Abstract
Prior to their fertilization, oocytes undergo asymmetric division, which is regulated by actin filaments. Recently, WASH complex were identified as actin nucleation promoting factors (NPF) that activated Arp2/3 complex. However, the roles of WASH complex remain uncertain, particularly for oocyte polarization and asymmetric division. Here, we examined the functions of two important subunits of a WASH complex, WASH1 and Strumpellin, during mouse oocyte meiosis. Depleting WASH1 or disrupting Strumpellin activity by WASH1 morpholino (MO) injection or Strumpellin antibody injection decreased polar body extrusion and caused oocyte symmetric division, and this may have been due to spindle formation and migration defects. Time lapse microscopy showed that actin filaments distribution and relative amount at the membrane and in the cytoplasm of oocytes was significantly decreased after disrupting WASH complex. In addition, Arp2/3 complex expression was reduced after WASH1 depletion. Thus, our data indicated that WASH complex regulated Arp2/3 complex and were required for cytokinesis and following polar body extrusion during mouse oocyte meiotic maturation.
In mammals, oocytes undergo asymmetric division prior to fertilization. Oocytes extrude half of their chromosomes in a small daughter cell called the polar body. Two steps are involved in regulating this asymmetric meiotic division: spindle positioning, which involves spindle migration and anchoring; and polar body extrusion, which involves cortical reorganization and cytokinesis1,2. This asymmetry is regulated by both microtubule and actin filaments.
Wiskott Aldrich Syndrome protein and scar homologue complex (WASH complex) is a ~500 kDa complex that includes WASH1, FAM21, SWIP (strumpellin- and WASH-interacting protein; also known as KIAA1033), Strumpellin, and CCDC53 (coiled-coil domain-containing protein 53) subunits3,4. The WASH complex was identified as a type I nucleation-promoting factor (NPF), which are Arp2/3 activators5. A previous study showed that WASH complex were in bundles of F-actin and microtubules in vitro and that, together with Rho, regulated both linear- and branched- actin networks6. In keeping with the role of these complex in regulating endosome al actin networks, WASH1 is a nucleation promoting factor (NPF) that activates Arp2/3 complex to regulate branched actin networks on endosome subdomains3,4. FAM21 contains an actin capping protein binding motif and has been proposed to control the activity of actin capping proteins, which regulate the stability and polymerization capacity of actin fibers7.
WASH1 is a member of the Wiskott-Aldrich syndrome protein (WASP) family comprising WASP/N-WASP, WAVE, WHAMM, JMY, and WASH18. As with other WASP family members, WASH contains a C-terminal VCA (verprolin homologous or WH2, central hydrophobic, and acidic) domain, which binds to actin and Arp2/3 complex to induce actin filament nucleation4,5,6,9,10. Recent studies have demonstrated that WASH1 was endosome-associated and functioned downstream of the Retromer complex to facilitate endosome-to-Golgi transport3,4,5,9. WASH1 is also essential for Arp2/3-mediated F-actin accumulation on endosomes4,11. Suppressing WASH1 expression resulted in the scission of membrane tubules emanating from endosomes3,4 and WASH knockout in MEFs resulted in the collapse of the early endosome and lysosome networks11. In addition, WASH1 was found to bind to Arp2/3 complex to locally nucleate and organize the polymerization of branched actin filaments4,10,12,13. WASH1 is maintained in an inhibited state by WASH complex. Although there are only weak sequence similarities between each component of these complex, several structural aspects of WASH complex are highly similar10. Because WASH1 apparently lacks intrinsic phospholipid-binding domains and has not been found to bind to endosome membranes, the other subunits of these WASH complex may aid in WASH1 targeting to endosomes10. Strumpellin is a component of the WASH complex, an actin-regulating complex that is recruited to endosomes by its interactions with the Retromer complex14. Moreover, Strumpellin activates Arp2/3-mediated actin polymerization and functions during the fission of endosomes4.
The Arp2/3 complex and nucleation-promoting factors (NPFs) that associate with Arp2/3 have been shown to regulate the branched actin filament networks that are required for various cellular processes, including cell migration and adhesion15,16,17, endocytosis18,19, and establishing polarity during oocyte meiosis20,21. Recent studies demonstrated that Arp2/3 complex and the associated NPFs WAVE2 and JMY regulated mammalian oocyte asymmetric division22,23. Furthermore, WASH1 was found to be essential for Drosophila oogenesis associated with Arp2/3-mediated actin nucleation activity6. However, the mechanisms of WASH complex regulation in mammalian oocytes are not well understood.
In this study, using injections of WASH1 morpholino and a Strumpellin antibody, we showed that WASH complex regulated Arp2/3 complex, which were involved in mouse oocyte cytokinesis and polar body extrusion.
Methods
Antibodies
Rabbit polyclonal anti-WASH1 antibody was from ECM Bioscience, rabbit polyclonal anti-Strumpellin antibody was from Abcam (Cambridge, UK), and rabbit polyclonal anti-ARP2 antibody was from Santa Cruz (Santa Cruz, CA). Phalloidin-TRITC and mouse monoclonal anti-α-tubulin-FITC antibody were from Sigma (St Louis, MO). FITC conjugated goat-anti-rabbit IgG and TRITC conjugated goat-anti-rabbit IgG were from Zhong Shan Jin Qiao, Beijing.
