Abstract
It is hypothesized that animals living in polluted environments possess antimicrobials to counter pathogenic microbes. The fact that snakes feed on germ-infested rodents suggests that they encounter pathogenic microbes and likely possess antimicrobials. The venom is used only to paralyze the rodent, but the ability of snakes to counter potential infections in the gut due to disease-ridden rodents requires robust action of the immune system against a broad range of pathogens. To test this hypothesis, crude lysates of different organs of Naja naja karachiensis (black cobra) were tested for antimicrobial properties. The antimicrobial activities of extracts were tested against selected bacterial pathogens (neuropathogenic Escherichia coli K1, methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, and Streptococcus pneumonia), protist (Acanthamoeba castellanii), and filamentous fungus (Fusarium solani). The findings revealed that plasma and various organ extracts of N. n. karachiensis exhibited antimicrobial activity against E. coli K1, MRSA, P. aeruginosa, S. pneumoniae, A. castellanii, and F. solani in a concentration-dependent manner. The results of this study are promising for the development of new antimicrobials.
Keywords: Infectious diseases, Antimicrobials, Black cobra, Acanthamoeba, Fungi, Protists
Introduction
Antimicrobial resistance presents a significant challenge to human and animal health. This is particularly important for developing countries where drug-resistant microbes are prevalent.1–3 For example, it is estimated that around 180 000 cases of multiple drug-resistant-tuberculosis (MDR-TB) occur annually in South-East Asia with more than 80% of these in Bangladesh, India, Indonesia, Myanmar, and Thailand.4 Streptococcus pneumoniae is one of the most common causative agents of pneumonias in children and adults in Asia.5 Multiple drug-resistant Klebsiella spp., Pseudomonas aeruginosa, Escherichia coli, methicillin-resistant Staphylococcus aureus (MRSA), and Acinetobacter species have given new dimensions to the problem of hospital-associated infections.6–9 In addition, protists such as Acanthamoeba are now recognized as a source of microbial infections.10 Acanthamoeba has been shown to act as a reservoir for microbial pathogens including viruses (Mimivirus), bacteria (Aeromonas, Coxiella, E. coli, Legionella, Vibrio, etc.), protists (Cryptosporidium), and yeast/fungi (Cryptococcus).11–13 Apart from its role as the Trojan horse of the microbial world, Acanthamoeba can produce blinding keratitis and fatal granulomatous amoebic encephalitis14,15 and showed resistance to a variety of anti-amoebic agents.16 Thus there is an urgent need to identify novel antimicrobials to counter pathogens.
Previously, we hypothesized that animals living in polluted environments possess antimicrobials to counter infections.17 In support, our studies identified potent antimicrobial properties in the brain lysates of cockroaches and locusts that intrigued the scientific community.17 Here, we tested this hypothesis further by examining other animals for antimicrobial activities.
The fact that snakes feed on germ-infested rodents (by swallowing the whole rodent) suggests their exposure to pathogenic microbes. The venom is used only to paralyze the rodent, but the ability of snakes to counter potential infections in the gut due to disease-ridden rodents requires robust action of the innate immune system against a broad range of pathogens.18 In many cases, the response (i.e., non-specific leucocytes, antimicrobial molecules, and the complement system) is highly effective,18 however this is a relatively untapped area of research that has the potential to provide pharmaceutical drug-leads for much needed antimicrobials. In this study, the plasma and lysates of various organs of black cobra were dissected out and tested for broad-spectrum antimicrobials against Gram positive and negative bacteria, protists, and fungal pathogens.
Materials and Methods
Snake sample and organ lysate preparation
The black Pakistani cobra (Naja naja karachiensis) is commonly found in southern parts of Pakistan.19 Snakes were provided routinely by M. Z. A. Khanzada, Dow Medical University of Health Sciences, Karachi, Pakistan. Snakes were anesthetized using chloroform and terminally bled through cardiac puncture. Blood was collected in EDTA vacutainers and subsequently plasma was separated through centrifugation at 1500 × g for 10 minutes. Next, snakes were dissected aseptically and their various organs such as lungs, liver, gut, stomach, gallbladder, kidneys, and testicles were collected. Each organ was weighed, homogenized (Tekmar homogenizing mixer), and sonicated (Branson Sonifier 450) in sterile distilled water, in 1∶1 ratio as previously described.17 Following sonication, tissue lysates were centrifuged at 12 000 × g for 30 minutes at 4°C. The supernatant containing soluble lysates were collected and filtered using a 0.2 μm pore size filters. All filtrates were stored at −80°C until tested. The concentrations of dissolved proteins were estimated using Bradford method.
Microbial cultures and growth conditions
Bacteria used in this study included neuropathogenic E. coli K1, MRSA, P. aeruginosa, and S. pneumoniae. E. coli K1 strains RS21820 and MRSA21 were isolated earlier from CSF and blood samples of neonatal meningitis and sepsis patients, respectively. Streptococcus pneumoniae was isolated from the blood culture of a pneumonia patient and P. aeruginosa was isolated from pus sample. All bacterial isolates are deposited in the departmental microbial collection and available upon request. All bacteria were grown aerobically at 37°C in nutrient broth except S. pneumoniae, which was grown on sheep blood agar plates and brain–heart infusion (BHI) broth.
