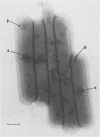
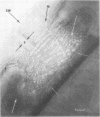
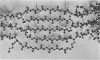
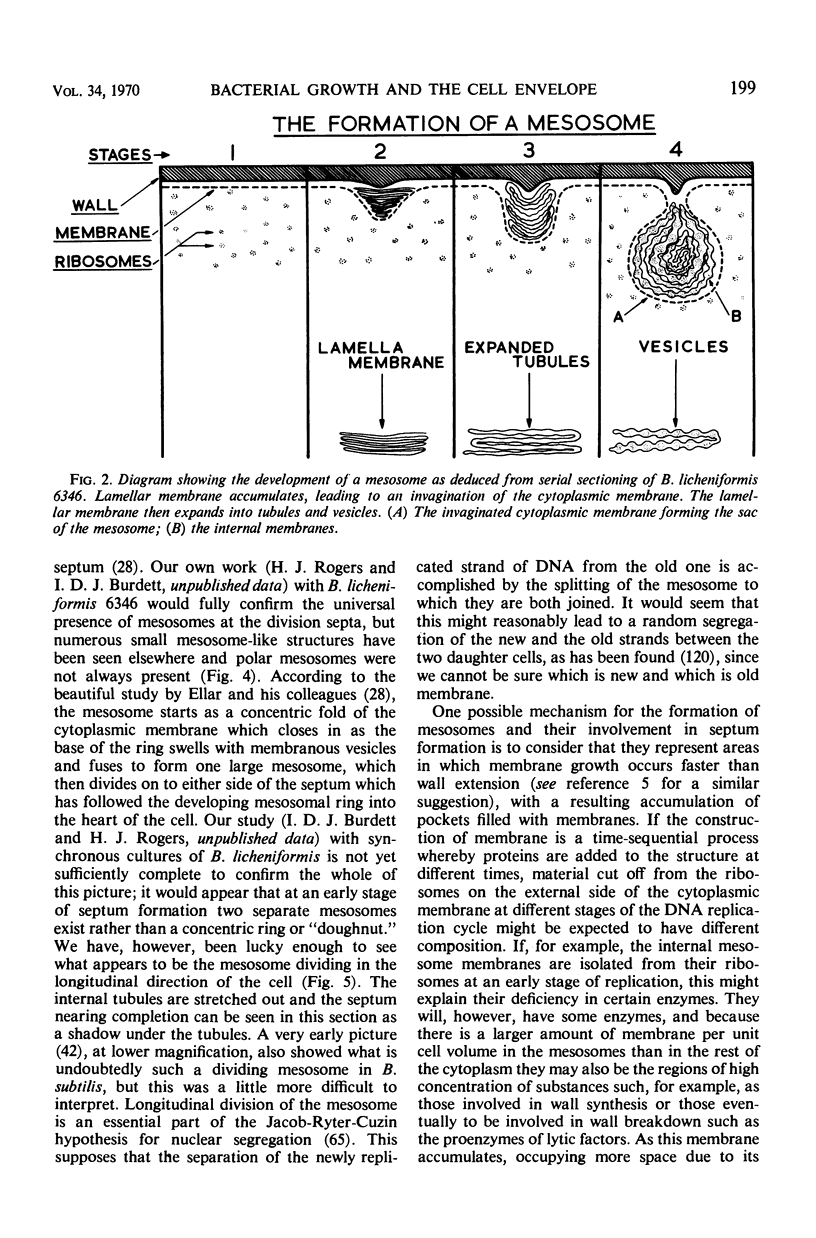
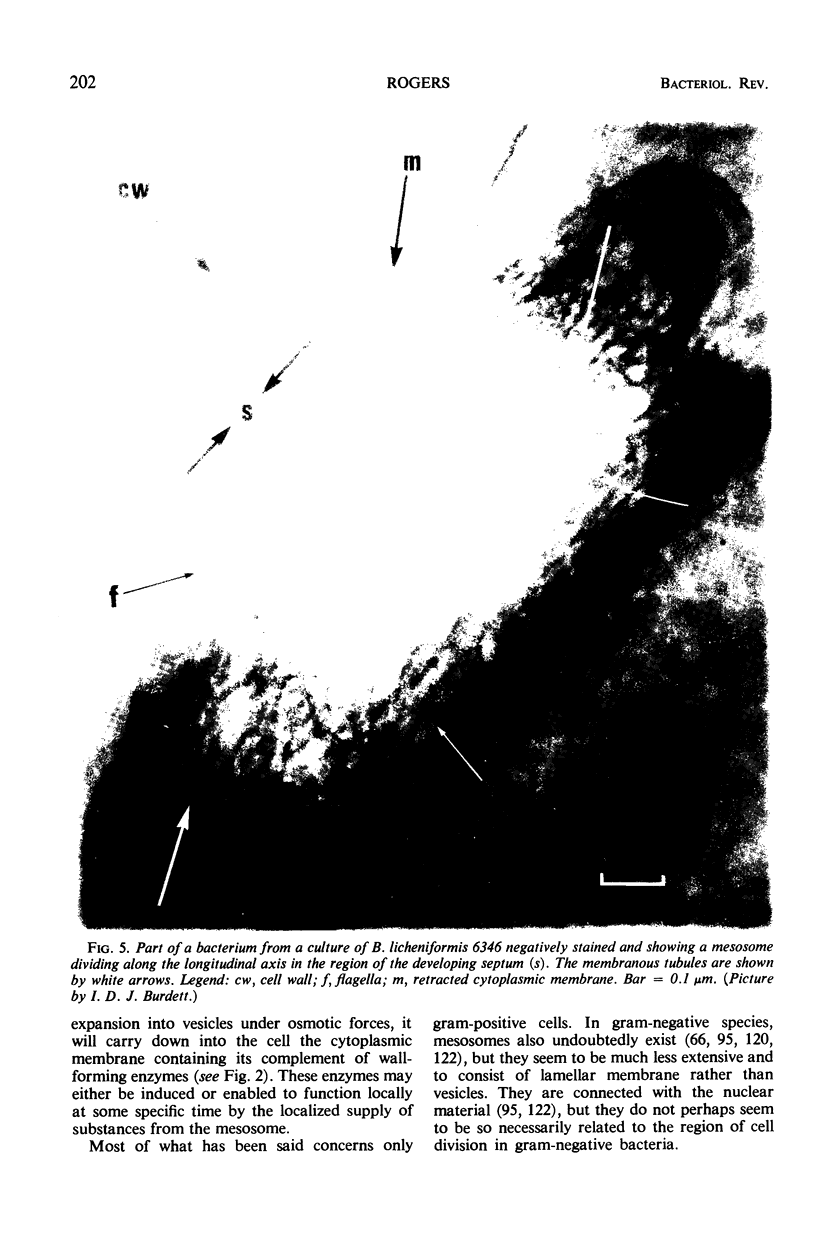
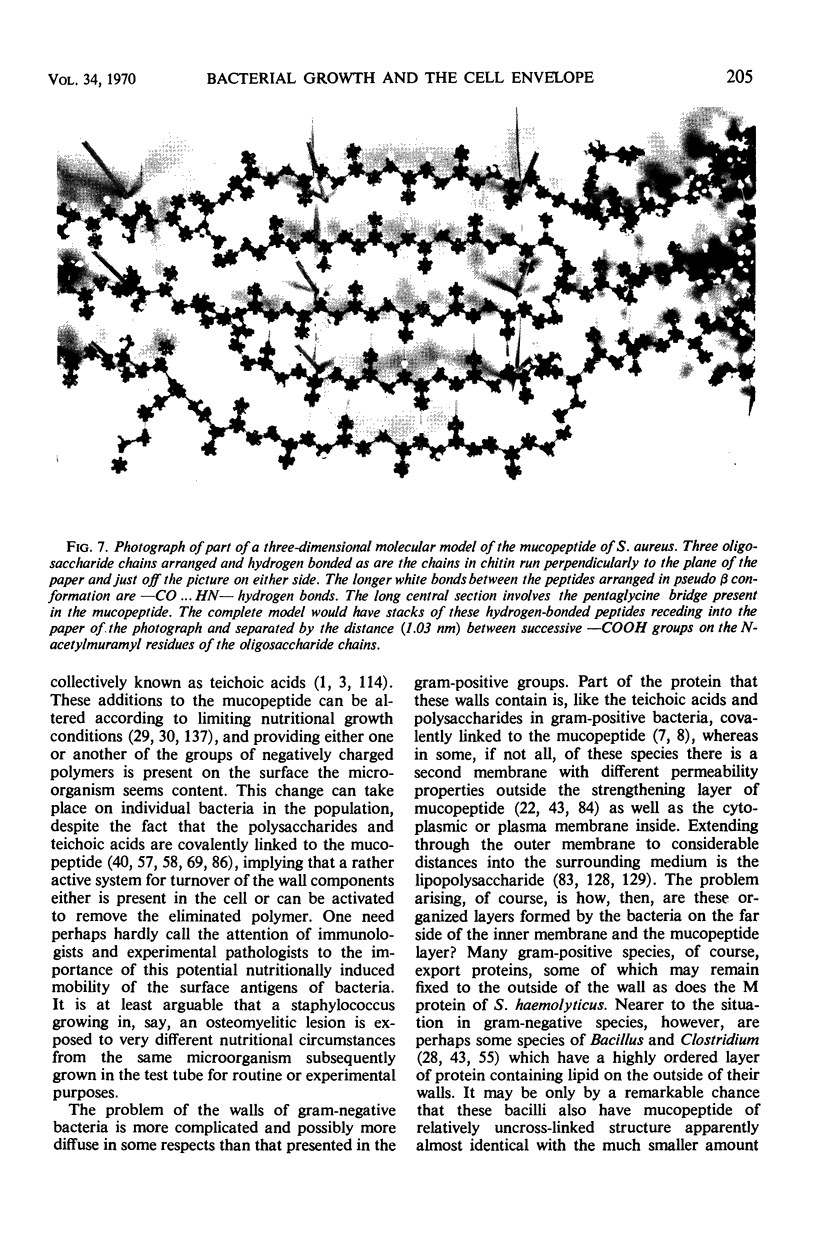
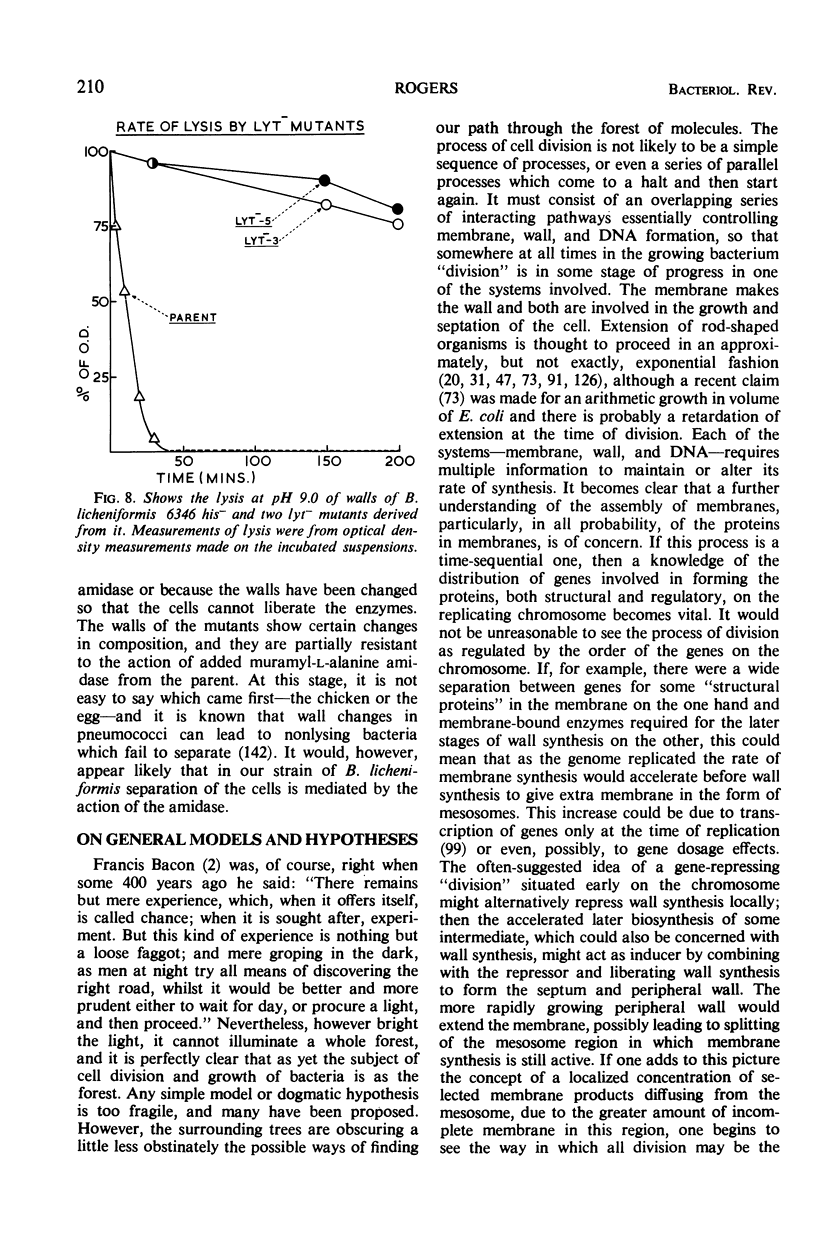
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald A. R., Baddiley J. The teichoic acids. Adv Carbohydr Chem Biochem. 1966;21:323–375. doi: 10.1016/s0096-5332(08)60320-3. [DOI] [PubMed] [Google Scholar]
- Baddiley J. The Leeuwenhoek lecture, 1967. Teichoic acids and the molecular structure of bacterial walls. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):331–348. doi: 10.1098/rspb.1968.0043. [DOI] [PubMed] [Google Scholar]
- Bayer M. E. Adsorption of bacteriophages to adhesions between wall and membrane of Escherichia coli. J Virol. 1968 Apr;2(4):346–356. doi: 10.1128/jvi.2.4.346-356.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazill G. W. Lethal unbalanced growth in bacteria. Nature. 1967 Oct 28;216(5113):346–349. doi: 10.1038/216346a0. [DOI] [PubMed] [Google Scholar]
- Beachey E. H., Cole R. M. Cell wall replication in Escherichia coli, studies by immunofluorescence and immunoelectron microscopy. J Bacteriol. 1966 Oct;92(4):1245–1251. doi: 10.1128/jb.92.4.1245-1251.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan R. J., Mendelson N. H. Initial characterization of a temperature-sensitive rod--mutant of Bacillus subtilis. J Bacteriol. 1969 Dec;100(3):1316–1321. doi: 10.1128/jb.100.3.1316-1321.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Brown W. C., Young F. E. Dynamic interactions between cell wall polymers, extracellular proteases and autolytic enzymes. Biochem Biophys Res Commun. 1970 Feb 20;38(4):564–568. doi: 10.1016/0006-291x(70)90618-2. [DOI] [PubMed] [Google Scholar]
- Burdett I. D., Rogers H. J. Modification of the appearance of mesosomes in sections of Bacillus licheniformis according to the fixation procedures. J Ultrastruct Res. 1970 Feb;30(3):354–367. doi: 10.1016/s0022-5320(70)80068-5. [DOI] [PubMed] [Google Scholar]
- CHAPMAN G. B., HILLIER J. Electron microscopy of ultra-thin sections of bacteria I. Cellular division in Bacillus cereus. J Bacteriol. 1953 Sep;66(3):362–373. doi: 10.1128/jb.66.3.362-373.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUNG K. L., HAWIRKO R. Z., ISAAC P. K. CELL WALL REPLICATION. I. CELL WALL GROWTH OF BACILLUS CEREUS AND BACILLUS MEGATERIUM. Can J Microbiol. 1964 Feb;10:43–48. doi: 10.1139/m64-007. [DOI] [PubMed] [Google Scholar]
- COLE R. M. CELL WALL REPLICATION IN SALMONELLA TYPHOSA. Science. 1964 Feb 21;143(3608):820–822. doi: 10.1126/science.143.3608.820. [DOI] [PubMed] [Google Scholar]
- COLE R. M., HAHN J. J. Cell wall replication in Streptococcus pyogenes. Science. 1962 Mar 2;135(3505):722–724. doi: 10.1126/science.135.3505.722. [DOI] [PubMed] [Google Scholar]
- COLLINS J. F., RICHMOND M. H. Rate of growth of Bacillus cereus between divisions. J Gen Microbiol. 1962 Apr;28:15–33. doi: 10.1099/00221287-28-1-15. [DOI] [PubMed] [Google Scholar]
- Chung K. L. Autoradiographic studies of bacterial cell wall replication. I. Cell wall growth of Bacillus cereus in the presence of chloramphenicol. Can J Microbiol. 1967 Apr;13(4):341–350. doi: 10.1139/m67-046. [DOI] [PubMed] [Google Scholar]
