Abstract
It is proposed that targeting the environmental host that harbour ‘superbugs’ is an effective strategy in our fight against infectious diseases.
Keywords: Superbugs, Infectious diseases, Antimicrobial resistance
Introduction
Drug-resistant microbes, i.e. superbugs, represent one of the gravest threats in the history of medicine. Because free-living amoebae such as Acanthamoeba spp. are heralded as the Trojan Horse of the microbial world,1 harbouring viral, bacterial, fungal. and protist pathogens, and provide them with protection against disinfectants, it has been suggested that targeting the host that harbour ‘terror cells’ may be an effective strategy in eliminating superbugs in hospitals.2 This area of research is both timely and topical. It is strongly believed that research is needed to demonstrate the efficacy of targeting the host that harbour ‘terror cells’ in our fight against infectious diseases.
Antibiotic-resistant ‘superbugs’
At present, a major concern in bacterial infections is their increasing resistance to the many available antibacterial agents. The resistance may be intrinsic (bacteria carry genes that allow them to survive exposure to the antibiotics), or acquired as part of natural selection (random changes or mutations occur in the genes of individual bacterial cells), and/or gene that carries antibiotic resistance are passed between bacteria. The result is creation of bacteria that carry resistance genes to different antibiotics, termed as superbugs.3–5 Antibiotic resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA), Klebsiella pneumoniae, Streptococcus pneumonia, Clostridium difficile, Neisseria gonorrhoeae, Acinetobacter baumannii, Mycobacterium tuberculosis, vancomycin-resistant Enterococcus, Salmonella, Escherichia coli, and Pseudomonas aeruginosa are some examples of the ‘superbugs’.3–5 According to the Centers for Disease Control and Prevention, superbugs infect at least two million people per year in the USA alone killing at least 23 000 people as a direct result of these infection.6
Superbugs present a significant challenge to human health, especially for developing countries where antibiotic-resistant bacteria may go unnoticed as observed in the case of metallo-beta-lactamase-1 containing Klebsiella pneumoniae bacterium commonly known as New Delhi Metallo-1.7–9 Globally, we are at the mercy of superbugs and heading to a pre-antibiotic era.10 Although many developed nations including UK, USA, Greece, Finland, Germany, Iceland, Japan, and The Netherlands have curtailed the use of antibiotics, and showed decrease in antibiotic resistance frequency following implementation of antibiotic control measures,11 other developing countries (e.g. Pakistan, India, Bangladesh) have ignored the emerging threat and continue the unregulated use of antibiotics, i.e. accessible over the counter, without a prescription, and with no drug resistance monitoring mechanisms in place.12,13
Because of the rising trends of infections in the community, schools and public places are being affected.14 The Centers for Disease Control and Prevention15 estimates that 12% of MRSA infections are now community-associated, which are on the rise compared with healthcare-associated MRSA infections. An example of community-associated infection was seen in June 2012, when an outbreak of Legionnaires’ disease was reported in Edinburgh, UK, leading to a distillery shutting down its cooling towers. The number of cases in this deadly outbreak stands at 95 people; three people have died so far.16 Not surprisingly, the World Health Organization has highlighted superbugs as the greatest threat to human health that we face today.17 The challenge is to develop new methods of preventing, diagnosing, and treating antibiotic-resistant infections.
Superbugs and Super-problems
The disturbing fact is that even the most robust mechanisms to eradicate superbugs had limited success.18,19 For example, a patient was diagnosed with a common bacterium, K. pneumoniae at the National Institutes of Health (NIH) Research Hospital.18 It appeared to be highly resistant to antibiotics. In an attempt to stop its spread to other patients, all transmission-based precautions were taken involving enhanced contact isolation, including strict enforcement of fastidious hand hygiene before entering and upon leaving patient rooms, universal use of gowns and gloves for all staff and visitors entering patient rooms, restrictions on patient activity outside rooms, restrictions on staff and visitor traffic, dedicating equipment for single-patient use (when feasible), extensive cleaning of shared equipment, and double-cleaning of vacated rooms with bleach. Despite all these efforts, it ended up spreading to other patients, and one by one, other patient’s started to show up with the same infection. Subsequent intervention measures included dismantling drainage systems, building new walls, and introduction of a robot to spray hydrogen peroxide to sterilize the empty rooms after patients had left. Overall, it spread to 17 other patients, six of whom died. Today, approximately 6% of all hospitals in the USA are fighting outbreaks with K. pneumoniae infection alone, let alone other superbugs such as MRSA, S. pneumonia, C. difficile, N. gonorrhoeae, A. baumannii, M. tuberculosis, vancomycin-resistant Enterococcus, Salmonella, E. coli, and P. aeruginosa. Similarly, approximately 8% of UK hospital patients suffer from hospital-acquired infections, while more than 37 000 people have died in the UK in the last decade alone from hospital-acquired infections caused by superbugs such as C. difficile and MRSA.18 According to the Center for Disease Control and Prevention, C. difficile and MRSA are killing 14 000 and 19 000 people respectively annually in the USA alone.15 The question arises that if superbugs can evade the robust health systems at the NIH that are equipped to deal with such a threat, the situation in the developing countries must be dire. During the outbreak at the NIH, it was not due to the inability of staff to respond rapidly to this threat, but possibly due to the ability of the bacterium to adapt and resist many available antibiotics.13,19 Unfortunately, despite well-established guidelines and robust interventional strategies, superbugs continue to spread, as evidenced by the NIH outbreak.18 Additionally, there is a lack of effective antimicrobials. For example, in four cases of the NIH outbreak, antibiotics were completely ineffective as the bacteria were resistant to all known antibiotics—a frightening scenario. There is an urgent need to do more research to develop interventional strategies to eliminate superbugs, as well as figuring out how to prevent their transmission. Other than fighting antibiotic-resistant infections, the situation has become an ethical battle for hospitals, as patients coming to hospitals for common disease or surgeries may be exposed to far-deadly and sometimes untreatable infections due to superbugs.20
