Abstract
Microfluidics, the technology that manipulates small amount of fluids in microscale complex devices, has undergone a remarkable development during the last decade, by targeting a significant range of applications, including biological tests and single-cell analysis, and by displaying many advantages such as reduced reagent consumption, decreased costs and faster analysis. Furthermore, the introduction of microfluidic tools has revolutionized the study of vascular functions, because the controlled three-dimensional environment and the continuous perfusion provided by the microdevice allow simulating the physiological characteristics of the circulatory system. Researchers interested in the study of vascular physiology, however, are often hampered by the difficulty in handling reduced number of cells after growth in these devices. This work shows how to apply different protocols commonly used in biology, such as the immunofluorescence technique, to cells grown in reversibly-bound microfluidic devices, obtaining results comparable to those retrieved under static conditions in multiwells. In this way, we are able to combine the advantages of microfluidic, i.e., application of continuous flow and shear stress, with classical protocols for the study of endothelial cells.
Keywords: microfluidic device, PDMS-peeled-off, endothelial cells, immunostaining
Introduction
The microvasculature mediates the interaction between blood and tissues, and defines the biological and physical characteristics of the microenvironment within tissues.1 In the last years, particular attention has been focused on understanding how the vascular system works, both in healthy and diseased states.2 Areas of particular interest include the investigation of endothelial cells lining the blood vessels to understand, for example, the angiogenesis process,3 the response of vascular tone to shear stress,4 the adhesion and transmigration of leukocytes during inflammation,5 and the regulation of vascular permeability.6 The large majority of in vitro studies are conventionally run by growing cells in multiwells or Petri dishes, whereby cells are just immersed in the growth medium, and only diffusion processes are present, in what from now on we define as standard static culture conditions. However, this static experimental setup does not mimic the complexity of the circulatory system and the physiological situation. A more complete model, to simulate the characteristics of the vascular system, should contemplate a directional continuous flow and the ensuing wall shear stress on the endothelial cell layer. To this end, microfluidic systems have gained popularity in the fields of cell biology and cell-based assay,7 because they can offer a larger likeness to the in vivo environment and physiological conditions, and provide continuous nutrition supply for cells.8
The large majority of microfluidic devices (MFDs) are built by engraving the circuit in the silicon elastomer PDMS (polydimethyilsiloxane) and sealing it to a glass surface, on which cells are grown. In addition to being cheap and biocompatible, PDMS has also optical transparency and high gas permeability, ensuring an oxygen supply in the closed circuit, and keeping a favorable environment to maintain cells alive and healthy for several days.9 PDMS MFDs have been successfully used in cell biology to investigate cells growth or interactions.10 In addition to the reproduction of some features of the real in vivo situation, miniaturization affords: low fabrication costs, reduced analysis time, and small amount of reagents. Nevertheless, there are difficulties to transfer well established techniques of cellular and molecular biology to cell cultures grown in MFDs. Given the small size of the device and the small number of cells, it is necessary to adapt existing protocols to the MFDs. In some cases it may be desirable to reversibly seal the PDMS from the glass surface, in order to detach the elastomer when needed, and use the glass surface as a coverslip, making the device easy-to-handle, and providing some advantages such as an easy storage of the sample, a lower consumption of reagents, and the easy realization of cellular and molecular analysis, such as for example cell viability assays (e.g., Live/Dead Fluorescence Assay), TEM (Transmission Electron Microscopy); analysis and immunofluorescence assays.
The classical procedure of MFD fabrication involves exposition of PDMS and glass to atomic oxygen and ozone atmosphere: PDMS includes repeating units of –O-Si(CH3)2 and the oxidizing treatment generates silanol groups (Si-OH) by the oxidation of methyl groups (Si-CH3). The presence of deprotonated silanols on the surfaces of the two materials makes them more reactive and prone to the condensation process, when set in contact.11 This irreversible type of bond has the advantage of withstanding high pressures (30-50 psi), but it usually makes difficult the removal of the PDMS from the glass slide without breaking the device. For this reason, methods have been developed to assembly MFDs with a reversible seal. One technique has been recently published by McDonald and Whitesides:12 the reversible seal is provided by simple van der Waals contact, it is watertight but cannot withstand pressures greater than 5 psi. Another simple method is described in many works, for example by Vitzthum et al.,13 in which PDMS microdevices are placed on poly-D-lysine coated glass coverslips with non-plasma and reversible bonding. This inexpensive method, however, presents some disadvantages: it involves a long-lasting protocol of fabrication, the devices need to be necessarily sterilized prior to use, and the liquids enter the device in a significantly longer time, because the two surfaces are hydrophobic.14
In this work, we fabricated PDMS MFDs characterized by a reversible seal with the glass surface, simply by modulating the time of exposition to atomic oxygen and ozone atmosphere: in this way we created a tight MFD capable to withstand high pressure flows, but at the same time having the advantage of a reversible seal between the two surfaces. After removal of PDMS we analyzed the cells grown on the glass slide by an immunofluorescence assay, following classical procedures. The procedure followed to open the MFD and prepare the cell sample can be used with many other protocols commonly used in cellular and molecular analysis.
