Abstract
Bone morphogenetic protein 2 (BMP-2) is a potent osteoinductive cytokine and a growing number of in vitro studies analyze its effects on human mesenchymal stem cells (hMSC) derived from aged or osteoporotic donors. In these studies the exact quantification of osteogenic differentiation capacity is of fundamental interest. Nevertheless, the experimental conditions for osteogenic differentiation of aged hMSC have not been evaluated systematically and vary to a considerable extend. Aim of the study was to assess the influence of cell density, osteogenic differentiation media (ODM) change intervals and duration of BMP-2 stimulation on osteoinduction. Furthermore, time series were carried out for osteogenic differentiation and BMP-2 concentration in ODM/BMP-2 cell culture supernatants. The experiments were performed using hMSC isolated from femoral heads of aged patients undergoing hip joint replacement. ODM change intervals of 96 hours resulted in significantly higher calcium deposition compared to shorter intervals. A cell density of 80% prior to stimulation led to stronger osteoinduction compared to higher cell densities. In ODM, aged hMSC showed a significant induction of calcium deposition after 9 days. Added to ODM, BMP-2 showed a stable concentration in the cell culture supernatants for at least 96 hours. Addition of BMP-2 to ODM for the initial 4 days led to a significantly higher induction of osteogenic differentiation compared to ODM alone. On the other hand, addition of BMP-2 for 21 days almost abrogated the osteoinductive effect of ODM. We could demonstrate that the factors investigated have a substantial impact on the extent of osteogenic differentiation of aged hMSC. Consequently, it is of upmost importance to standardize the experimental conditions in order to enable comparability between different studies. We here define standard conditions for osteogenic differentiation in regard to the specific features of aged hMSC. The finding that BMP-2 induces or inhibits osteogenic differentiation in a time dependent manner indicates an age related alteration in signal transduction of hMSC and requires further investigation.
Key words: mesenchymal stem cells, osteogenic differentiation, BMP-2, in vitro, age
Introduction
Osteogenesis is the differentiation of mesenchymal stem cells (MSC) into osteoblasts, which then deposit mineralized extracellular matrix of collagen, calcium, phosphorus and other minerals. In vivo, this process leads to the formation of new bone. Deregulations of osteogenesis underlie diseases of the bone such as osteoporosis or osteogenesis imperfecta. Thus, the precise understanding of the molecular alterations is of major clinical interest. To this end numerous in vitro models have been established. Most commonly, MSC are cultivated in osteogenic differentiation media (ODM), consisting of Dulbecco’s modified Eagle’s medium with high glucose and supplemented with fetal bovine serum, dexamethasone, β-glycerophosphate, as well as L-ascorbic acid 2-phosphate.1 The extracellular deposited calcium can be stained using alizarin red solution. Micrographs can be taken and quantitative analysis can be performed by determining the optical density (OD) values at 405 nm. Using this experimental set up, recent studies analyzed disease associated alterations in the osteogenic differentiation capacity of hMSC derived from aged donors.2-5
Proliferation and osteogenic differentiation capacity of hMSC seem to decrease with donor age.6-8 In order to identify disease associated alterations and to compare the results of different studies it is of crucial importance that experiments are carried out under standardized conditions adapted to the specific requirements of aged hMSC. Unfortunately, most studies inadequately address this issue and often lack a detailed description of the experimental setup. We therefore intended to answer the following basic questions for performing quantitative osteogenic differentiation analyses using hMSC derived from aged donors: i) what cell density ensures optimal osteogenic differentiation? ii) In which intervals should the ODM be changed in order to achieve optimal extracellular calcium deposition? iii) How long should the stimulation with ODM be carried out in order to achieve sufficient extracellular calcium deposition for comparative analyses?
Beyond the stimulation with osteogenic differentiation media, the bone morphogenic protein 2 (BMP-2) is a potent osteoinductive cytokine.9 BMP-2 physiologically contributes to the early phase of fracture healing,10,11 and is clinically approved for the treatment of distinct fracture entities.12 BMP-2 can be supplemented to the ODM in order to analyze its specific impact on osteoinduction of aged and diseased hMSC. The osteoinduction in response to BMP-2 seems to decrease with senescence of MSC.13 Nevertheless, BMP-2 still induces osteogenic differentiation of hMSC in a dose dependent manner with highest levels of calcium deposition after stimulation with a concentration of 100 ng/mL.14 Unfortunately, the optimal duration of stimulation has not been analyzed systematically. Therefore, the following question should also be addressed in this study: i) does the addition of BMP-2 to ODM ensure a constant concentration for the entire duration of one media change interval? ii) For how long should aged hMSC be stimulated with BMP-2 in order to achieve optimal extracellular calcium deposition?
