Full text
PDF




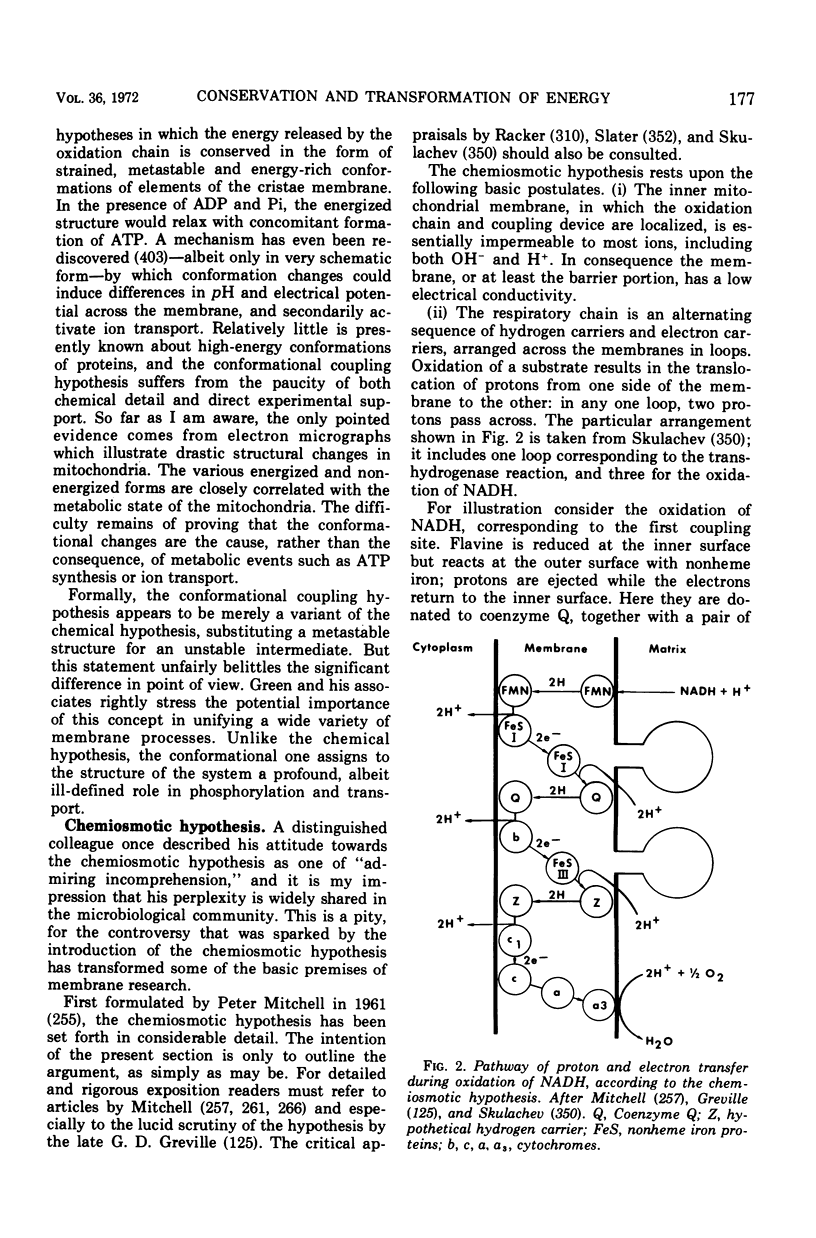





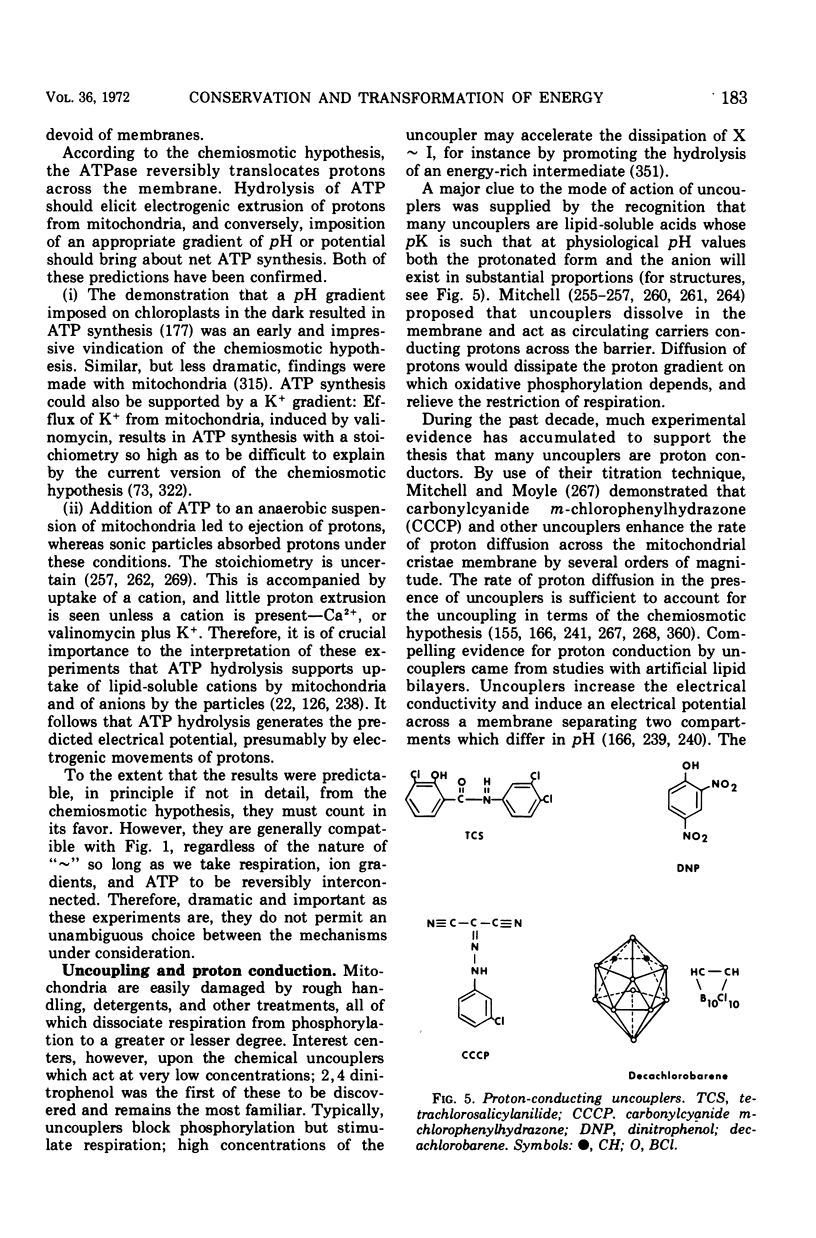




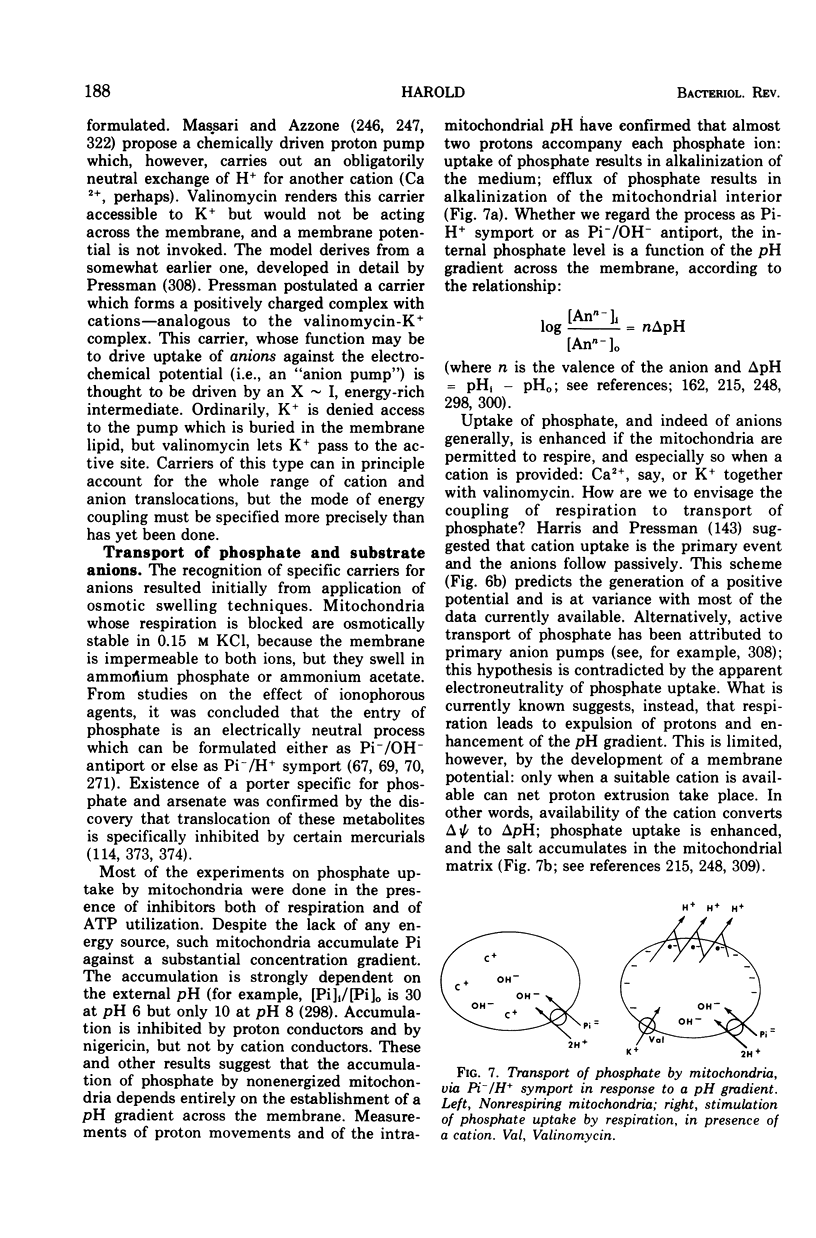








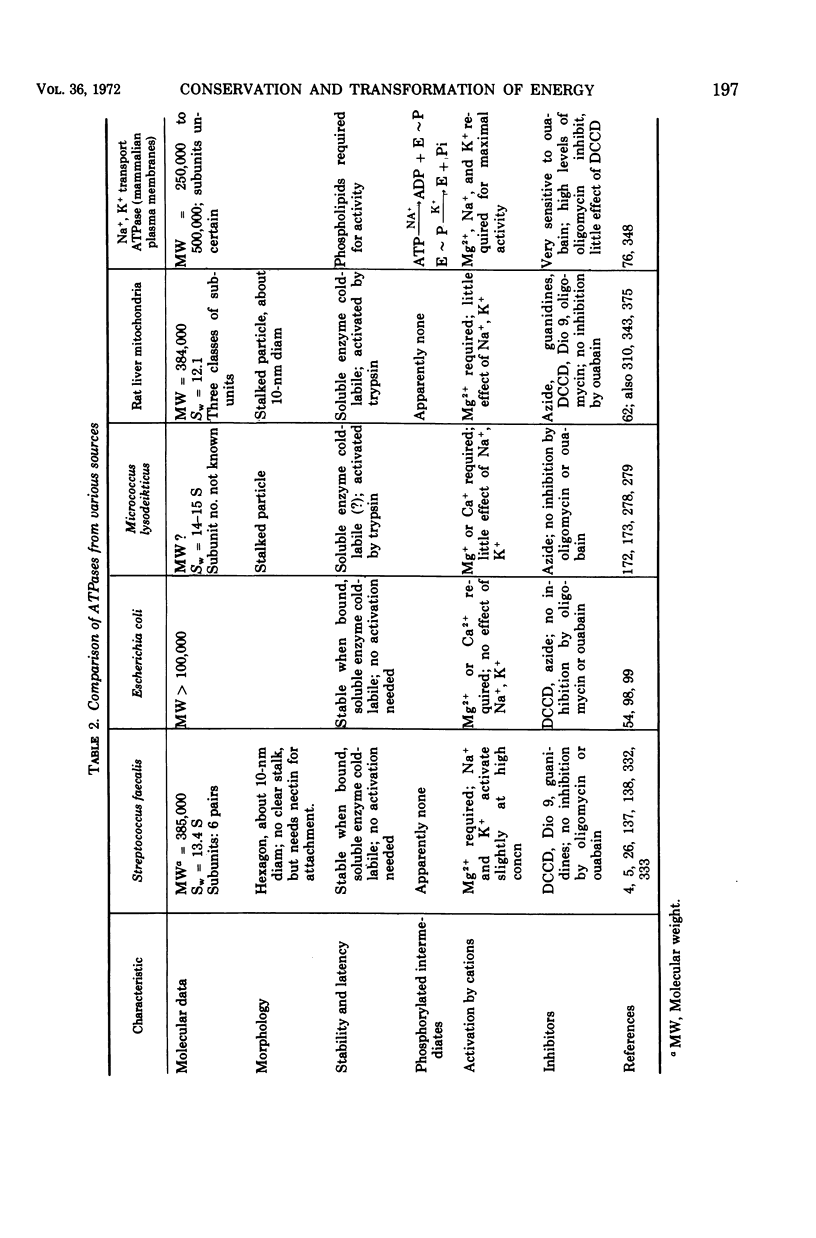

































Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D. ELECTRON MICROSCOPE OBSERVATIONS ON INTACT CELLS, PROTOPLASTS, AND THE CYTOPLASMIC MEMBRANE OF BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1965 Mar;89:855–873. doi: 10.1128/jb.89.3.855-873.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ABRAMS A. Reversible metabolic swelling of bacterial protoplasts. J Biol Chem. 1959 Feb;234(2):383–388. [PubMed] [Google Scholar]
- ASANO A., BRODIE A. F. OXIDATIVE PHOSPHORYLATION IN FRACTIONATED BACTERIAL SYSTEMS. XIV. RESPIRATORY CHAINS OF MYCOBACTERIUM PHLEI. J Biol Chem. 1964 Dec;239:4280–4291. [PubMed] [Google Scholar]
- Abrams A., Baron C. Inhibitory action of carbodiimides on bacterial membrane ATPase. Biochem Biophys Res Commun. 1970 Nov 25;41(4):858–863. doi: 10.1016/0006-291x(70)90162-2. [DOI] [PubMed] [Google Scholar]
- Abrams A., Baron C. The isolation and subunit structure of streptococcal membrane adenosine triphosphatase. Biochemistry. 1967 Jan;6(1):225–229. doi: 10.1021/bi00853a035. [DOI] [PubMed] [Google Scholar]
- Addanki A., Cahill F. D., Sotos J. F. Determination of intramitochondrial pH and intramitochondrial-extramitochondrial pH gradient of isolated heart mitochondria by the use of 5,5-dimethyl-2,4-oxazolidinedione. I. Changes during respiration and adenosine triphosphate-dependent transport of Ca++, Mg++, and Zn++. J Biol Chem. 1968 May 10;243(9):2337–2348. [PubMed] [Google Scholar]
- Adolfsen R., Moudrianakis E. N. Kinetic characterization of oxidative phosphorylation in Alcaligenes faecalis. Biochemistry. 1971 Feb 2;10(3):434–440. doi: 10.1021/bi00779a013. [DOI] [PubMed] [Google Scholar]
- Adolfsen R., Moudrianakis E. N. Purification and properties of 2 soluble coupling factors of oxidative phosphorylation from Alcaligenes faecalis. Biochemistry. 1971 Jun 8;10(12):2247–2253. doi: 10.1021/bi00788a010. [DOI] [PubMed] [Google Scholar]
- Adolfsen R., Moudrianakis E. N. Reconstitution of oxidative phosphorylation in Alcaligenes faecalis. Biochemistry. 1971 Feb 2;10(3):440–446. doi: 10.1021/bi00779a014. [DOI] [PubMed] [Google Scholar]
- Aithal H. N., Kalra V. K., Brodie A. F. Reversal of the effects of freezing on oxidative phosphorylation in the Mycobacterium phlei system. Biochem Biophys Res Commun. 1971 May 7;43(3):550–556. doi: 10.1016/0006-291x(71)90649-8. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Lever J. Components of histidine transport: histidine-binding proteins and hisP protein. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1096–1103. doi: 10.1073/pnas.66.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano A., Imai K., Sato R. Oxidative phosphorylation in Micrococcus denitrificans. 3. ATP-supported reduction of NAD+ by succinate. J Biochem. 1967 Aug;62(2):210–214. doi: 10.1093/oxfordjournals.jbchem.a128650. [DOI] [PubMed] [Google Scholar]
- Asano A., Imai K., Sato R. Oxidative phosphorylation in Micrococcus dentrificans. II. The properties of pyridine nucleotide transhydrogenase. Biochim Biophys Acta. 1967;143(3):477–486. doi: 10.1016/0005-2728(67)90053-9. [DOI] [PubMed] [Google Scholar]
- Azzi A., Chance B., Radda G. K., Lee C. P. A fluorescence probe of energy-dependent structure changes in fragmented membranes. Proc Natl Acad Sci U S A. 1969 Feb;62(2):612–619. doi: 10.1073/pnas.62.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi A. Redistribution of the electrical charge of the mitochondrial membrane during energy conservation. Biochem Biophys Res Commun. 1969 Oct 8;37(2):254–260. doi: 10.1016/0006-291x(69)90727-x. [DOI] [PubMed] [Google Scholar]
- Azzi Angelo, Baltscheffsky Margareta, Baltscheffsky Herrick, Vainio Harri. Energy-linked changes of the membrane of Rhodospirillum rubrum chromatophores detected by the fluorescent probe 8-anilinonaphthalene-1-sulfonic acid. FEBS Lett. 1971 Sep 15;17(1):49–52. doi: 10.1016/0014-5793(71)80561-6. [DOI] [PubMed] [Google Scholar]
- BOSE S. K., GEST H. PROPERTIES OF ADENOSINE TRIPHOSPHATASE IN A PHOTOSYNTHETIC BACTERIUM. Biochim Biophys Acta. 1965 Jan;96:159–162. doi: 10.1016/0005-2787(65)90621-0. [DOI] [PubMed] [Google Scholar]
- BOVELL C. R., PACKER L., HELGERSON R. PERMEABILITY OF ESCHERICHIA COLI TO ORGANIC COMPOUNDS AND INORGANIC SALTS MEASURED BY LIGHT-SCATTERING. Biochim Biophys Acta. 1963 Sep 24;75:257–266. doi: 10.1016/0006-3002(63)90604-8. [DOI] [PubMed] [Google Scholar]
- BRITTEN R. J., McCLURE F. T. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962 Sep;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK T. D., MOO-PENN G. An amino acid transport system in Streptococcus faecium. Arch Biochem Biophys. 1962 Aug;98:183–190. doi: 10.1016/0003-9861(62)90171-6. [DOI] [PubMed] [Google Scholar]
- BUCHWALD M., BRITTEN R. J. Incorporation of ribonucleic acid bases into the metabolic pool and RNA of E. coli. Biophys J. 1963 Mar;3:155–166. doi: 10.1016/s0006-3495(63)86811-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarini-Melandri A., Gest H., Pietro A. S. A coupling factor in bacterial photophosphorylation. J Biol Chem. 1970 Mar 10;245(5):1224–1226. [PubMed] [Google Scholar]
- Bakeeva L. E., Grinius L. L., Jasaitis A. A., Kuliene V. V., Levitsky D. O., Liberman E. A., Severina I. I., Skulachev V. P. Conversion of biomembrane-produced energy into electric form. II. Intact mitochondria. Biochim Biophys Acta. 1970 Aug 4;216(1):13–21. doi: 10.1016/0005-2728(70)90154-4. [DOI] [PubMed] [Google Scholar]
- Barnes E. M., Jr, Kaback H. R. Beta-galactoside transport in bacterial membrane preparations: energy coupling via membrane-bounded D-lactic dehydrogenase. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1190–1198. doi: 10.1073/pnas.66.4.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes E. M., Jr, Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. I. The site of energy coupling between D-lactic dehydrogenase and beta-galactoside transport in Escherichia coli membrane vesicles. J Biol Chem. 1971 Sep 10;246(17):5518–5522. [PubMed] [Google Scholar]
- Baron C., Abrams A. Isolation of a bacterial membrane protein, nectin, essential for the attachment of adenosine triphosphatase. J Biol Chem. 1971 Mar 10;246(5):1542–1544. [PubMed] [Google Scholar]
- Bennun A. Hypothesis for coupling energy transduction with ATP synthesis or ATP hydrolysis. Nat New Biol. 1971 Sep 1;233(35):5–8. doi: 10.1038/newbio233005a0. [DOI] [PubMed] [Google Scholar]
- Berlin R. D., Stadtman E. R. A possible role of purine nucleotide pyrophosphorylases in the regulation of purine uptake by Bacillus subtilis. J Biol Chem. 1966 Jun 10;241(11):2679–2686. [PubMed] [Google Scholar]
- Bhattacharyya P. Active Transport of Manganese in Isolated Membranes of Escherichia coli. J Bacteriol. 1970 Dec;104(3):1307–1311. doi: 10.1128/jb.104.3.1307-1311.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya P., Epstein W., Silver S. Valinomycin-induced uptake of potassium in membrane vesicles from Escherichia coli. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1488–1492. doi: 10.1073/pnas.68.7.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya P., Wendt L., Whitney E., Silver S. Colicin-tolerant mutants of Escherichia coli: resistance of membranes to colicin E1. Science. 1970 May 22;168(3934):998–1000. doi: 10.1126/science.168.3934.998. [DOI] [PubMed] [Google Scholar]
- Bogin E., Higashi T., Brodie A. F. Effects of heat treatment of electron-transport particles on bacterial oxidative phosphorylation. Proc Natl Acad Sci U S A. 1970 Sep;67(1):1–6. doi: 10.1073/pnas.67.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogin E., Higashi T., Brodie A. F. Interchangeability of coupling factors from bacterial and mammalian origin. Biochem Biophys Res Commun. 1970 Feb 6;38(3):478–483. doi: 10.1016/0006-291x(70)90738-2. [DOI] [PubMed] [Google Scholar]
- Bogin E., Higashi T., Brodie A. F. Oxidative phosphorylation in fractionated bacterial systems. 43. Coupling factors associated with the NAD+ linked electron transport pathway. Arch Biochem Biophys. 1970 Feb;136(2):337–351. doi: 10.1016/0003-9861(70)90204-3. [DOI] [PubMed] [Google Scholar]
- Bogin E., Higashi T., Brodie A. F. The effect of trypsin and heat treatment on oxidative phosphorylation in Mycobacterium phlei. Biochem Biophys Res Commun. 1970 Nov 25;41(4):995–1001. doi: 10.1016/0006-291x(70)90183-x. [DOI] [PubMed] [Google Scholar]
- Boos W., Gordon A. S. Transport properties of the galactose-binding protein of Escherichia coli. Occurrence of two conformational states. J Biol Chem. 1971 Feb 10;246(3):621–628. [PubMed] [Google Scholar]
- Boos W., Lengeler J., Hermann K. O., Unsöld H. J. The regulation of the beta-methylgalactoside transport system and of the galactose binding protein of Escherichia coli K12. Eur J Biochem. 1971 Apr 30;19(4):457–470. doi: 10.1111/j.1432-1033.1971.tb01336.x. [DOI] [PubMed] [Google Scholar]
- Boos W., Sarvas M. O. Close linkage between a galactose binding protein and the beta-methylgalactoside permease in Escherichia coli. Eur J Biochem. 1970 Apr;13(3):526–533. doi: 10.1111/j.1432-1033.1970.tb00956.x. [DOI] [PubMed] [Google Scholar]
- Borst-Pauwels G. W., Jager S. Inhibition of phosphate and arsenate uptake in yeast by monoiodoacetate, fluoride, 2,4-dinitrophenol and acetate. Biochim Biophys Acta. 1969 Apr 8;172(3):399–406. doi: 10.1016/0005-2728(69)90136-4. [DOI] [PubMed] [Google Scholar]