Materials and Methods
Fabrication of a microfluidic device for cell culture and manipulation
The microfluidic device (MFD) was prepared by replica molding in PDMS (polydimethyilsiloxane, Sylgard 184, Dow Corning) of a SU8 master, obtained through a mask polymerization process on a layer of photosensitive resin SU8 deposited on a silicon substrate using a UV lamp (UV400, Reinraumtechnik lanz). The internal dimensions of the device, suitable for cell culture, were 4 cm length, 2 mm width and 150 µm height. They were determined through the 3D reconstruction of confocal z-stack images of the channel filled with a fluorescein solution.15
Briefly, the PDMS pre-polymer was mixed with the curing agent in a 10:1 mass ratio, degassed and poured over the master on a hot plate at 150°C for 30 min, to allow the thermal polymerization. Then, the PDMS layer was peeled from the master with tweezers, and holes were punched to provide the channel inlet and outlet using a blunt needle. The PDMS replica and a coverglass were exposed to plasma atmosphere (atomic oxygen and ozone atmosphere, UV400, Jelight), and then brought into contact to permit sticking. Various exposition times were tested: i) 2 and 20 min; ii) 45 sec and 10 min; iii) 30 sec and 10 min, for PDMS and glass, respectively. Two LDPE (low-density polyethylene) tubes were inserted into the device, one in the inlet connected to a syringe pump, and one in the outlet, for waste discharge.
Cell culture in a MFD under flow conditions and in multiwells in static conditions
Human umbilical vein endothelial cells (HUVEC) were obtained from samples of the umbilical cord of consensual patients with procedures approved by the local ethic committee, isolated, and immunoseparated with Tosyl activated magnetic Dynabeads M-450 (Oxoid, Rodano, MI, Italy) coated with anti-human CD31 antibodies, following procedures set up by our group and previously described.16 Cells were maintained in endothelial basal medium (EBM-International PBI), supplemented with 2% FCS (fetal calf serum) and antibiotics (100 units/mL streptomycin and 100 units/mL penicillinG), and kept at 37°C in a humidified atmosphere containing 5% CO2.
Briefly, in static conditions, cells were plated (200 cells/mm2 in 24-multiwells fibronectin pre-coated containing a glass coverslip) and allowed to attach for 24 h.
For flow conditions, instead, the sterilized MFD – fabricated with exposition time iii), see before – was initially rinsed with PBS, and coated with fibronectin (1 µg/cm2, 30 min at 37°C). A HUVEC cell suspension (at a concentration in the range 200-300 cell/mm2, about 1.5-2×106 cell/mL) was injected through the inlet of the device, and allowed to attach at 37°C for 2 h. After this time, a syringe pump (World Precision Instruments, Inc., Sarasota, FL, USA) holding a sterile syringe connected to the device by the inlet tube, injected continuously culture medium for 22 h over the cells: 20 h at 2 µL min–1 and then 2 more h at 30 µL min–1 (Figure 1). The entire setup was placed in an incubator at 37°C and 5% CO2.
Figure 1.
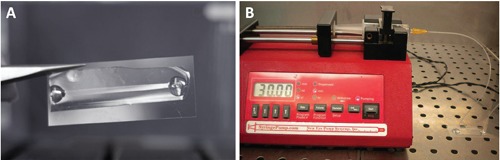
A) PDMS MFD for HUVEC cells culture under flow conditions. B) MFD connected to a syringe pump.