- Clark D. J. Regulation of deoxyribonucleic acid replication and cell division in Escherichia coli B-r. J Bacteriol. 1968 Oct;96(4):1214–1224. doi: 10.1128/jb.96.4.1214-1224.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. J. The regulation of DNA replication and cell division in E. coli B-r. Cold Spring Harb Symp Quant Biol. 1968;33:823–838. doi: 10.1101/sqb.1968.033.01.094. [DOI] [PubMed] [Google Scholar]
- Cole R. M. Symposium on the fine structure and replication of bacteria and their parts. 3. Bacterial cell-wall replication followed by immunofluorescence. Bacteriol Rev. 1965 Sep;29(3):326–344. doi: 10.1128/br.29.3.326-344.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Forsberg C., Matula T. I., Buckmire F. L., MacLeod R. A. Nutrition and metabolism of marine bacteria. XVI. Formation of protoplasts, spheroplasts, and related forms from a gram-negative marine bacterium. J Bacteriol. 1967 Nov;94(5):1764–1777. doi: 10.1128/jb.94.5.1764-1777.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D. Control of cell division in Escherichia coli: experiments with thymine starvation. J Bacteriol. 1969 Oct;100(1):260–268. doi: 10.1128/jb.100.1.260-268.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS M. R., STEVENS R. W. FINE STRUCTURE OF LISTERIA MONOCYTOGENES. J Bacteriol. 1963 Sep;86:414–428. doi: 10.1128/jb.86.3.414-428.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERRINGTON F. P., POWELL E. O., THOMPSON N. GROWTH CHARACTERISITICS OF SOME GRAM-NEGATIVE BACTERIA. J Gen Microbiol. 1965 Apr;39:109–123. doi: 10.1099/00221287-39-1-109. [DOI] [PubMed] [Google Scholar]
- Earhart C. F., Tremblay G. Y., Daniels M. J., Schaechter M. DNA replication studied by a new method for the isolation of cell membrane-DNA complexes. Cold Spring Harb Symp Quant Biol. 1968;33:707–710. doi: 10.1101/sqb.1968.033.01.079. [DOI] [PubMed] [Google Scholar]
- Ellar D. J., Freer J. H. The isolation and characterisation of mesosome material from Micrococcus lysodeikticus. J Gen Microbiol. 1969 Nov;58(3):vii–vii. [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G., Slepecky R. A. Fine structure of Bacillus megaterium during synchronous growth. J Bacteriol. 1967 Oct;94(4):1189–1205. doi: 10.1128/jb.94.4.1189-1205.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood D. C., Tempest D. W. Control of teichoic acid and teichuronic acid biosyntheses in chemostat cultures of Bacillus subtilis var. niger. Biochem J. 1969 Jan;111(1):1–5. doi: 10.1042/bj1110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood D. C., Tempest D. W. Influence of growth environment on the cell wall anionic polymers in some Gram-positive bacteria. J Gen Microbiol. 1969 Aug;57(3):xv–xv. [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhs G. W. Symposium on the fine structure and replication of bacteria and their parts. I. Fine structure and replication of bacterial nucleoids. Bacteriol Rev. 1965 Sep;29(3):277–293. doi: 10.1128/br.29.3.277-293.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHUYSEN J. M., TIPPER D. J., STROMINGER J. L. STRUCTURE OF THE CELL WALL OF STAPHYLOCOCCUS AUREUS, STRAIN COPENHAGEN. IV. THE TEICHOIC ACID-GLYCOPEPTIDE COMPLEX. Biochemistry. 1965 Mar;4:474–485. doi: 10.1021/bi00879a016. [DOI] [PubMed] [Google Scholar]
