Abstract
Since polyethylene is one of the most frequently used biomaterials as a liner in total hip arthroplasty, strong efforts have been made to improve design and material properties over the last 50 years. Antioxidants seems to be a promising alternative to further increase durability and reduce polyethylene wear in long term. As of yet, only in vitro results are available. While they are promising, there is yet no clinical evidence that the new material shows these advantages in vivo. To answer the question if vitamin-E enhanced ultra-high molecular weight polyethylene (UHMWPE) is able to improve long-term survivorship of cementless total hip arthroplasty we initiated a randomized long-term multicenter trial. Designed as a superiority study, the oxidation index assessed in retrieval analyses of explanted liners was chosen as primary parameter. Radiographic results (wear rate, osteolysis, radiolucency) and functional outcome (Harris Hip Scores, University of California-Los Angeles, Hip Disability and Osteoarthritis Outcome Score, Visual Analogue Scale) will serve as secondary parameters. Patients with the indication for a cementless total hip arthroplasty will be asked to participate in the study and will be randomized to either receive a standard hip replacement with a highly cross-linked UHMWPE-X liner or a highly cross-linked vitamin-E supplemented UHMWPE-XE liner. The follow-up will be 15 years, with evaluation after 5, 10 and 15 years. The controlled randomized study has been designed to determine if Vitamin-E supplemented highly cross-linked polyethylene liners are superior to standard XLPE liners in cementless total hip arthroplasty. While several studies have been started to evaluate the influence of vitamin-E, most of them evaluate wear rates and functional results. The approach used for this multicenter study, to analyze the oxidation status of retrieved implants, should make it possible to directly evaluate the ageing process and development of the implant material itself over a time period of 15 years.
Key words: total hip arthroplasty, antioxidants, PE, UHMWPE, vitamin-E, oxidation, wear, randomized trial
Introduction
The great clinical and functional benefit of total hip arthroplasty (THA) for patients undergoing total hip replacement, but also the socioeconomic significance of the restoration of the more and more young and still working patients with disabling hip diseases for health systems have been demonstrated in many studies during the last decades. It is therefore not amazing that THA was elected as operation of the 20th century.1
Relevant improvements in implant design, the progress in the biomaterials and articulating surfaces, the development of the instruments and the used surgical techniques have lead to excellent implant survivorship rates during the first 10 years after THR. However, aseptic loosening by wear debris particles still remains a major problem in long-term follow-up. The small particles generated by wear of the articulating surfaces initiate a local inflammation including release of IL-3, IL-6 and TNF-alpha, followed by an activation of the MAP kinase cascades, and the RANK/RANKL system. Beginning at the proximal part of the joint, activated osteoclasts promote osteolysis which may lead to micromotion or fracture resulting in implant failure.2
Metal-on-metal articulations demonstrated high rates of metal ion concentration, especially in large femoral head articulations and in cases of suboptimal implant positioning. Local immunoreactions have been observed [pseudotumors, adverse local tissue reaction (ALTR), aseptic lymphocytic vasculitis-associated lesion (ALVAL)],3-6 and have led to a heightened interest in alternative articulation materials like ceramics and polyethylene.
Polyethylene (PE) liners in hip arthroplasty have been introduced in the late 50ies and since that time, has been modified several times over the last decades (Figure 1).
Figure 1.
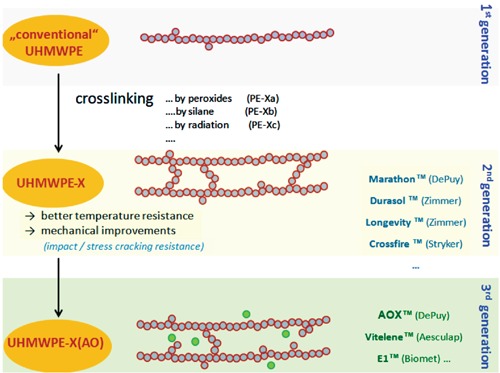
Evolution of polyeethylenes in total hip arthroplasty.
