Abstract
Background
Cockroaches are among the most common pests in public dwellings and health facilities. Their presence can raise safety concerns, especially as they maybe carriers of pathogenic organisms.
Methods
This study was carried out to isolate and identify the bacterial flora from German cockroaches (Blattella germanica). Cockroaches collected by hand catches from two public hospital environments in Tebessa city (northeast Algeria) were screened for microbial load from their external surfaces and alimentary tract using standard bacterial protocols.
Results
A total of 174 bacterial isolates were isolated from 39 German cockroach specimens. The most common and abundant bacterial species belonged to the Pseudomonas group (23.5%) and Serratia (13.2%). Pathogens like Staphylococcus aureus were also isolated, as well as opportunistic pathogens like Klebsiella species and food spoilage bacteria such as Enterobacter and Citrobacter species were isolated from both external surface and digestive tract of the insect. Generalized linear models (GLM) were performed to analyze the variation of abundances and occurrences of bacterial isolates harboured by B. germanica. The GLMs revealed that the main factors affecting variation of bacterial diversity and abundance were sex and hospital (P < 0.001).
Conclusion
The findings of this study suggest that German cockroach acts as reservoir and potential vector of some bacterial pathogens.
Keywords: German cockroach, Blattella germanica, Domiciliary fauna, Synanthropic organism, Hospital environment, Bacterial diversity, Pathogen bacteria
Introduction
Cockroaches are primitive and highly successful winged insects.1 Of the 4000 known species, only a dozen or so are considered as pests living in or around human structures and dwellings. Synanthropic animal species, including cockroaches, live in most human habitations, especially where food is stored, processed, prepared, or served. They breed in buildings and share human food and shelter. Apart from human food, they also consume putrefied and decaying organic matter.2,3 During the day, these insects hide in isolated locations or in gaps of the walls, but become active at night when they can move about unnoticed.4 The German cockroach, Blattella germanica (Dictyoptera: Blattellidae), is by far the most serious and predominant cosmopolitan pest in the world due to changes in human travel, commerce, and the urban environment.5–7
Cockroaches have been detected around hospitals, sick rooms, areas of intensive care, surgical sections, etc.8 Indeed, cockroaches are potential vectors of pathogenic organisms in the hospital environment.9 In addition, numerous published papers recognized the association of cockroaches with several infectious diseases and the spread of drug-resistant microbes worldwide.3,10–12
The medical importance of cockroaches is much greater than generally realized as they have been shown to carry diverse pathogenic and non-pathogenic bacterial flora, different protozoa, pathogenic helminthes, fungi, and viruses, but their role in the direct transmission of infection has seldom been established and is somewhat uncertain.2,12–15
It was reported that 98% of cockroaches found in medical facilities could carry pathogens on their integuments or digestive tracts.16 Indeed, many potential pathogens such as Escherichia coli, Enterobacter cloacae, Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella spp., Salmonella typhi, and Shigella dysenteriae were isolated from cockroaches collected in hospitals.11,16–18
Moreover, association of cockroach with the human environment can cause direct food contamination and several health problems such as allergic responses (skin rashes, watery eyes, and sneezing) particularly in patients who have a lung disease such as asthma.6,19–21
Domestic cockroaches exist in many human habitats, such as hospitals, restaurants, offices, homes, markets, and in the urban community together with the bacteria it harbours. They are important in the spread of diseases and dispersal of pathogenic agents and thus play a role of potential vectors of diseases due to their mobility and constant attendance with humans. Notwithstanding the importance of studies of cockroaches in the field of preventive medicine, there is no information on the status of the abundance of microorganisms and bacterial species harboured by the cockroaches in different habitats in Algeria or North Africa. The objective of this study is to investigate the bacterial load isolated from the alimentary tract (AT) and external surfaces of the German cockroach captured in two public hospitals in Tebessa, northeast Algeria.
