Abstract
Dietary supplementation with echium oil (EO) containing stearidonic acid (SDA) is a plant-based strategy to improve long-chain (LC) n–3 (ω-3) polyunsaturated fatty acid (PUFA) status in humans. We investigated the effect of EO on LC n–3 PUFA accumulation in blood and biochemical markers with respect to age, sex, and metabolic syndrome. This double-blind, parallel-arm, randomized controlled study started with a 2-wk run-in period, during which participants (n = 80) were administered 17 g/d run-in oil. Normal-weight individuals from 2 age groups (20–35 and 49–69 y) were allotted to EO or fish oil (FO; control) groups. During the 8-wk intervention, participants were administered either 17 g/d EO (2 g SDA; n = 59) or FO [1.9 g eicosapentaenoic acid (EPA); n = 19]. Overweight individuals with metabolic syndrome (n = 19) were recruited for EO treatment only. During the 10-wk study, the participants followed a dietary n–3 PUFA restriction, e.g., no fish. After the 8-wk EO treatment, increases in the LC n–3 metabolites EPA (168% and 79%) and docosapentaenoic acid [DPA (68% and 39%)] were observed, whereas docosahexaenoic acid (DHA) decreased (−5% and −23%) in plasma and peripheral blood mononuclear cells, respectively. Compared with FO, the efficacy of EO to increase EPA and DPA in blood was significantly lower (∼25% and ∼50%, respectively). A higher body mass index (BMI) was associated with lower relative and net increases in EPA and DPA. Compared with baseline, EO significantly reduced serum cholesterol, LDL cholesterol, oxidized LDL, and triglyceride (TG), but also HDL cholesterol, regardless of age and BMI. In the FO group, only TG decreased. Overall, daily intake of 15–20 g EO increased EPA and DPA in blood but had no influence on DHA. EO lowered cardiovascular risk markers, e.g., serum TG, which is particularly relevant for individuals with metabolic syndrome. Natural EO could be a noteworthy source of n–3 PUFA in human nutrition. This trial was registered at clinicaltrials.gov as NCT01856179.
Introduction
A high concentration of long-chain (LC)5 n–3 PUFA in human tissue is associated with a lower risk of cardiovascular disease (CVD) (1, 2). In mammals, the plant-based n–3 PUFA α-linolenic acid (ALA; 18:3n–3), which occurs naturally in linseed oil, serves as the essential precursor of its LC metabolites EPA (20:5n–3) and DHA (22:6n–3) (3).
To meet dietary EPA and DHA recommendations, the AHA recommends the consumption of 2 servings of fish (particularly oily fish) per week (4). However, there are problems associated with this recommendation, such as overfishing and pollution of the marine environment. Furthermore, some people either do not eat fish or have a fish protein allergy. Hence, there is a need to find alternative sources of LC n–3 PUFA for human nutrition and aquaculture feed. Promising strategies to obtain LC n–3 PUFA include the cultivation of macroalgae and microalgae (5) and metabolic engineering of LC n–3 PUFA-synthesizing transgenic plants and microbes (6–8).
Stearidonic acid (SDA; 18:4n–3), another plant-based n–3 PUFA, is an intermediate of ALA that occurs at high concentrations in some plant families as a result of the high activity of Δ6-desaturase, e.g., in Primulaceae and, in particular, Boraginaceae (9). Seed oils from echium species (Boraginaceae) are important by virtue of their uniquely high concentrations of SDA (10–15%) and ALA (30–40%), together with γ-linolenic acid (GLA; 18:3n–6; 10%) (9). The FDA declared refined echium oil (EO) as a novel food for the market, and oil from Echium plantagineum is now available as a food ingredient. EO is already used in aquaculture fish feed (10). In humans, the ability of SDA to increase EPA in blood is clearly higher than that of ALA, presumably because it bypasses the rate-limiting Δ6-desaturase (11). Although transgenic SDA-containing soybean, canola, and linseed oils have been developed in the past few years (12–14), the consumption of SDA oils remains limited. In some regions, especially in Europe, consumer antipathy to genetically modified foods for both human nutrition and animal feed is strong (5). For this reason, naturally occurring EO was chosen for the present study.
Metabolic syndrome comprises a cluster of cardiometabolic risk factors that include abdominal obesity, dyslipidemia, elevated blood pressure, and impaired glucose tolerance. Fish oil (FO) is known to decrease TG concentrations and improve other biochemical markers in individuals with metabolic syndrome (15). With respect to type 2 diabetes mellitus, SDA has been proven to possess potent novel therapeutic efficacy (15). The supplementation of EO could play a role in preventing the progression of CVD. To date, there have been no studies exploring the effects of EO in overweight individuals with the markers of metabolic syndrome.
The objective of this study was to investigate the effect of the combination of ALA, SDA, and GLA from EO on LC n–3 and n–6 PUFAs in plasma and cellular blood lipids. Several biochemical markers were also determined. The efficacy of EO was assessed in humans according to age, BMI, sex, and metabolic syndrome. Because EPA was expected to be the conversion product from SDA, EPA from FO was given to a control group.
Participants and Methods
The double-blind, parallel-arm, randomized controlled study was approved by the ethics committee of the Friedrich Schiller University, Jena, Germany (protocol no. 2270-04/08), and registered at clinicaltrials.gov as NCT01856179.
Participant recruitment and study design.
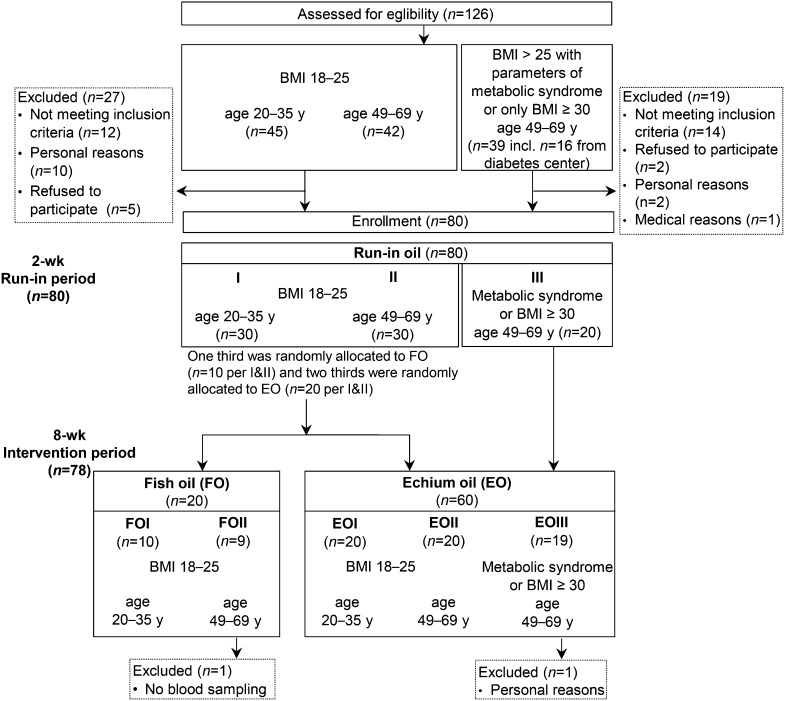
A sample of 20 individuals was estimated to be required to provide >95% power at α = 0.05 to detect a difference in the primary outcome plasma EPA (PASS version 6.0; NCSS Statistical Software). Volunteers were recruited via advertisement. Patients with markers of metabolic syndrome were mainly enlisted from the diabetes research center in Jena. Individuals were assessed and enrolled in subgroups according to the study design based on information collected via the phone, a preliminary meeting, or a questionnaire.
Normolipidemic and normal-weight (BMI of 18–25 kg/m2) individuals were recruited for 2 age groups: group I, 20–35 y; and group II, 49–69 y (Fig. 1). Older, overweight individuals were recruited for EO intervention only [49–69 y; BMI > 25 kg/m2 with markers of metabolic syndrome or BMI ≥ 30 kg/m2 (EOIII); Fig. 1].
FIGURE 1.
Flow diagram of individuals recruited and allocated to the treatment subgroups. Age is shown in years, and BMI is shown in kilograms per square meter. Sex was balanced in each subgroup. EO, echium oil; EOI, participants aged 20–35 y with BMI = 18–25 kg/m2, treated with EO; EOII, participants aged 49–69 y with BMI = 18–25 kg/m2, treated with EO; EOIII, participants aged 49–69 y with BMI > 25 kg/m2 and markers of metabolic syndrome or with BMI ≥ 30 kg/m2, treated with EO; FO, fish oil; FOI, participants aged 20–35 y with BMI = 18–25 kg/m2, treated with FO; FOII, participants aged 49–69 y with BMI = 18–25 kg/m2, treated with FO.
After the 2-wk run-in period, normal-weight individuals (n = 60) were randomly allocated to the treatment groups (FO or EO group). One third of the respective age group were administered FO, and two-thirds were administered EO (Fig. 1). For randomization, every third identification code was assigned to the FO group. Thus, 2 FO (FOI and FOII; each n = 10) and 2 EO (EOI and EOII, each n = 20) subgroups with similar BMI (18–25 kg/m2) but different age range (20–35 and 49–69 y) and the EOIII group with older, overweight individuals (n = 20; 49–69 y, BMI > 25 kg/m2) were investigated during the 8-wk intervention period (Fig. 1). Blood samples were collected after the run-in period (day 0), as well as on days 7 and 56 of the intervention period.
Exclusion criteria were vegetarianism; veganism; daily alcohol abuse; pregnancy; lactation; chronic diseases; or treatment with blood pressure–lowering drugs, cholesterol- and TG-lowering drugs, or dietary supplements. The intake of nonsteroidal anti-inflammatories (e.g., acetylsalicylic acid) had to be avoided, and participants were instructed to use other analgesics (e.g., acetaminophen) instead and to record this in the study protocol. All volunteers were informed of the purpose, course, and possible risks of the study and gave written consent before enrollment. All volunteers completed a questionnaire on health, lifestyle, and dietary factors (e.g., fish consumption) before entering the study. At the end of the study, participants completed a protocol relating to tolerance of the study oils and medication use during the study. Compliance with the study regimen was assessed via an anonymous questionnaire and by counting the number of cups of oil consumed.
Supplemented oils.
During the run-in period, all participants consumed the run-in oil with an FA distribution found in an average Western diet to obtain baseline values adapted to the daily dose of 17 g of oil during intervention (Fig. 1; Table 1). The run-in oil was mixed into a chocolate spread and contained various fats and oils (chocolate-spread fat, coconut fat, palm oil, palm kernel fat, olive oil, and common sunflower oil in the following proportions: 40:18:10:17:10:5.
TABLE 1.
FA profile of study oils and daily supplemented FA dose during the study1
| Daily dose |
||||||
| Study oils |
2-wk run-in period | 8-wk intervention period |
||||
| Run-in oil | Echium oil | Fish oil2 | Run-in oil | Echium oil | Fish oil | |
| g/100 g | g/d | |||||
| Oil | 17 | 17 | 17 | |||
| FAs | ||||||
| ∑SFA | 44 | 11 | 12 | 7.5 | 1.8 | 2.1 |
| ∑MUFA (mainly 18:1n–9) | 39 | 18 | 67 | 6.7 | 3.0 | 12 |
| ∑PUFA | 16 | 70 | 20 | 2.8 | 12 | 3.3 |
| 18:2n–6, LA | 16 | 17 | 5.7 | 2.8 | 2.9 | 0.9 |
| 18:3n–6, GLA | — | 11 | — | — | 1.8 | — |
| 18:3n–3, ALA | 0.2 | 30 | 0.6 | — | 5.0 | 0.1 |
| 18:4n–3, SDA | — | 12 | 1.2 | — | 2.0 [≙0.7 en%] | 0.2 |
| 20:5n–3, EPA | — | — | 11 | — | — | 1.9 [≙0.7 en%] |
| 22:5n–3, DPA | — | — | 0.2 | — | — | — |
| 22:6n–3, DHA | — | — | 1.4 | — | — | 0.2 |
Data are means of men and women. — indicates a concentration <0.1 g/100 g or <0.1 g/d. ALA, α-linolenic acid; DPA, docosapentaenoic acid; en%, % of total energy intake; GLA, γ-linolenic acid; LA, linoleic acid; SDA, stearidonic acid.