- GLAUERT A. M., BRIEGER E. M., ALLEN J. M. The fine structure of vegetative cells of Bacillus subtilis. Exp Cell Res. 1961 Jan;22:73–85. doi: 10.1016/0014-4827(61)90087-8. [DOI] [PubMed] [Google Scholar]
- GRULA E. A. Cell division in a species of Erwinia. I. Inhibition of division by D-amino acids. J Bacteriol. 1960 Sep;80:375–385. doi: 10.1128/jb.80.3.375-385.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan A. T. Studies on in vitro replication of Bacillus subtilis DNA. Cold Spring Harb Symp Quant Biol. 1968;33:45–57. doi: 10.1101/sqb.1968.033.01.010. [DOI] [PubMed] [Google Scholar]
- Garrett A. J. The effect of magnesium ion deprivation on the synthesis of mucopeptide and its precursors in Bacillus subtilis. Biochem J. 1969 Nov;115(3):419–430. doi: 10.1042/bj1150419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K., Murray R. G. Fine structure of Listeria monocytogenes in relation to protoplast formation. J Bacteriol. 1967 Jan;93(1):411–426. doi: 10.1128/jb.93.1.411-426.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- Giesbrecht P., Ruska H. Uber Veränderungen der Feinstrukturen von Bakterien unter der Einwirkung von Chloramphenicol. Klin Wochenschr. 1968 Jun 1;46(11):575–582. doi: 10.1007/BF01747836. [DOI] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- Gross J. D., Karamata D., Hempstead P. G. Temperature-sensitive mutants of B. subtilis defective in DNA synthesis. Cold Spring Harb Symp Quant Biol. 1968;33:307–312. doi: 10.1101/sqb.1968.033.01.034. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. J., Marr A. G., Painter P. R. Kinetics of growth of individual cells of Escherichia coli and Azotobacter agilis. J Bacteriol. 1967 Feb;93(2):605–617. doi: 10.1128/jb.93.2.605-617.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hash J. H., Davies M. C. Electron Microscopy of Staphylococcus aureus Treated with Tetracycline. Science. 1962 Nov 16;138(3542):828–829. doi: 10.1126/science.138.3542.828. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E., Pierucci O. Cell division during inhibition of deoxyribonucleic acid synthesis in Escherichia coli. J Bacteriol. 1968 May;95(5):1627–1633. doi: 10.1128/jb.95.5.1627-1633.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter C. E. Sequence of bacterial reproduction. Annu Rev Microbiol. 1969;23:223–238. doi: 10.1146/annurev.mi.23.100169.001255. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970 Feb;101(2):643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highton P. J. An electron microscopic study of cell growth and mesosomal structure of Bacillus licheniformis. J Ultrastruct Res. 1969 Jan;26(1):130–147. doi: 10.1016/s0022-5320(69)90040-9. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Leadbetter E. R. Comparative ultrastructure of selected aerobic spore-forming bacteria: a freeze-etching study. Bacteriol Rev. 1969 Jun;33(2):346–378. doi: 10.1128/br.33.2.346-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C., Pavlik J. G., Rogers H. J., Tanner P. J. Organization of polymers in the cell walls of some bacilli. Nature. 1968 Aug 10;219(5154):642–644. doi: 10.1038/219642a0. [DOI] [PubMed] [Google Scholar]