Alternative Strategies to Combat Superbugs: Thinking out of the Box
Our approach to prevent infections in patients and how to keep infections from transmitting from one patient to another, has been focused on people who actually come close to the patient and touch them, e.g. the doctors, the nurses, and the paraprofessionals.13,19,20 For example, the present strategy to stop bugs from spreading in the hospital is the use of disinfectants, and new anti-bacterials for hand washing and surface cleaning are regularly introduced to the market. By far, the most cost-effective disinfectant used commonly is chlorine bleach (a 5% solution of sodium hypochlorite), which is effective against many common pathogens, including many antibiotic-resistant bacterial strains.21 However, it is not effective against several cyst-forming protists such as Acanthamoeba22 and Cryptosporidium.23
Perhaps new drugs or disinfectants targeting bacteria are not the only weapon against antibiotic-resistant infections. For the development of novel prevention and infection control measures, we can no longer focus on a single etiological agent, where the link between exposure and infection is clearly defined. On the contrary, the disease is being considered as having multiple and confounding factors with different aetiologies and their environmental niches specific to the different affected subpopulations to enable us to fully understand the dynamics of disease outbreaks. Thus, more research is needed to study niches that provide sanctuary to the pathogenic microbes to enable us to design preventative measures, and identification of factors that allow transmission of pathogens to and between patients for innovative interventional measures.
Targeting the Amoeba Host that Harbours ‘superbugs’ should Be an Additive Strategy Against the Spread of Infectious Diseases
In a landmark observation of Rowbotham,24 it was observed that Legionella pneumophila can survive and multiply inside a free-living amoeba, Acanthamoeba castellanii. More recently, it has been shown that Acanthamoeba can harbour a variety of microbes including viruses, yeast, protists, and bacteria.1 The ability of pathogenic bacteria such as MRSA or L. pneumophila to survive and multiply inside Acanthamoeba suggests that amoeba acts not only as a vector, but also as a reservoir.
Acanthamoeba is one of the most ubiquitous protists. It has two stages in its life cycle: a vegetative trophozoite stage and a dormant cyst stage. Cysts are highly resistant to physical, chemical (antibiotics and biocides), and radiological conditions, and can be air-borne. For example, several studies have documented the acquisition of amoebic resistance to commonly used biocides (e.g. polyhexamethylene biguanide, benzalkonium chloride, propamidine isethionate, pentamidine isethionate, dibromopropamidine isethionate, hydrogen peroxide, hydrochloric acid, and chlorhexidine diacetate)25–27 as cysts are formed and mature.28 Acanthamoeba has gained increasing attention by the scientific and the medical community due to its ability to produce serious, and fatal, human and animal infections,29–32 while it can act as a host for a variety of microbes including viruses (mimivirus, coxsackieviruses, adenoviruses, poliovirus, echovirus, enterovirus, vesicular stomatitis virus, etc.), bacteria (Aeromonas, Bacillus, Bartonella, Burkholderia, Campylobacter, Chlamydophila, Coxiella, E. coli, Flavobacterium, Legionella, Listeria, Staphylococcus, Mycobacteria, Pasteurella, Prevotella, Porphyromonas, Pseudomonas, Rickettsia, Salmonella, Shigella, Vibrio, Streptomyces, etc.), protists (Cryptosporidium, Toxoplasma gondii), and yeast/fungi (Cryptococcus, Blastomyces, Sporothrix, Histoplasma, Exophiala, etc.).33,34 From a public health perspective, the ability of Acanthamoeba to harbour microbial pathogens, protect them in hostile environments (biocides, antibiotics), and assist in their environmental distribution and transmission to susceptible hosts is of particular concern. This ‘Trojan horse’ property of amoebae may contribute indirectly to human and animal infections caused by the pathogenic bacteria.33,34
The precise nature of this symbiosis is not clear and it is hypothesized that the fragility of non-spore-forming bacteria to survive hostile conditions and inability to disperse to favourable environments may have led to their evolutionary need to associate with a hardy host and/or a biological reservoir to remain viable intracellularly in the face of harsh conditions. The hardiness of Acanthamoeba cysts can protect microbial pathogens against harsh environments (antibiotics and biocides) and enable pathogenic microbes to survive in a variety of hostile conditions such as the use of chlorine-based disinfectants in a hospital setting, which may lead to their transmission to susceptible hosts to establish infection. Based on this, it is reasonable to speculate that Acanthamoeba may be a source of superbugs, and may play a role in transmission of superbugs to humans. To this end, research is needed to determine the prevalence of bacteria-containing Acanthamoeba in hospitals that will shed light on their potential role as transmission vehicles.