EPA-rich fish oil (EPA/DHA 9:1; fish oil was mixed in olive oil).
The EO group was administered ∼17 g/d EO (seed oil of E. plantagineum; Incromega V3; Croda) containing 5 g of ALA, 2 g of SDA, and 2 g of GLA (Table 1). The FO group (control) was administered EPA-rich FO (Croda EPA-TG-500) containing 1.9 g of EPA similar to the amount of SDA in EO (Table 1). For isocaloric supplementation in the FO group, the EPA-rich FO (∼3.5 g) was mixed with a refined n–3 PUFA–free olive oil (Gustav Heess). Hence, both the EO and FO groups were administered ∼17 g/d of the respective study oil (Table 1). All study oils contained the FA as TG. Given the generally lower intake of food by women, women were administered a slightly smaller quantity of oil than men (men, 18.5 g/d; women, 15.5 g/d). Therefore, in relation to total fat intake, both sexes were administered equal amounts of either SDA (EO) or EPA (FO) with ∼0.7% of total energy intake (Table 1).
The study oils were divided into 4-d portions in screw-lid cups under a nitrogen atmosphere, stored in the dark at 4–7°C and exposed to oxygen only when necessary. The volunteers and scientific staff involved in the study were unaware of treatment. The oil cups were labeled using a numeric code to ensure additional blinding.
Diets.
To reduce the intake of additional n–3 PUFA and linoleic acid (LA) in the diet during the 10-wk study, the volunteers were encouraged to avoid consuming the following foods: fish, FOs, n–3 PUFA-rich foods and oils (linseed and rapeseed oils), margarine, and common sunflower oil. Participants were advised to use olive oil to prepare foods. Furthermore, to reduce the variation in dietary FA intake and to allow exact calculation of FA intake before blood sampling at days 0 and 56, all volunteers were administered a defined diet for 3 successive days. This so-called standardized diet contained all foods required per participant/3 d. The standardized diet was n–3 PUFA–free and contained low amounts of LA. Individual requirements were assessed before starting the study by FFQ (7-d period) and analyzed by PRODI 4.4 (Nutri-Science). The general management of such a standardized diet and the methods used for the determination of food consumption and food components and for calculation of individual FA intake were described previously (16).
Blood sampling.
Blood samples were collected at the Institute of Nutrition in Jena. During the 3 d before each blood collection, all participants were asked to consume the supplements in the evening to achieve comparable times between intake and blood sampling. After overnight fasting, blood was collected from 0700 to 0800 by venipuncture into 2 EDTA vacutainers for plasma, peripheral blood mononuclear cell (PBMC), and RBC preparation and into lithium–heparin vacutainers for the determination of biochemical markers. EDTA blood was centrifuged (1000 × g, 15 min), and 1 mL of plasma was taken. Afterward, the EDTA tube was remixed for PBMC isolation using Histopaque-1077 (Sigma-Aldrich). After aspiration of the PBMC layer, the RBCs were dispersed in PBS (0.9%) and washed 3 times by centrifugation (1000 × g, 20 min) (17). Plasma, RBCs, and PBMCs were stored at −80°C.
Biochemical markers.
The concentrations of biochemical markers [total cholesterol (TC), HDL-C, LDL-C, TG, alanine aminotransferase, aspartate aminotransferase, γ-glutamyl transpeptidase, and total bilirubin] were ascertained by enzymatic means and high sensitivity C-reactive protein (hsCRP) by ELISA according to the methods of the International Federation of Clinical Chemistry and Laboratory Medicine, using commercially available kits and the Architect C16000 (Abbott Diagnostics Division) (18, 19). Insulin was determined in serum using a 2-site sandwich immunoassay and the ADVIA Centaur test instrument (Siemens). These variables were analyzed by the Institute of Clinical Chemistry and Laboratory Medicine, Jena University Hospital, Friedrich Schiller University. Oxidized LDL (oxLDL) was analyzed via ELISA (Mercodia) by an external laboratory (Amedes). The reference ranges of the biochemical markers were evaluated according to Thomas (20). The criteria for metabolic syndrome were based on the National Cholesterol Education Program Adult Treatment Panel III guidelines (21).
Anthropometric variables.
Waist circumference, blood pressure, and body weight were recorded at days 0 and 56 of intervention. Multiple blood pressure values were taken after the participants had rested for 15 min (Bosch & Sohn), and the final value was the mean of these values. Body composition was analyzed using a 50-kHz frequency impedance analyzer (Data Input).
FA analyses.
Preparation of plasma, RBCs, and PBMCs and lipid extraction with chloroform:methanol:water (2:1:1, v:v:v) were performed as described by Kuhnt et al. (16, 17). FAs were methylated with methanolic boron trifluoride into FAME. After purification by TLC, FAMEs were analyzed by GC with a flame ionization detector (column DB225MS; Agilent Technologies). In all analyzed blood fractions, the same 47 FAs were integrated (C10–C24). Individual FAMEs were expressed as a percentage of total identified FAME peak areas [percentage of total FAME (% FAME)]. Samples were blinded during FA analysis. The reference standards used as FAME included the following: no. 463,674 (Nu-Check Prep); BR2, BR4, and ME93 (Larodan); Supelco 37 Component FAME Mix (Supelco); and PUFA no. 3 (Matreya). For peak integration, LabSolutions for GC was used (Shimadzu).
Statistical analyses.
All statistical analyses were performed using SPSS software (version 19.0; IBM), with P < 0.05 indicating significant differences. In general, data are presented as means with their respective SDs, except for the mean changes of biochemical markers of total participants, which were shown as adjusted means with their SEMs. Data for variables that were not normally distributed according to the Kolmogorov-Smirnov test and had nonhomogeneous variance (i.e., hsCRP, TG) were log-transformed before analysis. The GLM procedure was used, and the statistical model contained control type I.
The effects over time of either EO or FO treatment within the subgroups were analyzed with repeated measures for 2 (biochemical markers) or 3 (FA analysis) time points (repeated-measures ANOVA). The effects over time in the total participants were analyzed with repeated measures with age, baseline BMI, and sex as covariates (repeated-measures ANCOVA). If Mauchly’s test showed no sphericity, the adjusted P value of Greenhouse-Geisser is presented. To test differences between the treatments at the end of the intervention at day 56 (treatment as fixed factor), the baseline value of the respective variable, age, baseline BMI, and sex were used in the model as covariates (univariate ANCOVA). All stated P values were of pairwise comparison, except for time effect over three time points in plasma and PBMC for which the overall P value for time was stated. P values were adjusted using the step-down Bonferroni method. Correlations were calculated using Pearson’s correlation analysis. Box plots were created with SigmaPlot 12.0, and outlying symbols indicated data points outside the 10th and 90th percentile.
Results
Characteristics of study subgroups.
As intended, the EO subgroups differed in terms of age (EOI < EOII = EOIII) and BMI (EOI = EOII < EOIII; Table 2). Significant differences were observed in anthropometric characteristics between the subgroups; e.g., higher age and BMI were associated with higher body fat, waist circumference, and blood pressure (Table 2). After 8 wk of intervention, BMI, body fat, and waist circumference increased similarly during both treatments because of the continuous additional calorie intake via the supplemented oils. Compared with baseline, mean diastolic blood pressure decreased with both treatments, regardless of age, BMI, and sex. There were no differences between EO and FO treatment (Table 2).
TABLE 2.
Characteristics of subgroups differing in age and/or BMI who were administered EO or FO treatment at days 0 and 561
| EO |
FO |
|||||||
| Total EO (n = 59) time effect, P2 | EOI (n = 20) | EOII (n = 20) | EOIII (n = 19) | Total FO (n = 19) time effect, P2 | FOI (n = 10) | FOII (n = 9) | EO vs. FO treatment effect, P3 | |
| Females/males | 31/28 | 10/10 | 11/9 | 10/9 | 10/9 | 5/5 | 5/4 | |
| BMI group, kg/m2 | 18–25 | 18–25 | >25 | 18–25 | 18–25 | |||
| Age group, y | 20–35 | 49–69 | 49–69 | 20–35 | 49–69 | |||
| Age, y | 28 ± 2.9b | 59 ± 5.7a | 61 ± 6.3a | 27 ± 2.5b | 60 ± 4.2a | |||
| Smokers, % | 35 | 15 | 5 | 20 | 22 | |||
| BMI, kg/m2 | 0.007 | 0.17 | 0.71 | |||||
| Day 0 | 22.0 ± 2.3b | 23.5 ± 2.4b | 30.1 ± 3.3a | 21.5 ± 2.6b | 24.8 ± 3.1a | |||
| Day 56 | 22.2 ± 2.2b | 23.6 ± 2.4b | 30.2 ± 3.3a | 21.5 ± 2.5b | 25.0 ± 3.1a | |||
| Body fat, % | 0.001 | 0.19 | 0.43 | |||||
| Day 0 | 20.5 ± 5.7b | 25.6 ± 7.7b | 30.6 ± 8.6a | 16.7 ± 6.6b | 28.3 ± 7.0a | |||
| Day 56 | 21.0 ± 5.8b* | 25.8 ± 7.5b | 30.9 ± 8.5a | 16.9 ± 6.8b | 28.7 ± 7.2a | |||
| Waist circumference, cm | 0.06 | 0.08 | 0.52 | |||||
| Day 0 | 80 ± 9.1b | 87 ± 9.2b | 104 ± 6.8a | 77 ± 9.6b | 91 ± 11a | |||
| Day 56 | 83 ± 7.3b‡ | 90 ± 6.6b* | 103 ± 8.1a | 84 ± 6.8a* | 89 ± 9.3a | |||
| Systolic blood pressure, mm Hg | 0.16 | 0.028 | 0.51 | |||||
| Day 0 | 125 ± 10.4c | 135 ± 19.9b | 141 ± 15.6a | 136 ± 13.6a | 136 ± 18.7a | |||
| Day 56 | 125 ± 12.3c | 131 ± 16.8b | 137 ± 10.3a | 130 ± 10.3a | 130 ± 18.8a | |||
| Diastolic blood pressure, mm Hg | 0.021 | 0.036 | 0.50 | |||||
| Day 0 | 82 ± 8.9c | 90 ± 12b | 91 ± 7.9a | 88 ± 7.7a | 88 ± 7.8a | |||
| Day 56 | 81 ± 8.4c | 86 ± 10b‡ | 88 ± 9.1a | 84 ± 7.7a‡ | 83 ± 11a | |||
Values are means ± SDs. *and ‡indicate differences from day 0 within a subgroup (repeated-measures ANOVA; *P ≤ 0.05, ‡0.05 < P ≤ 0.10). Within an oil treatment, means within a row without a common superscript letter differ (multivariate ANOVA; P ≤ 0.05). EO, echium oil; EOI, participants aged 20–35 y with BMI = 18–25 kg/m2, treated with EO; EOII, participants aged 49–69 y with BMI = 18–25 kg/m2, treated with EO; EOIII, participants aged 49–69 y with BMI > 25 kg/m2 and markers of metabolic syndrome or with BMI ≥ 30 kg/m2, treated with EO; FO, fish oil; FOI, participants aged 20–35 y with BMI = 18–25 kg/m2, treated with FO; FOII, participants aged 49–69 y with BMI = 18–25 kg/m2, treated with FO.
