Abstract
We demonstrate the feasibility of a time-resolved fluorescence spectroscopy (TRFS) technique for intraluminal investigation of arterial vessel composition under intravascular ultrasound (IVUS) guidance. A prototype 1.8-mm (5.4 Fr) catheter combining a side-viewing optical fiber (SVOF) and an IVUS catheter was constructed and tested with in vitro vessel phantoms. The prototype catheter can locate a fluorophore in the phantom vessel wall, steer the SVOF in place, perform blood flushing under flow conditions, and acquire high-quality TRFS data using 337-nm wavelength excitation. The catheter steering capability used for the coregistration of the IVUS image plane and the SVOF beam produce a guiding precision to an arterial phantom wall site location of 0.53±0.16 mm. This new intravascular multimodal catheter enables the potential for in vivo arterial plaque composition identification using TRFS.
Keywords: fluorescence spectroscopy, atherosclerosis, fluorescence lifetime, intravascular ultrasound
Catheter-based examination of arterial wall plaque characteristics is an important area of interest, especially as a means to study and diagnose atherosclerotic cardiovascular disease, which is considered the main cause of death in developed nations.1 We report the development of a new catheter-based approach for the detection of atherosclerotic plaques using intravascular ultrasound (IVUS) as an anatomical guidance method for the positioning of optical catheters capable of time-resolved fluorescence spectroscopy (TRFS).
TRFS itself, which can utilize both spectral and time-domain parameters, has been shown to detect elastin, collagen, lipids, and other sources of autofluorescence in normal and diseased arterial walls that exhibit inflammatory processes as well as to characterize the biochemical composition of human atherosclerotic plaques both ex vivo and in vivo. The comparison of various optically based methods for the analysis of plaques has produced a compelling rationale for a TRFS approach. On the basis of accuracy of plaque type identification, the following optical modalities have produced quality metrics, in terms of sensitivity and specificity against tissue histology, of: TRFS (85%, 95%)2 and (81%, 94%),3 optical coherence tomography (OCT; ranges of 71 to 79% and 97 to 98%),4 Raman spectroscopy (79 and 85%),5 and near-infrared (NIR) spectroscopy (ranges of 77 to 90% in sensitivity and 89% to 93% in specificity).6 OCT,7 Raman spectroscopy,8 and NIR spectroscopy9 have been implemented in catheter-based devices for use in coronary arteries. The multimodal, multifunctional device described here can provide the opportunity for enhanced plaque diagnosis. The current approach will not only facilitate intravascular use of TRFS as the high-quality means to determine plaque composition but will also enable assessment of the plaque morphology or structure.
A practical means to implement the TRFS technique involves first an intravascular means of visual guidance for the optical fiber and second a steering method to coregister the optical fiber close to a particular arterial site in a moving blood environment. The novel approach described here addresses these issues to enable the optical and ultrasound modalities to operate in a beneficial and complementary way in a single multimodal catheter (MMC).
IVUS is now a commonly used catheter-based ultrasonic imaging technique to diagnose and guide interventional therapeutic procedures with real-time arterial cross-section images. It can provide specific lesion site identification and can provide direct guidance to an intraluminal location, and subsequent relocation, of specific lesion sites of interest. IVUS, as a catheter standard for ease of use,10 is an obvious choice for the intraluminal guidance of the optical probe for use in obtaining TRFS data from sites of suspected vulnerable plaque.
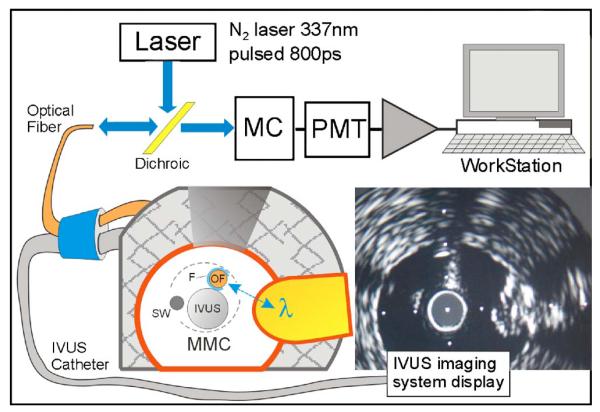
The optical fiber itself (RoMack, Inc., Williamsburg, Virginia) is a customized side-viewing optical fiber (SVOF), which is shown in Fig. 1 as OF. It is a 400-μm-diam fused silica fiber (multimode, NA=0.22) with a 45-deg polished metalized mirror termination for beam deflection at 90 deg to the fiber axis. The optical fiber is coupled to a dichroic mirror, which is coupled to a prototype TRFS apparatus in our lab. This apparatus is similar to a TRFS system we previously reported11 and allows for wavelength-resolved fluorescence lifetime measurements. In brief, a pulsed N2 laser (MNL205-C, LTB Lasertechnik Berlin, Germany, 337.1 nm, 800-ps pulse width, 30-Hz repetition rate) was used for autofluorescence excitation. The energy at the fiber tip was adjusted to 2 μJ/pulse by the adjustment of the iris in the fiber coupling port. The other major components of the optical system include a monochromator (MC; MicroHR, Horiba Jobin Yvon, Edison, New Jersey) for emission wavelength selection, a multichannel photomultiplier tube (MCP-PMT; R5916U-50, Hamamatsu, Bridgewater, New Jersey), a wideband amplifier (C-5594-12, 1.5-GHz bandwidth, Hamamatsu), and a fast digitizer (DPO 7254, 2.5-GHz bandwidth, 40 gigasamples/s, Tektronix, Richardson, Texas) with workstation. The single-fiber approach for TRFS data collection/analysis has been validated using fluorescence standard dyes and tissue endogenous fluorophores (elastin and collagen).