The Way Forward: How to Implement Anti-Acanthamoebic Strategies to Prevent Superbugs Attack
As Acanthamoeba harbour superbugs and form cysts that can remain viable for over 20 years while maintaining their pathogenicity35,36 and they can be air-borne,37 it poses a peculiar challenge to target this pathogen. Fortunately, the eradication of Acanthamoeba from contact lens disinfectants has been highlighted for the past few decades. Among a plethora of contact lens disinfecting solutions and constituents tested, many of them are effective in killing Acanthamoeba trophozoites but limited cysticidal properties.38,39 For example, studies have tested the effects of Alcon Opti-Clean II, Alcon Opti-Free Express, Alcon Opti-Free Replenish, AMO Complete MoisturePlus, AMO UltraCare, Bausch & Lomb Boston Simplus, Bausch & Lomb ReNu MoistureLoc, Bausch & Lomb ReNu MultiPlus, Ciba Vision Clear Care, Ciba Vision AQuify, Kirkland Signature Multipurpose Solution, and other chlorine-based solutions. It was found that none of the aforementioned contact lens disinfecting solutions showed effects against Acanthamoeba cysts.40–43 In contrast, contact lens disinfecting solutions based on hydrogen peroxide have proven to be most effective in killing Acanthamoeba trophozoites and effective against 1-week-old cysts.40–43 Among other benefits, hydrogen peroxide is considered relatively safe as an oxidizing agent against microbial pathogens by the US Food and Drug Administration. For example, 35% hydrogen peroxide is recommended to be used to prevent infection transmission in the hospital environment, and hydrogen peroxide vapour is registered with the US Environmental Protection Agency as a sporicidal sterilant, suggesting its cysticidal properties against Acanthamoeba. At this concentration, hydrogen peroxide can disinfect inanimate objects and may also target air-borne cysts. This may explain recent findings in which it was found that enhanced cleaning with the hydrogen peroxide vapour reduced by 80% a patient’s chances of becoming colonized by antibiotic-resistant bacteria.44,45 The multidrug-resistant organisms were found on room surfaces in 21% of rooms tested, but mostly in rooms that did not undergo enhanced cleaning. These findings suggested that acquiring a multidrug-resistant organism from a room previously occupied by a patient with multidrug-resistant organism is less likely, when the room was disinfected with hydrogen peroxide vapour than with standard methods.40,45 We postulate that the usefulness of enhanced cleaning with hydrogen peroxide vapour is highly effective in eradicating superbugs due to its anti-Acanthamoebic properties,30–43 in addition to anti-bacterial properties. In contrast, traditional hand-cleaning and mopping with other chlorine-based bleaching agents have limited effects against hardy protists cysts harbouring superbugs. More recently, ozone and hydrogen peroxide vapour gas mixture into a room were used to completely sterilize everything, including floors, walls, drapes, mattresses, chairs, and other surfaces.46 It was claimed that it is far more effective in killing bacteria than wiping down a room.46 Here, we rationalize that the efficacy of hydrogen peroxide disinfecting solutions is not just due to their antibacterial properties, but also attributed to their anti-amoebic properties.40–43 As hydrogen peroxide has been shown to be effective against bacterial pathogens and the host amoeba, it is likely that both terror cells and the host that harbour them are targeted.