In 1958, the first polymer liner which was successfully applied in THR was polytetrafluoroethylene (PTFE, teflon), a relatively soft material which showed relevant cold flow and poor resistance to wear and delamination in the 1960s.
Based on these poor material properties resulting in high wear and fracture rates, ultra-high molecular weight polyethylene (UHMWPE) sterilized by radiation in air was introduced in 1962 (traditional UHMWPE).7,8
While the traditional UHMWPE seemed to be superior to PTFE, the problem of implant failure caused by relevant wear was still unsolved. In the 1980s, further improvements in material properties have been achieved since UHMWPE was sterilized in an inert atmosphere (conventional UHMWPE).
Introduced in the late 1980s, UHMWPE has demonstrated a superior durability in mid- and long-term. With a growing understanding of the significance of wear debris at a nano- and micrometer level as cause of aseptic loosening (particle disease), hardening of the PE was believed to increase long-term implant survivorship.
In the 1990s, the first attempt to solve this problem by developing an enhanced UHMWPE (Hylamer®) failed. Produced with high pressure and temperature it was thought that changing the crystalline structure to extended chains might enhance the physical properties of UHMWPE, including an increased resistance to abrasion. However, based on extensive wear and associated peri-implant osteolysis, Hylamer® had to be recalled.9,10
The second attempt to make the material more resistant to wear resulted in additional cross-linking of UHMWPE (UHMPWE-X) by gamma irradiation or electrobeam in the early 2000.11 The positive or negative effects of UHMWPE-X versus conventional UHMWPE are still controversially discussed in the scientific community and strongly dependent on the manufacturing process.
Some authors report an increased wear during the first 2 years (running-in period), but afterwards the data correspond to in vitro data (simulator).12 Also the risk of fatigue cracks seems to be little higher in some types of UHMWPE-X compared to conventional UHMWPE. However, there is no evidence that smaller particles released from UHMWPE-X increase the risk of osteolysis. Therefore, besides UHMWPE-X conventional UHMWPE still is used in THA to date.
Although different optimization steps in polyethylene development have lead to excellent implant survivorship within the first 10-15 years, the problem of material ageing caused by progressive oxidation was still unsolved as has been shown by analysis of explanted liners.13-15 The clinical consequence of the oxidation is an increased wear rate starting approximately 10 years postoperative. Encouraged by promising in vitro results, today several acetabular inserts are supplemented with antioxidants.16-21 Different technical approaches have been realized for the manufacturing process (coating, diffusion, covalent binding, embedding/blending).22 Beside other substances, Vitamin-E (alpha-tocopherol) seems to be a promising candidate,23,24 as it is inert and has shown no relevant side effects in low concentrations in humans.
The oxidation is a relevant parameter concerning the long-term survival of the PE-material. Evaluation of standard PE-liners have shown that oxidative processes changes the material. While the PE evolves and gets more refractory, at first the wear rates improve in comparison to Vitamin-E stabilized UHMWPE-XE. In the following however, the growing brittleness leads to a higher wear and consequently higher implant failure rates.
It is yet unclear if antioxidants improve UHMWPE durability in vivo by reducing oxidation and show less abrasion in the long-term. Also the question of increased wear of UHMWPE-XE during the first years after implantation by modifications of the polymer texture arises.25 To date there are no clinical data available comparing UHMWPE-X with UHMWPE-XE in total hip arthroplasty liners. An in vivo evaluation of the oxidation of the PE material is not possible. Therefore, in this study, the oxidation index of PE-liners with and without Vitamin-E of revised cases shall be assessed, accompanied by a comparison of long-term clinical and functional results.
Materials and Methods
Patients
Patients, men and women, with the indication for a cementless total hip arthroplasty, aged 18 to 75 years are asked to participate in the study. Exclusion criteria are a heightened narcosis risk (ASA IV), tumors, drug or alcohol addiction, permanent cortisone therapy, clinically relevant infections, pregnancy or planned pregnancy, fractures, previous surgeries at the concerned hip (osteotomy, osteosynthesis, THA), a bone quality indicating a cemented implantation, relevant deformities (status after previous treatment like leg length differences >30 mm, offset reductions >30 mm), indications for non-ceramic heads or small cups needing 28 mm heads, treatments where a neck-extension is indicated. According to the Declaration of Helsinki informed consent of every patient is obtained prior to surgery in a written form.