Materials and Methods
Collection of cockroach specimens
Over a period of 2 months (April and May 2012), a set of 39 adult cockroaches were manually hand caught using sterile entomological forceps. These 2 months correspond to the end of winter and mid-spring season, where cockroaches exhibit high rate of growth and development. German cockroaches were caught once per week at night, from 18:00 to 23:00. Captures were carried out inside treatment and sick rooms of two public hospitals A and B in Tebessa (northeast Algeria). These two critical places (treatment and sick rooms) were selected because they are supposed to be high-septic area, and thus free of all kind of insects and pathogens. Hospital A is intended to cover a city with human population of 196 000 persons, while hospital B covers 85 000 people. The two hospitals are located 30 km apart. The caught cockroaches were kept in sterile test tubes until transported to the laboratory for species and sex identification, under a dissecting microscope, using standard taxonomic keys.22
Bacterial isolation and identification
Each trapped B. germanica was frozen at 0°C for 5–10 minutes. Then, a swab from the outer surface was performed to analyze the external microbial community. A homogenous enrichment suspension was prepared in nutrient broth. The next step was to remove the external contamination by washing the cockroach’s body with 70% ethyl alcohol for 2 minutes. Then, the body was washed in sterile normal saline (0.9%) for 2–3 minutes in order to remove the effect of alcohol. To evaluate the internal microbial flora, the digestive organ of each cockroach was dissected out under sterile conditions and a homogenous suspension was prepared in 5 ml of nutrient broth.
Aliquots (0.01 ml) of both prepared samples including internal and external suspensions were separately cultured on (i) plates of MacConkey agar (Fluka Analytical, St. Louis, USA), (ii) mannitol salt agar (Difco, New Jersey, USA), (iii) SS (Salmonella Shigella) agar (Fluka Analytical, St. Louis, USA), and (iv) cetrimide agar (Merck, Tunis, Tunisia) at 37°C overnight (24 hours). The isolated colonies were identified by standard bacteriological procedures. Each representative colony was characterized by (i) its macroscopic morphology, (ii) Gram stain, and (iii) several conventional approaches including production of catalase, coagulase, and respiratory type. Further identification of bacteria was achieved using a commercially available systems, i.e. Analytical Profile Index ‘API’ System (bioMérieux, France).
Data analyses
The distribution of bacterial isolate densities over the three different denominators (hospital, species sex, or organ position) was represented using relative abundance (RA), which was calculated as the percentage of abundance of each bacterial species isolate by the total number of isolates counted in a given observation.
Bacterial species diversity was assessed by the total specific richness ‘S’, which corresponds to the total number of identified bacterial species at each hospital, insect sex or organ position. Shannon’s index (H′ = −Σ(pi × log2 pi)) and evenness (E = H′/log2 S) were applied for the measurement of bacterial diversity (alpha diversity: that is defined as the species richness based on the number of species and the proportion in which each species is represented in the community) in each sampled hospital, species sex, or organ position based on the relative density pi of the ith species.23
The effects of hospital, insect sex, and organ position on the number of bacterial isolates and their occurrences were tested using generalized linear models (GLM).24 For the number of bacterial isolates, a Poisson distribution and log link were used, although a binomial distribution and logit link function were selected for their occurrences for GLM. Data used for the analyses were number of isolates and occurrences of the identified bacterial species. All variables and their interactions were included in the model. The GLM summary was given for each model including the Akaike Information Criterion (AIC).25,26 Then, the effect of ‘hospital’, ‘sex’, and ‘position’ for each model was tested using Type III ANOVAs, where the chi-square and P value were the outputs.25 All statistical tests were performed with the Rcmdr package version 2.0-0 of R software.26,27
Results
Bacterial isolation and abundance
All German cockroaches collected from both hospitals A and B contained various microorganisms, where 12 different bacterial species of Gram-negative bacilli and Gram-positive cocci were identified from the external surface and AT of the surveyed cockroaches (Table 1).
Table 1. Relative abundances of bacterial species isolates harboured by Blattella germanica (N = 39) collected in two hospitals in Tebessa (northeast Algeria).