- Borst-Pauwels G. W., Wolters G. H., Henricks J. J. The interaction of 2,4-dinitrophenol with anaerobic Rb+ transport across the yeast cell membrane. Biochim Biophys Acta. 1971 Feb 2;225(2):269–276. doi: 10.1016/0005-2736(71)90220-3. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Oxidative phosphorylation in Escherichia coli. Can J Biochem. 1968 Jul;46(7):631–641. doi: 10.1139/o68-099. [DOI] [PubMed] [Google Scholar]
- Brocklehurst J. R., Freedman R. B., Hancock D. J., Radda G. K. Membrane studies with polarity-dependent and excimer-forming fluorescent probes. Biochem J. 1970 Feb;116(4):721–731. doi: 10.1042/bj1160721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D. Formation of aromatic amino acid pools in Escherichia coli K-12. J Bacteriol. 1970 Oct;104(1):177–188. doi: 10.1128/jb.104.1.177-188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D. Maintenance and exchange of the aromatic amino acid pool in Escherichia coli. J Bacteriol. 1971 Apr;106(1):70–81. doi: 10.1128/jb.106.1.70-81.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan-Jones D. G., Whittenbury R. Haematin-dependent oxidative phosphorylation in Streptococcus faecalis. J Gen Microbiol. 1969 Oct;58(2):247–260. doi: 10.1099/00221287-58-2-247. [DOI] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPPELL J. B., CROFTS A. R. GRAMICIDIN AND ION TRANSPORT IN ISOLATED LIVER MITOCHONDRIA. Biochem J. 1965 May;95:393–402. doi: 10.1042/bj0950393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., MONOD J. Bacterial permeases. Bacteriol Rev. 1957 Sep;21(3):169–194. doi: 10.1128/br.21.3.169-194.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E., Balcavage W. X., Lehninger A. L., Mattoon J. R. Ca2+ metabolism in yeast cells and mitochondria. Biochim Biophys Acta. 1970 Apr 7;205(1):18–26. doi: 10.1016/0005-2728(70)90057-5. [DOI] [PubMed] [Google Scholar]
- Carafoli E., Hansford R. G., Sackton B., Lehninger A. L. Interaction of Ca2+ with blowfly flight muscle mitochondria. J Biol Chem. 1971 Feb 25;246(4):964–972. [PubMed] [Google Scholar]
- Carstensen E. L., Maniloff J., Einolf C. W., Jr Electrical properties and ultrastructure of Mycoplasma membranes. Biophys J. 1971 Jul;11(7):572–581. doi: 10.1016/S0006-3495(71)86236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. R., Fox C. F., Kennedy E. P. Interaction of sugars with the membrane protein component of the lactose transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Jun;60(2):725–732. doi: 10.1073/pnas.60.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell K. J., Lindop C. R., Knight I. G., Beechey R. B. The identification of the site of action of NN'-dicyclohexylcarbodi-imide as a proteolipid in mitochondrial membranes. Biochem J. 1971 Nov;125(1):169–177. doi: 10.1042/bj1250169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A., Pedersen P. L. Adenosine triphosphatase from rat liver mitochondria. I. Purification, homogeneity, and physical properties. J Biol Chem. 1971 Aug 25;246(16):4987–4994. [PubMed] [Google Scholar]
- Cavari B. Z., Avi-Dor Y., Grossowicz N. Induction by oxygen of respiration and phosphorylation of anaerobically grown Escherichia coli. J Bacteriol. 1968 Sep;96(3):751–759. doi: 10.1128/jb.96.3.751-759.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B. Fluorescent probe environment and the structural and charge changes in energy coupling of mitochondrial membranes. Proc Natl Acad Sci U S A. 1970 Oct;67(2):560–571. doi: 10.1073/pnas.67.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Lee C. P., Mela L. Control and conservation of energy in the cytochrome chain. Fed Proc. 1967 Sep;26(5):1341–1354. [PubMed] [Google Scholar]
- Chance B., Mela L. Energy-linked changes of hydrogen ion concentration in submitochondrial particles. J Biol Chem. 1967 Mar 10;242(5):830–844. [PubMed] [Google Scholar]
- Chavin S. I. Isolation and study of functional membrane proteins Present status and future prospects. FEBS Lett. 1971 May 20;14(5):269–282. doi: 10.1016/0014-5793(71)80278-8. [DOI] [PubMed] [Google Scholar]
- Cirillo V. P. Symposium on bioelectrochemistry of microorganisms. I. Membrane potentials and permeability. Bacteriol Rev. 1966 Mar;30(1):68–79. doi: 10.1128/br.30.1.68-79.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockrell R. S., Harris E. J., Pressman B. C. Synthesis of ATP driven by a potassium gradient in mitochondria. Nature. 1967 Sep 30;215(5109):1487–1488. doi: 10.1038/2151487a0. [DOI] [PubMed] [Google Scholar]
- Cole H. A., Wimpenny J. W., Hughes D. E. The ATP pool in Escherichia coli. I. Measurement of the pool using modified luciferase assay. Biochim Biophys Acta. 1967;143(3):445–453. doi: 10.1016/0005-2728(67)90050-3. [DOI] [PubMed] [Google Scholar]
- Contessa A. R., Bruni A. Different effects of oligomycin and dicyclohexylcarbodiimide on ATPases from mammalian cells. Biochim Biophys Acta. 1971 Aug 13;241(2):334–340. doi: 10.1016/0005-2736(71)90033-2. [DOI] [PubMed] [Google Scholar]
- Cope F. W., Damadian R. Cell potassium by 39K spin echo nuclear magnetic resonance. Nature. 1970 Oct 3;228(5266):76–77. doi: 10.1038/228076a0. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Butlin J. D., Gibson F. The energy-linked transhydrogenase reaction in respiratory mutants of Escherichia coli K12. Biochem J. 1971 Nov;125(2):489–493. doi: 10.1042/bj1250489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Gibson F., Snoswell A. M., Hamilton J. A. The function of ubiquinone in Escherichia coli. Biochem J. 1970 Apr;117(3):551–562. doi: 10.1042/bj1170551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer W. A., Phillips S. K. Response of an Escherichia coli-bound fluorescent probe to colicin E1. J Bacteriol. 1970 Nov;104(2):819–825. doi: 10.1128/jb.104.2.819-825.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts A. R., Wraight C. A., Fleischmann D. E. Energy conservation in the photochemical reactions of photosynthesis and its relation to delayed fluorescence. FEBS Lett. 1971 Jun 10;15(2):89–100. doi: 10.1016/0014-5793(71)80031-5. [DOI] [PubMed] [Google Scholar]
- Cross R. L., Cross B. A., Wang J. H. Detection of a phosphorylated intermediate in mitochondrial oxidative phosphorylation. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1155–1161. doi: 10.1016/0006-291x(70)90915-0. [DOI] [PubMed] [Google Scholar]
- Datta A., Penefsky H. S. Interaction of fluorescent probes with submitochondrial particles during oxidative phosphorylation. J Biol Chem. 1970 Apr 10;245(7):1537–1544. [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Attachment of flagellar basal bodies to the cell envelope: specific attachment to the outer, lipopolysaccharide membrane and the cyoplasmic membrane. J Bacteriol. 1971 Jan;105(1):396–407. doi: 10.1128/jb.105.1.396-407.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Fine structure and isolation of the hook-basal body complex of flagella from Escherichia coli and Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):384–395. doi: 10.1128/jb.105.1.384-395.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch R. N., Hageage G. J. Motility in procaryotic organisms: problems, points of view, and perspectives. Biol Rev Camb Philos Soc. 1968 Aug;43(3):317–362. doi: 10.1111/j.1469-185x.1968.tb00963.x. [DOI] [PubMed] [Google Scholar]
- Dreyfuss J., Pardee A. B. Regulation of sulfate transport in Salmonella typhimurium. J Bacteriol. 1966 Jun;91(6):2275–2280. doi: 10.1128/jb.91.6.2275-2280.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy A. A., Backen K., Watson G. The concentration of amino acids by yeast cells depleted of adenosine triphosphate. Biochem J. 1970 Dec;120(4):853–858. doi: 10.1042/bj1200853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy A. A., Indge K. J., Backen K., Nowacki J. A. Interctions between potassium ions and glycine transport in the yeast Saccharomyces carlsbergensis. Biochem J. 1970 Dec;120(4):845–852. doi: 10.1042/bj1200845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy A. A., Nowacki J. A. Stoicheiometrical proton and potassium ion movements accompanying the absorption of amino acids by the yeast Saccharomyces carlsbergensis. Biochem J. 1971 May;122(5):701–711. doi: 10.1042/bj1220701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellar D. J., Thomas T. D., Posgate J. A. Properties of mesosomal membranes isolated from Micrococcus lysodeikticus and Bacillus megaterium. Biochem J. 1971 May;122(5):44P–45P. doi: 10.1042/bj1220044p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M. Lipid bilayer structure in the membrane of Mycoplasma laidlawii. J Mol Biol. 1971 May 28;58(1):153–165. doi: 10.1016/0022-2836(71)90238-5. [DOI] [PubMed] [Google Scholar]
- Esfahani M., Limbrick A. R., Knutton S., Oka T., Wakil S. J. The molecular organization of lipids in the membrane of Escherichia coli: phase transitions. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3180–3184. doi: 10.1073/pnas.68.12.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr Membrane adenosine triphosphatase of Escherichia coli: activation by calcium ion and inhibition by monovalent cations. J Bacteriol. 1969 Nov;100(2):914–922. doi: 10.1128/jb.100.2.914-922.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J. Membrane Mg-(Ca)-Activated Adenosine Triphosphatase of Escherichia coli: Characterization in the Membrane-Bound and Solubilized States. J Bacteriol. 1970 Dec;104(3):1203–1212. doi: 10.1128/jb.104.3.1203-1212.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farron F. Isolation and properties of a chloroplast coupling factor and heat-activated adenosine triphosphatase. Biochemistry. 1970 Sep 15;9(19):3823–3828. doi: 10.1021/bi00821a023. [DOI] [PubMed] [Google Scholar]