The patients are recruited in all 6 participating hospitals. No definitions regarding a limitation of surgeons treating the study patients have been set up. The surgeon is informed by the study coordinator of his center on the randomization result, i.e. if standard highly crosslinked UHMWPE-X is used or Vitamin-E supported UHMWPE-XE (Figure 2). The study was approved by the ethics committee of the (#11-4845-BO) and is conducted according to the common guidelines for clinical trials (Declaration of Helsinki). The trial registration number is NCT01713062. Informed consent is obtained for all patients. The trial is in the recruitment phase.
Figure 2.

Different colors with UHMWPE-X and UHMWPE-XE (Vitelene™).
Study design
In 2011 we initiated a prospective and randomized multicenter study evaluating a CE-marked vitamin-E supplemented UHMWPE-XE (Vitelene®) versus UHMWPE-X (Figure 3), the Longterm-Evaluation of Vitelene® Against Standard (VITAS®, sponsor: B.Braun-Aesculap AG, Tuttlingen, Germany). Six study centers are participating, recruiting 400 patients within a planned recruiting till end of 2014. The randomization has been calculated as block randomization with random block length, stratified by center (SAS 9.2). The random numbers are accessed by the study centers via a SSL- secured online database. The study has a planned follow-up period of 15 years, aiming for long-term results.
Figure 3.
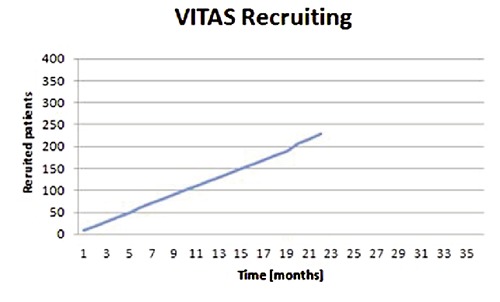
Patient recruitment.
Intervention
Patients treated with a cementless THA are randomized to receive either a Vitelene® liner or a standard PE liner. Plasmacup DC® (Aesculap, Tuttlingen, Germany), a cementless hemispheric cup with a microporous titanium coating (Plasmapore®) is used. Which cementless stem is used, if standard or short stem, the surgical technique and approach are left open to the choice of the surgeon. To eliminate the influence of different head materials or head sizes, Biolox® delta heads (Ceramtec, Plochingen, Germany) with 32 or 36 mm have been determined to be used for study patients. The intraoperative and postoperative medication and treatment of the study patients has not been defined and will correspond to the routine of the study centers.
Follow-up and outcome parameters
VITAS has been designed as prospective, randomized long-term evaluation of Vitamin-E supplemented highly cross-linked UHMWPE liners in comparison to standard highly cross-linked UHMWPE liners. The study has been set up for 15 years, with follow-ups after 1, 5, 10 and 15 years. Since projects with such long-term evaluation tend to have difficulties with high rates of loss of follow-up, it is planned to contact the study participants in the interim time by writing to stay informed on their status of health and to not loose the relationship.
The aim of the study is to evaluate the superiority of UHMWPE-XE in comparison to UHMWPE-X in regards to oxidation. To determine the efficiency of Vitamin-E as an antioxidant, the oxidation index (OI) in retrieved liners of revised patients has been set as primary parameter. To this end, all liners harvested during revision surgeries will be washed in sterile saline water, stored in special set up transport boxes and sent to the sponsor for the laboratory evaluation (oxidation index). The oxidation index is assessed with the Fourier-Transform-Infrared-Spectroscopy (FTIR – ISO 5834.1/.2 – Implants for surgery - Ultra-high molecular weight polyethylene), where the absorption of the infrared wavelength is measured.