Acanthamoeba castellanii, a keratitis isolate, belonging to the T4 genotype was purchased from American Type Culture Collection (ATCC 50492). Acanthamoeba castellanii trophozoites were grown in the PYG medium [0.75% (w/v) proteose peptone, 0.75% (w/v) yeast extract, and 1.5% (w/v) glucose] at 30°C in T-75 tissue culture flasks. To obtain amoebae in the trophozoite forms, the culture medium was refreshed 15–20 hours prior to experiments. Fusarium solani was purchased from First Fungal Culture Bank of Pakistan (FCBP0055) and grown aerobically on potato dextrose agar (PDA) plates at 30°C for 5–7 days. Conidiospores were collected by scraping the surface of fungal colonies in phosphate buffered saline (PBS) and hyphae were removed by filtering suspension through sterile gauze sieve. Spores in the filtrate were washed in PBS through centrifugation and used for experiments.22
Antibacterial assays
Antibacterial assays were performed as described previously.23 Briefly, approximately 106 colony forming units (cfu), suspended in 10 μl were incubated with various concentrations of snake plasma or different organ lysates (0.25, 0.5, 0.75, 1.0, 1.25 mg/ml; final volume adjusted to 200 μl) and incubated at 37°C for 2 hours After this incubation, bacterial cultures were 10-fold serially diluted and plated on nutrient/BHI agar plates and incubated further at 37°C overnight. Bacteria incubated with PBS alone served as negative control. For 100% kill, bacteria were incubated with appropriate antibiotics, 100 μg/ml gentamicin for E. coli K1 and P. aeruginosa; 100 μg/ml vancomycin for S. pneumonia and MRSA. Next day, colonies were enumerated. The percentage bactericidal effects were determined as follows: 100 – [(cfu in lysates/original inoculum) × 100].
Amoebicidal assays
Amoebicidal assays were performed in 24-well plates by incubating 106 A. castellanii trophozoites with lysates of various organs of black cobra (total volume was made up to 500 μl using PBS) and incubated at 30°C for 48 hours. Amoebae incubated with PBS alone served as controls. Following incubation, amoebae were counted microscopically using a heamocytometer. The percentage amoebicidal effects were determined as follows: 100 – [(amoebae count in lysates/amoebae count in control) × 100].
Fungicidal assays
To determine the fungicidal effects of various organs lysates of black cobra against F. solani, assays were performed similar to amoebicidal assays with minor modifications. Briefly, 106 conidiospores were incubated with different organ lysates in PBS in 500 μl at 30°C for 24 hours F. solani colonies in PBS alone were considered as control. Following incubation, spores were diluted and plated on PDA plates and incubated at 30°C until visible colonies appeared (∼48 hours). The percentage fungicidal effects were determined as follows: 100 – [(fungal colonies in lysates/fungal colonies in control) × 100].
Results
Black cobra plasma exhibited potent bactericidal activities against all bacteria tested except S. pneumonia
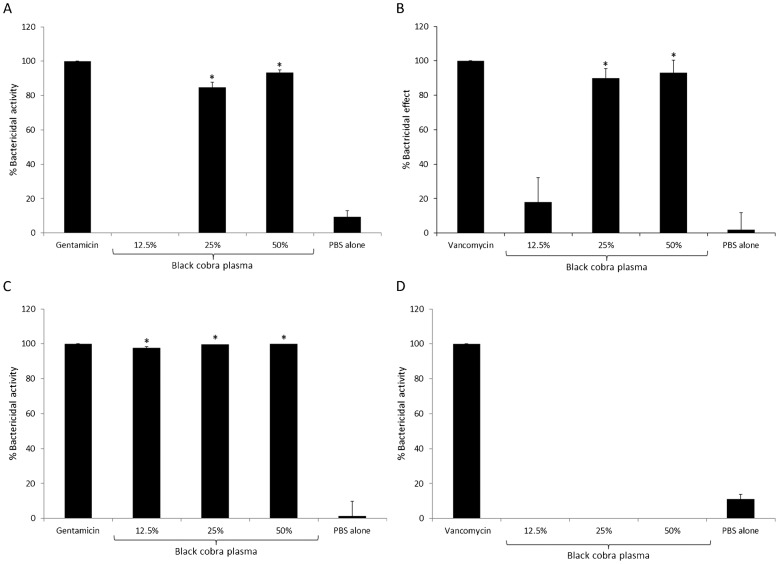
To determine the bactericidal activity of black cobra plasma against E. coli K1, MRSA, P. aeruginosa, and S. pneumoniae, 106 cfu were incubated with different concentration of snake plasma. The results showed that snake plasma exhibited potent bactericidal activity against all bacteria tested, except S. pneumoniae (Fig. 1). For E. coli K1, 25 and 50% snake plasma produced 85% ± 3 and 93% ± 1.8 bactericidal activities, respectively (Fig. 1A). Similarly, 25 and 50% snake plasma showed 90% ± 5.5 and 93% ± 7.5 bactericidal activities against MRSA, respectively (Fig. 1B). As low as 12.5% plasma showed 98% ± 0.87 killing of P. aeruginosa (Fig. 1C). Surprisingly, none of the snake plasma concentrations tested showed any effect on the viability of S. pneumoniae (Fig. 1D).
Figure 1.