| Bacteria | Hospital A (N = 29) | Hospital B (N = 10) | Overall | ||
| AT (%) | ES (%) | AT (%) | ES (%) | ||
| Pseudomonas aeruginosa | 0 | 1 (1.6) | 4 (13.8) | 2 (8.0) | 7 (4.0) |
| Pseudomonas sp. | 13 (22.4) | 10 (16.1) | 4 (13.8) | 7 (28.0) | 34 (19.5) |
| Staphylococcus aureus | 9 (15.5) | 13 (21.0) | 1 (3.4) | 1 (4.0) | 24 (13.8) |
| Non-pathogenic Staphylococcus | 2 (3.4) | 5 (8.1) | 1 (3.4) | 1 (4.0) | 9 (5.2) |
| Citrobacter freundii | 9 (15.5) | 12 (19.4) | 6 (20.7) | 3 (12.0) | 30 (17.2) |
| Enterobacter cloacae | 8 (13.8) | 7 (11.3) | 4 (13.8) | 3 (12.0) | 22 (12.6) |
| Enterobacter aerogenes | 1 (1.7) | 0 | 0 | 0 | 1 (0.6) |
| Enterobacter sp. | 1 (1.7) | 0 | 0 | 0 | 1 (0.6) |
| Klebsiella pneumoniae | 7 (12.1) | 8 (12.6) | 2 (6.9) | 4 (16.0) | 21 (12.1) |
| Pantoea sp. | 0 | 1 (1.6) | 1 (3.4) | 0 | 2 (1.1) |
| Serratia marcescens | 8 (13.8) | 5 (8.1) | 5 (17.2) | 4 (16.0) | 22 (12.6) |
| Serratia sp. | 0 | 0 | 1 (3.4) | 0 | 1 (0.6) |
| Overall | 58 (100) | 62 (100) | 29 (100) | 25 (100) | 174 (100) |
AT, alimentary tract; ES, external surface.
Five bacterial species appeared frequently, P. aeruginosa, S. aureus, Klebsiella pneumoniae, Citrobacter freundii, and E. cloacae. These are referred to as potential pathogens. The other identified pathogenic and potentially pathogenic bacterial species included Enterobacter aerogenes, Enterobacter sp. and Serratia marcescens.
Of the 174 bacterial isolates obtained from German cockroaches in the two hospitals, 41 (RA = 23.5%) belonged to the group of Gram-negative Pseudomonas bacteria. A large number of these bacteria was found in hospital A (RA = 24/41). P. aeruginosa strains isolated from B. germanica presented a minor group of Pseudomonas spp., whereas only seven different strains were isolated and identified with an RA of 4% of the total germs. Most P. aeruginosa were isolated from cockroaches captured in hospital B (Table 1).
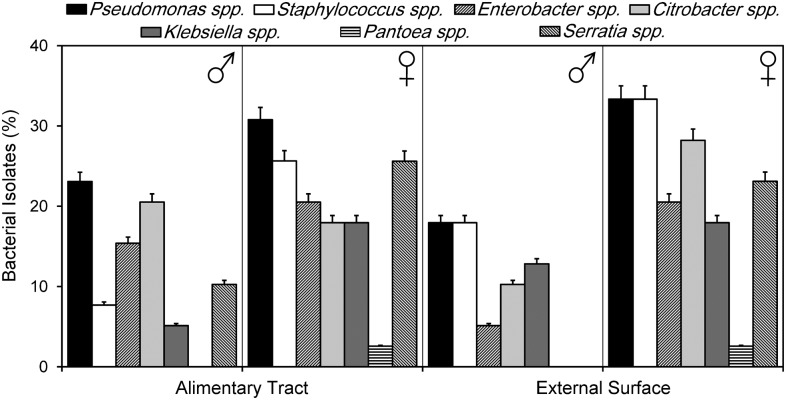
The Pseudomonas group dominated bacterial isolates obtained from both AT and external surface of female and male cockroaches. Overall, the RA of Pseudomonas spp. was quite similar internally and externally in males (RA = 23.1 and 17.9%, respectively) and females (30.8 and 33.3%, respectively). The Staphylococci group came in the second position in terms of isolate frequency (RA) with 33 isolated strains, distributed on both sides of the external surface and/or the gastrointestinal tract of both insect sexes. Female cockroaches exhibited the most important reservoirs of Staphylococcus with RA = 25.6 and 33.3% in both external surface and AT, respectively, versus males with 7.7% in AT and 17.9% on external surface (Fig. 1).
Figure 1.
Relative abundances of major bacterial groups isolated from males (N = 14) and females (N = 25) of Blattella germanica captured in hospitals of northeast Algeria.