Oocyte harvest and culture
All procedures with mice were conducted according to the Animal Research Institute Committee guidelines of Nanjing Agriculture University, China. Germinal vesicle-intact oocytes were harvested from the ovaries of 3–4 week old ICR mice and cultured in M medium (Sigma, MO) under paraffin oil at 37°C in an atmosphere of 5% CO2. Oocytes were harvested after different times in culture for subsequent experiments. All experimental protocols were approved by the Committee of Animal Research Institute, Nanjing Agricultural University, China.
Microinjection of WASH morpholino (MO) or Strumpellin antibody
For WASH1 knock-down in mouse oocytes, WASH1 morpholino (MO) 5′-TCT CAG CCA GGA AGT CCA ACA TGG T-3′ (Gene Tools, Philomath, OR) was diluted with reagent grade water (Sigma) to give a 2 mM stock concentration. Each fully grown GV oocyte was microinjected with 5–10 pl of WASH1 morpholino using an Eppendorf FemtoJet (Eppendorf AG, Hamburg, Germany) under an inverted microscope (Olympus IX71, Japan). After injection, oocytes were cultured for 22 h in M2 medium that contained 2.5 mM milrinone, then washed four times (2 min each wash) in fresh M2 medium. Oocytes were then transferred to fresh M2 medium and cultured under paraffin oil at 37°C in an atmosphere of 5% CO2 in air. Each control oocyte was microinjected with 5–10 pL of MO standard control (5′-CCT CTT ACC TCA GTT ACA ATT TAT A-3′).
For Strumpellin antibody injection, each fully grown GV oocyte was microinjected with 5–10 pl of Strumpellin antibody dope, and then cultured in fresh M2 medium.
Time-lapse microscopy
After microinjecting Alexa 488-Phalloidin (1 μM) and WASH1 MO, oocytes were incubated in M2 medium that contained Hoechst 33342 (5 ng/ml; Sigma) to acquire images of actin and chromosome dynamics during oocyte maturation. Images for actin dynamics were acquired using a Perkin Elmer precisely Ultra VIEW VOX confocal Imaging System. The exposure time was set to between 200 and 800 ms. Digital time-lapse images were acquired under the control of IP Lab (Scanalytics) or AQM6 (Andor/Kinetic-imaging) software. Confocal images of actin in viable oocytes were acquired with a 10x objective with a spinning disk confocal microscope (Perkin Elmer).
Western blot
Approximately 150 oocytes were lysed in Laemmli sample buffer that contained a protease inhibitor, and then subjected to 10% SDS-PAGE. Separated proteins were transferred to a PVDF membrane. Membranes were blocked in TBS (0.1% Tween 20 and 5% non- fat dry milk) for 1 hour, and then incubated with primary antibodies (1:1,000 monoclonal rabbit anti-WASH1 antibody or rabbit anti-ARP2 and 1:2,500 rabbit monoclonal anti-β-actin antibody) at 4°C overnight. After washing 3 times (10 min each wash) in PBST, membranes were incubated for at 37°C 1 h with 1:10,000 horseradish peroxidase conjugated secondary antibodies. Protein bands were visualized using an ECL Plus Western Blotting Detection System, Tanon-5500. A membrane was then washed and reblotted with an anti-β-actin antibody (1:10,000) as an internal control.
Immunofluorescent and confocal microscopy
For immunofluorescent staining, oocytes were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) at room temperature for 30 min and then transferred to a membrane permeabilization solution (0.5%Triton X-100) for 20 min. Oocytes were placed in blocking buffer (1% bovine serum albumin-supplemented PBS) for 1 h, after which oocytes were incubated at 4°C overnight or at room temperature for 4 h with a rabbit anti-WASH antibody (1:200), a rabbit anti-Strumpellin antibody (1:200), or a rabbit anti-ARP2 antibody (1:100). After three washes in wash buffer (0.1% Tween 20 and 0.01% Triton X-100 in PBS), oocytes were incubated with FITC-conjugated goat-anti-rabbit IgG (1:100) or TRITC conjugated goat-anti-rabbit IgG (1:100) at room temperature for 1 h. For Phalloidin-TRITC and α-tubulin-FITC staining, after incubation for 1 h, oocytes were washed 3 times (2 min each wash) in wash buffer, co-stained with Hoechst 33342 (10 mg/ml in PBS) for 10 min, and then washed 3 times in wash buffer. These oocytes were mounted on glass slides and examined with a confocal laser-scanning microscope (Zeiss LSM 700 META, Germany). At least 30 oocytes were examined for each experimental group.
Statistical analysis
At least three replicates were performed for each treatment with results expressed as means ± SEM's. Statistical comparisons were made by analysis of variance (ANOVA), followed by Duncan's multiple comparisons test. A p-value of < 0.05 was considered significant.
Fluorescence intensity statistics were assessed using Image J (NIH) software with at least 15 oocytes analyzed for each experiment.