Black cobra plasma exhibited potent antibacterial activities against Escherichia coli K1, methicillin-resistant Staphylococcus aureus (MRSA), and Pseudomonas aeruginosa but not Streptococcus pneumoniae. Various concentrations of black cobra plasma was incubated with approximately 106 cfu of E. coli K1 (A), MRSA (B), P. aeruginosa (C), and S. pneumoniae (D) at 37°C for 2 hours in 200 μl. For positive control, E. coli K1 and P. aeruginosa were incubated with 100 μg/ml gentamicin, while MRSA and S. pneumoniae were incubated with 100 μg/ml vancomycin. Bacteria incubated with PBS alone served as negative control. After incubation, bacterial colonies were enumerated and percentage bactericidal effects were determined in the Materials and Methods section. The data are presented as the mean ± standard error of three independent experiments performed in duplicate. P-values were calculated by comparing results of PBS alone with different plasma concentrations using student’s t-test. (*) indicates P < 0.0001.
Lysates of black cobra organs exhibited selective bactericidal activities
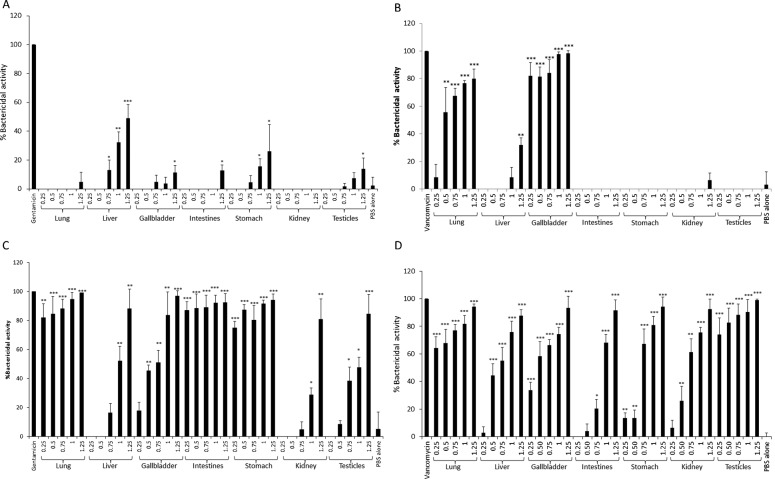
To determine the bactericidal activities of internal organs of black cobra, tissue lysates of different organs (lungs, liver, intestine, stomach, gallbladder, kidneys, and testicles) equivalent to protein concentration of 0.25, 0.5, 0.75, 1.0, and 1.25 mg/ml were incubated with E. coli K1, MRSA, P. aeruginosa, and S. pneumoniae. In the case of E. coli K1, among different organs tested, liver, gallbladder, intestine, stomach, and testicles lysates showed moderate to low level of bactericidal activities (Fig. 2A). Only liver lysate showed concentration-dependent bactericidal activity, where 0.75, 1.0, and 1.25 mg/ml lysate showed 13%±7.1, 32% ± 7.0, and 49% ± 9.6 bacterial kill, respectively (Fig. 2A). Lungs and gallbladder showed potent bactericidal activities at 0.5 mg/ml. Moderate bactericidal activities (32% ± 5.3) were observed at 1.25 mg/ml (Fig. 2B). The lysates of lungs, intestine, stomach, and gallbladder showed significant antibacterial activities against P. aeruginosa at 0.25 mg/ml (Fig. 2C). For S. pneumoniae, lysates of lungs, gallbladder, and testicles showed dose dependent bactericidal activities (Fig. 2D).
Figure 2.
Lysates of various internal organs of black cobra showed antibacterial activities against Escherichia coli K1, MRSA, Pseudomonas aeruginosa, and Streptococcus pneumoniae. Various internal organs of black cobra were collected and their lysates were prepared. Lysate equivalent to protein concentration of 0.25, 0.5, 0.75, 1.0, and 1.25 mg/ml, were incubated with ∼106 cfu of E. coli K1 (A), MRSA (B), P. aeruginosa (C), and S. pneumoniae (D) at 37°C for 2 hours in 200 μl. For positive controls, E. coli K1 and P. aeruginosa were incubated with100 μg/ml gentamicin, while MRSA and S. pneumoniae were incubated with 100 μg/ml vancomycin. Bacteria incubated with PBS alone served as negative control. After incubation, bacterial colonies were enumerated and percentage bactericidal effects were determined as described in the Materials and Methods section. The data are presented as the mean ± standard error of three independent experiments performed in duplicate. P-values were calculated by comparing results of PBS alone with different plasma concentrations using student’s t-test. (*), (**), and (***) indicate P < 0.05, P < 0.01, and P < 0.001, respectively.
Lysates of black cobra lungs and gallbladder exhibited potent anti-fungal and anti-protist activities, respectively
To study the fungicidal and amoebicidal activities of black cobra organ lysates, 106 conidiospores of F. solani and 106 A. castellanii were incubated with lysates of lungs, liver, gallbladder, and stomach. The results revealed that only lungs lysate showed potent anti-fungal activities (74% ± 5.76) against F. solani spores, while liver, gallbladder, and stomach lysates had no effects (8.4% ± 5.6, 5.3% ± 4.0, and 19.15% ± 12.2, respectively) (Fig. 3). For A. castellanii, gallbladder lysate showed potent amoebicidal activity (99% ± 1.02), whereas lung and stomach lysates showed limited amoebicidal activities (21% ± 4.65 and 20% ± 5.76, respectively) (Fig. 4).
Figure 3.