- Farron F., Racker E. Studies on the mechanism of the conversion of coupling factor 1 from chloroplasts to an active adenosine triphosphatase. Biochemistry. 1970 Sep 15;9(19):3829–3836. doi: 10.1021/bi00821a024. [DOI] [PubMed] [Google Scholar]
- Faust M. A., Doetsch R. N. Effect of drugs that alter excitable membranes on the motility of Rhodospirillum rubrum and Thiospirillum jenense. Can J Microbiol. 1971 Feb;17(2):191–196. doi: 10.1139/m71-033. [DOI] [PubMed] [Google Scholar]
- Faust M. A., Doetsch R. N. Effect of membrane-active antibiotics on motility and 42K permeability of Pseudomonas fluorescens. Can J Microbiol. 1971 Feb;17(2):183–189. doi: 10.1139/m71-032. [DOI] [PubMed] [Google Scholar]
- Faust M. A., Doetsch R. N. Effect of respiratory inhibitors on the motility of Pseudomonas fluorescens. J Bacteriol. 1969 Feb;97(2):806–811. doi: 10.1128/jb.97.2.806-811.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust P. J., Vandemark P. J. Phosphorylation coupled to NADH oxidation with fumarate in Streptococcus faecalis 10Cl. Arch Biochem Biophys. 1970 Apr;137(2):392–398. doi: 10.1016/0003-9861(70)90454-6. [DOI] [PubMed] [Google Scholar]
- Ferrandes B., Frehel C., Chaix P. Fractionment et purification des systèmes membranaires cytoplasmiques et mésosomiquees de lbacillus subtilis. Etude de quelques-unes de leurs propríetés oxydo-réductricwa associées à la chaine respiratoire. Biochim Biophys Acta. 1970 Dec 8;223(2):292–308. doi: 10.1016/0005-2728(70)90186-6. [DOI] [PubMed] [Google Scholar]
- Fields K. L., Luria S. E. Effects of colicins E1 and K on cellular metabolism. J Bacteriol. 1969 Jan;97(1):64–77. doi: 10.1128/jb.97.1.64-77.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields K. L., Luria S. E. Effects of colicins E1 and K on transport systems. J Bacteriol. 1969 Jan;97(1):57–63. doi: 10.1128/jb.97.1.57-63.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein A. Weak-acid uncouplers of oxidative phosphorylation. Mechanism of action on thin lipid membranes. Biochim Biophys Acta. 1970 Apr 7;205(1):1–6. doi: 10.1016/0005-2728(70)90055-1. [DOI] [PubMed] [Google Scholar]
- Fisher R. J., Chen J. C., Sani B. P., Kaplay S. S., Sanadi D. R. A soluble mitochondrial ATP synthetase complex catalyzing ATP-phosphate and ATP-ADP exchange. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2181–2184. doi: 10.1073/pnas.68.9.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. J., Sanadi D. R. Energy-linked nicotinamide adenine dinucleotide transhydrogenase in membrane particles from Escherchia coli. Biochim Biophys Acta. 1971 Aug 6;245(1):34–41. doi: 10.1016/0005-2728(71)90005-3. [DOI] [PubMed] [Google Scholar]
- Fisher R. R., Guillory R. J. Inhibition of the energy conservation reactions of Rhodospirillum rubrum by Dio-9. Biochim Biophys Acta. 1967;143(3):654–656. doi: 10.1016/0005-2728(67)90078-3. [DOI] [PubMed] [Google Scholar]
- Fonyo A. Phosphate carrier of rat-liver mitochondria: its role in phosphate outflow. Biochem Biophys Res Commun. 1968 Aug 21;32(4):624–628. doi: 10.1016/0006-291x(68)90283-0. [DOI] [PubMed] [Google Scholar]
- Forrest G., Edelstein S. J. On the subunit structure of the cold labile adenosine triphosphatase of mitochondria. J Biol Chem. 1970 Dec 10;245(23):6468–6470. [PubMed] [Google Scholar]
- Foulds J. Mode of action of a bacteriocin from Serratia marcescens. J Bacteriol. 1971 Sep;107(3):833–839. doi: 10.1128/jb.107.3.833-839.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank L., Hopkins I. Sodium-stimulated transport of glutamate in Escherichia coli. J Bacteriol. 1969 Oct;100(1):329–336. doi: 10.1128/jb.100.1.329-336.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehel C., Ferrandes B., Ryter A. Réactions d'oxydo-réduction au niveau des membranes cytoplasmiaues et mésosomiques de Bacillus subtilis. Biochim Biophys Acta. 1971 May 11;234(2):226–241. doi: 10.1016/0005-2728(71)90078-8. [DOI] [PubMed] [Google Scholar]
- Frenkel A. W. Multiplicity of electron transport reactions in bacterial photosynthesis. Biol Rev Camb Philos Soc. 1970 Nov;45(4):569–616. doi: 10.1111/j.1469-185x.1970.tb01177.x. [DOI] [PubMed] [Google Scholar]
- Gachelin G. Studies on the alpha-methylglucoside permease of Escherichia coli. A two-step mechanism for the accumulation of alpha-methylglucoside 6-phosphate. Eur J Biochem. 1970 Oct;16(2):342–357. doi: 10.1111/j.1432-1033.1970.tb01088.x. [DOI] [PubMed] [Google Scholar]
- Ghosh B. K., Sargent M. G., Lampen J. O. Morphological phenomena associated with penicillinase induction and secretion in Bacillus licheniformis. J Bacteriol. 1968 Oct;96(4):1314–1328. doi: 10.1128/jb.96.4.1314-1328.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. E., Asai J., Harris R. A., Penniston J. T. Conformational basis of energy transformations in membrane systems. 3. Configurational changes in the mitochondrial inner membrane induced by changes in functional states. Arch Biochem Biophys. 1968 May;125(2):684–705. doi: 10.1016/0003-9861(68)90626-7. [DOI] [PubMed] [Google Scholar]
- Grinius L. L., Jasaitis A. A., Kadziauskas Y. P., Liberman E. A., Skulachev V. P., Topali V. P., Tsofina L. M., Vladimirova M. A. Conversion of biomembrane-produced energy into electric form. I. Submitochondrial particles. Biochim Biophys Acta. 1970 Aug 4;216(1):1–12. doi: 10.1016/0005-2728(70)90153-2. [DOI] [PubMed] [Google Scholar]
- Groot G. S., Kovác L., Schatz G. Promitochondria of anaerobically grown yeast. V. Energy transfer in the absence of an electron transfer chain. Proc Natl Acad Sci U S A. 1971 Feb;68(2):308–311. doi: 10.1073/pnas.68.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R., Coles N. W. Adenosine triphosphatase in isolated membranes of Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1322–1326. doi: 10.1128/jb.95.4.1322-1326.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarraia L. J., Peck H. D., Jr Dinitrophenol-stimulated adenosine triphosphatase activity in extracts of Desulfovibrio gigas. J Bacteriol. 1971 Jun;106(3):890–895. doi: 10.1128/jb.106.3.890-895.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutnick D. L., Brodie A. F. Phosphate-dependent incorporation of tritium into a naphthoquinone during oxidative phosphorylation. J Biol Chem. 1965 Sep;240(9):3698–3699. [PubMed] [Google Scholar]
- HAROLD F. M., HAROLD R. L., ABRAMS A. A MUTANT OF STREPTOCOCCUS FAECALIS DEFECTIVE IN PHOSPHATE UPTAKE. J Biol Chem. 1965 Jul;240:3145–3153. [PubMed] [Google Scholar]
- HORECKER B. L., THOMAS J., MONOD J. Galactose transport in Escherichia coli. II. Characteristics of the exit process. J Biol Chem. 1960 Jun;235:1586–1590. [PubMed] [Google Scholar]
- Hadjipetrou L., Lilly M. D., Kourounakis P. Effect of ferricyanide on Escherichia coli. Antonie Van Leeuwenhoek. 1970;36(4):531–540. doi: 10.1007/BF02069055. [DOI] [PubMed] [Google Scholar]
- Hafkenscheid J. C., Bonting S. L. Studies on (Na+-K+)-activated ATPase. 23. A Mg2+-ATPase in Escherichia coli, activated by monovalent cations. Biochim Biophys Acta. 1969 Mar 18;178(1):128–136. doi: 10.1016/0005-2744(69)90139-9. [DOI] [PubMed] [Google Scholar]
- Hamilton W. A., Jeacock R. E. The ion-specific increases in membrane permeability with a group of membrane-active antibacterial agents. Biochem J. 1972 Apr;127(3):56P–57P. doi: 10.1042/bj1270056pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Dio 9 and chlorhexidine: inhibitors of membrane-bound ATPase and of cation transport in Streptococcus faecalis. Biochim Biophys Acta. 1969 Jun 3;183(1):129–136. doi: 10.1016/0005-2736(69)90136-9. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Inhibition of membrane-bound adenosine triphosphatase and of cation transport in Streptococcus faecalis by N,N'-dicyclohexylcarbodiimide. J Biol Chem. 1969 May 10;244(9):2261–2268. [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Effects of nigericin and monactin on cation permeability of Streptococcus faecalis and metabolic capacities of potassium-depleted cells. J Bacteriol. 1968 Mar;95(3):816–823. doi: 10.1128/jb.95.3.816-823.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of membrane transport in Streptococcus faecalis by uncouplers of oxidative phosphorylation and its relationship to proton conduction. J Bacteriol. 1968 Dec;96(6):2025–2034. doi: 10.1128/jb.96.6.2025-2034.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Pavlasova E. Extrusion of sodium and hydrogen ions as the primary process in potassium ion accumulation by Streptococcus faecalis. J Bacteriol. 1970 Jan;101(1):152–159. doi: 10.1128/jb.101.1.152-159.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Harold R. L., Baarda J. R., Abrams A. A genetic defect in retention of potassium by Streptococcus faecalis. Biochemistry. 1967 Jun;6(6):1777–1784. doi: 10.1021/bi00858a028. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Papineau D. Cation transport and electrogenesis by Streptococcus faecalis. I. The membrane potential. J Membr Biol. 1972;8(1):27–44. doi: 10.1007/BF01868093. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Papineau D. Cation transport and electrogenesis by Streptococcus faecalis. II. Proton and sodium extrusion. J Membr Biol. 1972;8(1):45–62. doi: 10.1007/BF01868094. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Pavlasová E., Baarda J. R. A transmembrane pH gradient in Streptococcus faecalis: origin, and dissipation by proton conductors and N,N'-dicyclohexylcarbodimide. Biochim Biophys Acta. 1970;196(2):235–244. doi: 10.1016/0005-2736(70)90011-8. [DOI] [PubMed] [Google Scholar]