Adjusted mean not shown; P value is for effect over time within an oil treatment of total participants (repeated-measures ANCOVA; sex, age, BMI).
Adjusted mean not shown; P value is for the difference between oil treatments of total participants at day 56 (univariate ANCOVA; sex, age, BMI, baseline value).
Dietary intake.
For those on the standardized diets (days 0 and 56), higher age and BMI were related to a greater intake of total energy, protein, fat, fibers, and cholesterol. Overall, no significant differences were seen compared with baseline or between the treatments (Table 3).
TABLE 3.
Daily intake of energy and macronutrients of participants administered EO or FO treatment before the study and on standardized diets at days 0 and 561
| EO (n = 59) |
FO (n = 19) |
||||
| Male (n = 28) | Female2 (n = 31) | Male (n = 10) | Female2 (n = 9) | EO vs. FO, P3 | |
| Energy, kcal | 0.39 | ||||
| Before study4 | 2420 ± 890 | 1990 ± 550 | 2580 ± 490 | 1870 ± 740 | |
| Day 05,6 | 2580 ± 220 | 2040 ± 240 | 2570 ± 310 | 2050 ± 320 | |
| Day 565,6 | 2620 ± 240 | 2070 ± 290 | 2340 ± 370 | 2060 ± 340 | |
| Protein, g | 0.89 | ||||
| Before study | 100 ± 40 | 78 ± 32 | 104 ± 21 | 85 ± 35 | |
| Day 0 | 94 ± 7 | 75 ± 7 | 93 ± 7 | 73 ± 12 | |
| Day 56 | 93 ± 8 | 75 ± 10 | 89 ± 11 | 74 ± 17 | |
| Carbohydrates, g | 0.79 | ||||
| Before study | 265 ± 95 | 216 ± 65 | 301 ± 83 | 215 ± 76 | |
| Day 0 | 314 ± 28 | 246 ± 28 | 308 ± 52 | 260 ± 30 | |
| Day 56 | 319 ± 40 | 251 ± 35 | 281 ± 58 | 263 ± 35 | |
| Fat, g | 0.11 | ||||
| Before study | 92 ± 44 | 72 ± 27 | 97 ± 22 | 70 ± 25 | |
| Day 06 | 102 ± 14 | 81 ± 13 | 104 ± 12 | 77 ± 18 | |
| Day 566 | 105 ± 11 | 82 ± 15 | 98 ± 17 | 76 ± 18 | |
| Fiber, g | 0.96 | ||||
| Before study | 25 ± 11 | 22 ± 7 | 29 ± 5 | 20 ± 10 | |
| Day 0 | 33 ± 3 | 27 ± 4 | 33 ± 5 | 27 ± 5 | |
| Day 56 | 34 ± 5 | 28 ± 4 | 31 ± 5 | 29 ± 3 | |
| Cholesterol, mg | 0.40 | ||||
| Before study | 388 ± 175 | 314 ± 140 | 340 ± 100 | 273 ± 120 | |
| Day 06 | 357 ± 44 | 284 ± 43 | 363 ± 37 | 269 ± 60 | |
| Day 566 | 363 ± 38 | 286 ± 48 | 325 ± 60 | 274 ± 79 | |
Values are means ± SDs. EO, echium oil; FO, fish oil.
Intake was significantly less in females vs. males (P ≤ 0.05).
Adjusted mean not shown; P value is for the difference between oil treatments of total participants at day 56 (univariate ANCOVA; sex, age, BMI, baseline value).
By means of an FFQ.
After a 2-wk run-in period (day 0) and 8-wk intervention period (day 56); mean of the 3-d standardized diet at the end of each study period; portions of the supplemented oils (run-in oil, treatment oils) were not included.
Intake was significantly greater in participants in the group aged 49–69 y vs. those aged 20–35 y (P ≤ 0.05).
FA distribution of plasma and PBMCs.
After 8 wk of EO treatment, the n–3 precursors ALA and SDA, as well as their conversion products eicosatetraenoic acid (ETA; 20:4n–3), EPA, and docosapentaenoic acid (DPAn–3; 22:5n–3), had significantly increased in all blood fractions of the EO groups (P < 0.05; RBCs not shown; Tables 4 and 5). For example, relative to baseline, ALA and SDA increased in plasma (230% and 730%) and PBMCs (87% and 387%), respectively. The mean EPA increased by 168% in plasma and 79% in PBMCs. The change in DPAn–3 was generally lower (plasma, 68% and PBMCs, 39%) but significant. Moreover, these increases varied between participants: e.g., plasma EPA, 10–328% and DPAn–3, 8–160%, respectively. The increases in EPA and DPAn–3 in both plasma and PBMCs from baseline were greater in the first week of intervention and comparatively lower between days 7 and 56. During EO treatment, DHA decreased significantly in plasma and PBMC, by −5% and −23%, respectively (Tables 4 and 5).
TABLE 4.
FA distribution of plasma of subgroups differing in age and/or BMI who were administered EO or FO on treatment days 0, 7, and 561
| EO |
FO |
|||||||
| FA | Total EO (n = 59) time effect, P2 | EOI (n = 20) | EOII (n = 20) | EOIII (n = 19) | Total FO (n = 19) time effect, P2 | FOI (n = 10) | FOII (n = 9) | EO vs. FO treatment effect, P3 |
| %FAME | %FAME | %FAME | %FAME | %FAME | ||||
| n–3 PUFA | ||||||||
| 18:3n–3, ALA | <0.001 | 0.16 | <0.001 | |||||
| Day 0 | 0.42 ± 0.07b | 0.51 ± 0.10a | 0.52 ± 0.08a | 0.50 ± 0.13a | 0.46 ± 0.09a | |||
| Day 7 | 1.57 ± 0.51a* | 1.52 ± 0.45a* | 1.66 ± 0.76a* | 0.47 ± 0.13a | 0.43 ± 0.08a | |||
| Day 56 | 1.59 ± 0.42a* | 1.54 ± 0.60a* | 1.59 ± 0.64a* | 0.43 ± 0.07a | 0.43 ± 0.09a | |||
| 18:4n–3, SDA | <0.001 | 0.010 | 0.021 | |||||
| Day 0 | 0.03 ± 0.01a | 0.04 ± 0.02a | 0.04 ± 0.01a | 0.02 ± 0.02a | 0.02 ± 0.02a | |||
| Day 7 | 0.34 ± 0.17a* | 0.28 ± 0.11a* | 0.34 ± 0.20a* | 0.04 ± 0.02a | 0.05 ± 0.02a | |||
| Day 56 | 0.31 ± 0.12a* | 0.29 ± 0.15a* | 0.32 ± 0.24a* | 0.04 ± 0.02a | 0.04 ± 0.02a | |||
| 20:4n–3, ETA | <0.001 | 0.001 | <0.001 | |||||
| Day 0 | 0.07 ± 0.02b | 0.11 ± 0.05a | 0.11 ± 0.04a | 0.09 ± 0.05a | 0.07 ± 0.05a | |||
| Day 7 | 0.41 ± 0.18a* | 0.49 ± 0.25a* | 0.43 ± 0.16a* | 0.11 ± 0.04a | 0.12 ± 0.05a | |||
| Day 56 | 0.41 ± 0.11a* | 0.48 ± 0.19a* | 0.42 ± 0.14a* | 0.12 ± 0.06a | 0.11 ± 0.03a | |||
| 20:5n–3, EPA | <0.001 | <0.001 | <0.001 | |||||
| Day 0 | 0.48 ± 0.17b | 0.72 ± 0.28a | 0.73 ± 0.18a | 0.43 ± 0.10b | 0.85 ± 0.32a | |||
| Day 7 | 1.24 ± 0.43b* | 1.67 ± 0.63a* | 1.53 ± 0.26ab* | 3.74 ± 0.80a* | 4.73 ± 1.47a* | |||
| Day 56 | 1.36 ± 0.41b* | 1.91 ± 0.64a* | 1.63 ± 0.27ab* | 3.87 ± 1.19a* | 3.95 ± 0.74a* | |||
| 22:5n–3, DPAn–3 | <0.001 | <0.001 | 0.018 | |||||
| Day 0 | 0.39 ± 0.11b | 0.47 ± 0.07a | 0.45 ± 0.06a | 0.36 ± 0.09b | 0.48 ± 0.13a | |||
| Day 7 | 0.55 ± 0.13a* | 0.63 ± 0.10a* | 0.61 ± 0.10a* | 0.75 ± 0.16a | 0.86 ± 0.32a | |||
| Day 56 | 0.68 ± 0.17a* | 0.77 ± 0.13a* | 0.69 ± 0.12a* | 0.83 ± 0.18a | 0.86 ± 0.29a | |||
| 22:6n–3, DHA | 0.028 | 0.001 | <0.001 | |||||
| Day 0 | 1.40 ± 0.39a | 1.60 ± 0.44a | 1.70 ± 0.49a | 1.30 ± 0.46a | 1.71 ± 0.67a | |||
| Day 7 | 1.34 ± 0.30a* | 1.56 ± 0.33a* | 1.55 ± 0.41a* | 1.79 ± 0.52a | 2.09 ± 0.71a | |||
| Day 56 | 1.40 ± 0.35a | 1.54 ± 0.47a* | 1.53 ± 0. 42a* | 1.89 ± 0.50a | 1.98 ± 0.63a | |||
| n–6 PUFA | ||||||||
| 18:2n–6, LA | 0.001 | <0.001 | 0.021 | |||||
| Day 0 | 31.1 ± 3.7a | 29.5 ± 3.2ab | 26.8 ± 4.0b | 31.5 ± 2.6a | 31.5 ± 4.5a | |||
| Day 7 | 30.4 ± 4.3a | 28.5 ± 2.5ab | 26.0 ± 4.0b | 27.5 ± 2.3a* | 30.0 ± 4.2a | |||
| Day 56 | 27.9 ± 2.3a*† | 26.4 ± 2.5ab*† | 25.2 ± 3.5b* | 26.7 ± 2.2a* | 28.4 ± 5.0a* | |||
| %FAME | %FAME | %FAME | %FAME | %FAME | ||||
| 18:3n–6, GLA | 0.001 | 0.009 | <0.001 | |||||
| Day 0 | 0.29 ± 0.07b | 0.48 ± 0.16a | 0.44 ± 0. 11a | 0.30 ± 0.09a | 0.37 ± 0.12a | |||
| Day 7 | 1.25 ± 0.25a* | 1.40 ± 0.25a* | 1.26 ± 0.25a* | 0.25 ± 0.08a | 0.32 ± 0.07a | |||
| Day 56 | 1.35 ± 0.27a* | 1.41 ± 0.34a* | 1.26 ± 0.35a* | 0.23 ± 0.07b* | 0.32 ± 0.08a | |||
| 20:3n–6, DGLA | 0.001 | <0.001 | <0.001 | |||||
| Day 0 | 1.49 ± 0.37a | 1.59 ± 0.21a | 1.57 ± 0.26a | 1.82 ± 0.30a | 1.20 ± 0.49b | |||
| Day 7 | 2.24 ± 0.65a* | 2.33 ± 0.35a* | 2.09 ± 0.44a* | 1.30 ± 0.28a* | 0.93 ± 0.34b* | |||
| Day 56 | 2.68 ± 0.69a*† | 2.58 ± 0. 37a*† | 2.39 ± 0.47a*† | 1.20 ± 0.37a* | 0.97 ± 0.33a* | |||
| 20:4n–6, AA | 0.001 | 1.00 | 0.002 | |||||
| Day 0 | 5.78 ± 1.19a | 6.38 ± 1.32a | 6.40 ± 1.29a | 6.41 ± 0.89a | 6.96 ± 1.23a | |||
| Day 7 | 6.24 ± 1.31a* | 6.89 ± 1.39a* | 6.45 ± 1.23a | 6.77 ± 1.20a | 7.76 ± 1.38a | |||
| Day 56 | 6.98 ± 1.39a*† | 6.92 ± 1.47a* | 6.91 ± 1.25a* | 6.53 ± 0.86a | 7.17 ± 1.01a | |||
| 22:4n–6 | 0.001 | <0.001 | 0.001 | |||||
| Day 0 | 0.14 ± 0.02b | 0.16 ± 0.04ab | 0.17 ± 0.02a | 0.18 ± 0.02a | 0.16 ± 0.06a | |||
| Day 7 | 0.13 ± 0.03a* | 0.14 ± 0.02a | 0.14 ± 0.02a* | 0.14 ± 0.03a* | 0.12 ± 0.05a* | |||
| Day 56 | 0.13 ± 0.02a* | 0.14 ± 0.02a | 0.14 ± 0.02a* | 0.10 ± 0.04a* | 0.11 ± 0.05a* | |||
| 22:5n–6 | 0.001 | <0.001 | 0.14 | |||||
| Day 0 | 0.12 ± 0.06a | 0.12 ± 0.02a | 0.12 ± 0.03a | 0.17 ± 0.04a | 0.11 ± 0.03b | |||
| Day 7 | 0.10 ± 0.03a | 0.10 ± 0.03a* | 0.10 ± 0.03a | 0.13 ± 0.03a | 0.10 ± 0.05a | |||
| Day 56 | 0.09 ± 0.02a | 0.09 ± 0.02a* | 0.09 ± 0.02a* | 0.09 ± 0.03a*† | 0.08 ± 0.04a* | |||
Values are means ± SDs for subgroups. Within an oil treatment, means within a row without a common superscript letter differ (multivariate ANOVA; P ≤ 0.05). Symbols indicate difference from day 0 (*) and day 7 (†) within a subgroup (repeated-measures ANOVA; P ≤ 0.05). AA, arachidonic acid; ALA, α-linolenic acid; DGLA, dihomo-γ-linolenic acid; DPA, docosapentaenoic acid; EO, echium oil; EOI, participants aged 20–35 y with BMI = 18–25 kg/m2, treated with EO; EOII, participants aged 49–69 y with BMI = 18–25 kg/m2, treated with EO; EOIII, participants aged 49–69 y with BMI > 25 kg/m2 and markers of metabolic syndrome or with BMI ≥ 30 kg/m2, treated with EO; ETA, eicosatetraenoic acid; FO, fish oil; FOI, participants aged 20–35 y with BMI = 18–25 kg/m2, treated with FO; FOII, participants aged 49–69 y with BMI = 18–25 kg/m2, treated with FO; GLA, γ-linolenic acid; LA, linoleic acid; SDA, stearidonic acid.