- Hughes R. C. The cell wall of Bacillus licheniformis N.C.T.C. 6346. Linkage between the teichuronic acid and mucopeptide components. Biochem J. 1970 Apr;117(3):431–439. doi: 10.1042/bj1170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst A., Stubbs J. M. Electron microscopic study of membranes and walls of bacteria and changes occurring during growth initiation. J Bacteriol. 1969 Mar;97(3):1466–1479. doi: 10.1128/jb.97.3.1466-1479.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M. Unlinking of cell division from deoxyribonucleic acid replication in a temperature-sensitive deoxyribonucleic acid synthesis mutant of Escherichia coli. J Bacteriol. 1969 Sep;99(3):842–850. doi: 10.1128/jb.99.3.842-850.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- Jacob F., Ryter A., Cuzin F. On the association between DNA and membrane in bacteria. Proc R Soc Lond B Biol Sci. 1966 Mar 22;164(995):267–278. doi: 10.1098/rspb.1966.0029. [DOI] [PubMed] [Google Scholar]
- KAYE J. J., CHAPMAN G. B. CYTOLOGICAL ASPECTS OF ANTIMICROBIAL ANTIBIOSIS. III. CYTOLOGICALLY DISTINGUISHABLE STAGES IN ANTIBIOTIC ACTION OF COLISTIN SULFATE ON ESCHERICHIA COLI. J Bacteriol. 1963 Sep;86:536–543. doi: 10.1128/jb.86.3.536-543.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Hall E. A. The linkage between the polysaccharide and mucopeptide components of the cell wall of Lactobacillus casei. Biochem J. 1965 Aug;96(2):302–309. doi: 10.1042/bj0960302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Vesk M., Work E. Relation between excreted lipopolysaccharide complexes and surface structures of a lysine-limited culture of Escherichia coli. J Bacteriol. 1966 Oct;92(4):1206–1217. doi: 10.1128/jb.92.4.1206-1217.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Ensign J. C. Isolation and chemical structure of the peptidoglycan of Spirillum serpens cell walls. J Bacteriol. 1968 Jan;95(1):201–210. doi: 10.1128/jb.95.1.201-210.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C., Tipper D. J., Strominger J. L. Sphere-rod morphogenesis in Arthrobacter crystallopoietes. I. Cell wall composition and polysaccharides of the peptidoglycan. J Bacteriol. 1967 Sep;94(3):734–740. doi: 10.1128/jb.94.3.734-740.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C., Tipper D. J., Strominger J. L. Sphere-rod morphogenesis in Arthrobacter crystallopoietes. II. Peptides of the cell wall peptidoglycan. J Bacteriol. 1967 Sep;94(3):741–750. doi: 10.1128/jb.94.3.741-750.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitschek H. E. Growth during the bacterial cell cycle: analysis of cell size distribution. Biophys J. 1969 Jun;9(6):792–809. doi: 10.1016/S0006-3495(69)86418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILLY M. D., CLARKE P. H., MEADOW P. M. THE ACCUMULATION OF NUCLEOTIDES BY ESCHERICHIA COLI STRAIN 26-26. J Gen Microbiol. 1963 Jul;32:103–116. doi: 10.1099/00221287-32-1-103. [DOI] [PubMed] [Google Scholar]
- LOMINSKI I., CAMERON J., WYLLIE G. Chaining and unchaining Streptococcus faecalis; a hypothesis of the mechanism of bacterial cell separation. Nature. 1958 May 24;181(4621):1477–1477. doi: 10.1038/1811477a0. [DOI] [PubMed] [Google Scholar]
- LOMINSKI I., GRAY S. Inhibition of lysozyme by 'Suramin'. Nature. 1961 Nov 18;192:683–683. doi: 10.1038/192683a0. [DOI] [PubMed] [Google Scholar]