Results
WASH1 and ARP2 localization during mouse oocyte meiotic maturation
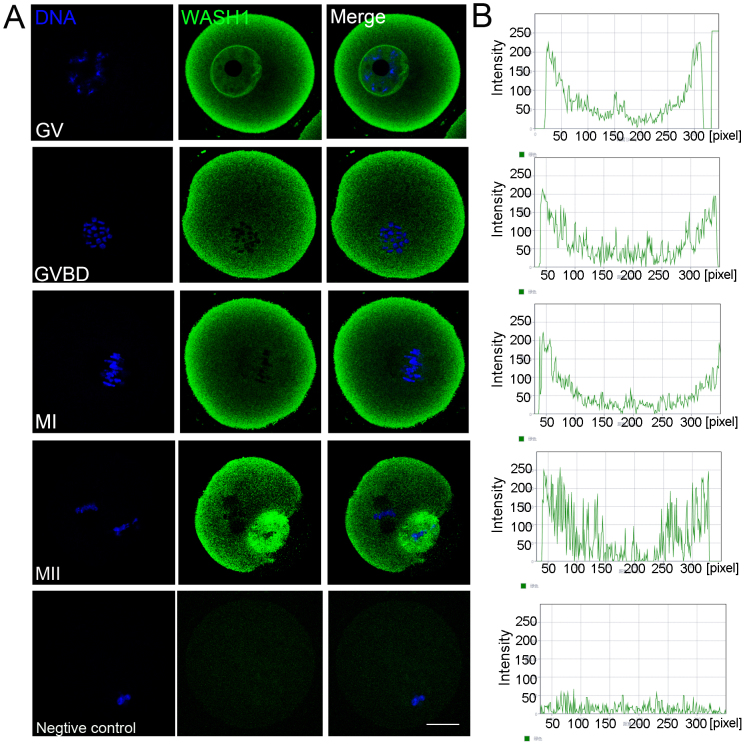
WASH1 subcellular localization at different stages of oocyte meiotic maturation was assessed using anti-WASH1 immunofluorescent staining. Oocyte samples were taken after culture for 0, 4, 8, or 12 h, which corresponded to the time points when most oocytes reached the GV, GVBD, MI, and MII stages, respectively. As shown in Fig. 1A, in GV oocytes, WASH1 was primarily distributed around the germinal vesicles and cortex of oocytes. After the GVBD stage, WASH1 was localized at the cortical region from the MI to the MII stages. We also assessed ARP2 localization in oocytes. As shown in Fig 1B, the linear fluorescence intensity analysis showed that a peak in the cortical region of each oocyte stage. The results proved WASH1 was mainly localized at the cortical region from the GV to the MII stage.
Figure 1. WASH1 and ARP2 localization during mouse meiotic maturation.
(A) Subcellular WASH1 localization during mouse oocyte meiosis based on staining with an anti-WASH1 antibody. From the GV stage to the MII stage, WASH1 staining was highest at the cortex of mouse oocytes. Green, WASH; blue, chromatin. Bar = 20 μm. (B) The linear fluorescence intensity analysis of each oocyte stage.
WASH complex affect mouse oocyte first polar body extrusion and asymmetric division
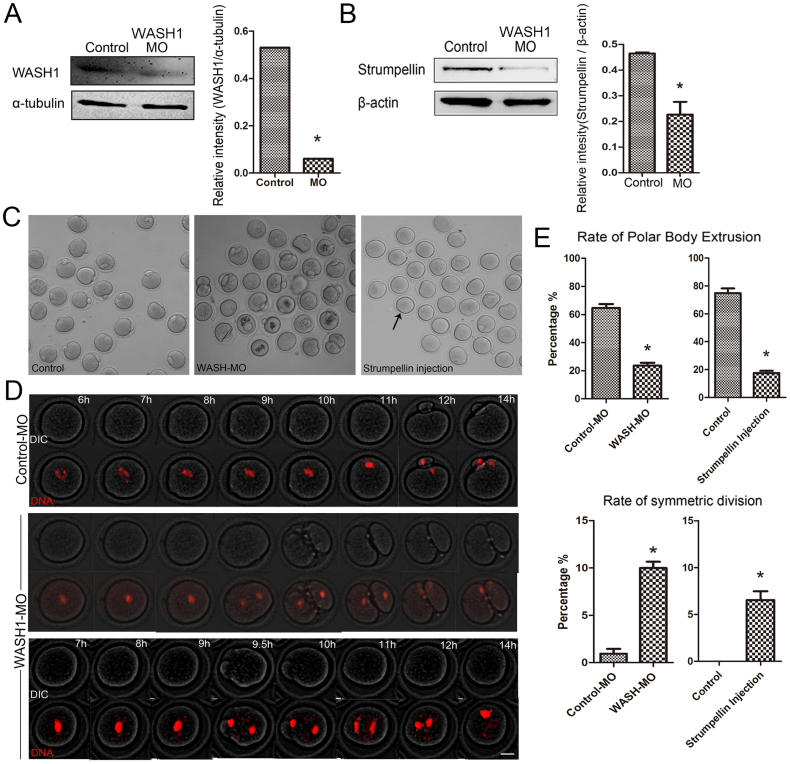
To further explore the functional role of WASH complex during oocyte meiotic maturation, we employed injections with WASH1 morpholino (MO) and a Strumpellin antibody. As shown in Fig. 2A, WASH1 expression was significantly reduced after WASH1 MO injection (0.53 vs 0.06 for current band, p<0.05). Moreover, Western blot and densitometry analysis showed that the Strumpellin expression level was also reduced after injection with WASH1 MO (0.45 ± 0.01 vs 0.22 ± 0.05, p<0.05) (Fig. 2B), which demonstrated that WASH1 regulated Strumpellin activity in the WASH complex of oocytes. As shown in Fig. 2C, after 12 h in culture, most WASH1 MO or Strumpellin antibody injected oocytes failed to extrude a first polar body and several oocytes underwent symmetric division.