- Harris E. J., Pressman B. C. The direction of polarity of the mitochondrial trans-membrane potential. Biochim Biophys Acta. 1969 Jan 14;172(1):66–70. doi: 10.1016/0005-2728(69)90092-9. [DOI] [PubMed] [Google Scholar]
- Harris R. A., Penniston J. T., Asai J., Green D. E. The conformational basis of energy conservation in membrane systems. II. Correlation between conformational change and functional states. Proc Natl Acad Sci U S A. 1968 Mar;59(3):830–837. doi: 10.1073/pnas.59.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. E., Maitra P. K. Control of respiration and metabolism in growing Klebsiella aerogenes. The role of adenine nucleotides. Biochem J. 1969 May;112(5):647–656. doi: 10.1042/bj1120647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechtman P., Scriver C. R. The isolation and properties of a beta-alanine permeaseless mutant of Pseudomonas fluorescens. Biochim Biophys Acta. 1970 Dec 1;219(2):428–436. doi: 10.1016/0005-2736(70)90220-8. [DOI] [PubMed] [Google Scholar]
- Hempfling W. P. Repression of oxidative phosphorylation in Escherichia coli B by growth in glucose and other carbohydrates. Biochem Biophys Res Commun. 1970 Oct 9;41(1):9–15. doi: 10.1016/0006-291x(70)90461-4. [DOI] [PubMed] [Google Scholar]
- Hempfling W. P. Studies of the efficiency of oxidative phosphorylation in intact Escherichia coli B. Biochim Biophys Acta. 1970;205(2):169–182. doi: 10.1016/0005-2728(70)90247-1. [DOI] [PubMed] [Google Scholar]
- Henderson P. J. Ion transport by energy-conserving biological membranes. Annu Rev Microbiol. 1971;25:393–428. doi: 10.1146/annurev.mi.25.100171.002141. [DOI] [PubMed] [Google Scholar]
- Henderson P. J., Lardy H. A. Antibiotic inhibition of mitochondrial energy-transfer reactions. Antimicrob Agents Chemother (Bethesda) 1969;9:18–27. [PubMed] [Google Scholar]
- Henderson P. J., McGivan J. D., Chappell J. B. The action of certain antibiotics on mitochondrial, erythrocyte and artificial phospholipid membranes. The role of induced proton permeability. Biochem J. 1969 Feb;111(4):521–535. doi: 10.1042/bj1110521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler R. W. Biological membrane ultrastructure. Physiol Rev. 1971 Jan;51(1):66–97. doi: 10.1152/physrev.1971.51.1.66. [DOI] [PubMed] [Google Scholar]
- Higashi T., Bogin E., Brodie A. F. Oxidative phosphorylation in fractionated bacterial systems. XLII. The effect of coupling factors on urea-treated particles from M. phlei. Arch Biochem Biophys. 1970 Feb;136(2):331–336. doi: 10.1016/0003-9861(70)90203-1. [DOI] [PubMed] [Google Scholar]
- Hinkle P. C., Horstman L. L. Respiration-driven proton transport in submitochondrial particles. J Biol Chem. 1971 Oct 10;246(19):6024–6028. [PubMed] [Google Scholar]
- Hinkle P., Mitchell P. Effect of membrane potential on equilibrium poise between cytochrome a and cytochrome c in rat liver mitochondria. J Bioenerg. 1970 Jun;1(1):45–60. doi: 10.1007/BF01516088. [DOI] [PubMed] [Google Scholar]
- Hirata H., Asano A., Brodie A. F. Respiration dependent transport of proline by electron transport particles from mycobacterium phlei. Biochem Biophys Res Commun. 1971 Jul 16;44(2):368–374. doi: 10.1016/0006-291x(71)90609-7. [DOI] [PubMed] [Google Scholar]
- Hirata H., Fukui S., Ishikawa S. Initial events caused by colicin K infection--cation movement and depletion of ATP pool. J Biochem. 1969 May;65(5):843–847. doi: 10.1093/oxfordjournals.jbchem.a129088. [DOI] [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Stadtman E. R. The regulation of purine utilization in bacteria. I. Purification of adenine phosphoribosyltransferase from Escherichia coli K12 and control of activity by nucleotides. J Biol Chem. 1971 Sep 10;246(17):5294–5303. [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Stadtman E. R. The regulation of purine utilization in bacteria. II. Adenine phosphoribosyltransferase in isolated membrane preparations and its role in transport of adenine across the membrane. J Biol Chem. 1971 Sep 10;246(17):5304–5311. [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Stadtman E. R. The regulation of purine utilization in bacteria. III. The involvement of purine phosphoribosyltransferases in the uptake of adenine and other nucleic acid precursors by intact resting cells. J Biol Chem. 1971 Sep 10;246(17):5312–5320. [PubMed] [Google Scholar]
- Holden J. T., Van Balgooy J. N., Kittredge J. S. Transport of aminophosphonic acids in Lactobacillus plantarum and Streptococcus faecalis. J Bacteriol. 1968 Oct;96(4):950–957. doi: 10.1128/jb.96.4.950-957.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer U., Lehninger A. L., Thompson T. E. Protonic conductance across phospholipid bilayer membranes induced by uncoupling agents for oxidative phosphorylation. Proc Natl Acad Sci U S A. 1968 Feb;59(2):484–490. doi: 10.1073/pnas.59.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio T., Kamen M. D. Bacterial cytochromes. II. Functional aspects. Annu Rev Microbiol. 1970;24:399–428. doi: 10.1146/annurev.mi.24.100170.002151. [DOI] [PubMed] [Google Scholar]
- Imai K., Asano A., Sato R. Oxidative phosphorylation in Micrococcus denitrificans. I. Preparation and properties of phosphorylating membrane fragments. Biochim Biophys Acta. 1967;143(3):462–476. doi: 10.1016/0005-2728(67)90052-7. [DOI] [PubMed] [Google Scholar]
- Imai K., Asano A., Sato R. Oxidative phosphorylation in Micrococcus denitrificans. IV. Further characterization of electron-transfer pathway and phosphorylation activity in NADH oxidation. J Biochem. 1968 Feb;63(2):207–218. doi: 10.1093/oxfordjournals.jbchem.a128763. [DOI] [PubMed] [Google Scholar]
- Isaev P. I., Liberman E. A., Samuilov V. D., Skulachev V. P., Tsofina L. M. Conversion of biomembrane-produced energy into electric form. 3. Chromatophores of Rhodospirillum rubrum. Biochim Biophys Acta. 1970 Aug 4;216(1):22–29. doi: 10.1016/0005-2728(70)90155-6. [DOI] [PubMed] [Google Scholar]
- Ishikawa S. Properties of an oxidative phosphorylation system reconstituted from coupling factors in Micrococcus lysodeikticus. J Biochem. 1970 Feb;67(2):297–312. doi: 10.1093/oxfordjournals.jbchem.a129253. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Crofts A. R. Energy-linked reduction of nicotinamide adenine dinucleotides in cells of Rhodospirillum rubrum. Biochem Biophys Res Commun. 1968 Sep 30;32(6):908–915. doi: 10.1016/0006-291x(68)90113-7. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Crofts A. R. The kinetics of light induced carotenoid changes in Rhodopseudomonas spheroides and their relation to electrical field generation across the chromatophore membrane. Eur J Biochem. 1971 Jan 1;18(1):120–130. doi: 10.1111/j.1432-1033.1971.tb01222.x. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Crofts A. R., von Stedingk L. V. Ion transport induced by light and antibiotics IN CHROMATOPHORES FROM Rhodospirillum rubrum. Eur J Biochem. 1968 Oct 17;6(1):41–54. doi: 10.1111/j.1432-1033.1968.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Jagendorf A. T., Uribe E. ATP formation caused by acid-base transition of spinach chloroplasts. Proc Natl Acad Sci U S A. 1966 Jan;55(1):170–177. doi: 10.1073/pnas.55.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966 Aug 27;211(5052):969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- Jasaitis A. A., Kuliene V. V., Skulachev V. P. Anilinonaphthalenesulfonate fluorescence changes induced by non-emzymatic generation of membrane potential in mitochondria and submitochondrial particles. Biochim Biophys Acta. 1971 Apr 6;234(1):177–181. doi: 10.1016/0005-2728(71)90144-7. [DOI] [PubMed] [Google Scholar]
- Jeacocke R. E., Niven D. F., Hamilton W. A. The protonmotive force in Staphylococcus aureus. Biochem J. 1972 Apr;127(3):57P–58P. doi: 10.1042/bj1270057p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John P., Hamilton W. A. Release of respiratory control in particles from Micrococcus denitrificans by ion-translocating antibiotics. Eur J Biochem. 1971 Dec 10;23(3):528–532. doi: 10.1111/j.1432-1033.1971.tb01650.x. [DOI] [PubMed] [Google Scholar]
- John P., Whatley F. R. Oxidative phosphorylation coupled to oxygen uptake and nitrate reduction in Micrococcus denitrificans. Biochim Biophys Acta. 1970 Sep 1;216(2):342–352. doi: 10.1016/0005-2728(70)90225-2. [DOI] [PubMed] [Google Scholar]
- John Philip, Hamilton W. A. Respiratory control in membrane particles from Micrococcus denitrificans. FEBS Lett. 1970 Oct 16;10(4):246–248. doi: 10.1016/0014-5793(70)80639-1. [DOI] [PubMed] [Google Scholar]
- KESSEL D., LUBIN M. Transport of proline in Escherichia coli. Biochim Biophys Acta. 1962 Feb 12;57:32–43. doi: 10.1016/0006-3002(62)91074-0. [DOI] [PubMed] [Google Scholar]
- KOCH A. L. THE ROLE OF PERMEASE IN TRANSPORT. Biochim Biophys Acta. 1964 Jan 27;79:177–200. doi: 10.1016/0926-6577(64)90050-6. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Barnes E. M., Jr Mechanisms of active transport in isolated membrane vesicles. II. The mechanism of energy coupling between D-lactic dehydrogenase and beta-galactoside transport in membrane preparations from Escherichia coli. J Biol Chem. 1971 Sep 10;246(17):5523–5531. [PubMed] [Google Scholar]
