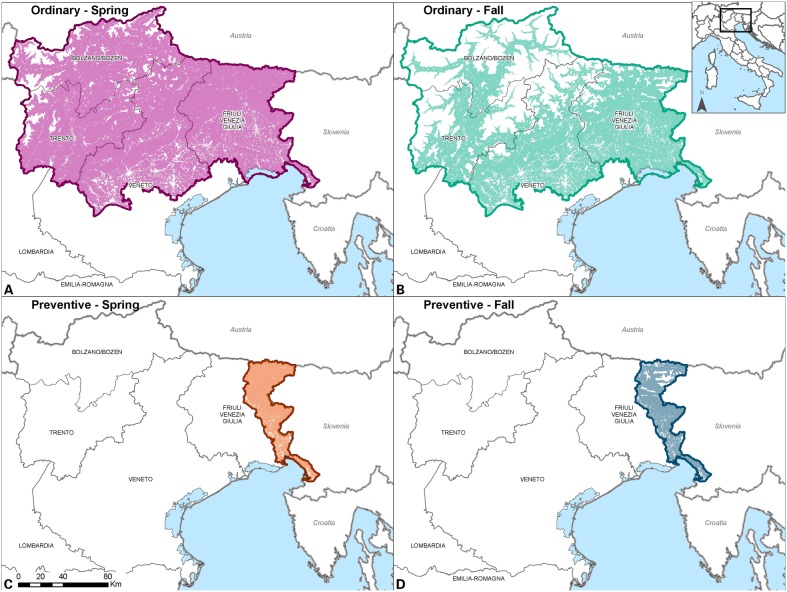
After the four emergency ORV campaigns, ordinary vaccination campaigns followed in 2011 and 2012 (Table 2). Similar to the previous ones, the ordinary ORV campaigns were performed by aerial distribution of vaccine baits with a biannual timeframe, in spring and fall. The ordinary vaccination campaigns covered the same areas as in the last two emergency campaigns, encompassing the mountainous part of Veneto region, and the whole territory of FVG, and Trento and Bolzano provinces (Table 2, Fig. 4).
Figure 4.
Geographical extension and suitable areas of the ordinary and preventive aerial vaccination.
Although the last rabies case was detected at the beginning of 2011, the acknowledged presence of rabies in the Balkan region prompted for continuing with ORV campaigns in Italy too. Therefore, preventive ORV campaigns were designed and approved for co-financing by the European Commission. The vaccination campaigns are planned to be carried out twice a year (spring and fall) for at least another 2 years (2013–2014). The area covered has been reduced with respect to the emergency and ordinary vaccination campaigns of 2009–2012, and consists of a 20 km belt on the border with Slovenia (Fig. 4). At the moment, the first preventive campaign has been concluded in May 2013 with procedures similar to those successfully applied in the 2009–2012 campaigns to distribute the vaccine baits (Table 2).
Constraints and Challenges to Rabies Control Measures Planning
Italy had already experienced rabies epizootics in the past, and was able to successfully eradicate the disease in the mid 1990s.2 Nevertheless due to the geographical features of the newly rabies-affected areas (i.e. orography, hydrography, and presence of urbanized areas), the limited experience with aerial distribution of vaccine baits, and the need for a timely emergency response, the 2009 outbreak posed new challenges for rabies control using ORV. Moreover, particular consideration was given to the stability of attenuated rabies vaccine viruses at low temperatures and the fact that the frozen vaccine cannot be released into the mouth cavity when the blister is punctured.10 The design and application of control measures and of vaccination programmes needed to take into account all of these geographical and environmental constraints. An approach strongly relying on GIS was selected, to manage all of the aspects related to the distribution of vaccine baits on the area,6 and geographical analyses allowed to re-modulate the vaccination activities on the basis of the distribution of rabies cases.11
Another important constraint for optimizing the vaccination activities consisted of the limited knowledge of the fox density and population dynamics in the affected areas. The latest census of fox population in north-eastern Italy traced back to the 1990s; however, drastic increases in the fox population density and activity were observed in the Alps region,12 stressing the impossibility to rely on old census data. This would impair both the definition of an optimal baits density to be released in the affected areas and the creation of fine-tuned models to study the effect of vaccination on the fox population. Following the European guidelines, a conservative target bait density for the vaccination operations was set to 20–30 baits/km2.7
To compensate for the lack of information on fox population, precise geographical data were collected for all the foxes gathered for assessing the epidemiological situation and the efficacy of the vaccination. For each animal submitted for rabies-related laboratory tests, information on the location of rabies positive and negative animals was based on administrative units where the foxes were found (province and municipality), other known geographical features (i.e. rivers, roads, forests, valleys, etc.) including their approximated distance to the place where the animals were found, or geographical coordinates, if available. This information was reported on a detailed map of the territory, at a nominal scale of 1:10 000, allowing the localization of the points of finding with sufficient accuracy. Data on the geographical location of animals were then transferred to the GIS software and combined with altitude data (extracted from a Digital Elevation Model) and laboratory tests. It was therefore possible to perform spatially explicit analyses to evaluate the efficacy of the vaccination, providing support for its possible re-modulation.