Materials and Methods
Cells and cell culture
Human MSC were isolated from femoral heads of aged patients with fractures to the proximal femur undergoing hip joint replacement as described before.15 The study was approved by the LMU ethical commission and performed according to the Declaration of Helsinki. A total of three patients were included. All donors were of female gender, patients’ ages were 77, 89, and 94 years. Cells were isolated using Ficoll gradient centrifugation and cultured in MEM Alpha GlutaMAX media (Invitrogen, Karlsruhe, Germany) supplemented with 10% fetal bovine serum (Sigma, Taufkirchen, Germany). Prior to experiments, cells were maintained at 60 to 80% confluency in T-75 plastic culture flasks at 37°C and 5% CO2.
The hMSC characteristics were verified according to Dominici et al.16 In brief, hMSC were plastic adherent as well as proven to be positive (>95%) for the hMSC-related markers CD105, CD90, CD73 and negative (<2%) for the hematopoiesis and leucocytes related markers CD45, CD34, CD19, CD14, HLA-DR using flow cytometric analyses.17,18 Furthermore, cells were differentiable into osteoblasts, adipocytes and chondroblasts in response to the specific differentiating conditions. Experiments were carried out using aged hMSC at first and second passage. Stimulation was performed using recombinant human BMP-2 (R&S Systems, Minneapolis, MN, USA) in a concentration of 100 ng/mL.
Osteogenic differentiation assay
Aged hMSC were plated in twelve-well dishes with 1000 cells/cm2 and cultured to the indicated cell density. Cell density was determined applying a custom build ImageJ (Version 1.47) algorithm to 10× magnification phase contrast images. Cell density was defined as the relative area covered by cell bodies. The algorithm consists of the following image processing tools using the indicated adjustments: run(“8-bit”);
run(“Median…”, “radius=3”);
run(“Enhance Contrast”, “saturated=1 normalize equalize”);
run(“Subtract Background…”, “rolling=20 light”);
run(“Enhance Contrast”, “saturated=1 normalize equalize”);
run(“Invert LUT”);
run(“Gaussian Blur…”, “sigma=10”);
run(“Enhance Contrast”, “saturated=1 normalize equalize”);
run(“Set Measurements…”, “ area_fraction limit display redirect=None decimal=0”);
The threshold was set manually. Osteogenic differentiation media consisting of Dulbecco’s modified Eagle’s medium with high glucose (PAA Laboratories, Cölbe, Germany) supplemented with 10% fetal bovine serum, 100 nM dexamethasone, 10 mM β-glycerophosphate, and 50 mM l-ascorbic acid 2-phosphate (Sigma) was applied for 21 days and changed as indicated. In some experiments BMP-2 was added to the osteogenic differentiation media for the initial 4 and 8 days or the entire duration of 21 days.
Osteogenic differentiation was evaluated by alizarin red (AR) staining, visualizing calcium-rich deposits produced by the cells.19 AR staining and quantification were performed using the Osteogenic Quantification Kit (Millipore, Billerica, MA, USA) according to the manufacturer’s instructions. Micrographs were taken with an Axiovert100 microscope and an AxioCam ICc3 camera (Carl Zeiss, Jena, Germany). Afterwards, AR was extracted with 10% acetic acid and neutralized with 10% NH4OH. Optical density measurements were taken at 405 nm on a microplate reader (Biotek, Bad Friedrichshall, Germany). The AR concentration was calculated against an AR standard curve. The time series experiments and the final testing of the here defined experimental set up were carried out analyzing aged hMSC of all three donors. The experiments on media change intervals, cell density, and BMP-stimulation were carried out analyzing aged hMSC of one donor. All experiments were carried out in triplicates.