Figure 2. WASH complex regulate first polar body extrusion and asymmetric division.
(A) Western blotting results for WASH1 in the WASH1 MO and control groups of oocytes were cropped gels. WASH1 molecular mass is 70 kDa and that of α-tubulin is 55 kDa. The relative staining intensity of WASH1 or α-tubulin was assessed by densitometry. (B) Western blotting results for Strumpellin in the WASH1 MO-injected and control MO-injected groups of oocytes were cropped gels. Strumpellin molecular mass is 134 kDa and that of β-actin is 43 kDa. Relative staining intensity of Strumpellin or β-actin was assessed by densitometry. (C) Oocyte meiotic maturation failure after WASH1 and Strumpellin inhibition. Bar = 80 μm. (D) Time lapse microscopic analysis of maturing oocytes in the control-MO injected and WASH1-MO injected groups of oocytes. In the controls, spindles moved to the cortex and extruded polar bodies, whereas in WASH1 MO-injected oocytes, oocytes underwent symmetric division or could not extrude a polar body. Bar = 20 μm. (E) Rates of first polar body extrusion and symmetric division after 12 h in culture for WASH1 MO and control MO injected groups and Strumpellin antibody injected and control groups. *, significantly different (p < 0.05).
Next, live cell imaging by time-lapse microscopy was used to examine the dynamic changes that occurred in maturing oocytes after injections with WASH1 MO. As shown in Fig. 2D, in the control MO-injected group, chromosomes moved toward the cortex, after which oocytes normally extruded a polar body. In contrast, in the WASH1 MO-injected group, two phenotypes were observed: (1) chromosomes separated in the central cytoplasm and symmetric division occurred; and (2) chromosomes segregated, although they re-joined, and the oocytes appeared to be shrunken, although they then returned to normal; during these events, a polar body was not extruded.
We also determined the rates of polar body extrusion and symmetric division in the control and treated oocytes. As shown in Fig. 2E, only a small proportion of oocytes extruded a first polar body in the WASH1 MO-injected group (23.6 ± 2.0%; n = 44), while a large proportion of oocytes extruded a first polar body in the control MO-injected group (64.7 ± 2.8%; n = 59; p < 0.05). Similarly, in the Strumpellin antibody injected group, only a small proportion of oocytes extruded a polar body (17.5 ± 1.5%; n = 60), while a large proportion of oocytes extruded a first polar body in the control group (74.8 ± 3.3%; n = 55; p<0.05). Moreover, the rates of symmetric division in the WASH1 MO and Strumpellin antibody injected groups (10.0 ± 0.7%, n = 65; and 6.5 ± 0.9%, n = 58, respectively) were significantly higher than those in the control MO-injected and control groups (1.0 ± 0.5%, n = 46; and 0, n = 38, respectively; both p < 0.05).
WASH complex affect spindle organization during oocyte meiosis
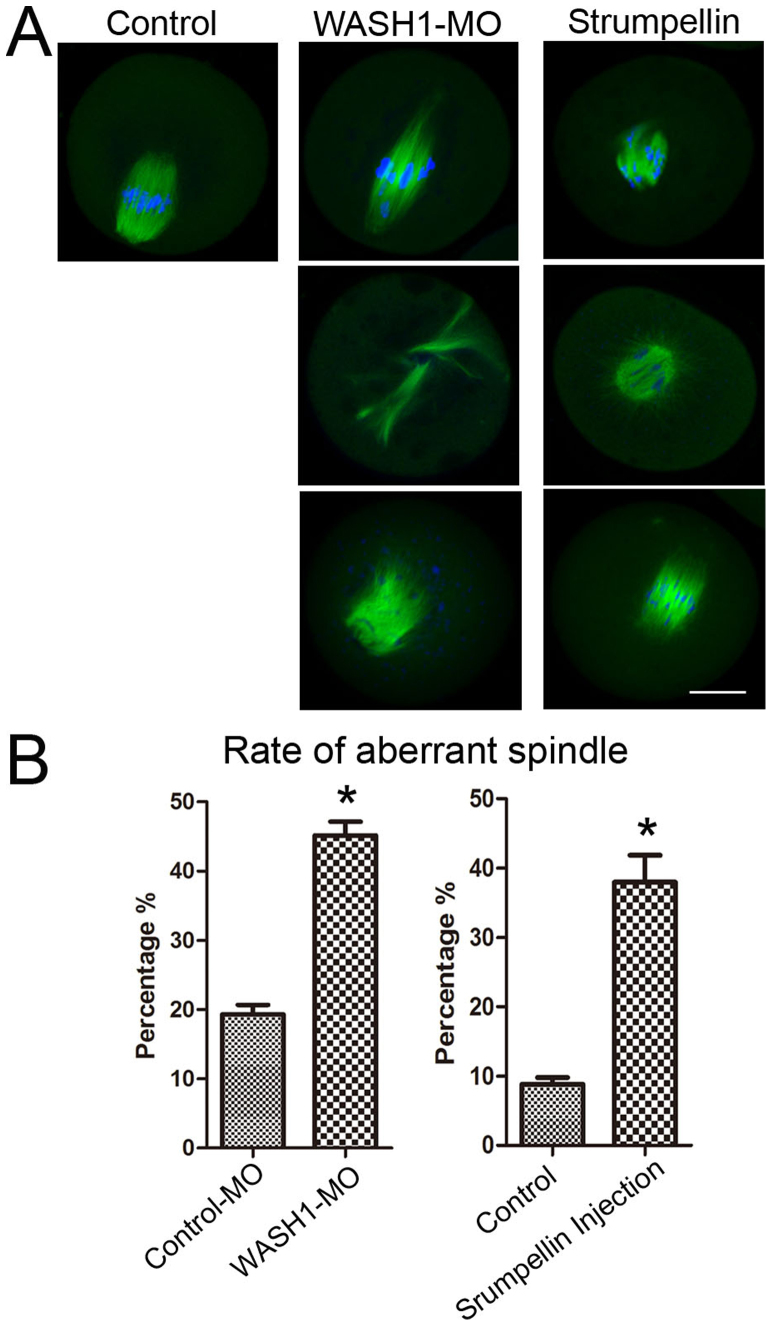