Fig. 1.
A simplified system diagram showing the optical and ultrasound pathways to support the MMC. A cross section of the distal end of the MMC is shown in a phantom artery with a fluorophore target plug placed in the wall. IVUS imaging can clearly orient the SVOF (OF) within the water flushing lumen (F) during the entire positioning procedure.
The IVUS imaging catheter used is a 3-Fr (1 mm) mechanical rotating type (SR Pro BSC, Fremont, California) catheter, which operates in the 30- to 40-MHz range with its ClearViewUltra (BSC) imaging system.
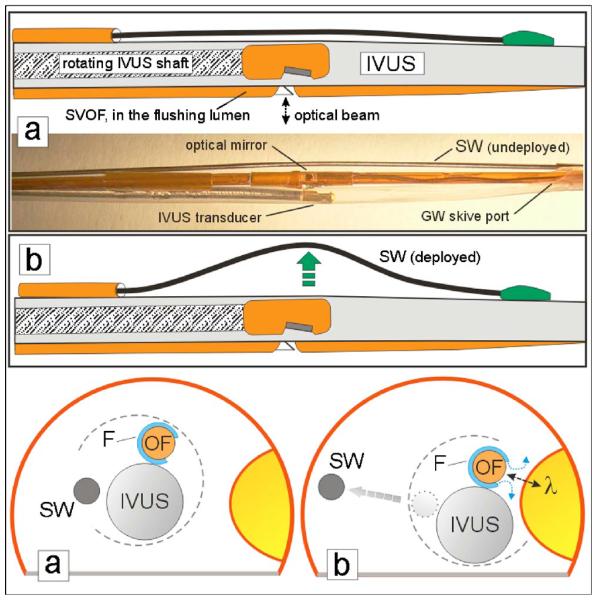
The prototype MMC device has three major components, which are assembled in parallel combination to perform image-guided TRFS, as shown in cross section in Figs. 1 and 2. The 1-mm-diam IVUS catheter is the largest component. The side-viewing optical fiber (SVOF) is assembled inside a 0.7-mm-diam water flushing lumen; it occupies the position of the IVUS monorail guide wire and, in this prototype design, is adhesively fixed in position at the distal end of the IVUS catheter. The third component is the steering wire (SW) which is housed in its own outboard lumen adhesively attached to the IVUS shaft. An operator push of the SW serves to move the MMC laterally (Fig. 2) toward the luminal wall site of interest.
Fig. 2.
Schematic and picture of the three-component construction of the MMC with deployment of lateral (steering) movement with the steering wire (SW). The normally compact MMC components are separated in the photo for clearer viewing. The SW in the undeployed state (a) is near the IVUS sheath and is mounted so that deployment (b) by pushing on the wire will move the MMC close to the fluorophore target in the phantom artery wall.
As shown in Figs. 1 and 3, the SVOF is rotated and adhesively positioned so that its optical mirror is directing light out of the fiber in a path at approximately 90 deg to the IVUS-SVOF axis. This allows the IVUS catheter to directly view the lumen wall target for TRFS investigation and also permits the mounting of the steering wire on the contralateral side of the beam, which allows more compact catheter assembly and permits more effective wire side-steering.
Fig. 3.