However, hydrogen peroxide is only used for terminal cleaning, once infection outbreak has been dealt with. It can have associated weaknesses such as instability and toxicity. Thus, other agents that are safe and effective with broad-spectrum anti-bacterial and anti-protist properties should be explored that address the needs of both developed nations, as well as of the developing countries. In particular, the latter have poor resources, but have rampant use of antibiotics, and are breeding grounds for superbugs. Other known anti-Acanthamoeba compounds are chlorhexidine or polyhexamethylene biguanide,1 which could be effective and should increase the shelf-life of the anti-bacterial disinfecting solutions, but they would prove ineffective against air-borne cysts. Other disinfecting solutions are Betadine® (10% povidone-iodine solution), which are broad-spectrum antiseptic, and possesses anti-viral, anti-bacterial, anti-protist, and anti-fungal properties.47 As opposed to hydrogen peroxide disinfecting solution that is effective against 1-week-old cysts but not 2-week-old cysts, povidone-iodine is highly effective against 2-week-old cysts. In addition to anti-viral, anti-bacterial, anti-protist, anti-fungal properties, and used routinely as a water disinfectant, povidone-iodine disinfecting solution has a better anti-Acanthamoebic activity compared with chlorhexidine.47 The use of high levels of radiation, such as concentrated ultraviolet light, X-ray, and gamma irradiation-fitted devices, may have the potential of eradicating amoeba plus bacteria from the operating theatres and intensive care units. Further research is needed to develop disinfecting strategies of anti-bacterial and anti-Acanthamoebic properties with long-term shelf-life, and are safe, cost-effective, and practical, to be applicable in a healthcare setting especially in developing countries. Such disinfectants should be tested against Acanthamoeba cysts containing superbugs, intracellularly, as well as superbugs alone in vitro. Because amoebae are shown to promote persistence of epidemic strains of multiple-drug resistant bacteria,48 it is expected that disinfecting solutions effective in killing both amoebae as well as superbugs would be of value for further testing in clinical settings.
What Makes Acanthamoeba Cysts So Hardy?
What makes Acanthamoeba cysts hardy is that they have the ability to resist chemotherapeutic agents, harsh environmental and physiological conditions, and high levels of radiation, making it a challenge to target ‘superbugs’ residing within? A likely explanation is that the majority of treatments/agents target functional aspects of living cells than affecting structure, as it is easier to terminate a function, than to demolish a structure. For example, beta-lactam antibiotics inhibit cell wall biosynthesis in bacteria, aminoglycosides inhibit cell membrane synthesis, quinolones interfere with DNA replication, tetracyclines are protein synthesis inhibitors, metronidazole interferes with electron transport and alters DNA in many intestinal protists, sulfonamide interferes with folate metabolism in Plasmodium and Toxoplasma, and azoles interfere with biosynthesis of ergosterol, leading to fungal membrane dysfunction. However, cysts are the dormant phase of the life cycle of Acanthamoeba with minimal metabolic activity (Fig. 1). How can we kill something that is already dead or pretends to be dead? Additionally, double-walled structure of cyst provides the physical barrier, resulting in amoebic resistance to biocides. Cysts only come to life (vegetative trophozoite phase), once harsh environments/conditions/agents have been lifted. Absence of the known function of Acanthamoeba cysts (e.g. protein synthesis, DNA/RNA synthesis, cell wall synthesis, or other metabolic pathways) counters our first line of attack and it has impeded disinfecting/therapeutic interventions, as well as facilitated transmission to susceptible hosts. The only other target left is its structure, which is far more difficult to destroy. Unfortunately, there is little information of the biochemical details of Acanthamoeba cysts, their proteins/polysaccharides/lipids, as well as the synthetic pathways induced during encystment (cyst wall formation). It is a double-walled structure (Fig. 2) that provides a permeability barrier (protective shell) for the trophozoite. Cyst walls are partly made of cellulose and a 21 kDa cyst-specific protein.49–51 More recently, gas chromatography combined with mass spectrometry determined the glycosyl composition of cyst walls of A. castellanii. It was shown that cyst walls are made of 4-linked glucopyranose (likely to be cellulose) (22.2%), but also unidentified sugars such as 3-linked galactopyranose (28.6%), 5-linked xylofuranose/4-linked xylopyranose (7.0%), mannopyranose (3.2%), 2,4-linked gluco or galactopyranose (4.4%), 3,4-linked galactopyranose (13.6%), 3,4-linked glucopyranose (6.0%), 4,6-linked mannopyranose (7.8%), and 3,6-linked galactopyranose (7.2%).52 Further research will determine the biochemical composition and linkage analyses of Acanthamoeba cysts, which will explain their resistance to harsh physical, chemical, and radiological conditions. Understanding the composition of Acanthamoeba cysts will offer insights, not only into cytodifferentiation, but also importantly into specific targets to attack the protective wall assembly. Consequently, a complete understanding of composition, structure, and permeability of the cyst wall will be valuable in determining the appropriate disinfection strategies to target the inhabitants within, i.e. ‘superbugs’.