Bone morphogenetic protein 2 immunoassay
BMP-2 stimulation experiments were carried out adding recombinant human BMP-2 in a calculated concentration of 100 ng/mL to the osteogenic differentiation media. Media was changed every 96 hours. In order to follow up on the BMP-2 concentration within these 96 hours, 50 μL of cell culture supernatant was gained at the following time points: 0, 24, 48 and 96 hours. BMP-2 quantification was performed using the Quantikine ELISA BMP-2 Immunoassay kit (R&S Systems, Minneapolis, MN, USA) according to the manufacture’s protocol. One-hundred μL of the Assay Diluent was added to each of the microplate wells. Samples were diluted 1:10. 50 μL of standard, control or sample was added per well. The microplate was incubated for two hours at room temperature on a horizontal shaker set at 500 rpm. Wells were washed four times using 400 μL of Wash Buffer. Two-hundred μL of BMP-2 conjugate was added to the dry wells. After incubation for two hours at room temperature on the shaker, the washing procedure was repeated. Using 200 μL of Substrate Solution, the microplate was incubated for 30 minutes at room temperature protected from light. Afterwards, 50 μL of Stop Solution was added to each well. Optical density was determined using a microplate reader set to 450 nm. The experiments were carried out on all three donor samples and in triplicates.
Statistics
Statistical evaluation was performed using the GraphPrism software (GraphPad, La Jolla, CA, USA). One-way ANOVA analyses with Newman-Keuls Multiple Comparison testing were applied. Student’s t-testing was carried out. A P-value <0.05 was considered significant. Graphs and bars charts show mean values and standard deviation.
Results
What cell density ensures optimal osteogenic differentiation?
Human MSC were cultured to a cell density of 80, 90 or 100% prior to osteogenic differentiation. Starting the stimulation with ODM at a cell density of 80% led to a maximum mean AR concentration of 0.89 mM (SD 0.16) after 21 days. Utilizing an initial cell density of 90% revealed a maximum mean AR concentration of 0.68 mM (SD 0.04). HMSC cultured to a cell density of 100% prior to starting the stimulation showed a maximum mean AR concentration of 0.64 mM (SD 0.14). Aged hMSC seem to favor a cell density of 80% rather than 90 or 100% at the starting point of osteogenic differentiation (Figure 1).
Figure 1.
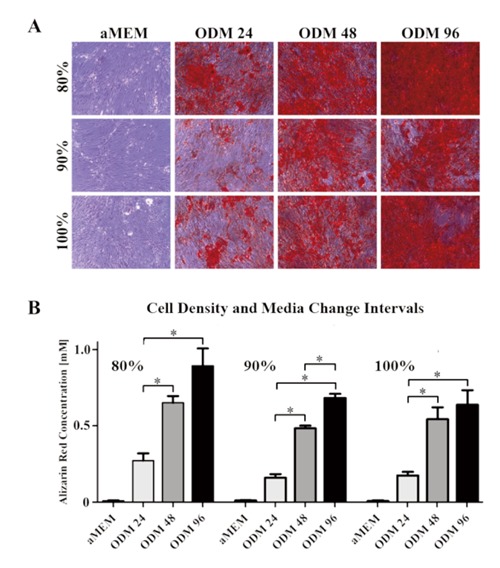
Effects of media change intervals and cell density at the starting time point of osteoinduction on extracellular calcium deposition. Highest concentrations of alizarin red were achieved when the osteogenic differentiation media (ODM) was applied to hMSC at a cell density of 80%. Changing the ODM every 96 hours ensured significantly higher calcium deposition compared to media changes every 24 or 48 hours. A) Micrographs of alizarin red stained aged hMSC cultures. B) Alizarin red quantification based on optical density measurements at 405 nm.
In which intervals should the osteogenic differentiation media be changed in order to achieve optimal extracellular calcium deposition?
During the osteogenic differentiation ODM was changed every 24, 48 or 96 hours. Despite the influence of the confluency, ODM change intervals of 24 hours revealed the lowest mean AR concentration levels after 21 days. There was a mean AR concentration of 0.27 mM (SD 0.07), 0.16 mM (SD 0.03) and 0.18 mM (SD 0.03) in the 80, 90 and 100% density groups, respectively. In contrast, the highest levels of extracellular calcium deposition were achieved by ODM change intervals of 96 hours. Here, mean AR concentrations of 0.89 mM (SD 0.16), 0.68mM (SD 0.04) and 0.64 mM (SD 0.14) were detected in the 80, 90% and 100% density groups, respectively. In all three groups, the increase of AR was significant when comparing media change intervals of 96 and 48 to 24 hours. In the 90% confluence group, the increase of calcium deposition also was significant when comparing media change intervals of 96 to 48 hours. Taken together, ODM change intervals of 96 hours improve the osteogenic differentiation conditions for aged hMSC (Figure 1).