We assessed the effects of WASH1 and Strumpellin on spindle organization and morphology. As shown in Fig. 3B, after 9 h of culture for control oocytes, chromosomes accumulated at the mid-plate and spindles formed and moved toward the cortex. However, in the WASH1 MO and Strumpellin antibody injected groups, oocytes exhibited various types of morphologically defective spindles. In the WASH1 MO-injected group, the major defects were two poles of elongated spindles. Other defects included spindles with multiple or no poles while chromosomes were dispersed or lagged. In the Strumpellin antibody injected group, the major defect was abnormal spindles with many misaligned chromosomes. Other defects included unformed spindles with astral microtubules as well as many cytoplasmic asters or spindles with no poles. The rates of abnormal spindles in the WASH MO and Strumpellin antibody injected groups were significantly higher than those of the corresponding control MO-injected and control antibody-injected groups (45.2 ± 2.0%, n = 38 versus 19.3 ± 1.4%, n = 46, p < 0.05; and 37.9 ± 3.9%, n = 49 versus 8.8 ± 1.0%, n = 51;all p < 0.05; Fig. 3C).
Figure 3. WASH complex regulate spindle formation.
(A) Spindle morphology after WASH1 and Strumpellin inhibition. In the WASH MO and Strumpellin injection groups, oocytes exhibited morphologically defective spindles. Bar = 20 μm. (B) Percentages of oocytes with abnormal spindles in the WASH1 MO injection and control MO groups and Strumpellin antibody injection and control groups. Results are means ± SEM's of 3 independent experiments. Different letters indicate significant differences (p < 0.05).
WASH complex affect actin filaments distribution during oocyte meiotic maturation
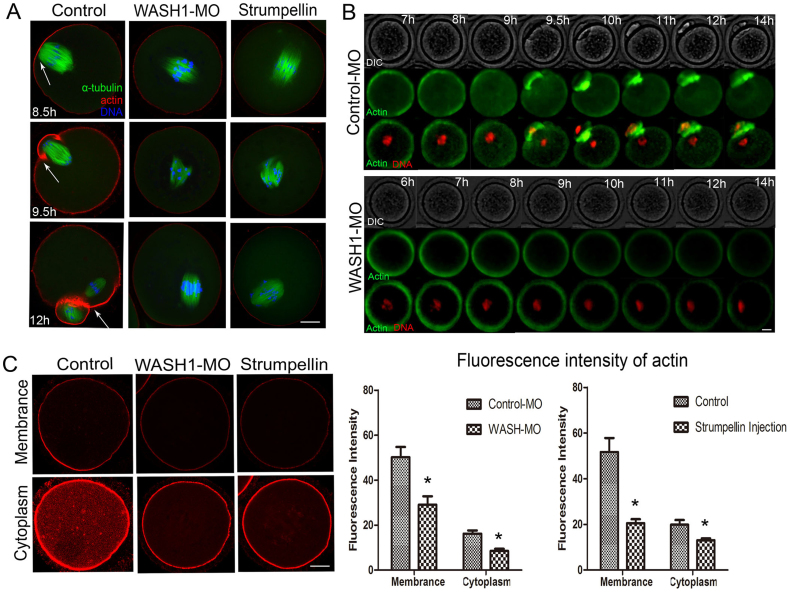
To further explore the reason of the aberrant progression of oocyte maturation, we first examined actin cap formation, a characteristic feature of oocyte polarization. As shown in Fig. 4A, chromosomes in the control group had moved to the cortex and formed an actin cap after 8.5 h of culture, whereas in the WASH1 MO or Strumpellin antibody injected group, most spindles stayed at the central positions of oocytes and no actin caps formed. After cultured of 9.5 h, most chromosomes segregated at the region of the cortex with a strong actin cap in the control oocytes, whereas in the WASH1 MO-injected or Strumpellin antibody injected group, most chromosomes also stayed in the central cytoplasm with no actin cap. After cultured of 12 h, in the control group, small polar bodies and large MII oocytes formed, and spindle microtubules were located under the region of the cortex where these actin caps had formed. In contrast, oocytes were arrested at the MI stage with no actin cap in the WASH MO-injected group or Strumpellin antibody injected group.
Figure 4. WASH complex regulate actin cap formation and actin filaments distribution.