The predominant bacterial species was S. aureus with a frequency of 13.8% followed by coagulase-negative Staphylococci. The external surface of cockroaches presented the most contaminated position with S. aureus. Female cockroaches contained more enterobacterial species isolates (69/100) with a frequency of 39.7% compared with the total isolated germs, while males included 17.8% of enterobacterial species isolates. A total of eight Gram-negative enterobacterial species were identified, namely C. freundii (RA = 17.2%), E. cloacae (RA = 12.6%), S. marcescens (RA = 12.6%), K. pneumoniae (RA = 12.1%), Pantoea sp. (RA = 1.2%), E. aerogenes (RA = 0.57%), Enterobacter sp. (RA = 0.57%), Serratia sp. (RA = 0.57%) (Table 1).
Enterobacter, Klebsiella, and Serratia had similar frequencies inside and outside insect’s body, with a larger tendency for females in both hospitals. Indeed, female cockroaches were found to be carrying more Citrobacter sp than male, both on the surface and in the digestive tract (Fig. 1).
Bacterial diversity and evenness
Relatively high diversity values were observed in the AT of Blattella females with 7 and 10 bacterial species identified in hospitals A and B, respectively. Generally, bacterial diversity values (species richness, Shannon index, and evenness) were almost closer to each other between the two sexes of cockroaches captured in each hospital. Bacterial populations isolated from the AT of female cockroaches captured in hospital B had the greater diversity values (H′ = 3.18 bits, E = 0.96), followed by the outer surface of females trapped at hospital A (H′ = 2.90 bits, E = 0.99) and the digestive tract of males at the same hospital (H′ = 2.86 bits, E = 0.90). Values of evenness were higher than 0.90 in both sampled hospitals, which indicates that species abundances at the two hospitals were evenly distributed (Table 2).
Table 2. Diversity indices of bacterial species identified in cockroaches associated with the surveyed hospitals.
| Hospital | Sex | Position | N | S | N/S | H′ | E |
| A | M | AT | 22 | 9 | 2.44 | 2.86 | 0.90 |
| ES | 13 | 5 | 2.6 | 2.30 | 0.99 | ||
| F | AT | 36 | 7 | 5.14 | 2.67 | 0.95 | |
| ES | 49 | 9 | 5.44 | 2.90 | 0.92 | ||
| B | M | AT | 10 | 5 | 2 | 2.25 | 0.97 |
| ES | 12 | 7 | 1.71 | 2.63 | 0.94 | ||
| F | AT | 19 | 10 | 1.9 | 3.18 | 0.96 | |
| ES | 13 | 6 | 2.16 | 2.32 | 0.90 |
AT, alimentary tract; ES, external surface; N, number of bacterial isolates; S, species richness; H′, Shannon’s index; E, evenness; M, male cockroach; F, female cockroach.
Factors affecting bacterial abundance
Number of bacterial isolates was significantly (P < 0.001) positively associated with external surfaces of female cockroaches collected in hospital A which represented the intercept of the GLM. However, bacterial isolates decreased significantly within hospital B, male insects and the interaction of Hospital B × male × AT. Isolate numbers were positively related with Hospital B × male and male × AT. Regarding the occurrence of isolates, the GLM revealed a negative relationship with the term ‘Hospital B × male × AT’, whereas the others terms of the model were not significant (Table 3).
Table 3. Results from the GLM for the effects of hospital, insect sex, and position on the number and occurrence of bacterial isolates in the hospital environments of Tebessa region, northeast Algeria.