How long should the stimulation with osteogenic differentiation media be carried out in order to achieve sufficient extracellular calcium deposition for comparative analyses?
Aged hMSC were cultured to a cell density of 80% and ODM was changed every 96 hours. A time series was carried out, stimulating aged hMSC with ODM for 6, 9, 12, 15, 18 and 21 days. Cell cultured in aMEM served as control and revealed mean AR concentrations of 0.011 mM (SD 0.004), 0.012 mM (SD 0.009), 0.025 mM (SD 0.015), 0.034 mM (SD 0.025), 0.038 mM (SD 0.024) and 0.033 mM (SD 0.032) for the designated time points, respectively. Stimulation with ODM led to a mean AR concentration of 0.018 mM (SD 0.008) after 6 days and significant increases of extracellular calcium deposition for the subsequent time points. Mean AR concentrations of 0.035 mM (SD 0.012), 0.148 mM (SD 0.082), 0.80 mM (SD 0.23), 1.41 mM (SD 0.41) and 1.72 mM (SD 0.91) were detected after 9, 12, 15, 18 and 21 days, respectively (Figure 2).
Figure 2.

Time series. Aged hMSC of all three donors were stimulated with osteogenic differentiation media (ODM) for 21 days. Media change was carried out every 96 hours. Micrographs were taken (A) and quantification of alizarin red was performed (B) at the designated time points. Starting with day 9 there was a significant increase in extracellular calcium deposition compared to standard growth media (aMEM) controls. The graph shows mean values and standard deviation error bars.
Does the addition of BMP-2 to osteogenic differentiation media ensure a constant concentration for the entire duration of one media change interval?
BMP-2 was added to the ODM in a concentration of 100 ng/mL. Supernatants of cell cultures were taken after 0, 24, 48 and 96 hours. The BMP-2 Immunoassay revealed a mean concentration of 91.8 (SD 2.4) and 89.9 ng/mL (SD 3.5) after 0 and 24 hours, respectively. After 48 hours the mean BMP-2 concentration was 91.6 (SD 2.1) and after 96 hours 92.7 ng/mL (SD 2.1). There was no significant alteration in the BMP-2 concentration at any time point investigated (Figure 3).
Figure 3.
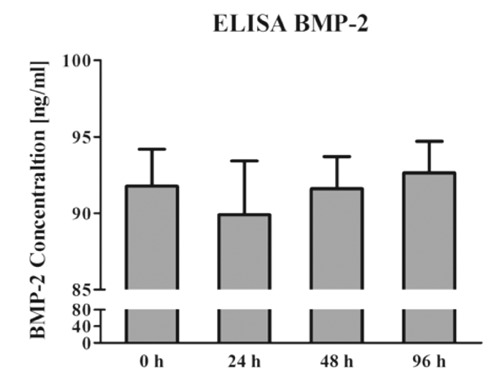
BMP-2 was added to the osteogenic differentiation media in a concentration of 100 ng/mL. Supernatants of hMSC cultures of all three donors were taken after 0, 24, 48 and 96 hours. ELISA analyses revealed no alterations of the BMP-2 concentration in the ODM for 96 hours. The graph shows mean values and standard deviation error bars.
For how long should aged human mesenchymal stem cells be stimulated with BMP-2 in order to achieve optimal extracellular calcium deposition?