- Kaback H. R., Deuel F. Proline uptake by disrupted membrane preparations from Escherichia coli. Arch Biochem Biophys. 1969 Jun;132(1):118–129. doi: 10.1016/0003-9861(69)90343-9. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Milner L. S. Relationship of a membrane-bound D-(-)-lactic dehydrogenase to amino acid transport in isolated bacterial membrane preparations. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1008–1015. doi: 10.1073/pnas.66.3.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R., Stadtman E. R. Proline uptake by an isolated cytoplasmic membrane preparation of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Apr;55(4):920–927. doi: 10.1073/pnas.55.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. The role of the phosphoenolpyruvate-phosphotransferase system in the transport of sugars by isolated membrane preparations of Escherichia coli. J Biol Chem. 1968 Jul 10;243(13):3711–3724. [PubMed] [Google Scholar]
- Kaback H. R. Transport. Annu Rev Biochem. 1970;39:561–598. doi: 10.1146/annurev.bi.39.070170.003021. [DOI] [PubMed] [Google Scholar]
- Kalckar H. M. The periplasmic galactose binding protein of Escherichia coli. Science. 1971 Nov 5;174(4009):557–565. doi: 10.1126/science.174.4009.557. [DOI] [PubMed] [Google Scholar]
- Kammen H. O., Strand M. Thymine metabolism in Escherichia coli. II. Altered uptake of thymine after bacteriophage infection. J Biol Chem. 1967 Apr 25;242(8):1854–1863. [PubMed] [Google Scholar]
- Kaplan D. M., Criddle R. S. Membrane structural proteins. Physiol Rev. 1971 Apr;51(2):249–272. doi: 10.1152/physrev.1971.51.2.249. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Role of metabolic energy in the transport of -galactosides by Streptococcus lactis. J Bacteriol. 1972 Feb;109(2):784–789. doi: 10.1128/jb.109.2.784-789.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T., Miyata I., Esaki K., Nose Y. Thiamine uptake in Escherichia coli. I. General properties of thiamine uptake system in Escherichia coli. Arch Biochem Biophys. 1969 Apr;131(1):223–230. doi: 10.1016/0003-9861(69)90125-8. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Gronlund A. F. Proline transport by Pseudomonas aeruginosa. Biochim Biophys Acta. 1969;193(2):444–455. doi: 10.1016/0005-2736(69)90203-x. [DOI] [PubMed] [Google Scholar]
- Keister D. L., Minton N. J. ATP synthesis driven by inorganic pyrophosphate in Rhodospirillum rubrum chromatophores. Biochem Biophys Res Commun. 1971 Mar 5;42(5):932–939. doi: 10.1016/0006-291x(71)90520-1. [DOI] [PubMed] [Google Scholar]
- Keister D. L., Minton N. J. Energy-linked reactions in photosynthetic bacteria. V. Relation of the light-induced proton uptake to photophosphorylation in R. rubrum chromatophores. Proc Natl Acad Sci U S A. 1969 Jun;63(2):489–495. doi: 10.1073/pnas.63.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keister D. L., Yike N. J. Energy-linked reactions in photosynthetic bacteria. I. Succinatelinked ATP-driven NAD reduction by Rhodospirillum rubrum chromatophores. Arch Biochem Biophys. 1967 Aug;121(2):415–422. doi: 10.1016/0003-9861(67)90095-1. [DOI] [PubMed] [Google Scholar]
- Kerwar G. K., Gordon A. S., Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. IV. Galactose transport by isolated membrane vesicles from Escherichia coli. J Biol Chem. 1972 Jan 10;247(1):291–297. [PubMed] [Google Scholar]
- Kimmich G. A. Active sugar accumulation by isolated intestinal epithelial cells. A new model for sodium-dependent metabolite transport. Biochemistry. 1970 Sep 15;9(19):3669–3677. doi: 10.1021/bi00821a004. [DOI] [PubMed] [Google Scholar]
- Klein K., Steinberg R., Fiethen B., Overath P. Fatty acid degradation in Escherichia coli. An inducible system for the uptake of fatty acids and further characterization of old mutants. Eur J Biochem. 1971 Apr;19(3):442–450. doi: 10.1111/j.1432-1033.1971.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Klemme B., Klemme J. H., San Pietro A. PPase, ATPase, and photophosphorylation in chromatophores of Rhodospirillum rubrum: inactivation by phospholipase A; reconstitution by phospholipids. Arch Biochem Biophys. 1971 May;144(1):339–342. doi: 10.1016/0003-9861(71)90486-3. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Metabolite transport in mitochondria: an example for intracellular membrane function. Essays Biochem. 1970;6:119–159. [PubMed] [Google Scholar]
- Klingenberg M., Palmieri F., Quagliariello E. Quantitative correlation between the distribution of anions and the pH difference across the mitochondrial membrane. Eur J Biochem. 1970 Dec;17(2):230–238. doi: 10.1111/j.1432-1033.1970.tb01158.x. [DOI] [PubMed] [Google Scholar]
- Knobloch K., Eley J. H., Aleem M. I. Generation of reducing power in bacterial photosynthesis. Rhodopseudomonas palustris. Biochem Biophys Res Commun. 1971 May 21;43(4):834–839. doi: 10.1016/0006-291x(71)90692-9. [DOI] [PubMed] [Google Scholar]
- Knobloch K., Ishaque M., Aleem M. I. Oxidative phosphorylation in Micrococcus denitrificans under autotrophic growth conditions. Arch Mikrobiol. 1971;76(2):114–125. doi: 10.1007/BF00411785. [DOI] [PubMed] [Google Scholar]
- Knowles A. F., Guillory R. J., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XXIV. A factor required for the binding of mitochondrial adenosine triphosphatase to the inner mitochondrial membrane. J Biol Chem. 1971 Apr 25;246(8):2672–2679. [PubMed] [Google Scholar]
- Knowles C. J., Smith L. The relationship between substrate-induced respiration and swelling in Azotobacter vinelandii. Biochim Biophys Acta. 1971 Apr 6;234(1):153–161. doi: 10.1016/0005-2728(71)90140-x. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Energy expenditure is obligatory for the downhill transport of galactosides. J Mol Biol. 1971 Aug 14;59(3):447–459. doi: 10.1016/0022-2836(71)90309-3. [DOI] [PubMed] [Google Scholar]
- Konings W. N., Barnes E. M., Jr, Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. 2. The coupling of reduced phenazine methosulfate to the concentrative uptake of beta-galactosides and amino acids. J Biol Chem. 1971 Oct 10;246(19):5857–5861. [PubMed] [Google Scholar]
- Konings W. N., Freese E. L-serine transport in membrane vesicles of Bacillus subtilis energized by NADH or reduced phenazine methosulfate. FEBS Lett. 1971 Apr 12;14(1):65–68. doi: 10.1016/0014-5793(71)80276-4. [DOI] [PubMed] [Google Scholar]
- Kovác L., Kuzela S. Effect of uncoupling agents and azide on the synthesis of beta-galactosidase in aerobically and anaerobically grown Escherichia coli. Biochim Biophys Acta. 1966 Oct 31;127(2):355–365. [PubMed] [Google Scholar]
- Kröger A., Dadák V., Klingenberg M., Diemer F. On the role of quinones in bacterial electron transport. Differential roles of ubiquinone and menaquinone in Proteus rettgeri. Eur J Biochem. 1971 Aug 16;21(3):322–333. doi: 10.1111/j.1432-1033.1971.tb01472.x. [DOI] [PubMed] [Google Scholar]
- Kröger A., Dadák V. On the role of quinones in bacterial electron transport. The respiratory system of Bacillus megaterium. Eur J Biochem. 1969 Dec;11(2):328–340. doi: 10.1111/j.1432-1033.1969.tb00776.x. [DOI] [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. I. Isolation of a phosphotransferase system from Escherichia coli. J Biol Chem. 1971 Mar 10;246(5):1393–1406. [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. II. Characterization of constitutive membrane-bound enzymes II of the Escherichia coli phosphotransferase system. J Biol Chem. 1971 Mar 10;246(5):1407–1418. [PubMed] [Google Scholar]
- Lardy H. A., Ferguson S. M. Oxidative phosphorylation in mitochondria. Annu Rev Biochem. 1969;38:991–1034. doi: 10.1146/annurev.bi.38.070169.005015. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L. A soluble, heat-labile, high-affinity Ca2 plus-binding factor extracted from rat liver mitochondria. Biochem Biophys Res Commun. 1971 Jan 22;42(2):312–318. doi: 10.1016/0006-291x(71)90104-5. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L., Carafoli E., Rossi C. S. Energy-linked ion movements in mitochondrial systems. Adv Enzymol Relat Areas Mol Biol. 1967;29:259–320. doi: 10.1002/9780470122747.ch6. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L., Carafoli E. The interaction of La 3+ with mitochondria in relation to respiration-coupled Ca 2+ transport. Arch Biochem Biophys. 1971 Apr;143(2):506–515. doi: 10.1016/0003-9861(71)90235-9. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L. Mitochondria and calcium ion transport. Biochem J. 1970 Sep;119(2):129–138. doi: 10.1042/bj1190129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levisohn R., Konisky J., Nomura M. Interaction of colicins with bacterial cells. IV. Immunity breakdown studied with colicins Ia and Ib. J Bacteriol. 1968 Sep;96(3):811–821. doi: 10.1128/jb.96.3.811-821.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman E. A., Skulachev V. P. Conversion of biomembrane-produced energy into electric form. IV. General discussion. Biochim Biophys Acta. 1970 Aug 4;216(1):30–42. doi: 10.1016/0005-2728(70)90156-8. [DOI] [PubMed] [Google Scholar]
- Liberman E. A., Topaly V. P. Selective transport of ions through bimolecular phospholipid membranes. Biochim Biophys Acta. 1968 Sep 17;163(2):125–136. doi: 10.1016/0005-2736(68)90089-8. [DOI] [PubMed] [Google Scholar]
- Liberman E. A., Topaly V. P., Silberstein A. Y. Charged and neutral ion carriers through bimolecular phospholipid membranes. Biochim Biophys Acta. 1970;196(2):221–234. doi: 10.1016/0005-2736(70)90010-6. [DOI] [PubMed] [Google Scholar]