Adjusted mean not shown; overall P value is for effect over time within an oil treatment of total participants (repeated-measures ANCOVA; sex, age, BMI).
Adjusted mean not shown; P value is for the difference between oil treatments of total participants at day 56 (univariate ANCOVA; sex, age, BMI, baseline value); EPA and DPA are log-transformed.
TABLE 5.
FA distribution of peripheral blood mononuclear cells of subgroups differing in age and/or BMI who were administered EO or FO treatment at days 0, 7, and 561
| EO |
FO |
|||||||
| FA | Total EO (n = 59) time effect, P2 | EOI (n = 20) | EOII (n = 20) | EOIII (n = 19) | Total FO (n = 19) time effect, P2 | FOI (n = 10) | FOII (n = 9) | EO vs. FO treatment effect, P3 |
| %FAME | %FAME | %FAME | %FAME | %FAME | ||||
| n–3 PUFA | ||||||||
| 18:3n–3, ALA | 0.001 | 0.18 | <0.001 | |||||
| Day 0 | 0.14 ± 0.09b | 0.32 ± 0.19a | 0.22 ± 0.12b | 0.12 ± 0.05a | 0.09 ± 0.05a | |||
| Day 7 | 0.31 ± 0.09a* | 0.37 ± 0.10a* | 0.35 ± 0.13a* | 0.10 ± 0.03a | 0.13 ± 0.08a | |||
| Day 56 | 0.35 ± 0.08a* | 0.46 ± 0.12a* | 0.45 ± 0.21a* | 0.15 ± 0.04a† | 0.20 ± 0.14a | |||
| 18:4n–3, SDA | 0.001 | 1.00 | 0.001 | |||||
| Day 0 | 0.01 ± 0.01a | 0.01 ± 0.01ab | 0.00 ± 0.00b | 0.01 ± 0.02a | 0.00 ± 0.01a | |||
| Day 7 | 0.06 ± 0.03a* | 0.04 ± 0.03a* | 0.04 ± 0.04a* | 0.01 ± 0.01a | 0.00 ± 0.00a | |||
| Day 56 | 0.06 ± 0.03a* | 0.04 ± 0.03a* | 0.04 ± 0.07a* | 0.01 ± 0.01a | 0.00 ± 0.01a | |||
| 20:4n–3, ETA | 0.001 | <0.001 | 0.001 | |||||
| Day 0 | 0.04 ± 0.03a | 0.05 ± 0.01a | 0.05 ± 0.03a | 0.05 ± 0.01a | 0.04 ± 0.01a | |||
| Day 7 | 0.18 ± 0.05ab* | 0.22 ± 0.08a* | 0.16 ± 0.05b* | 0.06 ± 0.01a* | 0.05 ± 0.01b | |||
| Day 56 | 0.19 ± 0.04ab* | 0.21 ± 0.06a* | 0.16 ± 0.05b* | 0.07 ± 0.02a* | 0.06 ± 0.01a* | |||
| 20:5n–3, EPA | 0.001 | <0.001 | 0.001 | |||||
| Day 0 | 0.35 ± 0.23a | 0.41 ± 0.14a | 0.39 ± 0.15a | 0.25 ± 0.07a | 0.41 ± 0.13b | |||
| Day 7 | 0.52 ± 0.13b* | 0.67 ± 0.19a* | 0.63 ± 0.21ab* | 1.67 ± 0.29a* | 1.80 ± 0.55a* | |||
| Day 56 | 0.61 ± 0.19b*† | 0.81 ± 0.23a*† | 0.63 ± 0.14b* | 1.95 ± 0.48a* | 1.85 ± 0.44a* | |||
| 22:5n–3, DPAn–3 | 0.001 | <0.001 | <0.001 | |||||
| Day 0 | 1.57 ± 0.35b | 1.86 ± 0.41a | 1.76 ± 0.41ab | 1.63 ± 0.27a | 1.84 ± 0.27a | |||
| Day 7 | 1.97 ± 0.44b* | 2.45 ± 0.37a* | 2.17 ± 0.58ab* | 2.73 ± 0.44a* | 2.63 ± 0.50a* | |||
| Day 56 | 2.20 ± 0.52b*† | 2.62 ± 0.36a* | 2.28 ± 0.66ab*† | 3.24 ± 0.27a*† | 3.34 ± 0.50a*† | |||
| 22:6n–3, DHA | 0.001 | 1.00 | 0.001 | |||||
| Day 0 | 1.62 ± 0.42a | 1.88 ± 0.47a | 1.92 ± 0.59a | 1.54 ± 0.40b | 2.03 ± 0.56a | |||
| Day 7 | 1.46 ± 0.29b | 1.87 ± 0.42a | 1.88 ± 0.56a* | 1.53 ± 0.25a | 1.74 ± 0.33a | |||
| Day 56 | 1.24 ± 0.28a* | 1.48 ± 0.42a*† | 1.42 ± 0.43a*† | 1.64 ± 0.22a | 1.80 ± 0.33a | |||
| n–6 PUFA | ||||||||
| 18:2n–6, LA | 0.07 | 0.43 | 0.86 | |||||
| Day 0 | 5.85 ± 1.19a | 5.90 ± 1.07a | 5.22 ± 1.12a | 5.94 ± 0.63a | 5.47 ± 0.46a | |||
| Day 7 | 6.09 ± 1.35a | 5.79 ± 0.64a | 5.35 ± 1.00a | 5.72 ± 0.46a | 5.41 ± 0.47a | |||
| Day 56 | 6.17 ± 0.56a | 5.98 ± 0.51a | 5.59 ± 1.11a | 6.19 ± 0.41a | 5.81 ± 1.03a | |||
| 18:3n–6, GLA | <0.001 | 0.96 | <0.001 | |||||
| Day 0 | 0.06 ± 0.02b | 0.13 ± 0.10a | 0.09 ± 0.02b | 0.06 ± 0.02a | 0.06 ± 0.02a | |||
| Day 7 | 0.15 ± 0.03a* | 0.17 ± 0.06a* | 0.16 ± 0.05a* | 0.05 ± 0.01a | 0.07 ± 0.03a | |||
| Day 56 | 0.16 ± 0.03a* | 0.18 ± 0.05a* | 0.17 ± 0.07a* | 0.05 ± 0.01a | 0.07 ± 0.02a | |||
| %FAME | %FAME | %FAME | %FAME | %FAME | ||||
| 20:3n–6, DGLA | <0.001 | 0.001 | 0.001 | |||||
| Day 0 | 1.42 ± 0.33a | 1.57 ± 0.26a | 1.44 ± 0.38a | 1.80 ± 0.42a | 1.18 ± 0.18b | |||
| Day 7 | 2.03 ± 0.38ab* | 2.29 ± 0.51a* | 1.91 ± 0.43b* | 1.50 ± 0.29a* | 0.96 ± 0.12b* | |||
| Day 56 | 2.18 ± 0.42ab* | 2.38 ± 0.47a* | 1.94 ± 0.36b* | 1.45 ± 0.50a* | 1.01 ± 0.16b* | |||
| 20:4n–6, AA | 0.28 | <0.001 | 0.010 | |||||
| Day 0 | 22.5 ± 4.00b | 23.5 ± 3.98a | 23.4 ± 6.49a | 22.9 ± 2.89a | 23.8 ± 2.17a | |||
| Day 7 | 21.5 ± 2.19b | 24.1 ± 1.96a | 23.4 ± 4.49ab | 21.8 ± 1.36a | 21.7 ± 2.14a* | |||
| Day 56 | 21.3 ± 2.35b | 23.3 ± 1.20a | 22.0 ± 3.44ab*† | 20.6 ± 1.01a*† | 20.8 ± 1.79a* | |||
| 22:4n–6 | 0.001 | <0.001 | 0.001 | |||||
| Day 0 | 1.92 ± 0.40b | 2.06 ± 0.40a | 2.26 ± 0.36a | 2.28 ± 0.23a | 2.13 ± 0.39a | |||
| Day 7 | 1.72 ± 0.29b* | 2.00 ± 0.30a | 2.06 ± 0.36a* | 1.85 ± 0.23a* | 1.68 ± 0.15a* | |||
| Day 56 | 1.68 ± 0.31b* | 1.78 ± 0.24ab*† | 1.94 ± 0.29a*† | 1.45 ± 0. 28a*† | 1.41 ± 0.20a*† | |||
| 22:5n–6 | 0.001 | <0.001 | 0.07 | |||||
| Day 0 | 0.21 ± 0.09b | 0.32 ± 0.18a | 0.28 ± 0.12ab | 0.33 ± 0.06a | 0.19 ± 0.03b | |||
| Day 7 | 0.18 ± 0.06b | 0.27 ± 0.14a* | 0.28 ± 0.15ab | 0.23 ± 0.05a* | 0.14 ± 0.03b* | |||
| Day 56 | 0.14 ± 0.04b*† | 0.22 ± 0.12ab*† | 0.24 ± 0.15a | 0.15 ± 0.05a*† | 0.11 ± 0.02a* | |||
Values are means ± SDs. Within an oil treatment, means within a row without a common superscript letter differ (multivariate ANOVA; P ≤ 0.05). Symbols indicate difference from day 0 (*) and day 7 (†) within a subgroup (repeated-measures ANOVA; P ≤ 0.05). AA, arachidonic acid; ALA, α-linolenic acid; DGLA, dihomo-γ-linolenic acid; DPA, docosapentaenoic acid; EO, echium oil; EOI, participants aged 20–35 y with BMI = 18–25 kg/m2, treated with EO; EOII, participants aged 49–69 y with BMI = 18–25 kg/m2, treated with EO; EOIII, participants aged 49–69 y with BMI > 25 kg/m2 and markers of metabolic syndrome or with BMI ≥ 30 kg/m2, treated with EO; ETA, eicosatetraenoic acid; FO, fish oil; FOI, participants aged 20–35 y with BMI = 18–25 kg/m2, treated with FO; FOII, participants aged 49–69 y with BMI = 18–25 kg/m2, treated with FO; GLA, γ-linolenic acid; LA, linoleic acid; SDA, stearidonic acid.