Figure 1.
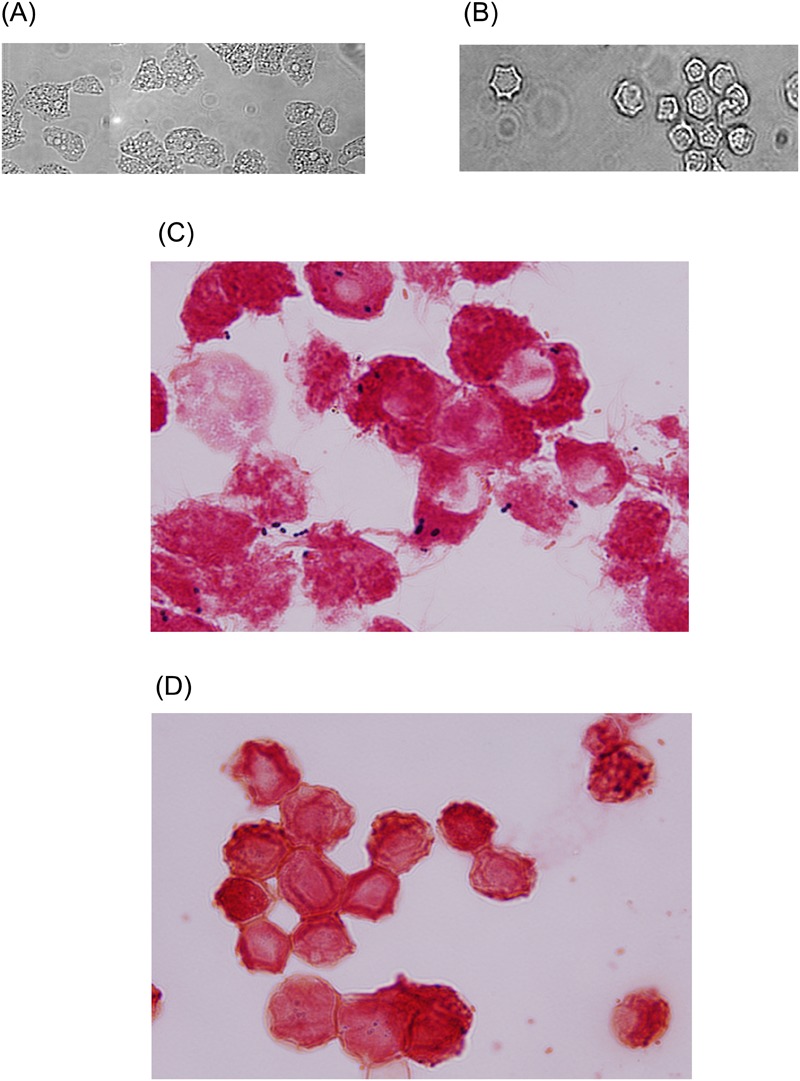
Representative micrograph of Acanthamoeba castellanii belonging to the T4 genotype. (A and C) A. castellanii trophozoites; (B and D) A. castellanii cysts. Images in A and B were taken using phase-contrast microscope (×250). For C and D, samples were stained using Gram staining and observed under microscope (×400). Note the presence of bacteria (dark blue) inside A. castellanii trophozoites, as well as A. castellanii cysts.
Figure 2.
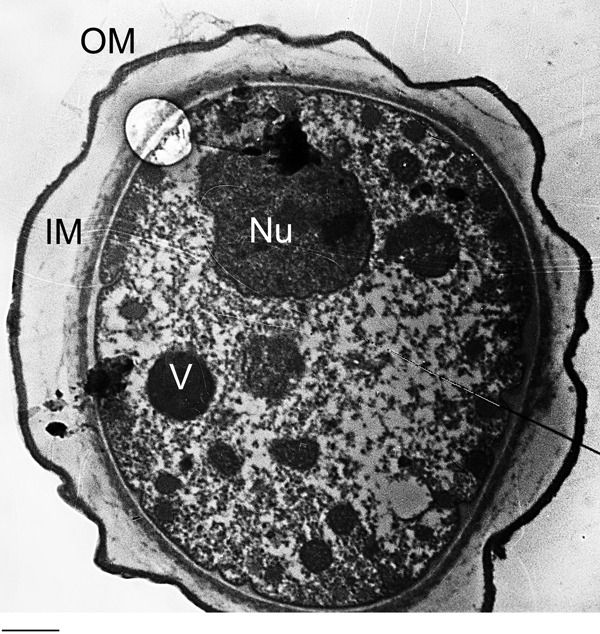
Representative transmission electron micrographs showing a complete double-walled cyst of Acanthamoeba castellanii belonging to the T4 genotype (American Type Culture Collection, ATCC 50492). Encystment was induced by inoculating trophozoites on non-nutrient agar plates and samples collected after 7 days of incubation and processed for TEM. V is vacuoles; Nu is nucleus; IM is inner membrane; and OM is 2 outer membrane. Bar = 2 μm.