In addition, clinical and radiological results will be obtained as secondary parameters. Linear wear (≥0.5 mm) will be evaluated on routinely taken conventional radiographs after 5, 10 and 15 years with the Martell Hip Analysis Suite®,12,26,27 that is able to detect cumulated linear wear rates of 0.3 mm. A recognized critical value for wear in regard to consequent osteolysis is 0.1 mm per year.28
Evaluation of standard radiographs for manifest osteolysis will be done additionally to the measurement of the cumulated wear rates. X-rays will be analyzed for osteolytic changes according to Gruen et al.29 Other radiological parameters like migration, implant position and ossifications according to Brooker et al.30 will be assessed and documented.
Functional outcome, the well-being of the patient in daily-life activities is assessed with the Harris Hip Scores (HHS) and with the Hip Disability and Osteoarthritis Outcome Score (HOOS).31,32 The level of activity is measured with the UCLA Score (University of California, Los Angeles).33,34 Table 1 summarizes different parameters.
Table 1.
Parameters and methods.
| Oxidation-index | Retrieval analysis |
|---|---|
| Implant survivorship | Amount of aseptic loosenings and revisions |
| Radiological results | Osteolysis; wear rates |
| Functional results | Harris Hip Score; Hip Disability and Osteoarthritis Score; Universisty of California-Los Angeles Score |
| Pain level | Visual Analogue Scale |
| Intra- and post-operative complications | Interview, medical reports and records |
| Surgical technique and implant components | Medical reports and records |
Statistical calculations and biometry
We expect a significant lower oxidation index for retrieved UHMWPE-XE liners in contrast to UHMWPE-X liners. Under the assumption of comparable revision rates in both groups, the two-sample t-test for mean difference with unequal variances gives a case number of 15 explants per group. Under consideration of the multicenter situation and the 15 years follow-up, a drop-out rate of 25% is assumed, leading to 20 explants per group. With a revision rate of 10% in 15 years, 400 patients will be needed to get 40 revision cases. Evaluation of endpoints is planned as per-protocol analysis. Statistical tests and presentation will be appropriate to the category and distribution of the respective variables. The test level for statistical significance is defined as P=0.05, two sided, for all tests.
Discussion
In vivo evaluation of implanted liners in THA is not possible without an invasive procedure. Therefore, the laboratory evaluation of explanted liners in revision cases has been chosen as primary criteria for the assessment of the influence of Vitamin-E on the oxidation process of the PE-material.
While a controlled setting is relevant to eliminate different influences on the outcome of the surgery i.e. the endpoints of the study, too strictly defined conditions generate an unrealistic situation. In this study, the high number of patients and participating clinics and surgeons and a recruiting time of almost 3 years should allow for a wide range of proceedings. Surgical technique, postoperative rehabilitation programs, medication etc. is not defined and will be done according to the regular standard in each hospital. Since head size and material are influencing the wear rate, size and material of the used head are the only specifications.
The prospective design and the randomization of the two study groups therefore should allow a scientific conclusion on the influence of the antioxidant in every day conditions.
Conclusions
The analysis of the retrieved implants should grant distinct results for the evaluation of highly cross-linked UHMWPE-X in comparison to Vitamin-E stabilized highly cross-linked UHMWPE-XE. Additional evaluation of radiographic wear, osteolysis and migration as well as the functional results of the every-day life of the patient will allow a long-term evaluation of the two biomaterials, one state of the art today, the other an option with promising, results.
Appendix: the VITAS Group
The VITAS™ study is financially supported by the sponsor (B.Braun-Aesculap AG, Tuttlingen, Germany). It is composed by: Christoph von Schulze Pellengahr (Director); Georgious Spyrou (Orthopaedic and Trauma Department, St. Josef Hospital, University of Bochum, GERMANY); Karl-Stefan Delank (Director); Sven Freche (Orthopaedic Department, University of Halle, Germany); Henning Windhagen (Director); Gabriele v. Lewinski, Thilo Flörkemeier, Stefan Budde (Orthopaedic Department, Hannover Medical Center, Germany); Axel Wilke (Orthopaedistin-Chief), Felix Hütter (Orthopaedic and Trauma Department, Elisabeth Hospital, Olsberg, Germany); Joern Michael (Orthopaedist-in-Chief); Dominik Mehlen (Orthoapedic and Trauma Department, St. Josef Hospital, Bendorf, Germany).