| Variable | Estimate | SE | Z | P | χ2 | P |
| Number of bacterial isolates | ||||||
| Intercept | 1.41 | 0.14 | 9.85 | <0.001 | ||
| Hospital B | −1.33 | 0.31 | −4.25 | <0.001 | ||
| Male | −1.33 | 0.31 | −4.25 | <0.001 | ||
| AT | −0.31 | 0.22 | −1.40 | 0.160 | ||
| Hospital B × male | 1.25 | 0.51 | 2.46 | 0.014 | ||
| Hospital B × AT | 0.69 | 0.42 | 1.63 | 0.103 | ||
| Male × AT | 0.83 | 0.41 | 2.02 | 0.043 | ||
| Hospital B × male × AT | −1.40 | 0.70 | −2.01 | 0.045 | ||
| Hospital | 25.67 | <0.001 | ||||
| Sex | 21.12 | <0.001 | ||||
| Position | <0.01 | 0.999 | ||||
| Hospital × sex | 2.08 | 0.149 | ||||
| Hospital × position | 0.28 | 0.595 | ||||
| Sex × position | 1.13 | 0.287 | ||||
| Hospital × sex × position | 4.09 | 0.043 | ||||
| Deviance | 8.19 | |||||
| Φ (df = 7) | 1.17 | |||||
| AIC | 382.06 | |||||
| Occurrence of bacterial isolates | ||||||
| Intercept | 1.10 | 0.67 | 1.65 | 0.099 | ||
| Hospital B | −1.10 | 0.88 | −1.25 | 0.213 | ||
| Male | −1.44 | 0.89 | −1.62 | 0.106 | ||
| AT | −0.76 | 0.89 | −0.86 | 0.390 | ||
| Hospital B × male | 1.77 | 1.21 | 1.46 | 0.143 | ||
| Hospital B × AT | 2.37 | 1.31 | 1.81 | 0.071 | ||
| Male × AT | 2.20 | 1.25 | 1.75 | 0.080 | ||
| Hospital B × male × AT | −4.48 | 1.79 | −2.51 | 0.012 | ||
| Hospital | 0.18 | 0.673 | ||||
| Sex | 1.59 | 0.208 | ||||
| Position | 0.71 | 0.399 | ||||
| Hospital × sex | 0.16 | 0.689 | ||||
| Hospital × position | <0.01 | 0.995 | ||||
| Sex × position | <0.01 | 0.965 | ||||
| Hospital × sex × position | 6.62 | 0.010 | ||||
| Deviance | 6.986 | |||||
| Φ (df = 7) | 0.998 | |||||
| AIC | 135.64 |
SE, standard error; AT, alimentary tract; Φ, dispersion (Φ = deviance/degree of freedom); AIC, Akaike Information Criterion; GLM, generalized linear models.
The GLMs revealed a very high significant effect (P < 0.001) of ‘hospital’ and ‘insect sex’ on the variation of numbers of bacterial isolates harboured by cockroaches. In general, the interactions of the factors ‘hospital’, ‘sex’, ‘organ position’ of the tested GLMs had no effect on variations of both abundance and occurrence of isolates; indeed all GLM terms were not significant (P > 0.05) except the interaction ‘Hospital × sex × position’ that was significant for both models P = 0.043 and P = 0.010, respectively (Table 3).
Discussion
The present results showed widespread bacterial contamination of all German cockroaches collected from the surveyed hospitals. Indeed, cockroaches captured in homes, hospitals, or other locals habitually contain a large number of micro-organisms.28,29 In hospital environments, cockroaches could be efficient carriers of nosocomial infections through dispersal and spread of pathogenic agents, especially to patients in intensive care, neonatal units, long-term care facilities, and nursing homes.14,18,30,31
In this study, 174 bacterial isolates of 12 bacterial species from 10 different genera were identified from external surfaces and ATs of B. germanica captured at two different hospitals in Tebessa region. The cockroaches harboured some bacterial species known as pathogenic or potentially pathogenic, including P. aeruginosa, S. aureus, and K. pneumoniae. These bacteria are opportunistic, known for causing several nosocomial sicknesses, such as lung, skin and eye infections, endocarditis, and digestive disorders. Cockroaches have previously been shown to be potential vectors of K. pneumoniae in the hospital environment.9
The predominant bacteria isolated from the captured cockroaches were Pseudomonas spp., Staphylococcus spp., C. Freundii, and K. pneumoniae. In contrast, the most common species reported from hospitals, domestic dwellings, and food establishments were Gram-negative E. coli, Klebsiella spp., Enterobacter spp., and Citrobacter spp.32 Although E. coli is normally present in the intestines of humans and other warm-blooded animals and reptiles,33 however, due to its low occurrence in cockroaches, it was completely absent in the current investigation, which is fairly similar to findings of other studies.34,35