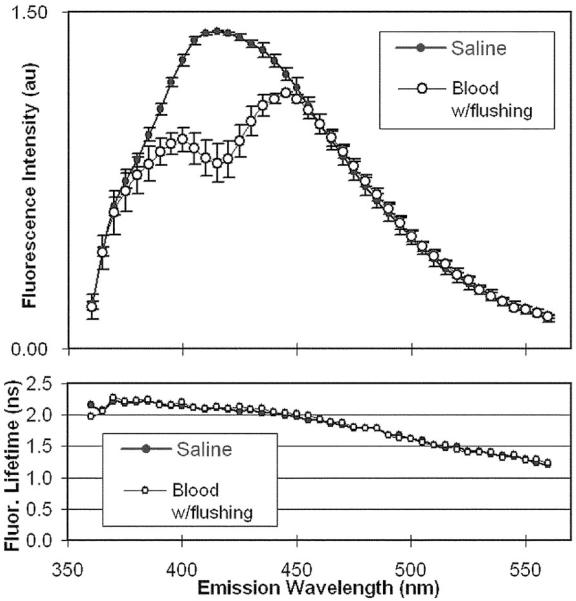
Top panel: Comparison of MMC-based fluorescence spectra from an ex vivo pig femoral artery for moving whole blood (N=7) conditions against the normalized spectral emission in diluted saline (N=5). Bottom panel: The corresponding fluorescence lifetimes along the emission spectrum.
A 356-μm-diam push-activated steering wire (SW; Fig. 2) is used to laterally direct the combination catheter to the wall; the proximal wire is confined in a small polyimide tube, which is adhesively fixed to the IVUS sheath. The small (0.7 mm diameter) fluid (saline) flushing tube surrounds the SVOF, except for a small saline exit window at the sideviewing optical beam path, to allow a local, low-volume water flush to achieve a large reduction in blood attenuation and scattering of the optical beam positioned close to the arterial wall.
Arterial phantoms made of polymethyl-methacrylate (PMMA) with wall-mounted fluorophore targets and ex vivo pig arteries were used to establish MMC performance metrics in a custom tank testing apparatus.
The optical beam width of the SVOF fundamentally determines the lower limit for optical spatial precision. The SVOF beam, exiting at 90 deg to the MMC length axis, was measured to be 0.84 mm (FWHM) at a distance of 1 mm from the fiber surface. Based on experimental data, the spatial convolution of the SVOF beam in water and a standard 0.5-mm fluorophore phantom were determined as a spatial reference standard to permit the subsequent coregistration accuracy of the MMC in an arterial phantom with the 0.5-mm fluorophore mounted on the inside wall of the phantom. Fifteen measurements using only IVUS guidance produced a spatial positioning accuracy of the SVOF beam of 0.53±0.16 mm.
An examination of the MMC performance in flowing whole blood conditions to assess (1) IVUS image guidance within either the phantom artery with a fluorescein isothiocyanate (FITC) fluorophore wall target or the pig femoral artery and (2) subsequent fluorescence spectroscopy was conducted in the following way. The arterial segment is mounted in the tank apparatus and blood introduced into the circulation system reservoir. The MMC catheter is then introduced through a hemostasis valve and advanced until the desired intraluminal wall position is in view with IVUS; the catheter is then rotated as needed to obtain the correct position (as shown in Figs. 1 and 2). The SW is then deployed to laterally shift the catheter for the optimal placement of the SVOF. With continuous low-volume (0.07 ml/s) saline flushing in the SVOF lumen, sample acquisitions of the intensity of fluorescence emission (470 nm for arterial data, 520 nm for FITC) are collected to gain feedback on good positioning. If the position yields a good fluorescence reading, the flushing is continued while the emission pulse transients across the entire fluorophore emission spectrum (360 to 560 nm, 5-nm interval) are recorded by the fast digitizing oscilloscope. The discrete spectral intensities were reconstructed by integrating each pulse as a function of time.
A pig femoral artery was used to test the effectiveness of saline flushing to diminish the optical attenuation effect of blood at 45% hematocrit. Using only IVUS for positional guidance, a calibration set of the TRFS data was collected first in water (N=5), followed by TRFS data collections with multiple catheter withdrawal and repositioning steps in bovine blood (N=7). The blood was circulated within the artery in a retrograde path with respect to the catheter direction at 5.9 cm/s. Saline flushing at 0.07 ml/s was continuously performed during the TRFS emission data collection across the emission spectrum at the rate of 0.77 s/wavelength (Fig. 3). We noted that the TRFS spectral intensity was modulated by the peak (at 415 nm) in the blood absorbance, but the timeresolved data (average lifetime values) were not affected. As anticipated, this demonstrates that fluorescence lifetime measurements are more robust than intensity measurements in the presence of the endogenous absorbers such as blood. A fast TRFS system, which has been fully tested and recently reported,12 will be used in the future to obtain the data from arteries in less than 1 second.