Aged hMSC were cultured to a density of 80% prior to stimulation. ODM was changed every 96 hours and aged hMSC were stimulated for 21 days. The stimulation was carried out with ODM alone or ODM supplemented with BMP-2 for the initial 4 and 8 days or the entire period of 21 days. Cell cultured in aMEM served as control and revealed a mean AR concentration of 0.011 mM (SD 0.003). Stimulation with ODM alone revealed a mean AR concentration of 0.47 mM (SD 0.05). The significantly highest levels of extracellular calcium deposition were observed in the ODM group supplemented with BMP-2 for the initial 4 days with a mean AR concentration of 0.85 mM (SD 0.1). Addition of BMP-2 for the initial 8 days led to a mean AR concentration of 0.54 mM (SD 0.16), which is significantly less compared to BMP-2 stimulation for 4 days and no significant increase compared to stimulation with ODM alone. Addition of BMP-2 for the entire 21 days almost abrogated the osteoinductive effect of ODM with a mean AR concentration of 0.039 mM (SD 0.01). This was significantly lower than the AR concentration due to ODM alone (Figure 4).
Figure 4.
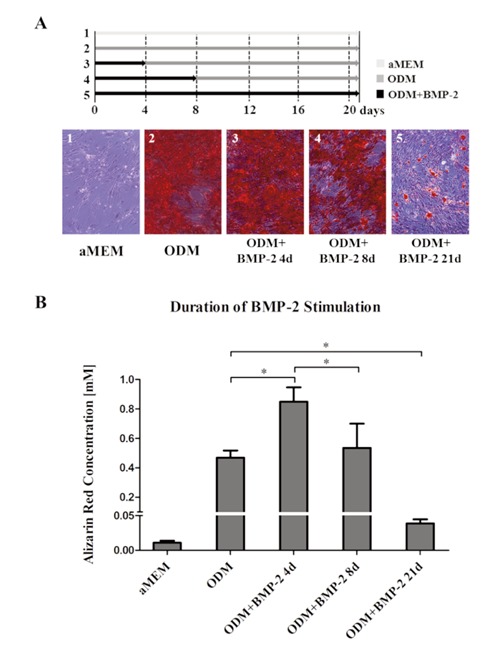
Aged hMSC were stimulated with osteogenic differentiation media (ODM) for 21 days. Media was changed every 96 hours. Stimulation was carried out with ODM alone or ODM supplemented with BMP-2 in a concentration of 100 ng/ml. BMP-2 was added for the initial 4 or 8 days or for the entire 21 days. A significantly higher deposition of calcium was observed due to BMP-2 addition for the initial 4 days while its addition for 21 days almost abrogated the osteoinduction. Cell cultivated in standard growth media (aMEM) served as control.
Testing the defined experimental set up on aged human mesenchymal stem cells of all three donors
Osteogenic differentiation assays were started at a cell density of 80% and carried out for 10 days. ODM was changed every 96 hours. Additional osteoinduction was achieved by adding BMP-2 for the initial 4 days of stimulation. Cell cultivated in standard growth media (aMEM) served as control. The control showed a mean AR concentration of 0.017 mM (SD 0.006). 10 days of ODM led to a significant osteoinduction with a mean AR concentration of 0.136 mM (SD 0.07). Adding BMP-2 to the ODM for the initial 4 days of stimulation resulted in a further significant increase of extracellular calcium deposition with a mean AR concentration of 0.329 mM (SD 0.21) (Figure 5).
Figure 5.
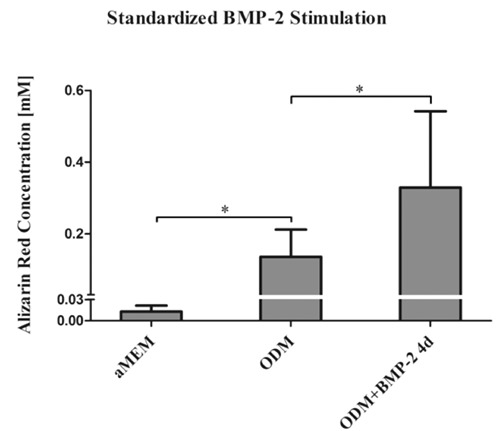
Testing the defined experimental set up on aged hMSC of all three donors. Osteogenic differentiation assays were started at a cell density of 80% and carried out for 10 days. ODM was changed every 96 hours. Additional osteoinduction was achieved by adding BMP-2 for the initial 4 days of stimulation. HMSC cultivated in standard growth media (aMEM) served as control.