(A) At the MI, ATI, and MII stages, actin caps formed in the control group, whereas no actin caps formed in the WASH1 MO or Strumpellin antibody injected oocytes. Arrowhead indicates an actin cap. Green, tubulin; blue, chromatin; red, actin. Bar = 20 μm. (B) Time lapse microscopy results for actin filaments distribution in control MO-injected and WASH1 MO-injected groups. In the control MO-injected group, chromosomes moved towards the cortex and extruded a polar body; actin signals were strong during this process. In contrast, in the WASH1 MO-injected group, actin signals gradually decreased in intensity during this process. Red, chromatin; green, actin; white, DIC. Bar = 40 μm. (C) Actin filaments distribution in oocyte membranes and cytoplasm after treatment. During the MI stage, actin amounts deceased in the cytoplasm and membranes of WASH1 MO or Strumpellin antibody injected oocytes. Green, tubulin; blue, chromatin; red, actin. Bar = 20 μm. (D) Average actin fluorescence intensities in mouse oocyte membranes and cytoplasm. *, significant difference (p < 0.05).
Next we examined actin filaments distribution and relative amount during mouse oocyte meiosis by time lapse microscopy. As show in Fig. 4B, in the control MO-injected group, chromosomes moved toward the cortex and extruded a polar body; actin signals were strong on the cortex near a chromosome. In the WASH1 MO-injected group, chromosomes moved toward the oocyte cortex but failed to extrude a polar body; actin signals at the cortex gradually decreased.
To further investigate the relationship between WASH1 and actin, we examined actin filaments distribution and relative amount in both the membrane and cytoplasm by Phalloidin-TRITC straining. As shown in Fig. 4C, the fluorescence intensity of membrane actin in the WASH1 MO or Strumpellin antibody injected group was significantly lower than that in the control MO-injected or control group (29.0 ± 3.8% versus 50.2 ± 4.5%, n = 45; and 20.5 ± 1.7% versus 51.7 ± 6.1%, n = 45, respectively; both p < 0.05; Fig. 4D). We also examined the average actin fluorescence intensity in the cytoplasm. The average fluorescence intensity of cytoplasmic actin in the WASH1 MO or Strumpellin antibody injected group was significantly lower compared with that in the control MO injected or control oocytes (8.5 ± 1.0% versus 16.2 ± 1.4%, n = 45; and 13.1 ± 0.8% versus 19.8 ± 2.1%, n = 45, respectively; both p < 0.05; Fig. 4D).
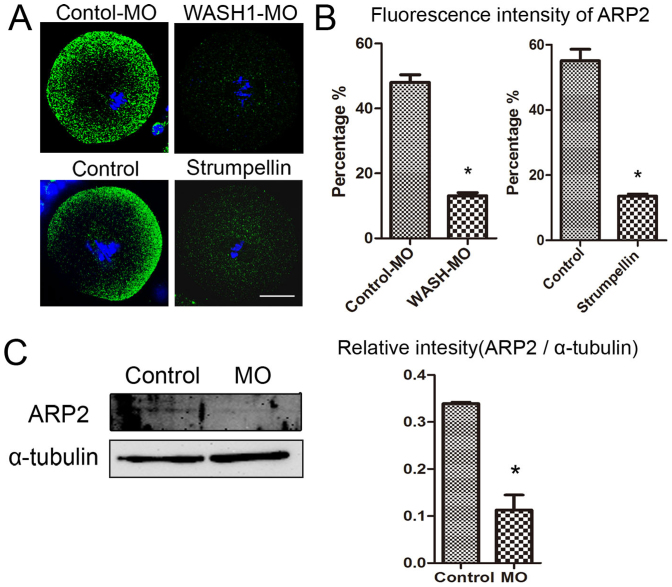
WASH complex regulate ARP2 expression during mouse oocytes meiosis
The involvement of Arp2/3 complex in the formation of new branched actin filaments depends on their interactions with nucleation-promoting factors (NPFs), which include WASH complex. To confirm the relationship between WASH complex and Arp2/3 complex during oocyte meiosis, we examined Arp2 expression by immunofluorescent staining after injecting oocytes with WASH1 morpholino or a Strumpellin antibody. As shown in Fig. 5A, ARP2 immunofluorescent staining was concentrated primarily in the cortex of the control MO-injected or control oocytes after culture for 9 h, whereas in WASH1 MO-injected or Strumpellin antibody injected oocytes, ARP2 was dispersed throughout the cytoplasm and with no specific localization. The fluorescence intensity of ARP2 in the WASH MO and Strumpellin antibody injected groups were significantly lower than those of the corresponding control MO-injected and control antibody-injected groups (13.1 ± 1.6% vs 48 ± 4.1%, p < 0.05; and 13.6 ± 1.1% vs 55.1 ± 6.1%, p < 0.05; Fig. 3B). Moreover, Western blot and densitometry analysis showed that the ARP2 expression level in WASH1 MO-injected oocytes was dramatically reduced as compared with that in control MO-injected oocytes (0.33 ± 0.01 vs 0.12 ± 0.05, p<0.05) (Fig. 5C).
Figure 5. WASH complex regulate ARP2 expression.