- Liberman E. A., Topaly V. P., Tsofina L. M., Jasaitis A. A., Skulachev V. P. Mechanism of coupling of oxidative phosphorylation and the membrane potential of mitochondria. Nature. 1969 Jun 14;222(5198):1076–1078. doi: 10.1038/2221076a0. [DOI] [PubMed] [Google Scholar]
- Lofrumento N. E., Hoek J. B., Meyer A. J., Tager J. M. Phosphate transport in rat-liver mitochondria. Biochim Biophys Acta. 1971 Mar 2;226(2):297–308. doi: 10.1016/0005-2728(71)90096-x. [DOI] [PubMed] [Google Scholar]
- Lusk J. E., Kennedy E. P. Magneisum transport in Escherichia coli. J Biol Chem. 1969 Mar 25;244(6):1653–1655. [PubMed] [Google Scholar]
- MARQUIS R. E., GERHARDT P. RESPIRATION-COUPLED AND PASSIVE UPTAKE OF ALPHA-AMINOISOBUTYRIC ACID, A METABOLICALLY INERT TRANSPORT ANALOGUE, BY BACILLUS MEGATERIUM. J Biol Chem. 1964 Oct;239:3361–3371. [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Manno J. A., Schachter D. Energy-coupled influx of thiomethylgalactoside into Escherichia coli. J Biol Chem. 1970 Mar 10;245(5):1217–1223. [PubMed] [Google Scholar]
- Massari S., Azzone G. F. The mechanism of ion translocation in mitochondria. 1. Coupling of K+ and H+ fluxes. Eur J Biochem. 1970 Feb;12(2):301–309. doi: 10.1111/j.1432-1033.1970.tb00851.x. [DOI] [PubMed] [Google Scholar]
- Massari S., Azzone G. F. The mechanism of ion translocation in mitochondria. 2. Active transport and proton pump. Eur J Biochem. 1970 Feb;12(2):310–318. doi: 10.1111/j.1432-1033.1970.tb00852.x. [DOI] [PubMed] [Google Scholar]
- McGivan J. D., Klingenberg M. Correlation between H+ and anion movement in mitochondria and the key role of the phosphate carrier. Eur J Biochem. 1971 Jun 11;20(3):392–399. doi: 10.1111/j.1432-1033.1971.tb01405.x. [DOI] [PubMed] [Google Scholar]
- Medveczky N., Rosenberg H. Phosphate transport in Escherichia coli. Biochim Biophys Acta. 1971 Aug 13;241(2):494–506. doi: 10.1016/0005-2736(71)90048-4. [DOI] [PubMed] [Google Scholar]
- Mela L. Inhibition and activation of calcium transport in mitochondria. Effect of lanthanides and local anesthetic drugs. Biochemistry. 1969 Jun;8(6):2481–2486. doi: 10.1021/bi00834a034. [DOI] [PubMed] [Google Scholar]
- Melandri B. A., Baccarini-Melandri A., San Pietro A., Gest H. Role of phosphorylation coupling factor in light-dependent proton translocation by Rhodopseudomonas capsulata membrane preparations. Proc Natl Acad Sci U S A. 1970 Oct;67(2):477–484. doi: 10.1073/pnas.67.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelson M. N. Phosphorylation and the reduced nicotinamide adenine dinucleotide oxidase reaction in Streptococcus agalactiae. J Bacteriol. 1969 Nov;100(2):895–901. doi: 10.1128/jb.100.2.895-901.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner L. S., Kaback H. R. The role of phosphatidylglycerol in the vectorial phosphorylation of sugar by isolated bacterial membrane preparations. Proc Natl Acad Sci U S A. 1970 Mar;65(3):683–690. doi: 10.1073/pnas.65.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky R., Barlow V. Purification and properties of ATPase from the cytoplasmic membrane of Bacillus megaterium KM. Biochim Biophys Acta. 1971 Sep 14;241(3):835–845. doi: 10.1016/0005-2736(71)90011-3. [DOI] [PubMed] [Google Scholar]
- Mirsky R. Membrane proteins from Bacillus megaterium KM. Biochemistry. 1969 Mar;8(3):1164–1169. doi: 10.1021/bi00831a050. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Acid-base titration across the membrane system of rat-liver mitochondria. Catalysis by uncouplers. Biochem J. 1967 Aug;104(2):588–600. doi: 10.1042/bj1040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Estimation of membrane potential and pH difference across the cristae membrane of rat liver mitochondria. Eur J Biochem. 1969 Feb;7(4):471–484. doi: 10.1111/j.1432-1033.1969.tb19633.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Proton translocation coupled to ATP hydrolysis in rat liver mitochondria. Eur J Biochem. 1968 May;4(4):530–539. doi: 10.1111/j.1432-1033.1968.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Translocation of some anions cations and acids in rat liver mitochondria. Eur J Biochem. 1969 Jun;9(2):149–155. doi: 10.1111/j.1432-1033.1969.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Proton-translocation phosphorylation in mitochondria, chloroplasts and bacteria: natural fuel cells and solar cells. Fed Proc. 1967 Sep;26(5):1370–1379. [PubMed] [Google Scholar]
- Mitchell P. Translocations through natural membranes. Adv Enzymol Relat Areas Mol Biol. 1967;29:33–87. doi: 10.1002/9780470122747.ch2. [DOI] [PubMed] [Google Scholar]
- Montal M., Nishimura M., Chance B. Uncoupling and charge transfer in bacterial chromatophores. Biochim Biophys Acta. 1970 Nov 3;223(1):183–188. doi: 10.1016/0005-2728(70)90143-x. [DOI] [PubMed] [Google Scholar]
- Morowitz H. J., Terry T. M. Characterization of the plasma membrane of Mycoplasma laidlawii. V. Effects of selective removal of protein and lipid. Biochim Biophys Acta. 1969 Jul 15;183(2):276–294. doi: 10.1016/0005-2736(69)90084-4. [DOI] [PubMed] [Google Scholar]
- Munoz E., Freer J. H., Ellar D. J., Salton M. R. Membrane-associated ATPase activity from Micrococcus lysodeikticus. Biochim Biophys Acta. 1968 Apr 29;150(3):531–533. doi: 10.1016/0005-2736(68)90156-9. [DOI] [PubMed] [Google Scholar]
- Muñoz E., Salton M. R., Ng M. H., Schor M. T. Membrane adenosine triphosphatase of Micrococcus lysodeikticus. Purification, properties of the "soluble" enzyme and properties of the membrane-bound enzyme. Eur J Biochem. 1969 Feb;7(4):490–501. [PubMed] [Google Scholar]
- Nachbar M. S., Salton M. R. Characteristics of a lipid-rich NADH dehydrogenase-containing particulate fraction obtained from Micrococcus lysodeikticus membranes. Biochim Biophys Acta. 1970 Dec 8;223(2):309–320. doi: 10.1016/0005-2728(70)90187-8. [DOI] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T., Simoni R. D., Hays J. B., Roseman S. Phosphorylation of a sugar-specific protein component of the lactose transport system in Staphylococcus aureus. Biochem Biophys Res Commun. 1971 Mar 5;42(5):836–843. doi: 10.1016/0006-291x(71)90506-7. [DOI] [PubMed] [Google Scholar]
- Newton N. A., Cox G. B., Gibson F. The function of menaquinone (vitamin K 2 ) in Escherichia coli K-12. Biochim Biophys Acta. 1971 Jul 20;244(1):155–166. doi: 10.1016/0304-4165(71)90132-2. [DOI] [PubMed] [Google Scholar]
- Nierlich D. P. Radioisotope uptake as a measure of synthesis of messenger RNA. Science. 1967 Dec 1;158(3805):1186–1188. doi: 10.1126/science.158.3805.1186. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Pressman B. C. Effects of ionophorous antibiotics on the light-induced internal and external hydrogen ion changes and phosphorylation in bacterial chromatophores. Biochemistry. 1969 Apr;8(4):1360–1370. doi: 10.1021/bi00832a009. [DOI] [PubMed] [Google Scholar]
- Niven D. F., Hamilton W. A. The mechanism of energy coupling in the active transport of amino acids by Staphylococcus aureus. Biochem J. 1972 Apr;127(3):58P–58P. doi: 10.1042/bj1270058pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M. Colicins and related bacteriocins. Annu Rev Microbiol. 1967;21:257–284. doi: 10.1146/annurev.mi.21.100167.001353. [DOI] [PubMed] [Google Scholar]
- Nomura M., Witten C. Interaction of colicins with bacterial cells. 3. Colicin-tolerant mutations in Escherichia coli. J Bacteriol. 1967 Oct;94(4):1093–1111. doi: 10.1128/jb.94.4.1093-1111.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny C. P., Englesberg E. The L-arabinose permease system in Escherichia coli B/r. Biochim Biophys Acta. 1966 Mar 28;117(1):217–230. doi: 10.1016/0304-4165(66)90169-3. [DOI] [PubMed] [Google Scholar]
- Ota N., Galsworthy P. R., Pardee A. B. Genetics of sulfate transport by Salmonella typhimurium. J Bacteriol. 1971 Mar;105(3):1053–1062. doi: 10.1128/jb.105.3.1053-1062.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Schairer H. U., Stoffel W. Correlation of in vivo and in vitro phase transitions of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1970 Oct;67(2):606–612. doi: 10.1073/pnas.67.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACKER L., PERRY M. Energy-linked light-scattering changes in Escherichia coli. Arch Biochem Biophys. 1961 Nov;95:379–388. doi: 10.1016/0003-9861(61)90163-1. [DOI] [PubMed] [Google Scholar]
- Packer L. Relation of structure to energy coupling in rat liver mitochondria. Fed Proc. 1970 Jul-Aug;29(4):1533–1540. [PubMed] [Google Scholar]
- Palmieri F., Prezioso G., Quagliariello E., Klingenberg M. Kinetic study of the dicarboxylate carrier in rat liver mitochondria. Eur J Biochem. 1971 Sep 13;22(1):66–74. doi: 10.1111/j.1432-1033.1971.tb01515.x. [DOI] [PubMed] [Google Scholar]
- Palmieri F., Quagliariello E. Correlation between anion uptake and the movement of K+ and H+ across the mitochondrial membrane. Eur J Biochem. 1969 Apr;8(4):473–481. doi: 10.1111/j.1432-1033.1969.tb00551.x. [DOI] [PubMed] [Google Scholar]
- Papa S., Lofrumento N. E., Kanduc D., Paradies G., Quagliariello E. The transport of citric-acid-cycle intermediates in rat-liver mitochondria. Electrical nature and coupling of the exchange-diffusion reactions with proton translocation. Eur J Biochem. 1971 Sep 13;22(1):134–143. doi: 10.1111/j.1432-1033.1971.tb01524.x. [DOI] [PubMed] [Google Scholar]