Discussion
The increasing scientific work analyzing aged hMSC with regard to disease associated alterations in osteogenic differentiation requires an optimized and standardized experimental set up. This set up should be adapted to the specific features of aged hMSC, especially as proliferation, osteogenic differentiation capacity and response to stimuli have been described to decrease with donor age.8,20,21 It is known for hMSC derived from young donors that higher plating densities have a positive effect on mineralized matrix deposition.1,22 We could demonstrate that this positive effect is limited as aged hMSC seem to favor a density of 80% at the starting point of osteoinduction while higher densities led to lower levels of calcium deposition. According to others, very high levels of cell density and especially complete confluence may induce apoptosis and inhibit differentiation.23 Furthermore, a less frequent change of ODM significantly improved the extracellular calcium deposition (Figure 1). The ODM media change interval has a fundamental impact on the extent of osteogenic differentiation.
Unfortunately, recent studies on aged hMSC and osteogenic differentiation do not state the media change intervals,4,5,14 or use suboptimal short intervals.21,24 Osteogenic differentiation is not only regulated by extrinsic stimulation but also depends on autocrine and paracrine factors.25-27 It is very likely that these autocrine and paracrine effects are constrained by excessive media changes. Utilizing aged hMSC we therefore defined a cell density of 80% at the starting point of osteoinduction and ODM change interval of four days as standard condition for further experiments.
Osteogenic differentiation assays with quantification of extracellular calcium deposition are often carried out for 21 or even 28 days.9,26,28,29 For future studies on disease associated alterations of aged hMSC we evaluated earlier time points with regard to significance of calcium deposition. If an earlier time point features a significant induction of calcium deposition one could shorten the osteogenic differentiation assay accordingly. Time series revealed that aged hMSC cultured in ODM showed a significant increase in extracellular calcium deposition as early as after 9 days (Figure 2). Jaiswal et al. showed comparable dynamics for calcium deposition of hMSC derived from young donors.1 Even though the osteogenic differentiation potential of hMSC has repeatedly been shown to decrease with donor age,20 the duration until aged hMSC feature a significant increase of calcium deposition is not prolonged.
BMP-2 is a potent osteoinductive cytokine and the number of studies analyzing its involvement in the pathophysiology of age and disease associated alterations is increasing.14,30-32 For in vitro experiments analyzing osteogenic differentiation capacities of hMSC recombinant human BMP-2 is routinely added to ODM. For osteoinductive experiments using BMP-2 a constant concentration of the cytokine over the course of the media change interval should be ensured in order to guarantee standardized experimental conditions. Unfortunately, the manufacturer does not provide information about the protein’s half-life and bioactivity in cell culture at 37°C. To this end, our ELISA analysis revealed a stable concentration of BMP-2 in the cell culture over 96 hours (Figure 3) while Degat et al. showed a sustained bioactivity in the cell culture over 6 days.33 Taken together, adding BMP-2 to the ODM seems to ensure a sufficient cytokine exposure to the hMSC, even if media changes are only carried out every 96 hours.
Interestingly, aged hMSC seem to differentially respond to short and long term stimulation with BMP-2. While the addition of BMP-2 to the ODM for the initial 4 days of osteogenic stimulation lead to significantly higher levels of extracellular calcium deposition, the continuous addition of BMP-2 to the ODM for 21 days almost abrogated the osteoinductive response (Figure 4). These observations go in line with findings by Fromigue et al.24 who also continuously stimulated aged hMSC with ODM and BMP-2 for 21 days. The authors could not detect a significant increase in collagen I synthesis at any time point. On the other hand, the continuous stimulation of hMSC derived from young donors with ODM and BMP-2 revealed a significant increase of collagen I synthesis after 21 days.9
Furthermore, in response to continuous BMP-2 stimulation young hMSC feature continuously increasing levels of alkaline phosphatase (ALP) activity with a peak level after 20 days,34 while aged hMSC respond with a peak ALP activity level already after 5 days and significantly decreasing levels thereafter.24 Taken together, the responsiveness of hMSC on BMP-2 seems to alter in an age dependent manner. Aged hMSC seem to be more prone to the timing and duration of BMP-2 stimulation. Only short term stimulation with BMP-2 leads to osteogenic differentiation of aged hMSC while prolonged stimulation induces the adverse response with inhibition of osteogenic differentiation. Further studies will have to confirm these findings and to analyse the molecular alterations underlying this observation. Nevertheless, BMP-2 seems to significantly induce osteogenic differentiation of aged hMSC as long as the stimulation protocol is adapted to the age specific requirements of these cells.