Adjusted mean not shown; overall P value is for effect over time within an oil treatment of total participants (repeated-measures ANCOVA; sex, age, BMI).
Adjusted mean not shown; P value is for the difference between oil treatments of total participants at day 56 (univariate ANCOVA; sex, age, BMI, baseline value); EPA and DPA are log-transformed.
The FO group demonstrated an increase in EPA in plasma and PBMC by 533% and 497%, respectively (Tables 4 and 5). FO increased the DPAn–3 (106%) and DHA (30%) in plasma, whereas DHA in PBMCs remained unchanged (Table 5).
Finally, at day 56 in the FO group, the concentrations of EPA, DPAn–3, and DHA in plasma and PBMCs were higher than those in the EO group (P ≤ 0.018; Tables 4 and 5). With respect to EPA accumulation in plasma and PBMCs, compared with FO, the efficacy of EO was 30% and 20%, and for DPA accumulation, efficacy reached 65% and 40%, respectively.
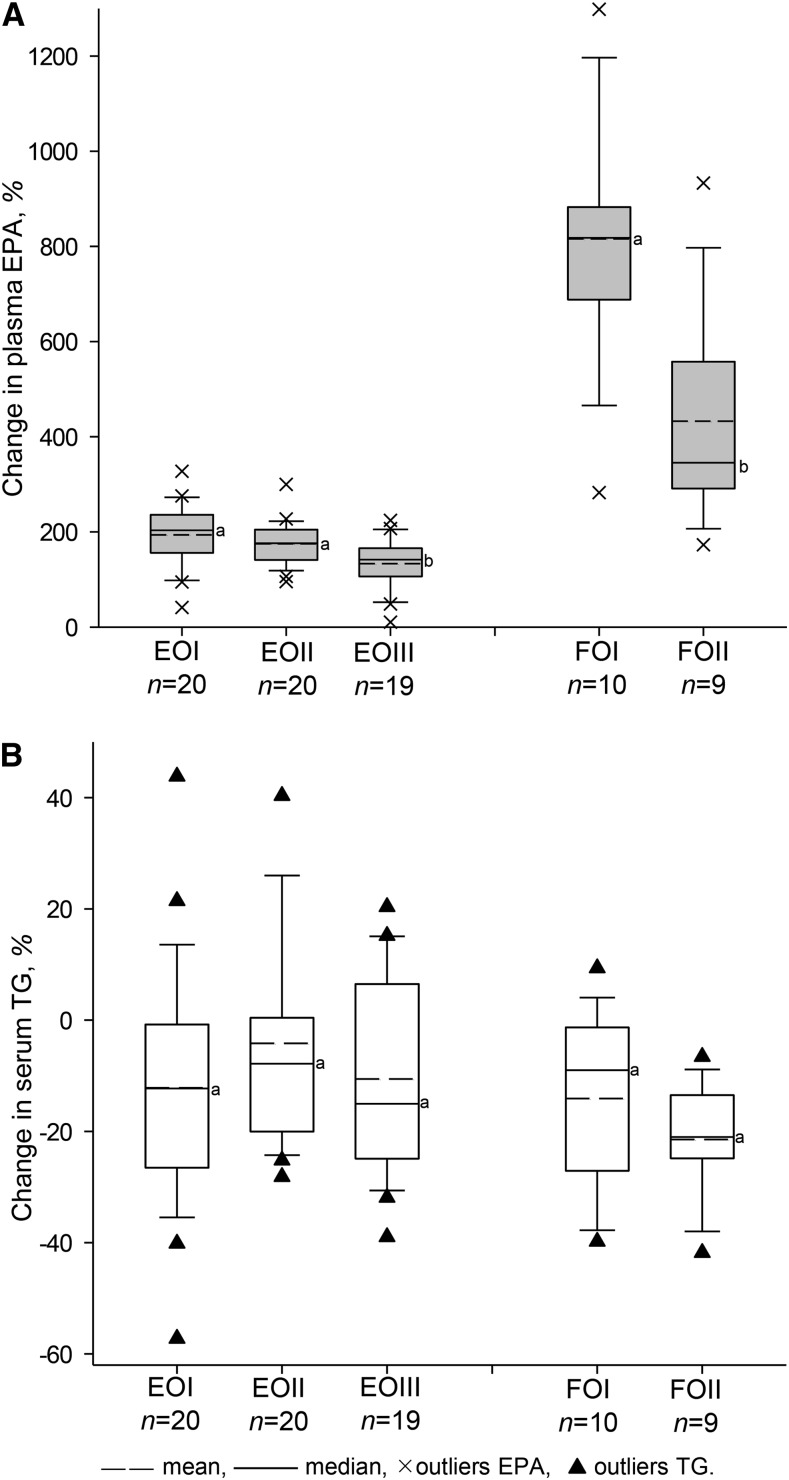
The effects of EO on n–3 PUFA in plasma and PBMCs were similar in the EO subgroups and were independent of age, BMI, and sex, with the exception of EPA and DPA increases. Here, higher age and BMI were associated with lower relative increases in plasma EPA and DPAn–3 (percentage of baseline), respectively (EPA%: age, r = −0.31, P = 0.018; BMI, r = −0.42, P = 0.001; DPA%: age, r = −0.34, P = 0.009; BMI, r = −0.45, P < 0.001). This was reflected in the differences between EOI, EOII, and EOIII in plasma [EPA: 194, 173, and 135%, P = 0.010 (Fig. 2); DPAn–3: 83, 65, and 56%, P = 0.016]. Hence, the overweight individuals (EOIII) showed significantly lower relative increases in plasma EPA and DPA, as confirmed by the significant time × BMI interaction for EPA and DPA accumulation during EO treatment for total participants (P < 0.001). In addition, the overweight individuals had lower net increases in plasma EPA (0.85 vs. 1.08 Δ% FAME; P = 0.039) and DPA (0.24 vs. 0.31 Δ% FAME; P = 0.057) than the normal-weight individuals after EO treatment (adjusted means for age and sex). No time × age interaction was found for plasma EPA and DPA. In PBMCs, no significant correlations and interactions were found (P > 0.05).
FIGURE 2.
Changes in plasma EPA (A) and serum TG (B) in subgroups I, II, and III differing in age and/or BMI who were administered EO or FO treatment for 8 wk. Each bar represents the means ± SDs. Within an oil treatment, labeled means without a common letter differ (P < 0.05). Outliers were outside the 10th and 90th percentile. EO, echium oil; EOI, participants aged 20–35 y with BMI = 18–25 kg/m2, treated with EO; EOII, participants aged 49–69 y with BMI = 18–25 kg/m2, treated with EO; EOIII, participants aged 49–69 y with BMI > 25 kg/m2 and markers of metabolic syndrome or with BMI ≥ 30 kg/m2, treated with EO; FO, fish oil; FOI, participants aged 20–35 y with BMI = 18–25 kg/m2, treated with FO; FOII, participants aged 49–69 y with BMI = 18–25 kg/m2, treated with FO.
In the older FOII individuals, the increases in relative plasma EPA and DPAn–3 were also lower than those in the younger FOI individuals [EPA: 433 vs. 816%, P = 0.007 (Fig. 2); DPAn–3: 80 vs. 138%, P = 0.020]. Age and BMI were inversely correlated with relative increases of plasma EPA and DPA (EPA%: age, r = −0.59, P = 0.010; BMI, r = −0.59, P = 0.010; DPA%: age, r = −0.58, P = 0.011; BMI, r = −0.63, P = 0.005). In FO groups, no significant time × BMI and time × age interactions were observed, possibly because of the smaller group and the absence of an age-matched subgroup with higher BMI.
Furthermore, EO supplementation enhanced GLA in plasma and PBMCs by 235% and 82%, respectively, and also its conversion product dihomo-γ-linolenic acid (DGLA; 20:3n–6) by 65% and 47%, respectively. Moreover, the plasma and PBMC GLA and DGLA concentrations of EO participants were higher than those in FO participants after intervention (P ≤ 0.001). During EO treatment, arachidonic acid increased in plasma but decreased in PBMCs (Tables 4 and 5). The effects of both treatments on n–6 PUFA in plasma and PBMCs were independent of age, sex, and BMI (with the exception of age for the increase in DGLA during EO treatment).
Biochemical markers.
In general, the baseline concentration of the biochemical markers differed according to age, BMI, and sex. For example, higher age was associated with higher serum concentrations of γ-glutamyl transpeptidase, hsCRP, insulin, TC, LDL-C, oxLDL, and TG (P < 0.05). A higher BMI was associated with a higher concentration of insulin, oxLDL, and TG, but lower HDL-C (P < 0.05).
At the time of our analysis, 13 of the overweight participants in the EOIII group completely fulfilled the criteria for metabolic syndrome (≥3 of the following criteria: waist circumference for females >88 cm, for males >102 cm; systolic and/or diastolic blood pressure ≥130/85 mm Hg; fasting plasma TG ≥1.69 mmol/L; HDL-C for males <1.04, for females <1.29 mmol/L) (21). The other 6 participants met 2 of these criteria with at least either increased TG or low HDL-C. At baseline, the EOIII subgroup had significantly higher serum concentrations of hsCRP, oxLDL, and TG compared with their normal-weight age group (EOII), whereas compared with the younger, normal-weight EOI subgroup, all analyzed biochemical markers were higher, except for HDL-C (Table 6).
TABLE 6.