Black cobra lung lysate exhibited potent activity against Fusarium solani conidiospores. Approximately, 106 F. solani conidiospores were incubated with 1.25 mg/ml lysate of black cobra lungs, liver, gallbladder, and stomach in PBS at 30°C for 48 hours. Next, fungal colonies were enumerated and percentage fungicidal activity was calculated as described in the Materials and Methods section. Fusarium solani spores incubated in PBS alone served as control. Among various organ lysates tested, lung lysates showed optimal fungicidal effects. The data are presented as the mean ± standard error of three independent experiments performed in duplicate. P-values were calculated by comparing results of PBS alone with different organ lysates using student’s t-test. (*) indicates P < 0.001.
Figure 4.
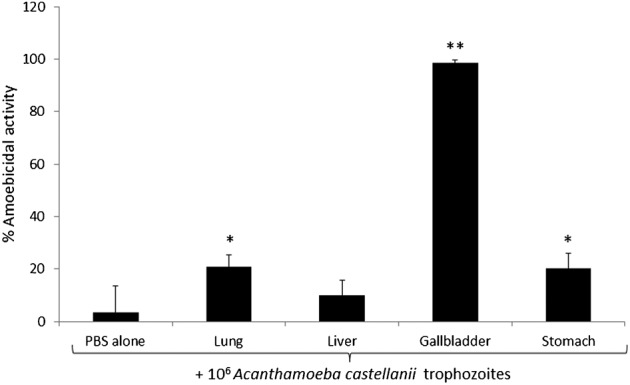
Potent anti-protist activity of black cobra gallbladder against Acanthamoeba castellanii trophozoites. Approximately, 106 amoebae were incubated with 1.25 mg/ml lysate of black cobra lungs, liver, gallbladder, and stomach in PBS at 30°C for 48 hours After incubation, A. castellanii trophozoites were counted microscopically using a haemocytometer and percentage amoebicidal activity was calculated as described in the Materials and Methods section. A. castellanii incubated in PBS alone served as control. Note that the gallbladder lysates showed optimal amoebicidal activity. The data are presented as the mean ± standard error of three independent experiments performed in duplicate. P-values were calculated by comparing results of PBS alone with different organ lysates using student’s t-test. (*) indicates P < 0.001.
Discussion
Widespread acquired resistance of bacteria against currently available antibiotics led the efforts to discover and design new antimicrobial agents. Previously, our group reported the discovery of antimicrobial activity from cockroaches and locusts.17 Both of these insects live in filthy environments and likely use these antimicrobials to protect their vital organs from invading bacterial pathogens. Given that snakes such as black cobra feed on germ-infested rodents, it was tempting to speculate that they possess antimicrobials to counter infections. A literature search revealed no reports on antibacterial activities of snake blood, plasma, or internal organs, albeit there are many reports on the antibacterial activity of snake venoms.24,25 Here for the first time, we show bactericidal activities in the blood/plasma as well as various body organs of the black cobra. Snake plasma showed potent activities against all bacteria tested except S. pneumoniae. Plasma, as high as 50%, failed to produce any antibacterial effect. Regardless, the remaining Gram negative and positive bacteria tested were effectively killed by the snake plasma suggesting the presence of robust antimicrobial molecules.
Lysates of snake lungs exhibited potent bactericidal activities. This is consistent with previous studies, which showed that surfactant proteins and peptides of vertebrate lungs possess antibacterial properties (Table 1), but importantly fungicidal activity was also observed. In addition to surfactant proteins and peptides, these activities might be induced by microbicidal factors of the innate immune system such as lysozyme and lectoferrin.26 It is noteworthy that lysates of the liver and gallbladder showed broad spectrum antibacterial activity against all bacteria tested. Gall bladder serves as a storage organ for bile, which is secreted by the liver. Bile is composed of proteins, ions, pigments, cholesterol, and various salts, collectively known to disrupt biological membrane and have selective antimicrobial activity against Gram positive bacteria.27 Our results are consistent with previous findings that show that liver and gall bladder lysates of fish, frogs, birds, and mice possess microbicidal activities against Gram positive and negative bacteria (Table 1), but we also noted amoebicidal activity. Compounds such as squalamine, peptidoglycan-associated protein, and defensin-like peptides have been suggested to be responsible for antibacterial activity (Table 1). Future studies will determine whether similar factors are responsible for antimicrobial activities of snake plasma or identify novel bioactive molecules.
Table 1. Known antimicrobial activities of various organs of selected vertebrates and possible active factors.