- Landman O. E., Ryter A., Fréhel C. Gelatin-induced reversion of protoplasts of Bacillus subtilis to the bacillary form: electron-microscopic and physical study. J Bacteriol. 1968 Dec;96(6):2154–2170. doi: 10.1128/jb.96.6.2154-2170.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark K. G. Initiation and control of DNA synthesis. Annu Rev Biochem. 1969;38:569–604. doi: 10.1146/annurev.bi.38.070169.003033. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J., ROGERS H. J. The incorporation of amino acids into the cell-wall mucopeptide of staphylococci and the effect of antibiotics on the process. Biochem J. 1959 Aug;72:654–662. doi: 10.1042/bj0720654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarotti G., Apirion D., Schlessinger D. Selection of sucrose-dependent Escherichia coli to obtain envelope mutants and fragile cultures. Science. 1966 Aug 19;153(3738):892–894. doi: 10.1126/science.153.3738.892. [DOI] [PubMed] [Google Scholar]
- Mendelson N. H., Gross J. D. Characterization of a temperature-sensitive mutant of Bacillus subtilis defective in deoxyribonucleic acid replication. J Bacteriol. 1967 Nov;94(5):1603–1608. doi: 10.1128/jb.94.5.1603-1608.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergenhagen S. E., Bladen H. A., Hsu K. C. Electron microscopic localization of endotoxic lipopolysaccharide in gram-negative organisms. Ann N Y Acad Sci. 1966 Jun 30;133(2):279–291. doi: 10.1111/j.1749-6632.1966.tb52371.x. [DOI] [PubMed] [Google Scholar]
- Miura T., Mizushima S. Separation by density gradient centrifugation of two types of membranes from spheroplast membrane of Escherichia coli K12. Biochim Biophys Acta. 1968 Jan 3;150(1):159–161. doi: 10.1016/0005-2736(68)90020-5. [DOI] [PubMed] [Google Scholar]
- Munoz E., Ghuysen J. M., Heymann H. Cell walls of Streptococcus pyogenes, type 14. C polysaccharide-peptidoglycan and G polysaccharide-peptidoglycan complexes. Biochemistry. 1967 Dec;6(12):3659–3670. doi: 10.1021/bi00864a007. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H., Stocker B. A. How genes determine the structure of the Salmonella lipopolysaccharide. J Gen Microbiol. 1969 Aug;57(3):vi–vi. [PubMed] [Google Scholar]
- NATHENSON S. G., STROMINGER J. L. Effects of penicillin on the biosynthesis of the cell walls of Escherichia coli and Staphylococcus aureus. J Pharmacol Exp Ther. 1961 Jan;131:1–6. [PubMed] [Google Scholar]
- Nanninga N. Structural features of mesosomes (chondrioids) of Bacillu subtilis after freeze-etching. J Cell Biol. 1968 Nov;39(2):251–263. doi: 10.1083/jcb.39.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J. Structure and biosynthesis of the bacterial cell wall. Annu Rev Biochem. 1969;38:501–538. doi: 10.1146/annurev.bi.38.070169.002441. [DOI] [PubMed] [Google Scholar]
- PARK J. T. Uridine-5'-pyrophosphate derivatives. III. Amino acid-containing derivatives. J Biol Chem. 1952 Feb;194(2):897–904. [PubMed] [Google Scholar]
- PELZER H. MUCOPEPTIDHYDROLASEN IN ESCHERICHIA COLI B. I. NACHWEIS UND WIRKUNGSSPEZIFITAET. Z Naturforsch B. 1963 Nov;18:950–956. [PubMed] [Google Scholar]
- Painter P. R., Marr A. G. Mathematics of microbial populations. Annu Rev Microbiol. 1968;22:519–548. doi: 10.1146/annurev.mi.22.100168.002511. [DOI] [PubMed] [Google Scholar]