Biochemical markers in serum of subgroups differing in age and/or BMI who were administered EO or FO treatment at days 0 and 561
| EO |
FO |
EO vs. FO |
|||||||||
| Total EO (n = 59) time effect, P2 | EOI (n = 20) | EOII (n = 20) | EOIII (n = 19) | Total FO (n = 19) time effect, P2 | FOI (n = 10) | FOII (n = 9) | Treatment effect, P3 | Total EO4 | Total FO4 | Treatment effect, P5 | |
| hsCRP | |||||||||||
| Day 0, mg/L | 0.76 ± 0.66b | 0.91 ± 0.57b | 2.60 ± 2.67a | 0.63 ± 0.64a | 1.12 ± 1.06a | ||||||
| Day 56, mg/L | 0.14 | 0.54 ± 0.36b* | 1.37 ± 1.36ab | 3.79 ± 5.18a | 0.06 | 0.74 ± 0.69a | 1.85 ± 1.73a‡ | 0.88 | |||
| Change, % | −13 ± 37a | 53 ± 118a | 40 ± 123a | 36 ± 117a | 75 ± 60a | 27 ± 14 | 58 ± 27 | 0.32 | |||
| Insulin | |||||||||||
| Day 0, mU/L | 6.03 ± 2.35b | 9.75 ± 5.89ab | 11.6 ± 4.77a | 6.08 ± 2.46a | 10.2 ± 7.44a | ||||||
| Day 56, mU/L | <0.001 | 4.21 ± 2.55b* | 5.87 ± 3.41ab* | 8.78 ± 6.07a‡ | 0.008 | 4.38 ± 2.29a* | 4.65 ± 3.15 a‡ | 0.41 | |||
| Change, % | −29 ± 28a | −30 ± 42a | −16 ± 65a | −27 ± 25a | −54 ± 34a | −26 ± 5.5 | −31 ± 10 | 0.65 | |||
| TC | |||||||||||
| Day 0, mmol/L | 4.52 ± 0.68b | 5.71 ± 1.24a | 6.30 ± 1.02a | 4.59 ± 0.62b | 5.73 ± 0.80a | ||||||
| Day 56, mmol/L | 0.001 | 4.21 ± 0.67b* | 5.57 ± 1.19a | 5.75 ± 0.82a* | 0.76 | 4.57 ± 0.78b | 5.85 ± 0.84a | 0.11 | |||
| Change, % | −6.2 ± 12a | −0.8 ± 13a | −8.2 ± 11a | −0.7 ± 9.6a | 2.6 ± 12a | −4.9 ± 1.6 | 0.6 ± 2.9 | 0.11 | |||
| HDL-C | |||||||||||
| Day 0, mmol/L | 1.43 ± 0.30a | 1.58 ± 0.47a | 1.33 ± 0.32a | 1.65 ± 0.34a | 1.92 ± 0.59a | ||||||
| Day 56, mmol/L | 0.001 | 1.30 ± 0.30ab* | 1.54 ± 0.54a | 1.24 ± 0.33b* | 0.96 | 1.63 ± 0.48a | 1.95 ± 0.49a | 0.06 | |||
| Change, % | −8.7 ± 12a | −1.4 ± 13a | −6.8 ± 10a | −1.2 ± 15a | 1.6 ± 13a | −5.7 ± 1.7 | 1.2 ± 3.1 | 0.06 | |||
| LDL-C | |||||||||||
| Day 0, mmol/L | 2.54 ± 0.61b | 3.54 ± 0.88a | 4.00 ± 0.81a | 2.49 ± 0.66a | 3.08 ± 0.70a | ||||||
| Day 56, mmol/L | <0.001 | 2.28 ± 0.54b* | 3.32 ± 0.71a‡ | 3.52 ± 0.79a* | 0.26 | 2.33 ± 0.66b‡ | 3.13 ± 0.82a | 0.17 | |||
| Change, % | −9.6 ± 14a | −4.8 ± 14a | −11 ± 11a | −3.9 ± 10a | 1.3 ± 12a | −8.3 ± 1.7 | −2.6 ± 3.1 | 0.11 | |||
| LDL-C/HDL-C | |||||||||||
| Day 0 | 1.87 ± 0.67b | 2.36 ± 0.66b | 3.08 ± 0.75a | 1.58 ± 0.56a | 1.81 ± 0.85a | ||||||
| Day 56 | 0.10 | 1.84 ± 0.68b‡ | 2.30 ± 0.71b | 2.98 ± 0.86a | 0.18 | 1.53 ± 0.60a | 1.72 ± 0.82a | 0.70 | |||
| Change, % | −1.6 ± 14a | −2.9 ± 10a | −3.8 ± 11a | −3.1 ± 12a | −4.4 ± 6.3a | −2.1 ± 1.5 | −4.0 ± 2.8 | 0.56 | |||
| Oxidized LDL | |||||||||||
| Day 0, μmol/L | 67.7 ± 12.7c | 80.2 ± 11.4b | 92.9 ± 13.1a | 59.0 ± 9.99b | 76.0 ± 14.7a | ||||||
| Day 56, μmol/L | <0.001 | 63.8 ± 11.5b‡ | 78.6 ± 11.4a | 85.7 ± 12.7a* | 0.48 | 61.2 ± 12.2b | 75.9 ± 12.2a | 0.22 | |||
| Change, % | −5.0 ± 12a | −1.6 ± 11a | −7.5 ± 8a | 4.5 ± 15b | 0.8 ± 8.5a | −4.5 ± 1.5 | 2.3 ± 2.7 | 0.032 | |||
| TG | |||||||||||
| Day 0, mmol/L | 0.89 ± 0.36b | 1.14 ± 0.42b | 1.82 ± 0.88a | 0.77 ± 0.21a | 0.90 ± 0.32a | ||||||
| Day 56, mmol/L | 0.001 | 0.74 ± 0.25c* | 1.08 ± 0.45b | 1.57 ± 0.70a* | 0.002 | 0.64 ± 0.13a* | 0.69 ± 0.21a* | 0.09 | |||
| Change, % | −12 ± 22a | −3.5 ± 21a | −11 ± 17a | −14 ± 17a | −22 ± 11a | −8.9 ± 2.7 | −17 ± 4.9 | 0.14 | |||
Values are means ± SDs of subgroups. Within an oil treatment, means within a row without a common superscript letter differ (multivariate ANOVA; P ≤ 0.05); TG is log-transformed. Symbols indicate differences from day 0 within a subgroup (repeated-measures ANOVA; *P ≤ 0.05; ‡0.05 < P ≤ 0.10). EO, echium oil; EOI, participants aged 20–35 y with BMI = 18–25 kg/m2, treated with EO; EOII, participants aged 49–69 y with BMI = 18–25 kg/m2, treated with EO; EOIII, participants aged 49–69 y with BMI > 25 kg/m2 and markers of metabolic syndrome or with BMI ≥ 30 kg/m2, treated with EO; FO, fish oil; FOI, participants aged 20–35 y with BMI = 18–25 kg/m2, treated with FO; FOII, participants aged 49–69 y with BMI = 18–25 kg/m2, treated with FO; HDL-C, HDL cholesterol; hsCRP, high sensitivity C-reactive protein; LDL-C, LDL cholesterol; TC, total cholesterol.
Adjusted mean not shown; P value is for effect over time within an oil treatment of total participants (repeated-measures ANCOVA; sex, age, BMI).
Adjusted mean not shown; P value is for the difference between oil treatments of total participants at day 56 (univariate ANCOVA; sex, age, BMI, baseline value); hsCRP is log-transformed.
Values are adjusted means ± SEMs of the change from day 0 to day 56 of total participants.
P value is for the difference of the change between oil treatments of total participants (univariate ANCOVA; sex, age, BMI).
Liver enzymes and bilirubin were within the physiologic range (20) in both study arms. Accordingly, liver dysfunction could be excluded during both treatments. Serum alanine aminotransferase and bilirubin concentration decreased during both treatments (P ≤ 0.008). The reduction in bilirubin by EO was greater than that achieved by FO (P = 0.05).
During EO supplementation, the mean serum concentrations of insulin, TC, HDL-C, LDL-C, and TG were significantly reduced compared with baseline. These effects were independent of age, BMI, and sex, with the exception of HDL-C and oxLDL. HDL-C was not reduced by EO in females in the EOII and EOIII groups. The reduction in oxLDL during EO treatment was dependent on sex (P = 0.038) and BMI (P = 0.06). Hence, oxLDL decreased to a greater extent in individuals with a higher BMI and especially in overweight women.
After the 8-wk FO treatment, only insulin and TG concentrations decreased, regardless of age, BMI, and sex (Table 6). The mean TG change resulting from FO did not significantly differ compared with EO (−17% vs. −9%, P = 0.14; Table 6). Surprisingly, after the 8-wk treatment, HDL-C was only reduced by EO and not by FO; therefore, the mean changes differed by trend (−5.7% vs. 1.2%, P = 0.06; Table 6).
In general, the net and relative increase in EPA in plasma and PBMCs showed no correlation with the reduction in serum TG with either EO or FO treatment. In addition, platelet function parameters, such as prothrombin time, activated partial thromboplastin time, fibrinogen, and antithrombin III, were unaffected by both treatments (data not shown).
Discussion
During EO supplementation, the precursors ALA and SDA rapidly increased in blood fractions. The concentrations of the LC n–3 PUFAs ETA, EPA, and DPA, which are direct metabolites of ALA and SDA, also increased significantly in plasma, RBCs (data not shown), and PBMCs. Similar increases in EPA and DPAn–3 in plasma and PBMCs after EO supplementation (3–8 wk) were reported previously (22, 23). The increase in SDA resulted from both Δ6-desaturation of ALA and from direct SDA intake. SDA was quickly metabolized into ETA and EPA, although SDA accumulation in cellular lipids was also detected. The daily intake of 17 g of EO with 2 g of SDA resulted in a 0.3-, 0.2-, and 0.3-fold EPA increase in plasma, RBCs, and PBMCs, respectively, compared with the FO group (1.9 g/d EPA). This confirms the effectiveness of SDA in increasing EPA in the blood compared with EPA (0.3:1) (11). The efficiency of formation of EPA from SDA as ethyl esters (1.5–4.0 g/d) was estimated to be 16–20% (24–26), whereas EPA formation was not directly proportional over the full SDA dosage range, suggesting that conversion of SDA becomes less efficient with increasing SDA intake (27).
In the EO groups, SDA was clearly metabolized to DPAn–3. In relation to the number of metabolic intermediate steps, EO was more efficient at increasing DPAn–3 than FO. Accumulation of DPAn–3 is important because it is a precursor of DHA and offers beneficial health effects (28). Because n–3 PUFA intake was restricted in the study diet, the increase in EPA and DPAn–3 can exclusively be attributed to the endogenous conversion of ALA and SDA from EO. However, ALA conversion into EPA was low in previous studies (0.2–8%) (29, 30), suggesting that SDA conversion has greater potential.
DHA is synthesized by elongation of DPAn–3 to 24:5n–3, an additional Δ6-desaturation to 24:6n–3, and finally β-oxidation in the peroxisomes (31). Nevertheless, despite the increased concentrations of DPAn–3 in the EO groups, DHA concentrations were reduced in the blood fractions after EO supplementation compared with baseline (Tables 4 and 5). Therefore, EO could not compensate for the lack of DHA in the diet during the 10-wk study. Moreover, the high amount of ALA in EO (5 g/d) could increase the competition for Δ6-desaturase, which is also needed for DHA synthesis in humans. In addition, as the ALA dosage increased, ALA conversion declined (32, 33). The restriction in dietary n–3 PUFAs and the larger study population compared with other SDA studies suggests that this decrease in DHA is of significance (11, 23, 24).
Because LA competes with ALA for the initial Δ6-desaturation, high dietary LA lowered ALA conversion into LC n–3 metabolites (34). It has been suggested that high LA intake, as in westernized countries, decreases the EPA content in tissues (35, 36). On this basis, the study participants were asked to minimize their consumption of oils and spreads rich in LA (e.g., common sunflower oil–based products) and to use olive oil instead when possible. Therefore, the LA intake during this study (2–3% of total energy intake) was low, similar to that reported by James et al. (11), suggesting a minor effect on ALA conversion.
The simultaneous occurrence of GLA in natural EO could interfere with the conversion of n–3 LC PUFA precursors by competition with the respective enzymes (elongase and Δ5-desaturase). This impeded the unambiguous evaluation of the extent to which n–3 and n–6 PUFA families influenced metabolic processes and contributed to the present results. However, the observed EPA enrichment in blood after EO intake was comparable with that after SDA supplementation alone (11, 25). Furthermore, the increase in DGLA concentrations via GLA conversion could facilitate the anti-inflammatory potential of natural EO (37).