| Organs | Animal | Antimicrobial activity | Active factor/s | References |
| Lung/Gills | Gadus morhua | G+, G− | ND | 33 |
| Siniperca chuatsi | G+, G− | ScBD peptide | 34 | |
| Crocodylus niloticus | G+ | ND | 32 | |
| Corvus corax | G+ | ND | 32 | |
| Gallus gallus domesticus | G− | Gal-7, Gal-9 peptide | 35 | |
| Meleagris gallopavo | G+ | ND | 32 | |
| Mus musculus | G+, G− | mBD-1 peptide | 36 | |
| G− | mBD-3 peptide | 37 | ||
| Rattus norvegicus | G+ | Surfactant containing fraction | 38 | |
| Liver | Squalus acanthias | G+, G−, Candida albicans, Paramecium caudatum | Squalamine | 39 |
| Sebastes schlegeli | G+, G− | SsPGRP-L2 (long chain of peptidoglycan related proteins) | 40 | |
| Oryzias latipes | G− | Medaka beta-defensin peptide | 41 | |
| Limnonectes fragilis | G+, G− | Cathelicidin like peptide | 42 | |
| C. corax | G+ | ND | 32 | |
| G. g. domesticus | G− | Gal-7 peptide | 35 | |
| M. musculus | G+, G− | mBD-1 peptide | 36 | |
| Gallbladder | S. acanthias | G+, G−, C. albicans, P. caudatum | Squalamine | 39 |
| G. morhua | G+, G− | ND | 33 | |
| Stomach | S. acanthias | G+, G−, C. albicans, P. caudatum | Squalamine | 39 |
| Xenopus laevis | G+, G−, C. albicans | PGQ (24 amino acids peptide with amino-terminal glycine and carboxylterminal glutamine) | 43 | |
| Bufo gargarizans | G+, G−, C. albicans, Cryptococcus neoformans, and Saccharomyces cerevisiae | Buforin 1, 2, 2b, and histonins peptides | 44 | |
| L. fragilis | G+, G− | Cathelicidin like peptide | 42 | |
| Rana catesbeiana | G+, G−, C. albicans, C. neoformans, and S. cerevisiae | bPaAP, bPcAP - peptide | 45 | |
| C. corax | G+ | ND | 32 | |
| G. g. domesticus | G− | Gal-7 peptide | 35 | |
| Intestines | S. acanthias | G+, G−, C. albicans, P. caudatum | Squalamine | 39 |
| G. morhua | G+, G− | ND | 33 | |
| S. schlegeli | G+, G− | SsPGRP-L1 (long chain of peptidoglycan related proteins) | 40 | |
| Myxine glutinosa | G+, G− | HFIAP 1, 2, and 3 peptides | 46 | |
| S. chuatsi | G+, G− | ScBD peptide | 34 | |
| C. corax | G+ | ND | 32 | |
| G. g. domesticus | G− | Gal-7 peptide | 35 | |
| M. musculus | G− | mBD-3 peptide | 37 | |
| M. musculus | G+, G− | Cryptdin 1 and 2 peptides | 47 | |
| Sus scrofa domesticus (extracellular matrix) | G+, G− | ND | 48 | |
| Kidney | S. chuatsi | G+, G− | ScBD peptide | 34 |
| G. morhua | G+, G− | ND | 33 | |
| Sparus aurata | G+, G− | SaBD (propeptide of 66 amino acids) | 49 | |
| L. fragilis | G+, G− | Cathelicidin like peptide | 42 | |
| G. g. domesticus | G− | Gal-7 peptide | 35 | |
| M. musculus | G+, G− | mBD-1 peptide | 36 | |
| Reproductive organs | S. acanthias (testes) | G+, G−, C. albicans, P. caudatum | Squalamine | 39 |
| R. norvegicus (testes) | G− | DEFB-21, 24, and 27 peptides | 50 | |
| G. g. domesticus (ovary) | G− | Gal-4 and Gal-7 peptide | 35 | |
| M. musculus (ovary) | G+, G− | mBD-1 peptide | 36 | |
| Skin | Pleuronectes americanus | G+, G− | Pleurocidin peptides | 51 |
| S. aurata | G+, G− | SaBD (propeptide of 66 amino acids) | 49 | |
| X. laevis | G+, G− | Pexiganan peptide | 52 | |
| Rana tigerina | G+, G− | Tegrin 1, 2, 3, and 4 peptides | 53 | |
| Litoria raniformis | G+, G− | ND | 54 | |
| L. fragilis | G+, G− | Cathelicidin like peptide | 42 | |
| X. laevis | G+, G−, C. albicans | Meganins | 55 | |
| G. g. domesticus | G− | Gal-7 peptide | 35 | |
| Blood/serum/plasma | Conger conger | G− | ND | 56 |
| Ictalurus punctatus (leucocytes) | G+, G− | Antimicrobial peptide (MW = 655 Da) | 57 | |
| G. morhua | G+, G− | ND | 33 | |
| Tiliqua rugosa | G− | ND | 56 | |
| Alligator mississippiensis | G+, G−, Naegleria gruberi | ND | 58 | |
| Crocodylus siamensis | G−, C. neoformans and Aspergillus niger | ND | 59 | |
| Bufo marinus | G− | ND | 56 | |
| Gallus gallus | G− | ND | 56 | |
| G. g. domesticus | G− | Gal-7 peptide | 35 | |
| G. gallus (leucocytes) | G+, G− | Gallinacins (Gal-1) | 60 | |
| G. gallus (heterophils) | G+, G− | CHP-1 and 2 peptides | 61 | |
| M. gallopavo (heterophils) | G+, G− | THP-1 peptide | 61 | |
| G+ | THP-2 and THP-3 peptides |
G+: Gram positive bacteria; G−: Gram negative bacteria; ND: not determined.
To test our hypothesis further, we tested antimicrobial activities of different internal organs of leaf-nose viper (Eristicophis macmahonii), a snake endemic to Pakistan–Iran border region. Our preliminary findings show that organs lysates of the leaf-nose viper exhibited antimicrobial activities similar to black cobra, suggesting that it is an important area for further research. Among other reptile species, the serum of American Alligator (Alligator mississippiensis),28–30 plasma of Siamese crocodile (Crocodylus siamensis),31 and tissue extracts of Nile crocodile (Crocodylus niloticus)32 have been shown to possess antibacterial as well as antiviral and anti-protist activities.
In summary, for the first time we report antimicrobial activity from plasma and internal body organs of the black cobra. Although the identification and characterization of active compounds in snake plasma and organ lysates will determine their usefulness as potential antimicrobials, the results of this study are promising and suggest that animals living in polluted environments and/or feeding on germ-infested organisms are a potential source of antimicrobials. The broad spectrum activities against bacterial, fungal, and protist pathogens suggest that the black cobra and other snakes may serve as a novel source of antimicrobial compounds.