Cell viability under flow conditions
Live/Dead cell staining kit (Sigma-Aldrich, St. Louis, MO, USA) was chosen to assess cell viability, by calcein-AM and propidium iodide (PI) fluorescence, which stain viable and dead cells, respectively. Briefly, stock solutions of calcein-AM and ethidium homodimer-1 were diluted in PBS to a final concentration of 10 µM and 5 µM, respectively. Cells seeded on a glass coverslip or in a MFD were firstly washed twice in PBS, further incubated with Live/Dead staining solution for 20 min at 37°C, and finally washed twice in PBS. The stained cells were then analyzed by fluorescence microscopy (Leica Microsystems, Wetzlar, Germany): the calcein generated from calcein-AM by esterase in viable cells emits green fluorescence (exc. 490 nm; em. 515 nm), whereas PI, intercalated with DNA by passing through disordered areas of dead cell membrane, emits red fluorescence (exc. 535 nm; em. 617 nm).
Immunofluorescence of α/β tubulin in HUVEC cells grown in flow and static conditions
Cells were washed three times in PBS, fixed with 4% formaldehyde in PBS for 15 min at room temperature, and then washed three times in PBS for 5 min. For cells grown in the MFD, the syringe pump was stopped and solutions were injected in the microchannel with a micropipette. The PDMS layer was carefully peeled from the coverglass with tweezers, as shown in Figure 2 A,B, and the cells area was outlined with a liquid repellent slide marker pen (Liquid blocker Pap pen, Cosmo Bio Co., Tokyo, Japan), as shown in Figure 2 C,D. A small volume (about 100 µL) of all the solutions mentioned below was carefully placed over the cells using a micropipette, covering the whole area of the channel, and vacuum was applied afterword to aspirate the liquid.
Figure 2.
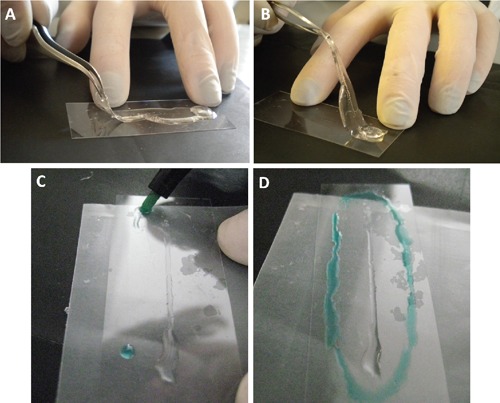
A-B) PDMS peeled from the glass. C-D) The area with cells attached to the glass is outlined with an hydrophobic barrier pen (in green) to allow the immunostaining.
Briefly, specimens were blocked in Blocking Buffer (1X PBS, 0.2% BSA, 1:100 normal goat serum, 0.3% Triton X-100) for 60 min, and incubated overnight at 4°C with primary antibody rabbit monoclonal anti α/β-tubulin (Cat. No. 2148S, Cell Signaling Technology, Danvers, MA, USA) diluted 1:50 dilution in Antibody Dilution Buffer (1X PBS, 1% BSA, 0.3% Triton X-100). Incubation in primary antibody was omitted in control samples. After 1 h at room temperature, samples were rinsed three times in PBS for 5 min each, and incubated, for 1 h at room temperature in dark, in fluorochrome-conjugated secondary antibody (DyLight 488 Affini Pure F(ab’)2 Fragment donkey anti-rabbit, Cat. No. 711-486-152, Jackson Immuno Research, Li StarFish Srl, Cernusco S/N, MI, Italy), diluted 1:800 in Antibody Dilution Buffer. Samples were then washed three times in PBS (for 5 min), once in distilled water and afterword in methanol solution (Sigma-Aldrich), and finally incubated at 37°C for 15 min with 4’,6-diamidino-2-phenylindole (DAPI) solution (Cell Signaling Technology) (1 µg/mL in Methanol). After one rinse in methanol, we let dry the glass and placed it inverted on a glass slide with a drop of Fluorescent Mounting Medium (Cat. No. S3023, DakoCytomation SpA, Milan, Italy), just before the analysis by fluorescence microscopy (DAPI: exc. BP 340-380 nm, em. LP 425 nm; DyLight 488: exc. BP 450-490 nm, em. 515 nm).
Results
Simple MFD in PDMS with a single linear channel (length 4 cm, width 2 mm and height 150 µm) was used to study endothelial cells under flow conditions. The structure of the final microdevice is depicted in Figure 1A.