Funding Statement
Funding: the study is financially supported by B.Braun-Aesculap AG, Germany; Trial registration: NCT01713062.
References
- 1.Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370:1508-19 [DOI] [PubMed] [Google Scholar]
- 2.Purdue PE, Koulouvaris P, Nestor BJ, Sculco TP. The central role of wear debris in periprosthetic osteolysis. Hss J. 2006;2:102-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurtz SM, Gawel HA, Patel JD. History and systematic review of wear and osteolysis outcomes for first-generation highly crosslinked polyethylene. Clin Orthop Relat Res. 2012;469:2262-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tailor H, Patel S, Patel RV, Haddad FS. Bearing couples in total hip arthroplasty. Br J Hosp Med (Lond). 2010;71:446-50 [DOI] [PubMed] [Google Scholar]
- 5.van der Veen HC, van den Akker-Scheek I, Bulstra SK, van Raay JJ. Wear, bone density, functional outcome and survival in vitamin E-incorporated polyethylene cups in reversed hybrid total hip arthroplasty: design of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiley KF, Ding K, Stoner JA, et al. Incidence of pseudotumor and acute lymphocytic vasculitis associated lesion (ALVAL) reactions in metal-on-metal hip articulations: a metaanalysis. J Arthroplasty. 2013;28:1238-45 [DOI] [PubMed] [Google Scholar]
- 7.Charnley J. Using Teflon in arthroplasty of the hip-joint. J Bone Joint Surg Am. 1966;48:819. [PubMed] [Google Scholar]
- 8.Good VD, Clarke IC, Gustafson GA, et al. Wear of ultra-high molecular weight polyethylene and polytetrafluoroethylene in a hip simulator: a dose-response study of protein concentration. Acta Orthop Scand. 2000;71:365-9 [DOI] [PubMed] [Google Scholar]
- 9.Cohen J. Catastrophic failure of the Elite Plus total hip replacement, with a Hylamer acetabulum and Zirconia ceramic femoral head. J Bone Joint Surg Br. 2004;86:148. [PubMed] [Google Scholar]
- 10.Visentin M, Stea S, De Clerico M, et al. Determination of crystallinity and crystal structure of Hylamer polyethylene after in vivo wear. J Biomater Appl. 2006;21:131-45 [DOI] [PubMed] [Google Scholar]
- 11.Puppulin L, Sugano N, Zhu W, Pezzotti G. Structural modifications induced by compressive plastic deformation in single-step and sequentially irradiated UHMWPE for hip joint components. J Mech Behav Biomed Mater. 2014;31:86-99 [DOI] [PubMed] [Google Scholar]
- 12.Jäger M, Behringer M, Zilkens C, et al. Initial increased wear debris of XLPE-Al(2)O(3) bearing in total hip arthroplasties. Arch Orthop Trauma Surg. 2010;130:1481-6 [DOI] [PubMed] [Google Scholar]
- 13.Wannomae KK, Christensen SD, Freiberg AA, et al. The effect of real-time aging on the oxidation and wear of highly crosslinked UHMWPE acetabular liners. Biomaterials. 2006;27:1980-7 [DOI] [PubMed] [Google Scholar]
- 14.MacDonald D, Sakona A, Ianuzzi A, et al. Do first-generation highly crosslinked polyethylenes oxidize in vivo? Clin Orthop Relat Res. 2011;469:2278-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Currier BH, Van Citters DW, Currier JH, Collier JP. In vivo oxidation in remelted highly cross-linked retrievals. J Bone Joint Surg Am. 2010;92:2409-18 [DOI] [PubMed] [Google Scholar]
- 16.Jarrett BT, Cofske J, Rosenberg AE, et al. In vivo biological response to vitamin E and vitamin-E-doped polyethylene. J Bone Joint Surg Am. 2010;92:2672-81 [DOI] [PubMed] [Google Scholar]