Disclaimer Statements
Contributors NK conceived the study. MS and RS designed and conducted all experiments under the supervision of NAK. MS, JI, and NAK contributed to the writing of the manuscript. All authors approved the final manuscript.
Funding Aga Khan Univerity.
Conflicts of interest None.
Ethics approval Not required.
Acknowledgments
This work was supported by the Aga Khan University.
References
- 1.Oberoi L, Singh N, Sharma P, Aggarwal A. ESBL, MBL and Ampc beta lactamases producing superbugs – havoc in the intensive care units of Punjab India. J Clin Diagn Res. 2013;7(1):70–3. doi: 10.7860/JCDR/2012/5016.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trivedi TH, Sabnis GR. Superbugs in ICU: is there any hope for solution? J Assoc Physicians India. 2009;57:623–5. [PubMed] [Google Scholar]
- 3.Yong DM, Toleman A, Giske CG, Cho HS, Sundman K, Lee K, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53(12):5046–54. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nair N, Wares F, Sahu S. Tuberculosis in the WHO South-East Asia Region. Bull World Health Organ. 2010;88(3):164. doi: 10.2471/BLT.09.073874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song JH, Oh WS, Kang CI, Chung DR, Peck KR, Ko KS, et al. Epidemiology and clinical outcomes of community-acquired pneumonia in adult patients in Asian countries: a prospective study by the Asian network for surveillance of resistant pathogens. Int J Antimicrob Agents. 2008;31(2):107–14. doi: 10.1016/j.ijantimicag.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamulitrat S, Arunpan P, Phainuphong P. Attributable mortality of imipenem-resistant nosocomial Acinetobacter baumannii bloodstream infection. J Med Assoc Thai. 2009;92(3):413–9. [PubMed] [Google Scholar]
- 7.Hsueh PR, Chen WH, Luh KT. Relationships between antimicrobial use and antimicrobial resistance in Gram-negative bacteria causing nosocomial infections from 1991-2003 at a university hospital in Taiwan. Int J Antimicrob Agents. 2005;26(6):463–72. doi: 10.1016/j.ijantimicag.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang CI, Baek JY, Jeon K, Kim SH, Chung DR, Peck KR, et al. Bacteremic pneumonia caused by extensively drug-resistant Streptococcus pneumoniae. J Clin Microbiol. 2012;50(12):4175–7. doi: 10.1128/JCM.01642-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rice LB. Antimicrobial resistance in Gram-positive bacteria. Am J Infect Control. 2006;34(5 Suppl 1):S11–9. doi: 10.1016/j.ajic.2006.05.220. discussion S64–73. [DOI] [PubMed] [Google Scholar]
- 10.Greub G, Raoult D. Microorganisms resistant to free-living amoebae. Clin Microbiol Rev. 2004;17:413–33. doi: 10.1128/CMR.17.2.413-433.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan NA. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol Rev. 2006;30(4):564–95. doi: 10.1111/j.1574-6976.2006.00023.x. [DOI] [PubMed] [Google Scholar]
- 12.La Scola B, Audic S, Robert C, Jungang L, de Lamballerie X, Drancourt M, et al. A giant virus in amoebae. Science. 2003;299(5615):2033. doi: 10.1126/science.1081867. [DOI] [PubMed] [Google Scholar]
- 13.La Scola B, Raoult D. Survival of Coxiella burnetii within free-living amoeba Acanthamoeba castellanii. Clin Microbiol Infect. 2001;7(2):75–9. doi: 10.1046/j.1469-0691.2001.00193.x. [DOI] [PubMed] [Google Scholar]
- 14.Marciano-Cabral F, Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev. 2003;16(2):273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007;50:1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 16.Schuster FL, Visvesvara GS. Opportunistic amoebae: challenges in prophylaxis and treatment. Drug Resist Updat. 2004;7(1):41–51. doi: 10.1016/j.drup.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Lee S, Siddiqui R, Khan NA. Animals living in polluted environments are potential source of antimicrobials against infectious agents. Pathog Glob Health. 2012;106(4):218–23. doi: 10.1179/2047773212Y.0000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmerman LM, Vogel LA, Bowden RM. Understanding the vertebrate immune system: insights from the reptilian perspective. J Exp Biol. 2010;213(5):661–71. doi: 10.1242/jeb.038315. [DOI] [PubMed] [Google Scholar]
- 19.Wuster W. Taxonomic changes and toxinology: systematic revisions of the Asiatic cobras (Naja naja species complex). Toxicon. 1996;34(4):399–406. doi: 10.1016/0041-0101(95)00139-5. [DOI] [PubMed] [Google Scholar]