Geographic Information Systems Approach in Orv Planning
The particular conformation of the rabies-affected regions in 2009, with broad mountain ranges, narrow valleys, and several sparse urbanized areas, called for a precise definition of the vaccination areas and of the optimal flight paths for the helicopters in use. Adverse climatic conditions during fall and winter required to avoid repeated freeze-thaw cycles of the oral RABV vaccine, due to vaccine stability and assumption issues.10 Furthermore, zones where foxes were unlikely to be found or where baits could not be dropped, such as cities, were also excluded from the bait distribution.6 An average freezing point (i.e. the altitude at which 0°C is reached) for the vaccination season was calculated and delineated for exclusion, and only areas below this point were considered suitable for vaccination. During the first emergency campaign in 2009, the threshold altitude was set at 1000 m above the sea level (asl).5,6 For the 2010 spring emergency ORV (April–June 2010), the maximum altitude was increased to 1500 m asl. In addition, the findings of spatial analyses performed during a forced stop of the vaccination operations allowed to re-modulate the suitable vaccination area.11 Spatial analyses were performed during the 2010 spring emergency vaccination campaign to investigate whether and where hotspots of rabid foxes might have occurred within the 2009 winter vaccination area. The analyses were based on defining cluster of rabies cases that were found at a similar elevation asl. Interestingly, all the hotspots were located above 1500 m, which was considered as the threshold altitude of the spring vaccination campaign in 2010. All the rabid foxes in the clusters were located above 1500 m (min: 1585; max: 2000; mean: 1675.14) and were found between 11 April and 14 May 2010.11 The results provided strong evidence that rabies may circulate in areas at elevations considered as unfavourable fox habitat. This supported the decision to increase the threshold altitude for vaccination to 2300 m asl for the remaining part of the 2010 spring emergency campaign and the future spring/summer ORV campaigns, while campaigns to be performed in fall/winter were set at 1500 m.11
For all the vaccination campaigns, flight paths were designed to optimize the helicopter mission, taking into account the threshold altitude, the general orography, and the presence of large urban settlements in the areas to be vaccinated. The distance between routes was set to be 500 m, to increase the efficacy of vaccination.13 Flight paths were designed though GIS methods and provided to the pilots to be uploaded on the on-board GPS present in the aircraft. Whereas for the flatlands the precise routes were provided, for mountainous areas a different approach was adopted. Sub-areas were defined in relation to the flight autonomy of the helicopters, and geographical information on the contours of these areas was provided to the pilots, allowing them to concentrically follow this trace to cover the entire mountainous sub-area.6
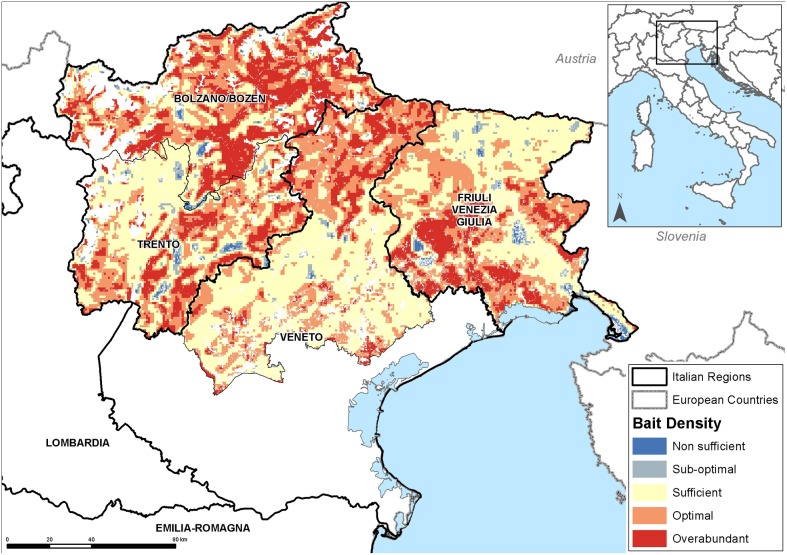
A satellite-navigated, computer-supported automatic system was used to adjust the spacing between the bait drops depending on the helicopter forward speed.14,15 This approach ensured a constant and homogeneous release of baits, allowing to record the precise geographical coordinates of each dropped baits on a file that was easily read with commonly used GIS software as ESRI® ArcMapTM (ESRI, Redlands, California, USA). Therefore, maps with the exact trace of baits distribution were easily created for a preliminary check of areas left uncovered. More reliable estimates of the territorial coverage were obtained by estimating the bait density at the soil level. A 1 km-step grid was superimposed over the vaccination areas, and the number of baits dropped into each cell; this estimate was then weighted by the fraction of suitable area present in the grid cell. Finally, the values estimated were reclassified to easily identify the areas where the bait density was not sufficient, scarce, or optimal6 (Fig. 5). The density analysis was performed on a daily basis to rapidly identify areas with non-optimal bait density and promptly intervene with complementary aerial redistribution of baits to reach the target density level. Bait density evaluation, performed at the end of the vaccination campaigns, allowed identifying areas where the bait density was still lower than optimal (e.g. in proximity to urban settlements), and therefore eligible for accessory manual bait distribution.