- Papa S., Lofrumento N. E., Loglisci M., Quagliariello E. On the transport of inorganic phosphate and malate in rat-liver mitochondria. Biochim Biophys Acta. 1969 Oct 21;189(2):311–314. doi: 10.1016/0005-2728(69)90060-7. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. Membrane transport proteins. Proteins that appear to be parts of membrane transport systems are being isolated and characterized. Science. 1968 Nov 8;162(3854):632–637. doi: 10.1126/science.162.3854.632. [DOI] [PubMed] [Google Scholar]
- Patterson P. H., Lennarz W. J. Novel protein composition of a bacterial membrane. Biochem Biophys Res Commun. 1970 Jul 27;40(2):408–415. doi: 10.1016/0006-291x(70)91024-7. [DOI] [PubMed] [Google Scholar]
- Pavlasova E., Harold F. M. Energy coupling in the transport of beta-galactosides by Escherichia coli: effect of proton conductors. J Bacteriol. 1969 Apr;98(1):198–204. doi: 10.1128/jb.98.1.198-204.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penniston J. T., Southard J. H., Green D. E., Luzzana M. Speed of respiration-dependent proton ejection by mitochondria. Application of a pH-measuring system with 10-msec resolution. Arch Biochem Biophys. 1971 Feb;142(2):638–644. doi: 10.1016/0003-9861(71)90529-7. [DOI] [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino acid transport systems in Escherichia coli K-12. J Biol Chem. 1968 Nov 25;243(22):5914–5920. [PubMed] [Google Scholar]
- Pressman B. C. Induced active transport of ions in mitochondria. Proc Natl Acad Sci U S A. 1965 May;53(5):1076–1083. doi: 10.1073/pnas.53.5.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. Mechanism of action of transport-mediating antibiotics. Ann N Y Acad Sci. 1969 Oct 31;147(19):829–841. doi: 10.1111/j.1749-6632.1969.tb41291.x. [DOI] [PubMed] [Google Scholar]
- Quagliariello E., Genchi G., Palmieri F. Respiration-dependent anion uptake by rat liver mitochondria. FEBS Lett. 1971 Mar 22;13(5):253–257. doi: 10.1016/0014-5793(71)80233-8. [DOI] [PubMed] [Google Scholar]
- Racker E. The two faces of the inner mitochondrial membrane. Essays Biochem. 1970;6:1–22. [PubMed] [Google Scholar]
- Reaveley D. A., Rogers H. J. Some enzymic activities and chemical properties of the mesosomes and cytoplasmic membranes of Bacillus licheniformis 6346. Biochem J. 1969 Jun;113(1):67–79. doi: 10.1042/bj1130067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves J. P. Transient pH changes during D-lactate oxidation by membrane vesicles. Biochem Biophys Res Commun. 1971 Nov;45(4):931–936. doi: 10.1016/0006-291x(71)90427-x. [DOI] [PubMed] [Google Scholar]
- Reid K. G., Utech N. M., Holden J. T. Multiple transport components for dicarboxylic amino acids in Streptococcus faecalis. J Biol Chem. 1970 Oct 25;245(20):5261–5272. [PubMed] [Google Scholar]
- Reid R. A. The synthesis of adenosine triphosphate by transmembrane ionic gradients. Biochem J. 1970 Feb;116(4):12P–12P. doi: 10.1042/bj1160012pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemersma J. C. Effects of sodium azide and 2,4-dinitrophenol on phosphorylation reactions and ion fluxes in Saccharomyces cerevisiae. Biochim Biophys Acta. 1968 Jan 15;153(1):80–87. doi: 10.1016/0005-2728(68)90148-5. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A. H., Eberhard S. J., Dingle S. L., McDowell T. D. Distribution of the phosphoenolpyruvate: glucose phosphotransferase system in bacteria. J Bacteriol. 1970 Nov;104(2):808–813. doi: 10.1128/jb.104.2.808-813.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S. P., Fox C. F. The -glucoside system of Escherichia coli. II. Kinetic evidence for a phosphoryl-enzyme II intermediate. Biochem Biophys Res Commun. 1971 Oct 15;45(2):376–380. doi: 10.1016/0006-291x(71)90829-1. [DOI] [PubMed] [Google Scholar]
- Rossi E., Azzone G. F. The mechanism of ion translocation in mitochondria. 3. Coupling of K+ efflux with ATP synthesis. Eur J Biochem. 1970 Feb;12(2):319–327. doi: 10.1111/j.1432-1033.1970.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Romeo D. Role of lipids in the biosynthesis of the bacterial cell envelope. Bacteriol Rev. 1971 Mar;35(1):14–38. doi: 10.1128/br.35.1.14-38.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Razin S. Electrophoretic patterns of membrane proteins of Mycoplasma. J Bacteriol. 1967 Aug;94(2):359–364. doi: 10.1128/jb.94.2.359-364.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. ATP synthesis and electrical membrane potential in mitochondria. Eur J Biochem. 1970 Jul;15(1):22–28. doi: 10.1111/j.1432-1033.1970.tb00971.x. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R. On the physical state of the intracellularly accumulates substrates of beta-galactoside-permease in Escherichia coli. Biochim Biophys Acta. 1958 Sep;29(3):579–587. doi: 10.1016/0006-3002(58)90015-5. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Young W. S., 3rd, Roseman S. Utilization and transport of hexoses by mutant strains of Salmonella typhimurium lacking enzyme I of the phosphoenolpyruvate-dependent phosphotransferase system. J Biol Chem. 1971 Sep 25;246(18):5838–5840. [PubMed] [Google Scholar]
- Salton M. R. Structure and function of bacterial cell membranes. Annu Rev Microbiol. 1967;21:417–442. doi: 10.1146/annurev.mi.21.100167.002221. [DOI] [PubMed] [Google Scholar]
- Sanwal B. D. Allosteric controls of amphilbolic pathways in bacteria. Bacteriol Rev. 1970 Mar;34(1):20–39. doi: 10.1128/br.34.1.20-39.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough G. A., Rumley M. K., Kennedy E. P. The function of adenosine 5'-triphosphate in the lactose transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Jul;60(3):951–958. doi: 10.1073/pnas.60.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Examination of the protein composition of the cell envelope of Escherichia coli by polyacrylamide gel electrophoresis. J Bacteriol. 1970 Nov;104(2):882–889. doi: 10.1128/jb.104.2.882-889.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnebli H. P., Abrams A. Membrane adenosine triphosphatase from Streptococcus faecalis. Preparation and homogeneity. J Biol Chem. 1970 Mar 10;245(5):1115–1121. [PubMed] [Google Scholar]
- Schnebli H. P., Vatter A. E., Abrams A. Membrane adenosine triphosphatase from Streptococcus faecalis. Molecular weight, subunit structure, and amino acid composition. J Biol Chem. 1970 Mar 10;245(5):1122–1127. [PubMed] [Google Scholar]
- Scholes P. B., McLain G., Smith L. Purification and properties of a c-type cytochrome from Micrococcus denitrificans. Biochemistry. 1971 May 25;10(11):2072–2076. doi: 10.1021/bi00787a017. [DOI] [PubMed] [Google Scholar]
- Scholes P. B., Smith L. Composition and properties of the membrane-bound respiratory chain system of Micrococcus denitrificans. Biochim Biophys Acta. 1968 Feb 12;153(2):363–375. doi: 10.1016/0005-2728(68)90081-9. [DOI] [PubMed] [Google Scholar]
- Scholes P. B., Smith L. The isolation and properties of the cytoplasmic membrane of Micrococcus denitrificans. Biochim Biophys Acta. 1968 Feb 12;153(2):350–362. doi: 10.1016/0005-2728(68)90080-7. [DOI] [PubMed] [Google Scholar]
- Scholes P., Mitchell P. Acid-base titration across the plasma membrane of Micrococcus denitrificans: factors affecting the effective proton conductance and the respiratory rate. J Bioenerg. 1970 Jun;1(1):61–72. doi: 10.1007/BF01516089. [DOI] [PubMed] [Google Scholar]
- Scholes P., Mitchell P., Moyle J. The polarity of proton translocation in some photosynthetic microorganisms. Eur J Biochem. 1969 Apr;8(3):450–454. doi: 10.1111/j.1432-1033.1969.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Scholes P., Mitchell P. Respiration-driven proton translocation in Micrococcus denitrificans. J Bioenerg. 1971 Sep;1(3):309–323. doi: 10.1007/BF01516290. [DOI] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Senior A. E., Brooks J. C. The subunit composition of the mitochondrial oligomycin-insensitive ATPase. FEBS Lett. 1971 Oct 1;17(2):327–329. doi: 10.1016/0014-5793(71)80178-3. [DOI] [PubMed] [Google Scholar]
- Short S. A., White D. C., Kaback H. R. Active transport in isolated bacterial membrane vesicles. V. The transport of amino acids by membrane vesicles prepared from Staphylococcus aureus. J Biol Chem. 1972 Jan 10;247(1):298–304. [PubMed] [Google Scholar]
- Silver S. Active transport of magnesium in escherichia coli. Proc Natl Acad Sci U S A. 1969 Mar;62(3):764–771. doi: 10.1073/pnas.62.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Johnseine P., King K. Manganese Active Transport in Escherichia coli. J Bacteriol. 1970 Dec;104(3):1299–1306. doi: 10.1128/jb.104.3.1299-1306.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulachev V. P. Electric fields in coupling membranes. FEBS Lett. 1970 Dec 18;11(5):301–308. doi: 10.1016/0014-5793(70)80554-3. [DOI] [PubMed] [Google Scholar]
- Slater E. C. The coupling between energy-yielding and energy-utilizing reactions in mitochondria. Q Rev Biophys. 1971 Feb;4(1):35–71. doi: 10.1017/s0033583500000391. [DOI] [PubMed] [Google Scholar]
- Snoswell A. M., Cox G. B. Piericidin A and inhibition of respiratory chain activity in Escherichia coli K12. Biochim Biophys Acta. 1968 Oct 1;162(3):455–458. doi: 10.1016/0005-2728(68)90132-1. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Fairbanks G., Wallach D. F. Disposition of the major proteins in the isolated erythrocyte membrane. Proteolytic dissection. Biochemistry. 1971 Jun 22;10(13):2617–2624. doi: 10.1021/bi00789a031. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Weinstein R. S., Straus J. H., Wallach D. F. Inside-out red cell membrane vesicles: preparation and purification. Science. 1970 Apr 10;168(3928):255–257. doi: 10.1126/science.168.3928.255. [DOI] [PubMed] [Google Scholar]