The high prevalence of bacteria harboured in the body and AT of the cockroaches is a public health risk, increasing the likelihood of transmission of nosocomial infections. Several authors reported that cockroaches collected from hospitals have more bacterial counts than cockroaches found in residential areas due to their permanent contact with infested sites.14,18,31 Hospital environments may be more conducive to accruing bacteria from many different contaminated sources such as water and food causing high rates of bacterial prevalence. Multidrug-resistant bacterial strains of medical importance have also been isolated from cockroaches in different hospitals and urban environments.18,36,37
Bacterial species richness statistically varied between hospitals, which provide information on their sanitation conditions and thus food dispersal and availability for insects, which have a direct impact on insect population density. It is known that sanitation-improved sites carry less pathogens and synanthropic organisms.15,38 The factor of ‘insect sex’ had a significant effect on variation of species richness, which may also be explained by phenological and behavioural differences in sexes of the species.39
The present results showed that there was no significant difference in bacterial abundance or in species richness between the outer surface and the digestive tract of cockroaches. Our findings were in concordance with those reported from hospitals of western Iran.40 While in Beijing, a significant difference was indicated between the external surfaces and the AT for the Gram-negative bacterial species in comparison with Gram-positive which exhibit no significant difference.28
This may be explained by the fact that there is a homogenization/contamination of the bacterial flora of the external environment with the inside of the body. Cockroaches use their mouthparts and legs for grooming increasing the likelihood of direct contact with contaminated surfaces. However, the occurrence of Gram-negative species on the surface was lower than in the tract, compared to Gram-positive species that showed low density in both body parts. These results are similar to those reported in the literature.28,34 This could be explained by the fact that Gram-negative enteric bacteria are better adapted to the digestive tract where the stability of conditions and nutrients are higher than outside.28
Cockroaches can readily move from contaminated zones (garbage) and create the opportunity to spread disease-causing organisms on food and food preparation surfaces.2 Many studies have highlighted a possible and potential risk of human contamination through bacteria carried by cockroaches in connection with human habitats.1,36,37
Conclusion
Poor hygiene is probably the most important factor in the spread of nosocomial pathogens. Cockroaches live in human structures and dwellings, including hospitals, where conditions are suited to their bio-ecological requirements (ambient temperature, humidity, food, etc.). Results of this study showed high prevalence of pathogenic organisms in the body and AT of female cockroaches. The findings of this study indicate that cockroaches in health and medical centres may play an important role as mechanical vectors of a wide range of pathogenic bacteria including several antibiotic-resistant species. B. germanica poses a serious risk factor to human health as it significantly decreases sanitation level by spreading pathogens as it moves. Therefore, further studies are clearly necessary to be carried out in order to investigate relevant control methods against cockroaches using effective health programs focusing on hygiene measures in medical institutions.
Disclaimer Statements
Contributors Taha Menasria, Fatima Moussa, Souad El-Hamza and Samir Tine designed and carried out the experimental study. Taha Menasria, Rochdi Megri and Haroun Chenchouni analysed data and wrote the manuscript. All authors approved the manuscript.
Funding This study did not receive any funding.
Conflicts of interest None.
Ethics approval Ethical approval was not required for the current study.
Acknowledgments
The authors thank Dr. Michael J. Barry (Department of Biology, Sultan Qaboos University) for his helpful linguistic editing.
References
- 1.Jeffery J, Sulaiman S, Oothuman P, Vellayan S, Zainol-Ariffin P, Paramaswaran S, et al. Domiciliary cockroaches found in restaurants in five zones of Kuala Lumpur Federal Territory, Peninsular Malaysia. Trop Biomed. 2012;29:180–6. [PubMed] [Google Scholar]