- Pontefract R. D., Bergeron G., Thatcher F. S. Mesosomes in Escherichia coli. J Bacteriol. 1969 Jan;97(1):367–375. doi: 10.1128/jb.97.1.367-375.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D. Relationship between the latent form and the active form of the autolytic enzyme of Streptococcus faecalis. J Bacteriol. 1969 Nov;100(2):617–624. doi: 10.1128/jb.100.2.617-624.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS H. J., GARRETT A. J. THE INTERRELATIONSHIP BETWEEN MUCOPEPTIDE AND RIBITOL TEICHOIC ACID FORMATION AS SHOWN BY THE EFFECT OF INHIBITORS. Biochem J. 1965 Jul;96:231–243. doi: 10.1042/bj0960231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS H. J., MANDELSTAM J. Inhibition of cell-wall-muco-peptide formation in Escherichia coli by benzylpenicillin and 6-[D(-)-alpha-aminophenylacetamido]penicillanic acid (ampicillin). Biochem J. 1962 Aug;84:299–303. doi: 10.1042/bj0840299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS H. J. The surface structures of bacteria. Biochem Soc Symp. 1963;22:55–104. [PubMed] [Google Scholar]
- RORSCH A., EDELMAN A., van der KAMP, COHEN J. A. Phenotypic and genotypic characterization of radiation sensitivity in Escherichia coli B. Biochim Biophys Acta. 1962 Aug 20;61:278–289. doi: 10.1016/0926-6550(62)90090-7. [DOI] [PubMed] [Google Scholar]
- RYTER A., JACOB F. ETUDE AU MICROSCOPE 'ELECTRONIQUE DE LA LIAISON ENTRE NOYAU ET M'ESOSOME CHEZ BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1964 Sep;107:384–400. [PubMed] [Google Scholar]
- RYTER A., LANDMAN O. E. ELECTRON MICROSCOPE STUDY OF THE RELATIONSHIP BETWEEN MESOSOME LOSS AND THE STABLE L STATE (OR PROTOPLAST STATE) IN BACILLUS SUBTILIS. J Bacteriol. 1964 Aug;88:457–467. doi: 10.1128/jb.88.2.457-467.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaveley D. A., Rogers H. J. Some enzymic activities and chemical properties of the mesosomes and cytoplasmic membranes of Bacillus licheniformis 6346. Biochem J. 1969 Jun;113(1):67–79. doi: 10.1042/bj1130067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaveley D. A. The isolation and characterisation of cytoplasmic membranes and mesosomes of Bacillus licheniformis 6346. Biochem Biophys Res Commun. 1968 Mar 27;30(6):649–655. doi: 10.1016/0006-291x(68)90562-7. [DOI] [PubMed] [Google Scholar]
- Remsen C. C. Fine structure of the mesosome and nucleoid in frozen-etched Bacillus subtilis. Arch Mikrobiol. 1968;61(1):40–47. doi: 10.1007/BF00704290. [DOI] [PubMed] [Google Scholar]
- Rogers H. J., McConnell M., Burdett I. D. Cell wall or membrane mutants of Bacillus subtilis and Bacillus licheniformis with grossly deformed morphology. Nature. 1968 Jul 20;219(5151):285–288. doi: 10.1038/219285a0. [DOI] [PubMed] [Google Scholar]
- Rogers H. J., McConnell M., Burdett I. D. The isolation and characterization of mutants of Bacillus subtilis and Bacillus licheniformis with disturbed morphology and cell division. J Gen Microbiol. 1970 May;61(2):155–171. doi: 10.1099/00221287-61-2-155. [DOI] [PubMed] [Google Scholar]