- Steim J. M., Tourtellotte M. E., Reinert J. C., McElhaney R. N., Rader R. L. Calorimetric evidence for the liquid-crystalline state of lipids in a biomembrane. Proc Natl Acad Sci U S A. 1969 May;63(1):104–109. doi: 10.1073/pnas.63.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J., Roseman S. A sodium-dependent sugar co-transport system in bacteria. Biochem Biophys Res Commun. 1971 Jul 2;44(1):132–138. doi: 10.1016/s0006-291x(71)80168-7. [DOI] [PubMed] [Google Scholar]
- Stockdale M., Selwyn M. J. Effects of ring substituents on the activity of phenols as inhibitors and uncouplers of mitochondrial respiration. Eur J Biochem. 1971 Aug 25;21(4):565–574. doi: 10.1111/j.1432-1033.1971.tb01502.x. [DOI] [PubMed] [Google Scholar]
- Storey B. T. Chemical hypothesis for energy conservation in the mitochondrial respiratory chain. J Theor Biol. 1970 Aug;28(2):233–259. doi: 10.1016/0022-5193(70)90054-8. [DOI] [PubMed] [Google Scholar]
- Sweetman A. J., Griffiths D. E. Studies on energy-linked reactions. Energy-linked reduction of oxidized nicotinamide-adenine dinucleotide by succinate in Escherichia coli. Biochem J. 1971 Jan;121(1):117–124. doi: 10.1042/bj1210117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetman A. J., Griffiths D. E. Studies on energy-linked reactions. Energy-linked transhydrogenase reaction in Escherichia coli. Biochem J. 1971 Jan;121(1):125–130. doi: 10.1042/bj1210125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Green M. L., Happold F. C. Cation-activated nucleotidase in cell envelopes of a marine bacterium. J Bacteriol. 1969 Sep;99(3):834–841. doi: 10.1128/jb.99.3.834-841.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., MacLeod R. A. Functions of Na+ and K+ in the active transport of -aminoisobutyric acid in a marine pseudomonad. J Biol Chem. 1971 Jun 25;246(12):4066–4074. [PubMed] [Google Scholar]
- Thorne K. J., Barker D. C. Bactoprenol, ATPase and acetate activating enzymes of a vesicular fraction from Lactobacillus casei. Eur J Biochem. 1969 Dec;11(3):582–591. doi: 10.1111/j.1432-1033.1969.tb00810.x. [DOI] [PubMed] [Google Scholar]
- Thorne K. J., Barker D. C. The occurrence of bactoprenol in the mesosome and plasma membranes of Lactobacillus casei and Lactobacillus plantarum. J Gen Microbiol. 1972 Apr;70(1):87–98. doi: 10.1099/00221287-70-1-87. [DOI] [PubMed] [Google Scholar]
- Ting H. P., Wilson D. F., Chance B. Effects of uncouplers of oxidative phosphorylation on the specific conductance of bimolecular lipid membranes. Arch Biochem Biophys. 1970 Nov;141(1):141–146. doi: 10.1016/0003-9861(70)90116-5. [DOI] [PubMed] [Google Scholar]
- Tourtellotte M. E., Branton D., Keith A. Membrane structure: spin labeling and freeze etching of Mycoplasma laidawii. Proc Natl Acad Sci U S A. 1970 Jul;66(3):909–916. doi: 10.1073/pnas.66.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukagoshi N., Fox C. F. Hybridization of membranes by sonic irradiation. Biochemistry. 1971 Aug 17;10(17):3309–3313. doi: 10.1021/bi00793a023. [DOI] [PubMed] [Google Scholar]
- Tupper J. T., Tedeschi H. Microelectrode studies on the membrane properties of isolated mitochondria. II. Absence of a metabolic dependence. Proc Natl Acad Sci U S A. 1969 Jul;63(3):713–717. doi: 10.1073/pnas.63.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupper J. T., Tedeschi H. Microelectrode studies on the membrane properties of isolated mitochondria. Proc Natl Acad Sci U S A. 1969 Jun;63(2):370–377. doi: 10.1073/pnas.63.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupper J. T., Tedeschi H. Mitochondrial membrane potentials measured with microelectrodes: probable ionic basis. Science. 1969 Dec 19;166(3912):1539–1540. doi: 10.1126/science.166.3912.1539. [DOI] [PubMed] [Google Scholar]
- Tyler D. D. Evidence of a phosphate-transporter system in the inner membrane of isolated mitochondria. Biochem J. 1969 Mar;111(5):665–678. doi: 10.1042/bj1110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler D. D. The inhibition of phosphate entry into rat liver mitochondria by organic mercurials and by formaldehyde. Biochem J. 1968 Mar;107(1):121–123. doi: 10.1042/bj1070121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagoloff A., Meagher P. Assembly of the mitochondrial membrane system. V. Properties of a dispersed preparation of the rutamycin-sensitive adenosine triphosphatase of yeast mitochondria. J Biol Chem. 1971 Dec 10;246(23):7328–7336. [PubMed] [Google Scholar]
- Vainio H., Mela L., Chance B. Energy dependent bivalent cation translocation in rat liver mitochondria. Eur J Biochem. 1970 Feb;12(2):387–391. doi: 10.1111/j.1432-1033.1970.tb00863.x. [DOI] [PubMed] [Google Scholar]
- Vernon L. P. Photochemical and electron transport reactions of bacterial photosynthesis. Bacteriol Rev. 1968 Sep;32(3):243–261. doi: 10.1128/br.32.3.243-261.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. A., Crofts A. R. Photosynthesis. Annu Rev Biochem. 1970;39:389–428. doi: 10.1146/annurev.bi.39.070170.002133. [DOI] [PubMed] [Google Scholar]
- Wallace W., Nicholas D. J. The biochemistry of nitrifying microorganisms. Biol Rev Camb Philos Soc. 1969 Jul;44(3):359–391. doi: 10.1111/j.1469-185x.1969.tb01216.x. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Brodie A. F. Enzymatic formation of a phosphorylated derivative of vitamin K. Proc Natl Acad Sci U S A. 1966 Sep;56(3):940–945. doi: 10.1073/pnas.56.3.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbach E. C., Garbus J. Structural changes in mitochondria induced by uncoupling reagents. The response to proteolytic enzymes. Biochem J. 1968 Feb;106(3):711–717. doi: 10.1042/bj1060711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach H., Thomas E., Kaback H. R. Studies on the metabolism of ATP by isolated bacterial membranes: formation and metabolism of membrane-bound phosphatidic acid. Arch Biochem Biophys. 1971 Nov;147(1):249–254. doi: 10.1016/0003-9861(71)90332-8. [DOI] [PubMed] [Google Scholar]
- Wendt L. Mechanism of colicin action: early events. J Bacteriol. 1970 Dec;104(3):1236–1241. doi: 10.1128/jb.104.3.1236-1241.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West I. C. Lactose transport coupled to proton movements in Escherichia coli. Biochem Biophys Res Commun. 1970 Nov 9;41(3):655–661. doi: 10.1016/0006-291x(70)90063-x. [DOI] [PubMed] [Google Scholar]
- West I. C., Mitchell P. Proton movements coupled to the transport of -galactosides into escherichia coli. Biochem J. 1972 Apr;127(3):56P–56P. doi: 10.1042/bj1270056pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West I. C. The site of action of adenosine-5'-triphosphate on beta-galactoside transport in Escherischia coli. FEBS Lett. 1969 Jul;4(2):69–71. doi: 10.1016/0014-5793(69)80198-5. [DOI] [PubMed] [Google Scholar]
- White D. C., Sinclair P. R. Branched electron-transport systems in bacteria. Adv Microb Physiol. 1971;5:173–211. doi: 10.1016/s0065-2911(08)60407-5. [DOI] [PubMed] [Google Scholar]
- Wilkins M. H., Blaurock A. E., Engelman D. M. Bilayer structure in membranes. Nat New Biol. 1971 Mar 17;230(11):72–76. doi: 10.1038/newbio230072a0. [DOI] [PubMed] [Google Scholar]
- Willecke K., Pardee A. B. Inducible transport of citrate in a Gram-positive bacterium, Bacillus subtilis. J Biol Chem. 1971 Feb 25;246(4):1032–1040. [PubMed] [Google Scholar]
- Wilson D. F., Koppelman M., Erecinska M., Dutton P. L. Energy conservation in detergent-treated mitochondria and purified succinate-cytochrome c reductase. Biochem Biophys Res Commun. 1971 Aug 20;44(4):759–766. doi: 10.1016/0006-291x(71)90775-3. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Ting H. P., Koppelman M. S. Mechanism of action of uncouplers of oxidative phosphorylation. Biochemistry. 1971 Jul 20;10(15):2897–2902. doi: 10.1021/bi00791a016. [DOI] [PubMed] [Google Scholar]
- Wilson T. H., Kusch M., Kashket E. R. A mutant in Escherichia coli energy-uncoupled for lactose transporta defect in the lactose-operon. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1409–1414. doi: 10.1016/0006-291x(70)90024-0. [DOI] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. The role of energy coupling in the transport of beta-galactosides by Escherichia coli. J Biol Chem. 1966 May 25;241(10):2200–2211. [PubMed] [Google Scholar]
- Wong J. T., Pincock A., Bronskill P. M. Site of energy coupling in the carrier mechanism for beta-galactoside transport. Biochim Biophys Acta. 1971 Mar 9;233(1):176–188. doi: 10.1016/0005-2736(71)90370-1. [DOI] [PubMed] [Google Scholar]
- Wong P. T., Kashket E. R., Wilson T. H. Energy coupling in the lactose transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Jan;65(1):63–69. doi: 10.1073/pnas.65.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. T., Thompson J., MacLeod R. A. Nutrition and metabolism of marine bacteria. XVII. Ion-dependent retention of alpha-aminoisobutyric acid and its relation to Na+ dependent transport in a marine pseudomonad. J Biol Chem. 1969 Feb 10;244(3):1016–1025. [PubMed] [Google Scholar]
- Young J. H., Blondin G. A., Green D. E. Conformational model of active transport: role of protons. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1364–1368. doi: 10.1073/pnas.68.6.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stedingk L. V., Baltscheffsky H. The light-induced, reversible pH change in chromatophores from Rhodospirillum rubrum. Arch Biochem Biophys. 1966 Nov;117(2):400–404. doi: 10.1016/0003-9861(66)90428-0. [DOI] [PubMed] [Google Scholar]