- 20.Iqbal J, Rajani M, Siddiqui R, Khan NA. Neuropathogenic Escherichia coli K1 does not exhibit proteolytic activities to exert its pathogenicity. J Neg Res Biomed. 2013;12:8. doi: 10.1186/1477-5751-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardas M, Khan NA, Alsam S. Staphylococcus aureus exhibit similarities in their interactions with Acanthamoeba and ThP1 macrophage-like cells. Exp Parasitol. 2012;132:513–8. doi: 10.1016/j.exppara.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Retuerto MA, Szczotka-Flynn L, Ho D, Mukherjee P, Ghannoum MA. Efficacy of care solutions against contact lens-associated Fusarium biofilms. Optom Vis Sci. 2012;89:382–91. doi: 10.1097/OPX.0b013e31824cb754. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Duce I, Atkins H, Khan NA. Cockroaches and locusts: physicians’ answer to infectious diseases. Int J Antimicrob Agents. 2011;37(3):279–80. doi: 10.1016/j.ijantimicag.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 24.de Oliveira Junior NG, e Silva Cardoso MH, Franco O. Snake venoms: attractive antimicrobial proteinaceous compounds for therapeutic purposes. Cell Mol Life Sci. 2013;70(24):4645–58. doi: 10.1007/s00018-013-1345-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perumal Samy R, Gopalakrishnakone P, Thwin MM, Chow TK, Bow H, Yap EH, et al. Antibacterial activity of snake, scorpion and bee venoms: a comparison with purified venom phospholipase A2 enzymes. J Appl Microbiol. 2007;102(3):650–9. doi: 10.1111/j.1365-2672.2006.03161.x. [DOI] [PubMed] [Google Scholar]
- 26.Rogan MP, Geraghty P, Greene CM, O’Neill SJ, Taggart CC, McElvaney NG. Antimicrobial proteins and polypeptides in pulmonary innate defence. Respir Res. 2006;7:29. doi: 10.1186/1465-9921-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29(4):625–51. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Merchant ME, Roche C, Elsey RM, Prudhomme J. Antibacterial properties of serum from the American alligator (Alligator mississippiensis). Comp Biochem Physiol B Biochem Mol Biol. 2003;136(3):505–13. doi: 10.1016/s1096-4959(03)00256-2. [DOI] [PubMed] [Google Scholar]
- 29.Merchant M, Thibodeaux D, Loubser K, Elsey RM. Amoebacidal effects of serum from the American alligator (Alligator mississippiensis). J Parasitol. 2004a;90(6):1480–3. doi: 10.1645/GE-3382. [DOI] [PubMed] [Google Scholar]
- 30.Merchant ME, Pallansch M, Paulman RL, Wells JB, Nalca A, Ptak R. Antiviral activity of serum from the American alligator (Alligator mississippiensis). Antiviral Res. 2005;66(1):35–8. doi: 10.1016/j.antiviral.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Kommanee J, Preecharram S, Daduang S, Temsiripong Y, Dhiravisit A, Yamada Y, et al. Antibacterial activity of plasma from crocodile (Crocodylus siamensis) against pathogenic bacteria. Ann Clin Microbiol Antimicrob. 2012;11(1):22. doi: 10.1186/1476-0711-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaharabany M, Gollop N, Ravin S, Golomb E, DeMarco L, Ferreira PC, et al. Naturally occurring antibacterial activities of avian and crocodile tissues. J Antimicrob Chemother. 1999a;44(3):416–8. doi: 10.1093/jac/44.3.416. [DOI] [PubMed] [Google Scholar]
- 33.Ruangsri J, Fernandes JM, Brinchmann M, Kiron V. Antimicrobial activity in the tissues of Atlantic cod (Gadus morhua L.). Fish Shellfish Immunol. 2010;28(5–6):879–86. doi: 10.1016/j.fsi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Wang G, Li J, Zou P, Xie H, Huang B, Nie P, et al. Expression pattern, promoter activity and bactericidal property of β-defensin from the mandarin fish Siniperca chuatsi. Fish Shellfish Immunol. 2012;33(3):522–31. doi: 10.1016/j.fsi.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Milona P, Townes CL, Bevan RM, Hall J. The chicken host peptides, gallinacins 4, 7, and 9 have antimicrobial activity against Salmonella serovars. Biochem Biophys Res Commun. 2007;356(1):169–74. doi: 10.1016/j.bbrc.2007.02.098. [DOI] [PubMed] [Google Scholar]
- 36.Bals R, Goldman MJ, Wilson JM. Mouse beta-defensin 1 is a salt-sensitive antimicrobial peptide present in epithelia of the lung and urogenital tract. Infect Immun. 1998;66(3):1225–32. doi: 10.1128/iai.66.3.1225-1232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bals R, Wang X, Meegalla RL, Wattler S, Weiner DJ, Nehls MC, et al. Mouse beta-defensin 3 is an inducible antimicrobial peptide expressed in the epithelia of multiple organs. Infect Immun. 1999;67(7):3542–7. doi: 10.1128/iai.67.7.3542-3547.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coonrod JD, Yoneda K. Detection and partial characterization of antibacterial factor(s) in alveolar lining material of rats. J Clin Invest. 1983;71(1):129–41. doi: 10.1172/JCI110741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore KS, Wehrli S, Roder H, Rogers M, Forrest JN, McCrimmon D, et al. Squalamine: an aminosterol antibiotic from the shark. Proc Natl Acad Sci U S A. 1993;90(4):1354–8. doi: 10.1073/pnas.90.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim MY, Jang JH, Lee JW, Cho JH. Molecular cloning and characterization of peptidoglycan recognition proteins from the rockfish, Sebastes schlegeli. Fish Shellfish Immunol. 2010;28(4):632–9. doi: 10.1016/j.fsi.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 41.Zhao JG, Zhou L, Jin JY, Zhao Z, Lan J, Zhang YB, et al. Antimicrobial activity-specific to Gram-negative bacteria and immune modulation-mediated NF-kappaB and Sp1 of a medaka beta-defensin. Develop Comp Immunol. 2009;33(4):624–37. doi: 10.1016/j.dci.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Yu H, Cai S, Gao J, Zhang S, Lu Y, Qiao X, et al. Identification and polymorphism discovery of the cathelicidins, Lf-CATHs in ranid amphibian (Limnonectes fragilis). FEBS J. 2013;280(23):6022–32. doi: 10.1111/febs.12521. [DOI] [PubMed] [Google Scholar]