We tested various exposure times of PDMS and glass to atomic oxygen and ozone atmosphere: i) 2 and 20 min; ii) 45 sec and 10 min; iii) 30 sec and 10 min, for PDMS and glass, respectively. The first two options generated an irreversible seal between the two substrates, whereas the third one resulted in a reversible bond, while ensuring the tightness of circuit even after 48h at 37°C with high applied flow-rates (up to mL min–1). A reproducible protocol was developed to inject, seed, and grow HUVEC cells inside them. The cells were attached to the glass wall of the MFD after the first hour and they were evenly distributed in the area of the microfluidics compartment after 24 h, with a maximum tolerated flow-rate of 30 µL min–1 (Figure 3 C,D,E). The wall shear-stress in the device can be roughly estimated using the relation reported by Son:17 for a rectangular channel with width W infinitely larger than the height H and for a Newtonian fluid, the wall shear-stress can be estimated as equal to: SS = 6ηQ/WH2 ≈ 6ηv/H is the applied flow-rate, η the fluid viscosity and v the maximum fluid velocity in the center of the channel (measured by Fluorescence Correlation Spectroscopy technique). In a MFD with the dimensions reported above and with an applied flow-rate of 30 µL min–1, v corresponds to a maximum velocity in the channel of about 1.5 mm s–1, and SS is approximately 0.06 Pa, similar to physiological conditions.18,19
Figure 3.
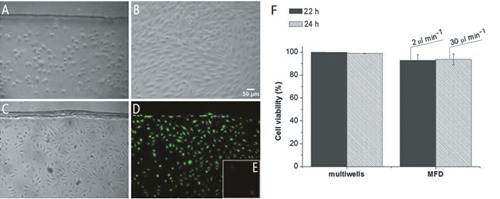
HUVEC in the channel of a MFD. A) 2 h, flow absent in order to promote cellular adhesion. B) 20 h with 2 µL min–1 medium flow rate; cells subjected to a flow rate of 30 µL min–1 for 2 h (C) are analyzed by Live/Dead assay. D) Green fluorescence of calcein in viable cells. E) Red fluorescence of PI in dead cells; images recorded with a DM-IRE2 microscope (phase contrast images) and a fluorescence microscope (Leica Microsystems). F) Percentage of live cells in multiwells or in MFD after 22 h (2 h flow absent + 20 h 2 µL min–1) and 24h (2 h flow absent + 20 h 2 µL min–1 + 2 h 30 µL min–1). The data represent mean±SD (2≤n≤4).
We demonstrated, as shown in Figure 3F, that HUVEC cells remained alive in MFD, also after 24 h (2 h flow absent + 20 h at 2 µL min–1 flow-rate + 2 h at 30 µL min–1 flow-rate): the flow condition did not cause a significant decrease of cell viability when compared with the standard static cultures.
A standard immunofluorescence protocol was applied to HUVEC cells grown under usual static conditions in multiwells as well as in a reversibly-bound MFD, and then the results were compared, to verify if protocols commonly used in biology can be easily exported to microfluidics. The PDMS layer was easily peeled from the coverglass with tweezers, and the cells area was outlined as shown in Figure 2. The results (Figure 4) showed that the immunofluorescence of α/β tubulin was carried out successfully in the MFD following the method described above: cell images displayed intact tubulin filament and cytoskeleton orientation with respect to standard static sample.
Figure 4.
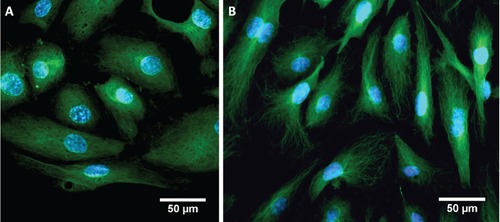
Immunofluorescence of HUVEC cells: nuclei are stained in blue, using DAPI fluorescent dye; α/β tubulin is in green, in flow conditions in MFD with flow rate of 2 µL min–1 for the first 20 h and then 30 µL min–1 for 2 h (A) and in static conditions in multiwells (B).