This 5.4-F prototype MMC design demonstrates the feasibility of the IVUS guided approach for intravascular TRFS and provides the means for future studies in vivo in animal models of the effects of drug therapies on the atherosclerotic plaque composition. The next generation design will further integrate the optical fiber into the IVUS construction as a smaller, more flexible, and multifunction catheter with a size of 4.6 F (1.5 mm).
Acknowledgments
The authors gratefully acknowledge Tat-Jin Teo at Boston Scientific Corp. for his cooperation and help with experimental materials. This work was supported in part by the NIH Grant No. R01 HL 067377 and the Philip Morris (USA and International) External Research Grant No. 07004732.
References
- 1.Honda Y, Fitzgerald PJ. Frontiers in intravascular imaging technologies. Circulation. 2008;117(15):2024–2037. doi: 10.1161/CIRCULATIONAHA.105.551804. [DOI] [PubMed] [Google Scholar]
- 2.Marcu L, Fang QY, Jo JA, Papaioannou T, Dorafshar A, Reil T, Qiao J, Baker JD, Freischlag JA, Fishbein MC. In vivo detection of macrophages in a rabbit atherosclerotic model by timeresolved laser-induced fluorescence spectroscopy. Atherosclerosis. 2005;181(2):295–303. doi: 10.1016/j.atherosclerosis.2005.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcu L, Jo JA, Fang QY, Papaioannou T, Reil T, Qiao J, Baker JD, Freischlag JA, Fishbein MC. Detection of ruptureprone atherosclerotic plaques by time-resolved laser-induced fluorescence spectroscopy. Atherosclerosis. 2009;204(1):156–164. doi: 10.1016/j.atherosclerosis.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Kang DH, Halpern EF, Tearney GJ. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106(13):1640–1645. doi: 10.1161/01.cir.0000029927.92825.f6. [DOI] [PubMed] [Google Scholar]
- 5.Motz JT, Fitzmaurice M, Miller A, Gandhi SJ, Haka AS, Galindo LH, Dasari RR, Kramer JR, Feld MS. In vivo Raman spectral pathology of human atherosclerosis and vulnerable plaque. J. Biomed. Opt. 2006;11(2):021003–1. doi: 10.1117/1.2190967. [DOI] [PubMed] [Google Scholar]
- 6.Moreno PR, Lodder RA, Purushothaman KR, Charash WE, O’Connor WN, Muller JE. Detection of lipid pool, thin fibrous cap, and inflammatory cells in human aortic atherosclerotic plaques by near-infrared spectroscopy. Circulation. 2002;105(8):923–927. doi: 10.1161/hc0802.104291. [DOI] [PubMed] [Google Scholar]
- 7.Tearney GJ, Boppart SA, Bouma BE, Brezinski ME, Weissman NJ, Southern JF, Fujimoto JG. Scanning single-mode fiber optic catheter-endoscope for optical coherence tomography. Opt. Lett. 1996;21(7):543–545. doi: 10.1364/ol.21.000543. [DOI] [PubMed] [Google Scholar]
- 8.Komachi Y, Sato H, Aizawa K, Tashiro H. Micro-optical fiber probe for use in an intravascular Raman endoscope. Appl. Opt. 2005;44(22):4722–4732. doi: 10.1364/ao.44.004722. [DOI] [PubMed] [Google Scholar]
- 9.Waxman S, Ishibashi F, Caplan JD. Rationale and use of near-infrared spectroscopy for detection of lipid-rich and vulnerable plaques. J. Nucl. Cardiol. 2007;14(5):719–728. doi: 10.1016/j.nuclcard.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Waxman S, Ishibashi F, Muller JE. Detection and treatment of vulnerable plaques and vulnerable patients—novel approaches to prevention of coronary events. Circulation. 2006;114(22):2390–2411. doi: 10.1161/CIRCULATIONAHA.105.540013. [DOI] [PubMed] [Google Scholar]
- 11.Fang QY, Papaioannou T, Jo JA, Vaitha R, Shastry K, Marcu L. Time-domain laser-induced fluorescence spectroscopy apparatus for clinical diagnostics. Rev. Sci. Instrum. 2004;75(1):151–162. doi: 10.1063/1.1634354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y, Liu R, Elson DS, Hollars CW, Jo JA, Park J, Sun Y, Marcu L. Simultaneous time- and wavelength-resolved fluorescence spectroscopy for near real-time tissue diagnosis. Opt. Lett. 2008;33(6):630–632. doi: 10.1364/ol.33.000630. [DOI] [PMC free article] [PubMed] [Google Scholar]