The low number of donor samples displays a limitation of the study and the validity of the presented data. MSC derived from aged humans are highly valuable samples and their distinct biological properties can reliably be analyzed in very early passages only. The major aim of the study was to assess age specific features of hMSC in order to define standard experimental set ups for further investigations on osteogenic differentiation of aged and diseased hMSC. To this end, the collected data provided a feasible foundation for the validity of subsequently conducted experiments.15
Conclusions
Taken together, cell density and the media change intervals have a substantial impact on the extent of osteogenic differentiation of aged hMSC. Using optimized conditions with a cell density of 80% and a media change interval of 96 hours, aged hMSC show a significant induction of calcium deposition as early as 9 days. Consequently, the osteogenic differentiation assay can be shortened accordingly. Whenever BMP-2 is added one must be aware that short term stimulation induces while long term stimulation inhibits osteogenic differentiation of aged hMSC. Apparently, it is of upmost importance to standardize the experimental conditions in order to enable comparability between different studies. We here define standard conditions for osteogenic differentiation in regard to the specific features of aged hMSC.
Acknowledgements
The authors would like to thank Christine Opels for her technical assistance.
Funding Statement
Funding: Wolf Christian Prall acknowledges the support of the Faculty of Medicine, LMU Munich (FöFoLe, project No. 565).
References
- 1.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295-312 [PubMed] [Google Scholar]
- 2.Astudillo P, Rios S, Pastenes L, et al. Increased adipogenesis of osteoporotic human-mesenchymal stem cells (MSCs) characterizes by impaired leptin action. J Cell Biochem. 2008;103:1054-65 [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez JP, Garat S, Gajardo H, et al. Abnormal osteogenesis in osteoporotic patients is reflected by altered mesenchymal stem cells dynamics. J Cell Biochem. 1999;75:414-23 [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez JP, Montecinos L, Rios S, et al. Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J Cell Biochem. 2000;79:557-65 [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez JP, Rios S, Fernandez M, Santibanez JF. Differential activation of ERK1,2 MAP kinase signaling pathway in mesenchymal stem cell from control and osteoporotic postmenopausal women. J Cell Biochem. 2004;92:745-54 [DOI] [PubMed] [Google Scholar]
- 6.Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950-3 [DOI] [PubMed] [Google Scholar]
- 7.Baxter MA, Wynn RF, Jowitt SN, et al. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675-82 [DOI] [PubMed] [Google Scholar]
- 8.Chen HT, Lee MJ, Chen CH, et al. Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J Cell Mol Med. 2012;16:582-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jager M, Fischer J, Dohrn W, et al. Dexamethasone modulates BMP-2 effects on mesenchymal stem cells in vitro. J Orthop Res. 2008;26:1440-8 [DOI] [PubMed] [Google Scholar]
- 10.Gerstenfeld LC, Cullinane DM, Barnes GL, et al. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873-84 [DOI] [PubMed] [Google Scholar]
- 11.Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Min Res. 2002;17:513-20 [DOI] [PubMed] [Google Scholar]
- 12.Govender S, Csimma C, Genant HK, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am 2002;84A:2123-34 [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa H, Kitoh H, Sugiura F, Ishiguro N. The effect of recombinant human bone morphogenetic protein-2 on the osteogenic potential of rat mesenchymal stem cells after several passages. Acta Orthop. 2007;78:285-92 [DOI] [PubMed] [Google Scholar]
- 14.Pountos I, Georgouli T, Henshaw K, et al. The effect of bone morphogenetic protein-2, bone morphogenetic protein-7, parathyroid hormone, and platelet-derived growth factor on the proliferation and osteogenic differentiation of mesenchymal stem cells derived from osteoporotic bone. J Orthop Trauma. 2010;24:552-6 [DOI] [PubMed] [Google Scholar]