- Rogers H. J., McConnell M. The role of L-glutamine in the phenotypic change of a rod mutant derived from Bacillus subtilis 168. J Gen Microbiol. 1970 May;61(2):173–181. doi: 10.1099/00221287-61-2-173. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. The inhibition of mucopeptide synthesis by benzylpenicillin in relation to irreversible fixation of the antibiotic by staphylococci. Biochem J. 1967 Apr;103(1):90–102. doi: 10.1042/bj1030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg B. H., Cavalieri L. F. Shear and the melting of DNA: an especially sensitive portion of the E. coli genome. Biophys J. 1968 Oct;8(10):1138–1145. doi: 10.1016/s0006-3495(68)86545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg B. H., Cavalieri L. F. Shear sensitivity of the E. coli genome: multiple membrane attachment points of the E. coli DNA. Cold Spring Harb Symp Quant Biol. 1968;33:65–72. doi: 10.1101/sqb.1968.033.01.012. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- Ryter A. Association of the nucleus and the membrane of bacteria: a morphological study. Bacteriol Rev. 1968 Mar;32(1):39–54. doi: 10.1128/br.32.1.39-54.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A., Jacob F. Etude morphologique de la liaison du noyau à la membrane chez E. coli et chez les protoplastes de B. subtilis. Ann Inst Pasteur (Paris) 1966 Jun;110(6):801–812. [PubMed] [Google Scholar]
- SCHAECHTER M., WILLIAMSON J. P., HOOD J. R., Jr, KOCH A. L. Growth, cell and nuclear divisions in some bacteria. J Gen Microbiol. 1962 Nov;29:421–434. doi: 10.1099/00221287-29-3-421. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Shands J. W. Localization of Somatic Antigen on Gram-Negative Bacteria by Electron Microscopy. J Bacteriol. 1965 Jul;90(1):266–270. doi: 10.1128/jb.90.1.266-270.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shands J. W. Localization of somatic antigen on gram-negative bacteria using ferritin antibody conjugates. Ann N Y Acad Sci. 1966 Jun 30;133(2):292–298. doi: 10.1111/j.1749-6632.1966.tb52372.x. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Cheney M. C. Autolytic enzyme system of Streptococcus faecalis. V. Nature of the autolysin-cell wall complex and its relationship to properties of the autolytic enzyme of Streptococcus faecalis. J Bacteriol. 1969 Jun;98(3):1199–1207. doi: 10.1128/jb.98.3.1199-1207.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Martin J. T. Autolytic enzyme system of Streptococcus faecalis. IV. Electron microscopic observations of autolysin and lysozyme action. J Bacteriol. 1968 Nov;96(5):1803–1810. doi: 10.1128/jb.96.5.1803-1810.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Pooley H. M., Thompson J. S. Autolytic enzyme system of Streptococcus faecalis. 3. Localization of the autolysin at the sites of cell wall synthesis. J Bacteriol. 1967 Nov;94(5):1525–1530. doi: 10.1128/jb.94.5.1525-1530.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D. Symposium on the fine structure and replication of bacteria and their parts. IV. Unbalanced cell-wall synthesis: autolysis and cell-wall thickening. Bacteriol Rev. 1965 Sep;29(3):345–358. doi: 10.1128/br.29.3.345-358.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry. 1967 Apr;6(4):1054–1065. doi: 10.1021/bi00856a014. [DOI] [PubMed] [Google Scholar]
- Smith D. W., Hanawalt P. C. Properties of the growing point region in the bacterial chromosome. Biochim Biophys Acta. 1967 Dec 19;149(2):519–531. doi: 10.1016/0005-2787(67)90180-3. [DOI] [PubMed] [Google Scholar]
- Sueoka N., Quinn W. G. Membrane attachment of the chromosome replication origin in Bacillus subtilis. Cold Spring Harb Symp Quant Biol. 1968;33:695–705. doi: 10.1101/sqb.1968.033.01.078. [DOI] [PubMed] [Google Scholar]