- 43.Moore KS, Bevins CL, Brasseur MM, Tomassini N, Turner K, Eck H, et al. Antimicrobial peptides in the stomach of Xenopus laevis. J Biol Chem. 1991;266(29):19851–7. [PubMed] [Google Scholar]
- 44.Cho JH, Sung BH, Kim SC. Buforins: histone H2A-derived antimicrobial peptides from toad stomach. Biochim Biophys Acta. 2009;1788(8):1564–9. doi: 10.1016/j.bbamem.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 45.Minn I, Kim HS, Kim SC. Antimicrobial peptides derived from pepsinogens in the stomach of the bullfrog, Rana catesbeiana. Biochim Biophys Acta. 1998;1407(1):31–9. doi: 10.1016/s0925-4439(98)00023-4. [DOI] [PubMed] [Google Scholar]
- 46.Uzzell T, Stolzenberg ED, Shinnar AE, Zasloff M. Hagfish intestinal antimicrobial peptides are ancient cathelicidins. Peptides. 2003;24(11):1655–67. doi: 10.1016/j.peptides.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 47.Eisenhauer PB, Harwig SS, Lehrer RI. Cryptdins: antimicrobial defensins of the murine small intestine. Infect Immun. 1992;60(9):3556–65. doi: 10.1128/iai.60.9.3556-3565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarikaya A, Record R, Wu CC, Tullius B, Badylak S, Ladisch M. Antimicrobial activity associated with extracellular matrices. Tissue Eng. 2002;8(1):63–71. doi: 10.1089/107632702753503063. [DOI] [PubMed] [Google Scholar]
- 49.Cuesta A, Meseguer J, Esteban MÃ. Molecular and functional characterization of the gilthead seabream β-defensin demonstrate its chemotactic and antimicrobial activity. Mol Immunol. 2011;48(12–13):1432–8. doi: 10.1016/j.molimm.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 50.Yenugu S, Chintalgattu V, Wingard CJ, Radhakrishnan Y, French FS, Hall SH. Identification, cloning and functional characterization of novel beta-defensins in the rat (Rattus norvegicus). Reprod Biol Endocrinol. 2006;4:7. doi: 10.1186/1477-7827-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cole AM, Weis P, Diamond G. Isolation and characterization of pleurocidin, an antimicrobial peptide in the skin secretions of winter flounder. J Biol Chem. 1997;272(18):12008–13. doi: 10.1074/jbc.272.18.12008. [DOI] [PubMed] [Google Scholar]
- 52.Ge Y, MacDonald DL, Holroyd KJ, Thornsberry C, Wexler H, Zasloff M. In vitro antibacterial properties of pexiganan, an analog of magainin. Antimicrob Agents Chemother. 1999;43:782–8. doi: 10.1128/aac.43.4.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sai KP, Jagannadham MV, Vairamani M, Raju NP, Devi AS, Nagaraj R, et al. Tigerinins: novel antimicrobial peptides from the Indian frog Rana tigerina. J Biol Chem. 2001;276(4):2701–7. doi: 10.1074/jbc.M006615200. [DOI] [PubMed] [Google Scholar]
- 54.Schadich E, Mason D, Cole AL. Neutralization of bacterial endotoxins by frog antimicrobial peptides. Microbiol Immunol. 2012;57(2):159–61. doi: 10.1111/1348-0421.12012. [DOI] [PubMed] [Google Scholar]
- 55.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987;84(15):5449–53. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwab GE, Reeves PR. Comparison of the bactericidal activity of different vertebrate sera. J Bacteriol. 1966;91(1):106–12. doi: 10.1128/jb.91.1.106-112.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ourth DD, Chung KT. Purification of antimicrobial factor from granules of channel catfish peripheral blood leucocytes. Biochem Biophys Res Commun. 2004;313(1):28–36. doi: 10.1016/j.bbrc.2003.11.093. [DOI] [PubMed] [Google Scholar]
- 58.Merchant ME, Leger N, Jerkins E, Mills K, Pallansch MB, Paulman RL, et al. Broad spectrum antimicrobial activity of leukocyte extracts from the American alligator (Alligator mississippiensis). Vet Immunol Immunopathol. 2006;110(3–4):221–8. doi: 10.1016/j.vetimm.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Leelawongtawon R, Siruntawineti J, Chaeychomsri W, Sattaponpan C. Antibacterial and antifungal activities from Siamese crocodile blood. J Med Assoc Thai. 2011;93(Suppl 7):S58–64. [PubMed] [Google Scholar]
- 60.Harwig SS, Swiderek KM, Kokryakov VN, Tan L, Lee TD, Panyutich EA, et al. Gallinacins: cysteine-rich antimicrobial peptides of chicken leukocytes. FEBS Lett. 1994;342(3):281–5. doi: 10.1016/0014-5793(94)80517-2. [DOI] [PubMed] [Google Scholar]
- 61.Evans EW, Beach GG, Wunderlich J, Harmon BG. Isolation of antimicrobial peptides from avian heterophils. J Leukoc Biol. 1994;56(5):661–5. doi: 10.1002/jlb.56.5.661. [DOI] [PubMed] [Google Scholar]