Conclusions
Overall these findings suggest that anti-amoebic approaches in eradicating ‘superbugs’ from clinical settings must be considered in addition to the currently used disinfectants. These have shown to reduce the overall number of episodes of infections. Research is needed to demonstrate the efficacy of targeting the host amoebae that harbour ‘terror cells’ to confront the spread of superbugs in our fight against infectious diseases.
Contributors
NAK has a lifelong interest in the field of Acanthamoeba and became interested in its role in the spread of infectious agents. RS is a microbiology researcher. All authors contributed equally to the manuscript and will act as guarantors.
Competing of Interests
The authors declare (1) no financial support for the submitted work from anyone other than their employer; (2) no financial relationships with commercial entities that might have an interest in the submitted work; (3) no spouses, partners, or children with relationships with commercial entities that might have an interest in the submitted work; and (4) no non-financial interests that may be relevant to the submitted work.
Acknowledgments
The authors are grateful for the kind support provided by the Aga Khan University, Karachi, Pakistan.
References
- 1.Khan NA. Acanthamoeba: biology and pathogenesis. Norfolk: Caister Academic Press; 2009. [Google Scholar]
- 2.Siddiqui R, Khan NA. Infection control strategy by killing drug-resistant bacteria. Pathog Glob Health. 2013;107:215–6. doi: 10.1179/2047773213Y.0000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordmann P, Naas T, Fortineau N, Poirel L. Superbugs in the coming new decade; multidrug resistance and prospects for treatment of Staphylococcus aureus, Enterococcus spp. and Pseudomonas aeruginosa in 2010. Curr Opin Microbiol. 2007;10:436–40. doi: 10.1016/j.mib.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Alanis AJ. Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res. 2005;36:697–705. doi: 10.1016/j.arcmed.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Blondeau J. Gram-negative superbugs: inappropriate antimicrobial therapy and mortality. Expert Rev Clin Pharmacol. 2013;6:347–9. doi: 10.1586/17512433.2013.811831. [DOI] [PubMed] [Google Scholar]
- 6.CDC. Antibiotic resistance threats in the United States, 2013. Atlanta (GA): CDC; 2013. Available from: http://www.cdc.gov/drugresistance/threat-report-2013/ (accessed 22 May 2013). [Google Scholar]
- 7.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53:5046–54. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oberoi L, Singh N, Sharma P, Aggarwal A. ESBL, MBL and Ampc β lactamases producing superbugs — havoc in the intensive care units of Punjab India. J Clin Diagn Res. 2013;7:70–3. doi: 10.7860/JCDR/2012/5016.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trivedi TH, Sabnis GR. Superbugs in ICU: is there any hope for solution? J Assoc Physicians India. 2009;57:623–5. [PubMed] [Google Scholar]
- 10.Appelbaum PC. 2012 and beyond: potential for the start of a second pre-antibiotic era? J Antimicrob Chemother. 2012;67:2062–8. doi: 10.1093/jac/dks213. [DOI] [PubMed] [Google Scholar]
- 11.Barbosa TM, Levy SB. The impact of antibiotic use on resistance development and persistence. Drug Resist Updat. 2000;3:303–11. doi: 10.1054/drup.2000.0167. [DOI] [PubMed] [Google Scholar]
- 12.Marshall BM, Levy SB. Food Animals and Antimicrobials: Impacts on Human Health. Clin Microbiol Rev. 2011;24:718–33. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy SB. The 2000 Garrod lecture. Factors impacting on the problem of antibiotic resistance. J Antimicrob Chemother. 2002;49:25–30. doi: 10.1093/jac/49.1.25. [DOI] [PubMed] [Google Scholar]
- 14.Klein E, Smith DL, Laxminarayan R. Community-associated methicillin-resistant Staphylococcus aureus in outpatients, United States, 1999–2006. Emerg Infect Dis. 2009;15:1925–30. doi: 10.3201/eid1512.081341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Atlanta (GA): CDC. Available from: http://www.cdc.gov/ (accessed 6 June 2013). [Google Scholar]