- Szulmajster J., Arnaud M., Young F. E. Some properties of a sporulating Bacillus subtilis mutant containing heavy DNA. J Gen Microbiol. 1969 Jul;57(1):1–10. doi: 10.1099/00221287-57-1-1. [DOI] [PubMed] [Google Scholar]
- Tempest D. W., Dicks J. W., Ellwood D. C. Influence of growth condition on the concentration of potassium in Bacillus subtilis var. niger and its possible relationship to cellular ribonucleic acid, teichoic acid and teichuronic acid. Biochem J. 1968 Jan;106(1):237–243. doi: 10.1042/bj1060237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike J., Park J. T. A method for demonstrating the stepwise addition of glycine from transfer RNA into the murein precursor of Staphylococcus aureus. Biochem Biophys Res Commun. 1969 Jun 6;35(5):642–647. doi: 10.1016/0006-291x(69)90452-5. [DOI] [PubMed] [Google Scholar]
- Tipper D. J. Mechanism of autolysis of isolated cell walls of Staphylococcus aureus. J Bacteriol. 1969 Feb;97(2):837–847. doi: 10.1128/jb.97.2.837-847.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L., Ensign J. C. Structure of the cell wall of Staphylococcus aureus, strain Copenhagen. VII. Mode of action of the bacteriolytic peptidase from Myxobacter and the isolation of intact cell wall polysaccharides. Biochemistry. 1967 Mar;6(3):906–920. doi: 10.1021/bi00855a035. [DOI] [PubMed] [Google Scholar]
- Tipper D. J. Structures of the cell wall peptidoglycans of Staphylococcus epidermidis Texas 26 and Staphylococcus aureus Copenhagen. II. Structure of neutral and basic peptides from hydrolysis with the Myxobacter al-1 peptidase. Biochemistry. 1969 May;8(5):2192–2202. doi: 10.1021/bi00833a061. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay G. Y., Daniels M. J., Schaechter M. Isolation of a cell membrane-DNA-nascent RNA complex from bacteria. J Mol Biol. 1969 Feb 28;40(1):65–76. doi: 10.1016/0022-2836(69)90296-4. [DOI] [PubMed] [Google Scholar]
- VAN ITERSON W. Some features of a remarkable organelle in Bacillus subtilis. J Biophys Biochem Cytol. 1961 Jan;9:183–192. doi: 10.1083/jcb.9.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Walker J. R., Pardee A. B. Evidence for a relationship between deoxyribonucleic acid metabolism and septum formation in Escherichia coli. J Bacteriol. 1968 Jan;95(1):123–131. doi: 10.1128/jb.95.1.123-131.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D., Dworkin M., Tipper D. J. Peptidoglycan of Myxococcus xanthus: structure and relation to morphogenesis. J Bacteriol. 1968 Jun;95(6):2186–2197. doi: 10.1128/jb.95.6.2186-2197.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H. DNA synthesis during germination of Bacillus subtilis spores. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1476–1483. doi: 10.1073/pnas.53.6.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Iterson W. Symposium on the fine structure and replication of bacteria and their parts. II. Bacterial cytoplasm. Bacteriol Rev. 1965 Sep;29(3):299–325. doi: 10.1128/br.29.3.299-325.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Iterson W., den Kamp J. A. Bacteria-shaped gymnoplasts (protoplasts) of Bacillus subtilis. J Bacteriol. 1969 Jul;99(1):304–315. doi: 10.1128/jb.99.1.304-315.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]