In general, the FAs in PBMCs consistently reflected the changes in plasma and RBCs in this study. In most studies, PBMC FAs were only analyzed at the end of the intervention on the assumption that PBMCs were more resistant to FA intervention than plasma (11, 37–39). In fact, FA precursors and their LC metabolites had increased in PBMCs after only 1 wk of EO and FO treatment. Another study confirmed the rapid response of FA in PBMCs after FO intake, reflecting their fast turnover time (40). In contrast to other studies on SDA (11) and EO (23), higher proportions of SDA and ETAn–3 were detected in PBMCs, suggesting that the EO supplement containing 2 g of SDA and 5 g of ALA used in the current study promotes SDA and ETAn–3 accumulation, partly as ALA metabolites (22).
Several factors may influence the n–3 PUFA status in humans. In addition to increasing with fish intake, concentrations of n–3 PUFAs in the blood increased with age but were lower in individuals with a higher BMI and diabetes (41). In the current study, younger participants had a lower baseline concentration of n–3 PUFAs. Therefore, younger participants showed greater relative increases in EPA and DPAn–3 compared with older participants after EO treatment (Fig. 2). However, the net increases in EPA and DPA were lower in younger participants compared with older participants, as confirmed by PBMCs. In contrast, n–3 PUFA concentrations at baseline did not differ between lean and overweight participants. However, during EO treatment, both the relative and net increases in EPA and DPA were lower in overweight participants compared with lean participants. Hence, lower EPA and DPA accumulation could indicate the beginning of disrupted FA metabolism. In addition, a reduction in Δ6-desaturase activity with increasing age (42) and limited activity of Δ5- and Δ6-desaturases is considered to be involved in the initiation and progression of atherosclerosis (43). In this respect, a limitation of this study was that no younger overweight subgroup was included, which could have strengthened this observation. Regardless of diet, women showed higher DHA but lower EPA and DPAn–3 concentrations (42, 44), as confirmed by the higher DHA and lower DPAn–3 concentrations in all blood fractions in the present study. However, the reduction in DHA did not differ between men and women during EO treatment. In general, previous SDA studies did not report interactions between BMI, sex, and age (11, 22–26). In this study, no labeled FAs were used, and the proportions of FA in the blood fractions were dependent on various factors, such as oxidation rate, individual enzyme activity, physical activity, and hormone status. Thus, to evaluate the impact of BMI, sex, and age on LC PUFA metabolism as the primary outcome, more participants and matched subgroups would be required.
The TG-lowering effect (25–35%) of EPA and DHA supplementation has been observed in various studies (45–47) and is associated with a greater reduction in participants with a higher initial TG concentration (48). The present 1.9 g/d EPA achieved a TG decrease of 17% in the FO group, whereas EO intake decreased TG by 9%. In participants with very high TG concentrations (4.1 mmol/L), supplementation with 15 g/d of EO decreased TG by 21% compared with baseline, although no other changes in the lipid profile were reported (22). In contrast, in addition to the TG decrease, the present EO treatment also reduced TC, LDL-C, oxLDL, insulin, and blood pressure compared with baseline, providing additional benefits especially for participants with metabolic syndrome. In participants with metabolic syndrome, even low doses of EPA and DHA (180 and 120 mg) improved lipid profile and blood pressure compared with baseline (49). However, both a previous study and the present study observed a TG-lowering effect by EO and FO even in participants with initially low TG concentrations (50). In individual participants, the TG concentration remained unchanged or even increased with either EO or FO (Fig. 2), as observed previously (22). In contrast, the intake of only ALA (4.5 g/d) in hyperlipidemic participants achieved no reduction in TG (51), indicating that the present effects of EO could be SDA related or may also be attributable to the combination with GLA in EO.
The underlying mechanisms of LC n–3 PUFAs are primarily related to reducing the hepatic production of VLDL, along with increased plasma lipolytic activity through lipoprotein lipase-mediated clearance and increased FA oxidation mediated by PPARα activation (45, 46, 52). EO treatment in mildly hypertriglyceridemic mice decreased plasma TG and VLDL in association with the downregulation of several genes involved in hepatic TG biosynthesis, as well as with a reduction in hepatic TG and TC, similar to the results achieved by FO (52). Another study in mice confirmed a greater reduction in TC, VLDL, and TG by EO than by marine oils (53). New data show that plant and marine oils affect the activation of PPARα and PPARγ in a different way (54). For instance, the effects on genes involved in FA hepatic synthesis (e.g., PPARα) clearly differed in the EO treatment (54).
Furthermore, all cholesterol blood fractions decreased after the present EO treatment, as did mean HDL-C concentrations; such reductions were not observed with the FO treatment. FO is generally known to raise HDL-C but only minimally (3–5%) (48). Other SDA studies observed no reduction in HDL-C compared with baseline (11, 22–26). In addition, oxLDL decreased only with EO treatment, with a clear reduction in participants with metabolic syndrome, who had higher initial oxLDL concentrations. In contrast, in the smaller normal-weight FO group, oxLDL remained unchanged after 8 wk, as reported previously (50). Accumulating evidence indicates that elevated oxLDL is a marker for coronary artery disease (55). To our knowledge, this is the first study to have analyzed oxLDL during EO intervention. The reduction of oxLDL in EO participants could be associated with a decreased LDL protein fraction in the liver and plasma, as found in mice after EO intake (52, 53). Furthermore, EO is naturally rich in tocopherols, which could prevent the oxidation of LDL particles.
In terms of the observed effects of EO on biochemical markers related to baseline, the higher number of participants involved in the current study was presumably responsible for the more significant effects compared with previous SDA studies (11, 22, 23). In addition, the smaller number of participants in the control group (FO) may have precluded significant treatment effects. However, the main aim of this work was to investigate EO treatment according to age, sex, and metabolic syndrome.
Large clinical trials with moderate doses of n–3 PUFAs (1–2 g) have proved clinically beneficial by reducing the risk of CVD despite minimal changes in TG or other blood lipids (56, 57). Overall, because SDA supplementation has been shown to effectively increase EPA in blood, it would appear that SDA has a protective role in cardiac events (24) and type 2 diabetes mellitus (15). However, the preventive and therapeutic implications of SDA-rich EO remain to be fully determined. It is further possible that EO treatment affects eicosanoids and their derived metabolites, adipokines, and cytokines, especially in metabolic syndrome.
It can be concluded that EO effectively increases LC n–3 PUFAs, such as ETA, EPA, and DPA, in blood fractions. However, it cannot replace dietary DHA. A higher BMI was associated with lower increases in EPA and DPAn–3. EO alters the serum lipid profile, i.e., lowers TC, LDL-C, oxLDL, and TG, but also HDL-C. Those individuals at higher risk of CVD and type 2 diabetes, i.e., individuals with metabolic syndrome, would profit from a daily intake of 15–20 g of EO. EO as vegetable oil also could be a noteworthy source of n–3 PUFAs for vegetarians and vegans. Its unique combination of SDA, ALA, and GLA is considered to be relevant in health-related nutrition.
Acknowledgments
The authors thank Cora Richert for analyzing the biochemical markers, Dr. Ulrich Schäfer for proofreading, and Nasim Krögel for language editing of the manuscript. The authors also thank Dr. Jens Schumacher from the Institute of Stochastics for assistance in the statistical analysis. As study investigator, K.K. designed the study, conducted the power analysis and statistical analyses, wrote the manuscript, and had primary responsibility for the final content; K.K., C.F., and M.Köhler conducted the study and performed the FA analyses; M.Kiehntopf was responsible for the analysis of biochemical markers; and G.J. was the head of the Department of Nutritional Physiology. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ALA, α-linolenic acid; CVD, cardiovascular disease; DGLA, dihomo-γ-linolenic acid; DPA, docosapentaenoic acid; EO, echium oil; EOI, participants aged 20–35 y with BMI = 18–25 kg/m2, treated with EO; EOII, participants aged 49–69 y with BMI = 18–25 kg/m2, treated with EO; EOIII, participants aged 49–69 y with BMI > 25 kg/m2 and markers of metabolic syndrome or with BMI ≥ 30 kg/m2, treated with EO; ETA, eicosatetraenoic acid; FO, fish oil; FOI, participants aged 20–35 y with BMI = 18–25 kg/m2, treated with FO; FOII, participants aged 49–69 y with BMI = 18–25 kg/m2, treated with FO; GLA, γ-linolenic acid; hsCRP, high sensitivity C-reactive protein; LA, linoleic acid; LC, long-chain; oxLDL, oxidized LDL; PBMC, peripheral blood mononuclear cell; SDA, stearidonic acid; TC, total cholesterol.
Literature Cited
- 1.Mozaffarian D, Wu JH. Omega-3 fatty acids and CVD: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–67 [DOI] [PubMed] [Google Scholar]
- 2.Harris WS. The omega-3 index: from biomarker to risk marker to risk factor. Curr Atheroscler Rep. 2009;11:411–7 [DOI] [PubMed] [Google Scholar]
- 3.Barceló-Coblijn G, Murphy EJ. Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog Lipid Res. 2009;48:355–74 [DOI] [PubMed] [Google Scholar]
- 4.American Heart Association. Fish 101 [updated 2013 Mar 20; cited 2013 Feb 26]. Available from: http://www.heart.org/HEARTORG/GettingHealthy/NutritionCenter/Fish-101_UCM_305986_Article.jsp
- 5.Venegas-Calerón M, Sayanova O, Napier JA. An alternative to fish oils: metabolic engineering of oil-seed crops to produce omega-3 long chain polyunsaturated fatty acids. Prog Lipid Res. 2010;49:108–19 [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-López N, Sayanova O, Napier JA, Haslam RP. Metabolic engineering of the omega-3 long chain polyunsaturated fatty acids biosynthetic pathway into transgenic plants. J Exp Bot. 2012;63:2397–410 [DOI] [PubMed] [Google Scholar]
- 7.Guil-Guerrero JL. Stearidonic acid (18:4n-3): metabolism, nutritional importance, medical uses and natural sources. Eur J Lipid Sci Technol. 2007;109:1226–36 [Google Scholar]
- 8.Hemaiswarya S, Raja R, Ravi Kumar R, Ganesan V, Anbazhagan C. Microalgae: a sustainable feed source for aquaculture. World J Microbiol Biotechnol. 2011;27:1737–46 [Google Scholar]
- 9.Kuhnt K, Degen C, Jaudszus A, Jahreis G. Searching for health beneficial n-3 and n-6 fatty acids in plant seeds. Eur J Lipid Sci Technol. 2012;114:153–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaz-Lopez M, Perez MJ, Acosta NG, Tocher DR, Jerez S, Lorenzo A, Rodriguez C. Effect of dietary substitution of fish oil by echium oil on growth, plasma parameters and body lipid composition in gilthead seabream (Sparus aurata L.). Aquacult Nutr. 2009;15:500–12 [Google Scholar]
- 11.James MJ, Ursin VM, Cleland LG. Metabolism of stearidonic acid in human subjects: comparison with the metabolism of other n-3 fatty acids. Am J Clin Nutr. 2003;77:1140–5 [DOI] [PubMed] [Google Scholar]
- 12.Ursin VM. Modification of plant lipids for human health: development of functional land-based omega-3 fatty acids. J Nutr. 2003;133:4271–4 [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-López N, Haslam RP, Venegas-Caleron M, Larson TR, Graham IA, Napier JA, Sayanova O. The synthesis and accumulation of stearidonic acid in transgenic plants: a novel source of “heart-healthy” omega-3 fatty acids. Plant Biotechnol J. 2009;7:704–16 [DOI] [PubMed] [Google Scholar]
- 14.Harris WS. Stearidonic acid-enhanced soybean oil: a plant-based source of (n-3) fatty acids for foods. J Nutr. 2012;142:600S–4S [DOI] [PubMed] [Google Scholar]
- 15.Banz WJ, Davis JE, Clough RW, Cheatwood JL. Stearidonic acid: is there a role in the prevention and management of type 2 diabetes mellitus? J Nutr. 2012;142:635S–40S [DOI] [PubMed] [Google Scholar]
- 16.Kuhnt K, Kraft J, Moeckel P, Jahreis G. Trans-11–18:1 is effectively Delta9-desaturated compared with trans-12–18:1 in humans. Br J Nutr. 2006;95:752–61 [DOI] [PubMed] [Google Scholar]
- 17.Kuhnt K, Kraft J, Vogelsang H, Eder K, Kratzsch J, Jahreis G. Dietary supplementation with trans11 and trans12 18:1 increases cis9,trans11 conjugated linoleic acid in human immune cells, but without effects on biomarkers of immune function and inflammation. Br J Nutr. 2007;97:1196–205 [DOI] [PubMed] [Google Scholar]
- 18.Quinn FA, Armbruster DA. Abbott ARCHITECT® family of analyzers. In: Wild D, editor. The immunoassay handbook. Theory and applications of ligand binding, ELISA and related techniques. 4th ed. Oxford (United Kingdom): Elsevier; 2013:561–6.