Figure 5.
Spatial evaluation of the bait territorial coverage; Spring Ordinary ORV 2012.
Conclusions
The risk of rabies introduction through the northern and eastern border regions of Italy has long been acknowledged. Infected foxes appeared indeed to have crossed the border from Slovenia and Austria during previous epidemics.2,3 Although the introduction of rabies into Italy in 2008 was not totally unexpected, the rapidity and extent of the disease spread were unpredictable, and ultimately proved the capacity of the Italian veterinary public health authority to promptly respond to a new and rapidly growing threat. Passive surveillance systems may not be completely reliable in areas with non-recent history of rabies or at the beginning of an epidemic.16,17 Once a sufficiently large fraction of the population was infected, the virus become more likely to be noticed and reported. An effective surveillance system is paramount, especially in areas exposed at a higher risk of disease introduction, as it allows a rapid detection of rabies and consequently prompts implementation of control measures.
Our review focussed essentially on how the control measures were programmed and put into action, with particular attention to the aerial vaccination campaigns. The technical and scientific support included the automated aerial bait distribution, which allowed to reduce the effort for obtaining a satisfying territorial coverage, considering both temporal and the personnel involvements, and the implementation of a well-structured GIS system for the management and evaluation of the distribution activities. The collection of ancillary information on the foxes found dead (e.g. geographical location) allowed to obtain an interesting set of data to better evaluate not only the disease dynamics but also the efficacy of the vaccination campaigns. Furthermore, the spatial analyses performed on these data allowed to observe a vertical wave of rabies spreading towards areas usually considered to be less favourable for foxes. The results could also be exploited in the definition of control measures also in other countries, which share similar orographic and environmental characteristics as Italy. The identification of rabies foci at high altitudes contrasts previous common assumption of limited fox activities in mountainous areas18 and stresses that sources of re-infection may persist if vaccination activities are not accurately planned.
The intensive efforts that were put in the vaccination campaigns and other supporting control measures proved to be highly effective. The proportion of immunized foxes in the populations resulted satisfying after almost all the vaccination campaigns (Table 3), proving effective in avoiding the uncontrolled spread of the disease. The low percentage of immunized fox in the 2013 preventive ORV likely derives from the scant number of foxes that were delivered during the monitoring activities, which also explains the wide confidence intervals (Table 3), more than a low efficacy of the vaccination.
The last rabid fox was detected on 14 February 2011. Since in the last 2 years, no further cases were identified during the surveillance programme for rabies in wildlife, according to Article 8.10.2 of Chapter 8.10 of the OIE Terrestrial Animal Health Code,19 the rabies-free status has been acknowledged to Italy as of 14 February 2013.
The 2008–2011 Italian experience with rabies stresses that re-emergence of neglected diseases is still an important issue for the public veterinary health services, deserving appropriate attention and contingency measures. The presence of the infection in the wild fox populations in near areas bordering with Italy, such as the Balkan region,18,20 needs to be not overlooked. The preventive vaccination to be carried out in 2013–2014 has been planned within this context, to immunize the fox populations on the border with Slovenia, in order to decrease the probability of uncontrolled spread in the event of a new rabies introduction.
Acknowledgments
The authors wish to thank the veterinary services of FVG, Veneto and of the provinces of Trento and Bolzano, for their collaboration and the organization of the on-field operations.