- 17.Oral E, Muratoglu OK. Vitamin E diffused, highly crosslinked UHMWPE: a review. Int Orthop. 2011;35:215-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oral E, Wannomae KK, Rowell SL, Muratoglu OK. Migration stability of alphatocopherol in irradiated UHMWPE. Biomaterials. 2006;27:2434-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner A, Okubo Y, Teramura S, et al. The antioxidant and non-antioxidant contributions of vitamin E in vitamin E blended ultra-high molecular weight polyethylene for total knee replacement. J Mech Behav Biomed Mater. 2014;31:21-30 [DOI] [PubMed] [Google Scholar]
- 20.Wolf C, Krivec T, Blassnig J, et al. Examination of the suitability of alpha-tocopherol as a stabilizer for ultra-high molecular weight polyethylene used for articulating surfaces in joint endoprostheses. J Mater Sci Mater Med. 2002;13:185-9 [DOI] [PubMed] [Google Scholar]
- 21.Wolf C, Macho C, Lederer K. Accelerated ageing experiments with crosslinked and conventional ultra-high molecular weight polyethylene (UHMW-PE) stabilised with alpha-tocopherol for total joint arthroplasty. J Mater Sci Mater Med. 2006;17:1333-40 [DOI] [PubMed] [Google Scholar]
- 22.Oral E, Neils AL, Rowell SL, et al. Increasing irradiation temperature maximizes vitamin E grafting and wear resistance of ultrahigh molecular weight polyethylene. J Biomed Mater Res B Appl Biomater. 2013;101:436-40 [DOI] [PubMed] [Google Scholar]
- 23.Oral E, Ghali BW, Rowell SL, et al. A surface crosslinked UHMWPE stabilized by vitamin E with low wear and high fatigue strength. Biomaterials. 2010;31:7051-60 [DOI] [PubMed] [Google Scholar]
- 24.Kurtz SM, Dumbleton J, Siskey RS, et al. Trace concentrations of vitamin E protect radiation crosslinked UHMWPE from oxidative degradation. J Biomed Mater Res A. 2009;90:549-63 [DOI] [PubMed] [Google Scholar]
- 25.Affatato S, Bracco P, Costa L, et al. In vitro wear performance of standard, crosslinked, and vitamin-E-blended UHMWPE. J Biomed Mater Res A. 2012;100:554-60 [DOI] [PubMed] [Google Scholar]
- 26.Matthew J, Kraay MJ, Moore RD, et al. Reassessment of computerized wear measurement for total hip arthroplasty with correction for projectional image distortion: a brief follow-up report. J Bone Joint Surg Am. 2010;92:1858-67 [DOI] [PubMed] [Google Scholar]
- 27.Martell JM, Berkson E, Berger R, Jacobs J. Comparison of two and three-dimensional computerized polyethylene wear analysis after total hip arthroplasty. J Bone Joint Surg Am 2003;85A:1111-7 [DOI] [PubMed] [Google Scholar]
- 28.Dowd JE, Sychterz CJ, Young AM, Engh CA. Characterization of long-term femoral-head-penetration rates. Association with and prediction of osteolysis. J Bone Joint Surg Am 2000;82A:1102-7 [DOI] [PubMed] [Google Scholar]
- 29.Gruen TA, McNeice GM, Amstutz HC. Modes of failure of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res 1979:17-27 [PubMed] [Google Scholar]
- 30.Brooker AF, Bowerman JW, Robinson RA, Riley LH., Jr Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Joint Surg Am. 1973;55:1629-32 [PubMed] [Google Scholar]
- 31.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737-55 [PubMed] [Google Scholar]
- 32.Nilsdotter AK, Lohmander LS, Klassbo M, Roos EM. Hip disability and osteoarthritis outcome score (HOOS)--validity and responsiveness in total hip replacement. BMC Musculoskelet Disord. 2003;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahman WA, Garbuz DS, Masri BA. Total hip arhtroplasty in steroid-induced osteonecrosis: early functional and radiological outcomes. Can J Surg. 2013;56:41-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amstutz HC, Thomas BJ, Jinnah R, et al. Treatment of primary osteoarthritis of the hip. A comparison of total joint and surface replacement arthroplasty. J Bone Joint Surg Am. 1984;66:228-41 [PubMed] [Google Scholar]