- 19.Tamimi W, Albanyan E, Altwaijri Y, Tamim H, Alhussein F. Age- and gender-specific reference intervals for fasting blood glucose and lipid levels in school children measured with Abbott Architect c8000 chemistry analyzer. Indian J Clin Biochem. 2012;27:141–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas L. Labor und Diagnose. Indikation und Bewertung von Laborbefunden für die medizinische Diagnostik. 6th ed. Frankfurt/Main, Germany: TH-Books Verlagsgesellschaft; 2005.
- 21.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults—findings from the third national health and nutrition examination survey. JAMA. 2002;287:356–9 [DOI] [PubMed] [Google Scholar]
- 22.Surette ME, Edens M, Chilton FH, Tramposch KM. Dietary echium oil increases plasma and neutrophil long-chain (n-3) fatty acids and lowers serum triacylglycerols in hypertriglyceridemic humans. J Nutr. 2004;134:1406–11 [DOI] [PubMed] [Google Scholar]
- 23.Miles EA, Banerjee T, Calder PC. The influence of different combinations of gamma-linolenic, stearidonic and eicosapentaenoic acids on the fatty acids composition of blood lipids and mononuclear cells in human volunteers. Prostaglandins Leukot Essent Fatty Acids. 2004;70:529–38 [DOI] [PubMed] [Google Scholar]
- 24.Harris WS, Lemke SL, Hansen SN, Goldstein DA, DiRienzo MA, Su H, Nemeth MA, Taylor ML, Ahmed G, George C. Stearidonic acid enriched soybean oil increased the omega-3 index, an emerging cardiovascular risk marker. Lipids. 2008;43:805–11 [DOI] [PubMed] [Google Scholar]
- 25.Lemke SL, Vicini JL, Su H, Goldstein DA, Nemeth MA, Krul ES, Harris WS. Dietary intake of stearidonic acid-enriched soybean oil increases the omega-3 index: randomized, double-blind clinical study of efficacy and safety. Am J Clin Nutr. 2010;92:766–75 [DOI] [PubMed] [Google Scholar]
- 26.Krul ES, Lemke SL, Mukherjea R, Taylor ML, Goldstein DA, Su H, Liu P, Lawless A, Harris WS, Maki KC. Effects of duration of treatment and dosage of eicosapentaenoic acid and stearidonic acid on red blood cell eicosapentaenoic acid content. Prostaglandins Leukot Essent Fatty Acids. 2012;86:51–9 [DOI] [PubMed] [Google Scholar]
- 27.Maki KC, Rains TM. Stearidonic acid raises red blood cell membrane eicosapentaenoic acid. J Nutr. 2012;142:626S–9S [DOI] [PubMed] [Google Scholar]
- 28.Kaur G, Cameron-Smith D, Garg M, Sinclair AJ. Docosapentaenoic acid (22:5n-3): a review of its biological effects. Prog Lipid Res. 2011;50:28–34 [DOI] [PubMed] [Google Scholar]
- 29.Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N. Physiological compartmental analysis of α-linolenic acid metabolism in adult humans. J Lipid Res. 2001;42:1257–65 [PubMed] [Google Scholar]
- 30.Goyens PL, Spilker M, Zock P, Katan M, Mensink R. Conversion of alpha-linolenic acid in humans is influenced by the absolute amounts of alpha-linolenic acid and linoleic acid in the diet and not by their ratio. Am J Clin Nutr. 2006;84:44–53 [DOI] [PubMed] [Google Scholar]
- 31.Sprecher H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim Biophys Acta. 2000;1486:219–31 [DOI] [PubMed] [Google Scholar]
- 32.Vermunt SHF, Mensink RP, Simonis MMG, Hornstra G. Effects of dietary alpha-linolenic acid on the conversion and oxidation of C-13-alpha-linolenic acid. Lipids. 2000;35:137–42 [DOI] [PubMed] [Google Scholar]
- 33.Cleland LG, Gibson RA, Pedler J, James MJ. Paradoxical effect of n-3 containing vegetable oils on long-chain n-3 fatty acids in rat heart. Lipids. 2005;40:995–8 [DOI] [PubMed] [Google Scholar]
- 34.Emken EA, Adlof RO, Gulley RM. Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim Biophys Acta. 1994;1213:277–88 [DOI] [PubMed] [Google Scholar]
- 35.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93:950–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liou YA, King DJ, Zibrik D, Innis SM. Decreasing linoleic acid with constant alpha-linolenic acid in dietary fats increases (n-3) eicosapentaenoic acid in plasma phospholipids in healthy men. J Nutr. 2007;137:945–52 [DOI] [PubMed] [Google Scholar]
- 37.Miles EA, Banerjee T, Dooper MM, M’Rabet L, Graus YM, Calder PC. The influence of different combinations of gamma-linolenic acid, stearidonic acid and EPA on immune function in healthy young male subjects. Br J Nutr. 2004;91:893–903 [DOI] [PubMed] [Google Scholar]
- 38.Kew S, Mesa MD, Tricon S, Buckley R, Minihane AM, Yaqoob P. Effects of oils rich in eicosapentaenoic and docosahexaenoic acids on immune cell composition and function in healthy humans. Am J Clin Nutr. 2004;79:674–81 [DOI] [PubMed] [Google Scholar]
- 39.Thies F, Nebe-von-Caron G, Powell JR, Yaqoob P, Newsholme EA, Calder PC. Dietary supplementation with eicosapentaenoic acid, but not with other long-chain n-3 or n-6 polyunsaturated fatty acids, decreases natural killer cell activity in healthy subjects aged >55 y. Am J Clin Nutr. 2001;73:539–48 [DOI] [PubMed] [Google Scholar]
- 40.Faber J, Berkhout M, Vos PV, Sijben JWC, Calder PC, Garssen J, van Helvoort A. Supplementation with a fish oil-enriched, high-protein medical food leads to rapid incorporation of EPA into white blood cells and modulates immune responses within one week in healthy men and women. J Nutr. 2011;141:964–70 [DOI] [PubMed] [Google Scholar]
- 41.Sands SA, Reid KJ, Windsor SL, Harris WS. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids. 2005;40:343–7 [DOI] [PubMed] [Google Scholar]
- 42.Bolton-Smith C, Woodward M, Tavendale R. Evidence for age-related differences in the fatty acids composition of human adipose tissue, independent of diet. Eur J Clin Nutr. 1997;51:619–24 [DOI] [PubMed] [Google Scholar]
- 43.Das UN. A defect in the activity of Delta6 and Delta5 desaturases may be a factor in the initiation and progression of atherosclerosis. Prostaglandins Leukot Essent Fatty Acids. 2007;76:251–68 [DOI] [PubMed] [Google Scholar]
- 44.Childs CE, Romeu-Nadal M, Burdge GC, Calder PC. Gender differences in the n-3 fatty acid content of tissues. Proc Nutr Soc. 2008;67:19–27 [DOI] [PubMed] [Google Scholar]
- 45.Jacobson TA. Role of n-3 fatty acids in the treatment of hypertriglyceridemia and CVD. Am J Clin Nutr. 2008;87:1981S–90S [DOI] [PubMed] [Google Scholar]
- 46.Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr. 2012;142:592S–9S [DOI] [PubMed] [Google Scholar]
- 47.Poudyal H, Panchal SK, Diwan V, Brown L. Omega-3 fatty acids and metabolic syndrome: effects and emerging mechanisms of action. Prog Lipid Res. 2011;50:372–87 [DOI] [PubMed] [Google Scholar]
- 48.Harris WS. n-3 Fatty acids and serum lipoproteins: Human studies. Am J Clin Nutr. 1997;65:1645S–54S [DOI] [PubMed] [Google Scholar]
- 49.Ebrahimi M, Ghayour-Mobarhan M, Rezaiean S, Hoseini M, Parizade SMR, Farhoudi F, Hosseininezhad SJ, Tavallaei S, Vejdani A, Azimi-Nezhad M, et al. Omega-3 fatty acid supplements improve the cardiovascular risk profile of subjects with metabolic syndrome, including markers of inflammation and auto-immunity. Acta Cardiol. 2009;64:321–7 [DOI] [PubMed] [Google Scholar]
- 50.Damsgaard CT, Frøkiaer H, Andersen AD, Lauritzen L. Fish oil in combination with high or low intakes of linoleic acid lowers plasma triacylglycerols but does not affect other cardiovascular risk markers in healthy men. J Nutr. 2008;138:1061–6 [DOI] [PubMed] [Google Scholar]
- 51.Finnegan YE, Minihane AM, Leigh-Firbank EC, Kew S, Meijer GW, Muggli R, Calder PC, Williams CM. Plant- and marine-derived n-3 polyunsaturated fatty acid have differential effects on fasting and postprandial blood lipid concentrations and on the susceptibility of LDL to oxidative modification in moderately hyperlipidemic subjects. Am J Clin Nutr. 2003;77:783–95 [DOI] [PubMed] [Google Scholar]
- 52.Zhang P, Boudyguina E, Wilson MD, Gebre AK, Parks JS. Echium oil reduces plasma lipids and hepatic lipogenic gene expression in apoB100-only LDL receptor knockout mice. J Nutr Biochem. 2008;19:655–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Botelho PB, Mariano Kda R, Rogero MM, de Castro IA. Effect of echium oil compared with marine oils on lipid profile and inhibition of hepatic steatosis in LDLr knockout mice. Lipids Health Dis. 2013;12:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campioli E, Rustichelli C, Avallone R. n-3 Dietary supplementation and lipid metabolism: differences between vegetable- and fish-derived oils. J Funct Foods. 2012;4:207–12 [Google Scholar]
- 55.Suzuki T, Kohno H, Hasegawa A, Toshima S, Amaki T, Kurabayashi M, Nagai R, Suzuki T, Amaki T, Nagai R, et al. Diagnostic implications of circulating oxidized low density lipoprotein levels as a biochemical risk marker of coronary artery disease. Clin Biochem. 2002;35:347–53 [DOI] [PubMed] [Google Scholar]
- 56.GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–55 [PubMed] [Google Scholar]
- 57.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–8 [DOI] [PubMed] [Google Scholar]