References
- 1.Bellani L, Gagliardi G, Irsara A, Mantovani A, Prosperi S. Situation and control of rabies in Italy. Bull Off Int Epizoot. 1976;86:243–52. [Google Scholar]
- 2.Mutinelli F, Stankov S, Hristovski M, Seimenis A, Theoharakou H, Vodopija I. Paris: OIE; 2004. Rabies in Italy, Yugoslavia, Croatia, Bosnia, Slovenia, Macedonia, Albania and Greece; pp. 93–118. In: King A, Fooks A, Aubert M, Wandeler A, editors. Historical perspectives of rabies in Europe and the Mediterranean Basin. [Google Scholar]
- 3.De Benedictis P, Gallo T, Iob A, Coassin R, Squecco G, Ferri G, et al. Emergence of fox rabies in north-eastern Italy. Euro Surveill. 2008;13(45):pii):19033. [PubMed] [Google Scholar]
- 4.Fusaro A, Monne I, Salomoni A, Angot A, Trolese M, Ferrè N, et al. The introduction of fox rabies into Italy (2008–2011) was due to two viral genetic groups with distinct phylogeographic patterns. Infect Genet Evol. 2013;17:202–9. doi: 10.1016/j.meegid.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 5.Capello K, Mulatti P, Comin A, Gagliazzo L, Guberti V, Citterio C, et al. Impact of emergency oral rabies vaccination of foxes in northeastern Italy, 28 December 2009–20 January 2010: preliminary evaluation. Euro Surveill. 2010;15(28):pii):19617. [PubMed] [Google Scholar]
- 6.Mulatti P, Ferrè N, Patregnani T, Bonfanti L, Marangon S. Geographical information systems in the management of the 2009–2010 emergency oral anti-rabies vaccination of foxes in north-eastern Italy. Geospat Health. 2011;5(2):217–26. doi: 10.4081/gh.2011.174. [DOI] [PubMed] [Google Scholar]
- 7.European Commission. The oral vaccination of foxes against rabies – report of the Scientific Committee on Animal Health and Animal Welfare, Europe; 2002. Adopted on 23 October 2002 [Google Scholar]
- 8.Italian Ministry of Health. Ministerial Decree of 26 November 2009, containing measures to prevent rabies spreading in the north-eastern Italian Regions. OJ Gen Ser. 2009285 [Google Scholar]
- 9. OIE. Rabies, Ch. 2.1.13. Manual of diagnostic tests and vaccines for terrestrial animals, 7th edn. Paris, France: OIE; 2012. [Google Scholar]
- 10.Pastoret PP, Brochier B, Languet B, Duret C, Chappuis G, Desmettre P. Stability of recombinant vaccinia-rabies vaccine in veterinary use. Dev Biol Stand. 1996;87:245–9. [PubMed] [Google Scholar]
- 11.Mulatti P, Müller T, Bonfanti L, Marangon S. Emergency oral rabies vaccination of foxes in Italy in 2009–2010: identification of residual rabies foci at higher altitudes in the Alps. Epidemiol Infect. 2012;140:591–8. doi: 10.1017/S0950268811001282. [DOI] [PubMed] [Google Scholar]
- 12.Gloor S, Bontadina F, Hegglin D, Deplazes P, Breitenmoser U. The rise of urban fox populations in Switzerland. Mamm Biol. 2001;66:155–64. [Google Scholar]
- 13.Thulke H-H, Selhorst T, Müller T, Wyszomirski T, Müller U, Breitenmoser U. Assessing anti-rabies baiting – what happens on the ground? BMC Infect Dis. 2004;4:9. doi: 10.1186/1471-2334-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vos A, Mührke H, Holzhofer E, Gschwender P, Schuster P. A satellite navigated and computer-supported fully automatic system for distributing oral vaccine-baits against rabies: SURVIS. Proc. 12th Rabies American Conf., Peterborough, Ont., Canada; 2011. p. 66. [Google Scholar]
- 15.Selhorst T, Müller T, Bätza HJ. Epidemiological analysis of setbacks in oral vaccination in the final stage of fox rabies elimination in densely populated areas in Germany. Dev Biol (Basel). 2006;125:127–32. [PubMed] [Google Scholar]
- 16.Bacon P. Consequences of unreported rabies. J Environ Manage. 1981;13(2):195–200. [Google Scholar]
- 17.Townsend SE, Lembo T, Cleaveland S, Meslin FX, Miranda ME, Putra AA, et al. Surveillance guidelines for disease elimination: a case study of canine rabies. Comp Immunol Microbiol Infect Dis. 2013;36(3):249–61. doi: 10.1016/j.cimid.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hostnik P, Toplak I, Barlic-Maganja D, Grom J, Bidovec A. Control of rabies in Slovenia. J Wildl Dis. 2006;42(2):459–65. doi: 10.7589/0090-3558-42.2.459. [DOI] [PubMed] [Google Scholar]
- 19. OIE. Chapter 8.10. Infection with rabies virus. Terrestrial animal health code, 21st edn. Paris, France: OIE; 2012. [Google Scholar]
- 20. World Health Organization. WHO rabies bulletin Europe – 2010. Geneve, Switzerland: World Health Organization. 2010. [Google Scholar]