- 15.Prall WC, Haasters F, Heggebo J, et al. Mesenchymal stem cells from osteoporotic patients feature impaired signal transduction but sustained osteoinduction in response to BMP-2 stimulation. Biochem Biophys Res Commun. 2013;440:617-22 [DOI] [PubMed] [Google Scholar]
- 16.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8: 315-7 [DOI] [PubMed] [Google Scholar]
- 17.Kohler J, Popov C, Klotz B, et al. Uncovering the cellular and molecular changes in tendon stem/progenitor cells attributed to tendon aging and degeneration. Aging Cell. 2013;12:988-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haasters F, Prall WC, Anz D, et al. Morphological and immunocytochemical characteristics indicate the yield of early progenitors and represent a quality control for human mesenchymal stem cell culturing. J Anatomy. 2009;214:759-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popov C, Radic T, Haasters F, et al. Integrins alpha2beta1 and alpha11beta1 regulate the survival of mesenchymal stem cells on collagen I. Cell Death Dis. 2011;2:e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5:91-116 [DOI] [PubMed] [Google Scholar]
- 21.Zaim M, Karaman S, Cetin G, Isik S. Donor age and long-term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann Hematol. 2012;91:1175-86 [DOI] [PubMed] [Google Scholar]
- 22.Sumanasinghe RD, Osborne JA, Loboa EG. Mesenchymal stem cell-seeded collagen matrices for bone repair: effects of cyclic tensile strain, cell density, and media conditions on matrix contraction in vitro. J Biomed Mater Res A. 2009;88:778-86 [DOI] [PubMed] [Google Scholar]
- 23.Song IH, Caplan AI, Dennis JE. Dexamethasone inhibition of confluence-induced apoptosis in human mesenchymal stem cells. J Orthop Res. 2009;27:216-21 [DOI] [PubMed] [Google Scholar]
- 24.Fromigue O, Marie PJ, Lomri A. Bone morphogenetic protein-2 and transforming growth factor-beta2 interact to modulate human bone marrow stromal cell proliferation and differentiation. J Cell Biochem. 1998;68:411-26 [PubMed] [Google Scholar]
- 25.Briolay A, Lencel P, Bessueille L, et al. Autocrine stimulation of osteoblast activity by Wnt5a in response to TNF-alpha in human mesenchymal stem cells. Biochem Biophys Res Commun. 2013;430:1072-7 [DOI] [PubMed] [Google Scholar]
- 26.Hodgkinson CP, Naidoo V, Patti KG, et al. Abi3bp is a multifunctional autocrine/paracrine factor that regulates mesenchymal stem cell biology. Stem Cells. 2013;31:1669-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li F, Whyte N, Niyibizi C. Differentiating multipotent mesenchymal stromal cells generate factors that exert paracrine activities on exogenous MSCs: implications for paracrine activities in bone regeneration. Biochem Biophys Res Commun. 2012;426:475-9 [DOI] [PubMed] [Google Scholar]
- 28.Lai CF, Cheng SL. Alphavbeta integrins play an essential role in BMP-2 induction of osteoblast differentiation. J Bone Min Res. 2005;20:330-40 [DOI] [PubMed] [Google Scholar]
- 29.Bailey Dubose K, Zayzafoon M, Murphy-Ullrich JE. Thrombospondin-1 inhibits osteogenic differentiation of human mesenchymal stem cells through latent TGF-beta activation. Biochem Biophys Res Commun. 2012;422:488-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Islam AA, Rasubala L, Yoshikawa H, et al. Healing of fractures in osteoporotic rat mandible shown by the expression of bone morphogenetic protein-2 and tumour necrosis factor-alpha. Br J Oral Maxillofac Surg. 2005;43:383-91 [DOI] [PubMed] [Google Scholar]
- 31.Styrkarsdottir U, Cazier JB, Kong A, et al. Linkage of osteoporosis to chromosome 20p12 and association to BMP2. PLoS Biol. 2003;1:E69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tranah GJ, Taylor BC, Lui LY, et al. Genetic variation in candidate osteoporosis genes, bone mineral density, and fracture risk: the study of osteoporotic fractures. Calcif Tissue Int. 2008;83:155-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Degat MC, Dubreucq G, Meunier A, et al. Enhancement of the biological activity of BMP-2 by synthetic dextran derivatives. J Biomed Mater Res A. 2009;88:174-83 [DOI] [PubMed] [Google Scholar]
- 34.Schwartz Z, Simon BJ, Duran MA, et al. Pulsed electromagnetic fields enhance BMP-2 dependent osteoblastic differentiation of human mesenchymal stem cells. J Orthop Res. 2008;26:1250-5 [DOI] [PubMed] [Google Scholar]