- 2.Bennett GW. Cockroaches and disease. In: Capinera JL, editor. Encyclopedia of entomology. Dordrecht: Springer; 2008. p. 948–52. [Google Scholar]
- 3.Pavlik I, Falkinham JO. The occurrence of pathogenic and potentially pathogenic mycobacteria in animals and the role of the environment in the spread of infection. In: Kazda J, Pavlik I, Falkinham JO III, Hruska K, editors. The ecology of mycobacteria: impact on animal’s and human’s health. Dordrecht: Springer; 2009. p. 199–281. [Google Scholar]
- 4.Cochran DG. Cockroaches: their biology, distribution and control. Geneva, Switzerland: World Health Organization; Document No.: WHO/CDS/CPC/WHOPES/99.3; 1999. [Google Scholar]
- 5.Milner RJ, Pereira RM. Microbial control of urban pests – cockroaches, ants and termites. In: Lacey LA, Kaya HK, editors. Field manual of techniques in invertebrate pathology. Dordrecht: Springer; 2007. p. 695–711. [Google Scholar]
- 6.Bonnefoy X, Kampen H, Sweeney K. Public health significance of urban pests. Geneva, Switzerland: WHO publications; 2008. pp. 53–84. [Google Scholar]
- 7.Lihoreau M, Costa JT, Rivault C. The social biology of domiciliary cockroaches: colony structure, kin recognition and collective decisions. Insect Soc. 2012;59:445–52. [Google Scholar]
- 8.Dubus J, Guerra M, Bodiou A. Cockroach allergy and asthma. Allergy. 2001;56:351–2. doi: 10.1034/j.1398-9995.2001.00109.x. [DOI] [PubMed] [Google Scholar]
- 9.Cotton MF, Wasserman E, Pieper CH, Theron DC, Van-Tubbergh D, Campbell G, et al. Invasive disease due to extended spectrum beta lactamase-producing Klebsiella pneumoniae in a neonatal unit: the possible role of cockroaches. J Hosp Infect. 2000;44:13–7. doi: 10.1053/jhin.1999.0650. [DOI] [PubMed] [Google Scholar]
- 10.Prado MA, Pimenta FC, Hayashid M, Souza PR, Pereira MS, Gir E. Enterobacteria isolated from cockroaches (Periplaneta americana) captured in a Brazilian Hospital. Rev Panam Salud Publica. 2002;11:93–8. doi: 10.1590/s1020-49892002000200005. [DOI] [PubMed] [Google Scholar]
- 11.Tachebe J, Erku W, Gerbe-Michae LT, Ashenafi M. Cockroach associated food-borne bacteria from hospital and restaurant in Addis Ababa, Ethiopia: distribution and antibiograms. J Rural Trop Public Health. 2006;5:34–41. [Google Scholar]
- 12.Tatfeng YM, Usuanle A, Orukpe A, Digban AK, Okodua M, Oviasogie F, et al. Mechanical transmission of pathogenic organisms: the role of cockroaches. J Vector Borne Dis. 2005;42:129–34. [PubMed] [Google Scholar]
- 13.Fotedar R, Banerjee UV, Verma A. Cockroaches (Blattella germanica) as carriers of microorganisms of medical importance in hospitals. Epidemiol Infect. 1991;107:181–7. doi: 10.1017/s0950268800048809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fotedar R, Nayar E, Samantray JC, Shriniwas UB, Dogra V, Kumar A. Cockroaches as vectors of pathogenic bacteria. J Commun Dis. 1989;21:318–22. [PubMed] [Google Scholar]
- 15.Marriott NG, Gravani RB, editors. Pest control. Principles of food sanitation (Food science texts series) New York: Springer; 2006235–56. [Google Scholar]
- 16.Cloarec A, Rivault C, Fontaine F, Le Guyader A. Cockroaches as carriers of bacteria in multi-family dwellings. Epidemiol Infect. 1992;109:483–90. doi: 10.1017/s0950268800050470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivault C, Cloarec A, Le Guyader A. Bacterial load of cockroaches in relation to urban environment. Epidemiol Infect. 1993;110:317–25. doi: 10.1017/s0950268800068254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salehzadeh A, Tavacol P, Mahjub H. Bacterial, fungal and parasitic contamination of cockroaches in public hospitals of Hamadan. Iran J Vector Borne Dis. 2007;44:105–10. [PubMed] [Google Scholar]
- 19.Chew GL, Carlton EJ, Kass D, Hernandez M, Clarke B, Tiven J, et al. Determinants of cockroach and mouse exposure and associations with asthma in families and elderly individuals living in New York City public housing. Ann Allergy Asthma Immunol. 2006;97:502–13. doi: 10.1016/S1081-1206(10)60942-8. [DOI] [PubMed] [Google Scholar]