- 16.McCormick D, Thorn S, Milne D, Evans C, Stevenson J, Llano M, et al. Incident Management Team. Public health response to an outbreak of Legionnaires’ disease in Edinburgh, United Kingdom, June 2012. Euro Surveill. 2012;17(28):pii):20216. doi: 10.2807/ese.17.28.20216-en. [DOI] [PubMed] [Google Scholar]
- 17.WHO. Use of antimicrobials outside human medicine and resultant antimicrobial resistance in humans. Geneva: WHO; 2002. Available from: http://www.who.int/mediacentre/factsheets/fs268/en/index.html (accessed 6 June 2013). [Google Scholar]
- 18.Snitkin ES, Zelazny AM, Thomas PJ, Stock F NISC Comparative Sequencing Program Group, Henderson DK. Palmore TN, Segre JA. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med. 2012;4:148. doi: 10.1126/scitranslmed.3004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy SB. The challenge of antibiotic resistance. Sci Am. 1998;278:46–53. doi: 10.1038/scientificamerican0398-46. [DOI] [PubMed] [Google Scholar]
- 20.Madeo M.Cleaning the hospital environment — a focus on Difficil-S. Br J Nurs. 201120688, 690–93. [DOI] [PubMed] [Google Scholar]
- 21.Eckstein BC, Adams DA, Eckstein EC, Rao A, Sethi AK, Yadavalli GK, et al. Reduction of Clostridium difficile and vancomycin-resistant Enterococcus contamination of environmental surfaces after an intervention to improve cleaning methods. BMC Infect Dis. 2007;7:61. doi: 10.1186/1471-2334-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coulon C, Collignon A, McDonnell G, Thomas V. Resistance of Acanthamoeba cysts to disinfection treatments used in health care settings. J Clin Microbiol. 2010;48:2689–97. doi: 10.1128/JCM.00309-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Briancesco R, Veschetti E, Ottaviani M, Bonadonna L. Peracetic acid and sodium hypochlorite effectiveness in reducing resistant stages of microorganisms. Cent Eur J Public Health. 2005;13:159–62. [PubMed] [Google Scholar]
- 24.Rowbotham TJ. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J Clin Pathol. 1980;33:1179–83. doi: 10.1136/jcp.33.12.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lloyd D, Turner NA, Khunkitti W, Hann AC, Furr JR, Russell AD. Encystation in Acanthamoeba castellanii: development of biocide resistance. J Eukaryot Microbiol. 2001;48:11–6. doi: 10.1111/j.1550-7408.2001.tb00410.x. [DOI] [PubMed] [Google Scholar]
- 26.Turner NA, Russell AD, Furr JR, Lloyd D. Emergence of resistance to biocides during differentiation of Acanthamoeba castellanii. J Antimicrob Chemother. 2000;46:27–34. doi: 10.1093/jac/46.1.27. [DOI] [PubMed] [Google Scholar]
- 27.Turner NA, Russell AD, Furr JR, Lloyd D. Resistance, biguanide sorption and biguanide-induced pentose leakage during encystment of Acanthamoeba castellanii. J Appl Microbiol. 2004;96:1287–95. doi: 10.1111/j.1365-2672.2004.02260.x. [DOI] [PubMed] [Google Scholar]
- 28.Connell C, Rutter A, Hill B, Suller M, Lloyd D. Encystation of Acanthamoeba castellanii: dye uptake for assessment by flow cytometry and confocal laser scanning microscopy. J Appl Microbiol. 2001;90:706–12. doi: 10.1046/j.1365-2672.2001.01296.x. [DOI] [PubMed] [Google Scholar]
- 29.Martinez AJ, Visvesvara GS. Free-living, amphizoic and opportunistic amebas. Brain Pathol. 1997;7:583–98. doi: 10.1111/j.1750-3639.1997.tb01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marciano-Cabral F, Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev. 2003;16:273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan NA. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol Rev. 2006;30:564–95. doi: 10.1111/j.1574-6976.2006.00023.x. [DOI] [PubMed] [Google Scholar]
- 32.Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007;50:1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 33.La Scola B, Audic S, Robert C, Jungang L, de Lamballerie X, Drancourt M, et al. A giant virus in amoebae. Science. 2003;299:2033. doi: 10.1126/science.1081867. [DOI] [PubMed] [Google Scholar]
- 34.Greub G, Raoult D. Microorganisms resistant to free-living amoebae. Clin Microbiol Rev. 2004;17:413–33. doi: 10.1128/CMR.17.2.413-433.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazur T, Hadaś E, Iwanicka I. The duration of the cyst stage and the viability and virulence of Acanthamoeba isolates. Trop Med Parasitol. 1995;46:106–8. [PubMed] [Google Scholar]