(A) ARP2 expression after culture for 9 h. In the control group, ARP2 was expressed mainly at the membrane, whereas ARP2 expression was significantly reduced in oocytes that were injected with WASH1 MO or a Strumpellin antibody. Green, ARP2; blue, chromatin. Bar = 20 μm. (B) Average ARP2 fluorescence intensities were determined in mouse oocytes after injection of WASH1 MO or a Strumpellin antibody. *, significant difference (p<0.05). (C) Cropped gels were used for our western blot results. Western blot results showed that ARP2 expression was significantly reduced after injection with WASH1 MO. ARP2 molecular mass is 43 kDa and that of α-tubulin is 55 kDa. Relative ARP2 or α-tubulin staining intensity was assessed by densitometry.
Discussion
In this study, we investigated the localization patterns and possible functions of two important subunits of WASH complex, WASH1 and Strumpellin, during mouse oocyte meiotic maturation. We showed that WASH1 MO injection or Strumpellin antibody injection disrupted polar body extrusion, asymmetric division, cytokinesis, and Arp2 expression during oocyte meiotic maturation. Thus, our data indicated that WASH complex were involved in mouse oocyte asymmetric division and cytokinesis mediated through their effect on Arp2/3 complex.
During oocyte meiotic maturation, WASH1 was found to be mainly localized at the cortex of oocytes, and this localization pattern was similar to that of ARP2 in oocytes23. Moreover, because ARP2 was concentrated primarily in the cortex of oocytes and co-localized with actin during mouse oocyte meiotic maturation, we verified that WASH1 co-localized with actin. Furthermore, during meiosis, Arp2/3 complex were shown to regulate oocyte asymmetric division23. Based on the localization pattern of WASH1, we speculated that WASH complex was involved in oocyte meiosis based on their actin regulation.
To confirm this hypothesis, WASH1 MO injection and Strumpellin antibody injection were used to investigate the roles of WASH complex during mouse oocyte meiotic maturation. These results demonstrated that inhibiting the activities of WASH1 and Strumpellin resulted in disrupting oocyte polar body extrusion. The results showed that inhibiting WASH1 and Strumpellin activities caused the aberrant progression of oocyte maturation.
A previous study showed that actin flow drove oocytes progressed to MII stage which resulted in oocyte asymmetric division24. To further confirm this, we examined actin cap formation and actin filaments distribution during meiosis.
Staining with phalloidin-TRITC showed that actin filaments distribution was significantly reduced in the membrane and cytoplasm after injecting WASH1 MO or a Strumpellin antibody, and that no clear actin caps had formed. These results indicated that WASH complex might affect actin assembly, followed by disrupted polar body extrusion. Similar results were also obtained with the actin nucleation factors for Arp2/3 complex25, including the associated NPFs JMY26, N-WASP27, WAVE223, and Formin-228 in mouse oocytes, which molecules were shown to regulate polar body extrusion mediated through their effects on actin-based spindle migration during mouse oocyte meiosis. Thus, our results suggest that WASH complex participate in polar body extrusion mediated through their regulation of actin assembly.
WASH1 is an endosome-associated protein and facilitates endosome-to-Golgi transport3,4,5,9. Actin and its associated proteins also play significant roles in the structure and function of Golgi and endocytic sites29. Moreover, actin structures are dynamically organized to assist in the remodeling of the cell surface to allow the inward movement of vesicles30. A recent study showed that endosome or Golgi associated proteins, such as GM130, were also involved in oocyte meiosis31. It was shown that GM130 was involved in oocyte meiosis through its effects on actin-based spindle migration and actin cap formation. This led us to speculate that WASH1 acts as an endosome-associated protein that might regulate endosome-to-Golgi transport through its effects on F-actin. However, this requires further verification.
A previous report indicated that WASH complex activated Arp2/3 complex, which regulate actin nucleation during mitosis. Based on this, we hypothesized that WASH complex may participate in actin-mediated oocyte meiosis in conjunction with Arp2/3 complex. The decreased ARP2 expression in WASH1 MO or Strumpellin antibody injected oocytes confirmed that WASH complex regulated actin assembly during oocyte meiotic maturation through their effects on Arp2/3 complex.
Because both WASH1 and Strumpellin, which are components of WASH complex, had similar effects on oocyte meiosis, we explored the relationship between these two. Interestingly, WASH1 knock-down caused a reduced Strumpellin expression level. This indicated that WASH1 could regulate Strumpellin activity. Thus, based on our results, we speculate that WASH1 and Strumpellin in WASH complex might have a synergistic effect on oocyte meiosis.
In conclusion, our study results indicate that a WASH complex-Arp2/3 complex-actin pathway is involved in mouse oocyte cytokinesis and following polar body extrusion.
Author Contributions
F.W., S.C.S. conceived and designed the experiments; F.W., L.Z. and G.L.Z. performed the experiments; F.W., S.C.S. analyzed the data; Z.B.W., X.S.C. and N.H.K. contributed reagents/materials/analysis tools; F.W., S.C.S. wrote the paper.
Supplementary Material
Supplementary information
Acknowledgments
This work was supported by the National Basic Research Program of China (2014CB138503), the Natural Science Foundation of Jiangsu Province (BK20130671), and the Biogreen 21 Program (PJ009594, PJ009080 and PJ00909801), RDA, Republic of Korea.