- 20.Berenji F, Fata A, Hosseininejad Z. A case of Moniliformis moniliformis (Acanthocephala) infection in Iran. Korean J Parasitol. 2007;45:145–8. doi: 10.3347/kjp.2007.45.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safari M, Amin R, Kashef S, Aleyasin S, Ayatollahi M. Cockroaches sensitivity in Iranian asthmatic children under the age of five years. Turk Thoracic J. 2009;10:26–30. [Google Scholar]
- 22.Appel AG. Blattella and related species. In: Rust MK, Owens JM, Reierson DA, editors. Understanding and controlling the German cockroach. New York: Oxford University Press; 1995. p. 1–20. [Google Scholar]
- 23.Magurran AE. Measuring biological diversity. Oxford: Wiley-Blackwell; 2004. [Google Scholar]
- 24.Zeileis A, Kleiber C, Jackman S. Regression models for count data in R. Research Report series. Vienna: Department of Statistics and Mathematics, Vienna University of Economics and Business Administration; 2007. [Google Scholar]
- 25.Fox J. Generalized linear models. In: Fox J, editor. Applied regression analysis and generalized linear models, Ch. 15, 2nd edn. Thousand Oaks: SAGE Publications; 2008. p. 379–424. [Google Scholar]
- 26.Fox J. Getting started with the R commander: a basic-statistics graphical user interface to R. J Stat Softw. 2005;14:1–42. [Google Scholar]
- 27.Anonymous R. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 28.Fu X, Ye L, Ge F. Habitat influences on diversity of bacteria found on German cockroach in Beijing. J Environ Sci (China). 2009;21:249–54. doi: 10.1016/s1001-0742(08)62259-7. [DOI] [PubMed] [Google Scholar]
- 29.Bouamama L, Sorlozano A, Laglaoui A, Lebbadi M, Aarab A, Gutierrez J. Antibiotic resistance patterns of bacterial strains isolated from Periplaneta americana and Musca domestica in Tangier, Morocco. J Infect Dev Ctries. 2010;4(4):194–201. doi: 10.3855/jidc.336. [DOI] [PubMed] [Google Scholar]
- 30.Fakoorziba MR, Eghbal F, Hassanzadeh J, Moemenbellah-Fard MD. Cockroaches (Periplaneta americana and Blattella germanica) as potential vectors of the pathogenic bacteria found in nosocomial infections. Ann Trop Med Parasitol. 2010;104:521–8. doi: 10.1179/136485910X12786389891326. [DOI] [PubMed] [Google Scholar]
- 31.Pai H-H. Multidrug resistant bacteria isolated from cockroaches in long-term care facilities and nursing homes. Acta Trop. 2012;125:18–22. doi: 10.1016/j.actatropica.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Chaichanawongsaroj N, Vanichayatanarak K, Pipatkullachat T, Polrojpanya M, Somkiatcharoen S. Isolation of Gram-negative bacteria from cockroach trapped from urban environment. Southeast Asian J Trop Med Public Health. 2004;35:681–4. [PubMed] [Google Scholar]
- 33.Tenaillon O, Skurnik D, Picard B, Denamur E. The population genetics of commensal Escherichia coli. Nat Rev Microbiol. 2010;8:207–17. doi: 10.1038/nrmicro2298. [DOI] [PubMed] [Google Scholar]
- 34.Pai H-H, Chen W-C, Peng C-F. Isolation of bacteria with antibiotic resistance from household cockroaches (Periplaneta americana and Blattella germanica). Acta Trop. 2005;93:259–65. doi: 10.1016/j.actatropica.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Wannigama DL, Dwivedi R, Zahraei-Ramazani A. Prevalence and antibiotic resistance of gram-negative pathogenic bacteria species isolated from Periplaneta americana and Blattella germanica in Varanasi, India. J Arthropod Borne Dis. 2014;8:10–20. [PMC free article] [PubMed] [Google Scholar]
- 36.Oliva GR, Díaz C, Fuentes González O, Martínez MD, Fernández C, Cordovi R, et al. Blattella germanica as a possible cockroach vector of microorganisms in a hospital. J Hosp Infect. 2010;74:93–5. doi: 10.1016/j.jhin.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Pai H-H, Chen W-C, Peng C-F. Cockroaches as potential vectors of nosocomial infections. Infect Control Hosp Epidemiol. 2004;25:979–84. doi: 10.1086/502330. [DOI] [PubMed] [Google Scholar]
- 38.Carling PC, Bartley JM. Evaluating hygienic cleaning in health care settings: what you do not know can harm your patients. Am J Infect Control. 2010;38:41–50. doi: 10.1016/j.ajic.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Schal C, Gautier JY, Bell WJ. Behavioural ecology of cockroaches. Biol Rev. 2008;59:209–54. [Google Scholar]
- 40.Nejati J, Keyhani A, Moosa-Kazemi SH, Mohammadi M, Mahjoob M, Boostanbakhsh A. Cockroaches’ bacterial infections in wards of hospitals, Hamedan city, west of Iran. Asian Pac J Trop Dis. 2012;2:381–4. [Google Scholar]