- 36.Sriram R, Shoff M, Booton G, Fuerst P, Visvesvara GS. Survival of Acanthamoeba cysts after desiccation for more than 20 years. J Clin Microbiol. 2008;46:4045–8. doi: 10.1128/JCM.01903-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez-Zaragoza S, Magana-Becerra A. Prevalence of pathogenic Acanthamoeba (Protozoa: Amoebidae) in the atmosphere of the city of San Luis Potosi, Mexico. Toxicol Ind Health. 1997;13:519–26. doi: 10.1177/074823379701300404. [DOI] [PubMed] [Google Scholar]
- 38.Borazjani RN, Kilvington S. Efficacy of multipurpose solutions against Acanthamoeba species. Cont Lens Anterior Eye. 2005;28:169–75. doi: 10.1016/j.clae.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Buck SL, Rosenthal RA, Schlech BA. Methods used to evaluate the effectiveness of contact lens care solutions and other compounds against Acanthamoeba: a review of the literature. CLAO J. 2000;26:72–84. [PubMed] [Google Scholar]
- 40.Kobayashi T, Gibbon L, Mito T, Shiraishi A, Uno T, Ohashi Y. Efficacy of commercial soft contact lens disinfectant solutions against Acanthamoeba. Jpn J Ophthalmol. 2011;55:547–7. doi: 10.1007/s10384-011-0062-y. [DOI] [PubMed] [Google Scholar]
- 41.Johnston SP, Sriram R, Qvarnstrom Y, Roy S, Verani J, Yoder J, et al. Resistance of Acanthamoeba cysts to disinfection in multiple contact lens solution. J Clin Microbiol. 2009;47:2040–5. doi: 10.1128/JCM.00575-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shoff ME, Joslin CE, Tu EY, Kubatko L, Fuerst PA. Efficacy of contact lens systems against recent clinical and tap water Acanthamoeba isolates. Cornea. 2008;27:713–9. doi: 10.1097/QAI.0b013e31815e7251. [DOI] [PubMed] [Google Scholar]
- 43.Verani JR, Lorick SA, Yoder JS, Beach MJ, Braden CR, Roberts JM, et al. Acanthamoeba Keratitis Investigation Team. National outbreak of Acanthamoeba keratitis associated with use of a contact lens solution, United States. Emerg Infect Dis. 2009;15:1236–42. doi: 10.3201/eid1508.090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doan L, Forrest H, Fakis A, Craig J, Claxton L, Khare M. Clinical and cost effectiveness of eight disinfection methods for terminal disinfection of hospital isolation rooms contaminated with Clostridium difficile 027. J Hosp Infect. 2012;82:114–21. doi: 10.1016/j.jhin.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 45.Passaretti CL, Otter JA, Reich NG, Myers J, Shepard J, Ross T, et al. An evaluation of environmental decontamination with hydrogen peroxide vapor for reducing the risk of patient acquisition of multidrug-resistant organisms. Clin Infect Dis. 2013;56:27–35. doi: 10.1093/cid/cis839. [DOI] [PubMed] [Google Scholar]
- 46.Zoutman D, Shannon M, Mandel A. Effectiveness of a novel ozone-based system for the rapid high-level disinfection of health care spaces and surfaces. Am J Infect Control. 2011;39:873–9. doi: 10.1016/j.ajic.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 47.Gatti S, Cevini C, Bruno A, Penso G, Rama P, Scaglia M. In vitro effectiveness of povidone-iodine on Acanthamoeba isolates from human cornea. Antimicrob Agents Chemother. 1998;42:2232–4. doi: 10.1128/aac.42.9.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huws SA, Smith AW, Enright MC, Wood PJ, Brown MR. Amoebae promote persistence of epidemic strains of MRSA. Environ Microbiol. 2006;8:1130–3. doi: 10.1111/j.1462-2920.2006.00991.x. [DOI] [PubMed] [Google Scholar]
- 49.Tomlinson G, Jones EA. Isolation of cellulose from the cyst wall of a soil amoeba. Biochim Biophys Acta. 1962;63:194–200. doi: 10.1016/0006-3002(62)90353-0. [DOI] [PubMed] [Google Scholar]
- 50.Neff RJ, Neff RH. The biochemistry of amoebic encystment. Symp Soc Exp Biol. 1969;23:51–81. [PubMed] [Google Scholar]
- 51.Hirukawa Y, Nakato H, Izumi S, Tsuruhara T, Tomino S. Structure and expression of a cyst specific protein of Acanthamoeba castellanii. Biochim Biophys Acta. 1998;1398:47–56. doi: 10.1016/s0167-4781(98)00026-8. [DOI] [PubMed] [Google Scholar]
- 52.Dudley R, Jarroll EL, Khan NA. Carbohydrate analysis of Acanthamoeba castellanii. Exp Parasitol. 2009;122:338–343. doi: 10.1016/j.exppara.2009.04.009. [DOI] [PubMed] [Google Scholar]