References
- Brunet S. & Verlhac M. H. Positioning to get out of meiosis: the asymmetry of division. Hum Reprod Update 17, 68–75. [DOI] [PubMed] [Google Scholar]
- Azoury J., Verlhac M. & Dumont J. Actin filaments: key players in the control of asymmetric divisions in mouse oocytes. Biol Cell 101, 69–76 (2009). [DOI] [PubMed] [Google Scholar]
- Gomez T. S. & Billadeau D. D. A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev Cell 17, 699–711 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derivery E. et al. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev Cell 17, 712–723 (2009). [DOI] [PubMed] [Google Scholar]
- Linardopoulou E. V. et al. Human subtelomeric WASH genes encode a new subclass of the WASP family. PLoS Genet 3, e237 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R. et al. Wash functions downstream of Rho and links linear and branched actin nucleation factors. Development 136, 2849–2860 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Valladares M. et al. Structural characterization of a capping protein interaction motif defines a family of actin filament regulators. Nat Struct Mol Biol 17, 497–503 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campellone K. G. & Welch M. D. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol 11, 237–251 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duleh S. N. & Welch M. D. WASH and the Arp2/3 complex regulate endosome shape and trafficking. Cytoskeleton 67, 193–206 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia D. et al. WASH and WAVE actin regulators of the Wiskott–Aldrich syndrome protein (WASP) family are controlled by analogous structurally related complexes. Proc Natl Acad Sci U S A 107, 10442–10447 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez T. S., Gorman J. A., de Narvajas A. A.-M., Koenig A. O. & Billadeau D. D. Trafficking defects in WASH-knockout fibroblasts originate from collapsed endosomal and lysosomal networks. Mol Biol Cell 23, 3215–3228 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltman D. M. & Insall R. H. WASP family proteins: their evolution and its physiological implications. Mol Biol Cell 21, 2880–2893 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotty J. D., Wu C. & Bear J. E. New insights into the regulation and cellular functions of the ARP2/3 complex. Nat Rev Mol Cell Biol 14, 7–12 (2013). [DOI] [PubMed] [Google Scholar]
- Freeman C., Seaman M. N. & Reid E. The hereditary spastic paraplegia protein strumpellin: characterisation in neurons and of the effect of disease mutations on WASH complex assembly and function. Biochim Biophys Acta 1832, 160–173 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly M. et al. The F-actin side binding activity of the Arp2/3 complex is essential for actin nucleation and lamellipod extension. Curr Biol 11, 620–625 (2001). [DOI] [PubMed] [Google Scholar]
- Rogers S. L., Wiedemann U., Stuurman N. & Vale R. D. Molecular requirements for actin-based lamella formation in Drosophila S2 cells. J Cell Biol 162, 1079–1088 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen A. et al. Filopodia formation in the absence of functional WAVE-and Arp2/3-complexes. Mol Biol Cell 17, 2581–2591 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau V., Galan J., Devilliers G., Haguenauer-Tsapis R. & Winsor B. The yeast actin-related protein Arp2p is required for the internalization step of endocytosis. Mol Biol Cell 8, 1361 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaerer-Brodbeck C. & Riezman H. Functional interactions between the p35 subunit of the Arp2/3 complex and calmodulin in yeast. Mol Biol Cell 11, 1113–1127 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goley E. D. & Welch M. D. The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol 7, 713–726 (2006). [DOI] [PubMed] [Google Scholar]
- Rouiller I. et al. The structural basis of actin filament branching by the Arp2/3 complex. J Cell Biol 180, 887–895 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. C. et al. Arp2/3 complex regulates asymmetric division and cytokinesis in mouse oocytes. PLoS One 6, e18392 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. C. et al. WAVE2 regulates meiotic spindle stability, peripheral positioning and polar body emission in mouse oocytes. Cell Cycle 10, 1853–1860 (2011). [DOI] [PubMed] [Google Scholar]
- Yi K. & Li R. Actin cytoskeleton in cell polarity and asymmetric division during mouse oocyte maturation. Cytoskeleton 69, 727–737. [DOI] [PubMed] [Google Scholar]
- Boldogh I. R. et al. Arp2/3 complex and actin dynamics are required for actin-based mitochondrial motility in yeast. Proc Natl Acad Sci U S A 98, 3162–3167 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. C., Sun Q. Y. & Kim N. H. JMY is required for asymmetric division and cytokinesis in mouse oocytes. Mol Hum Reprod 17, 296–304 (2011). [DOI] [PubMed] [Google Scholar]
- Lommel S. et al. Actin pedestal formation by enteropathogenic Escherichia coli and intracellular motility of Shigella flexneri are abolished in N-WASP-defective cells. EMBO Rep 2, 850–857 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J. et al. Formin-2 is required for spindle migration and for the late steps of cytokinesis in mouse oocytes. Dev Biol 301, 254–265 (2007). [DOI] [PubMed] [Google Scholar]
- Harris K. P. & Tepass U. Cdc42 and vesicle trafficking in polarized cells. Traffic 11, 1272–1279 (2010). [DOI] [PubMed] [Google Scholar]
- Smythe E. & Ayscough K. R. Actin regulation in endocytosis. J Cell Sci 119, 4589–4598 (2006). [DOI] [PubMed] [Google Scholar]
- Zhang C. H. et al. GM130, a cis-Golgi protein, regulates meiotic spindle assembly and asymmetric division in mouse oocyte. Cell Cycle 10, 1861–1870 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information