Discussion
Microfluidics has achieved a great development during the last decade, with a significant range of applications, and with new revolutionary tools for the study of vascular functions. Indeed, these dynamic experiments in a MFD add a physical parameter i.e. flow, present in in vivo physiological conditions of the circulatory system, to in vitro studies. MFDs then offer the possibility to observe the influence of flow and the ensuing wall shear stress on cells, as the endothelial ones, that are normally in presence to these phenomena because of the blood flux. Inside a microfluidic device HUVEC cells can be subjected to different flow-rates of culture medium by means of a syringe pump to trigger a variable flow-induced shear stress. We demonstrated that the reversibly-bound MFD described in this work tolerate high flow-rates (up to mL min–1), but the maximum flow-rate at which HUVEC cells remain attached to the MFD glass wall and alive in this device is 30 µL min–1. Under these conditions, the viability is not significantly different with respect to multiwells experiments. With 50 µL min–1 flowrate, instead, HUVEC cells detach from the glass and flow out from the device (data not shown). With the technique described in this work we easily fabricated an MFD in PDMS and glass with a reversible seal and with dimensions suitable for cell culture. Following the procedure outlined in the paper we were able to grow cells on the glass support and to remove and eliminate the PDMS layer when needed, with no waste of time and money. We therefore demonstrated how protocols commonly used in biology and cell analysis are then easily transferred to cell samples grown in MFDs, preserving at the same time the advantages of this new technology, such as reduced reagent consumption and decreased costs. In this work, as an example, an immunofluorescence test was performed on the glass of the MFD after delimitation of the cells area with a liquid repellent slide marker pen, which permitted to use low volume of reagents (about 100 µL, versus 200 µL in the well) and to perform an efficient incubation with antibodies that would be difficult to accomplish inside the closed microfluidic device. The cell images demonstrated that our system properly operates and revealed no substantial differences between flow and static conditions, as expected for the low flow-induced shear stress imposed by the syringe pump settings.
Acknowledgments
The authors gratefully acknowledge financial support from PRIN2011 n. 2010C4R8M8, FIRB RBPR05JH2P and FIRB RBAP11X42L (MIUR). C. Fede acknowledges the support of an FSE Scholarship n. 2105/101/4/1103/2010.
References
- 1.Zheng Y, Chen J, Cravenc M, Choi NW, Totorica S, Diaz-Santana A, et al. , In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci USA 2012;109:9342-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong KHK, Chan JM, Kamm RD, Tien J. Microfluidic models of vascular functions. Annu Rev Biomed Eng 2012;14:205-30 [DOI] [PubMed] [Google Scholar]
- 3.XiaoZhen D, Shao C, QunFang Y, JiaHuan J, XiaoQing Y, Xin X, et al. , A novel in vitro angiogenesis model based on a microfluidic device. Chin Sci Bull 2011;56:3301-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, et al. , Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci 2004;101:14871-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alcaide P, Auerbach S, Luscinskas FW. Neutrophil recruitment under shear flow: it’s all about endothelial cell rings and gaps. Microcirculation 2009;16:43-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curry FR, Adamson RH. Vascular permeability modulation at the cell, microvessel, or whole organ level: towards closing gaps in our knowledge. Cardiovasc Res 2010;87:218-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeon JH, Park JK. Microfluidic cell culture systems for cellular analysis. Biochip J 2007;1:17-27 [Google Scholar]
- 8.Whitesides GM. The origins and the future of microfluidics. Nature 2006;442:368-73 [DOI] [PubMed] [Google Scholar]
- 9.Leclerc E, Sakai Y, Fujii T. Cell culture in 3-dimensional microfluidic structure of PDMS (polydimethylsiloxane). Biomed Microdev 2003;5:109-14 [Google Scholar]
- 10.Sia SK, Whitesides GM. Microfluidic devices fabricated in poly(dimethylsiloxane) for biological studies. Electrophoresis 2003;24:3563-76 [DOI] [PubMed] [Google Scholar]
- 11.Mc Donald JC, Duffy DC, Anderson JR, Chiu DT, Wu H, Schueller OJA, et al. , Fabrication of microfluidic systems in poly (dimethylsiloxane). Electrophoresis 2000; 21 :27-40 [DOI] [PubMed] [Google Scholar]
- 12.Mc Donald JC, Whitesides GM. Poly (dimethylsiloxane) as a material for fabricating microfluidic devices. Acc Chem Res 2002;35:491-9 [DOI] [PubMed] [Google Scholar]
- 13.Vitzthum L, Chen X, Kintner DB, Huang Y, Chiu SY, Williams J, et al. , Study of Na+/H+ exchange-mediated pHi regulations in neuronal soma and neurites in compartmentalized microfluidic devices. Integr Biol 2010;2:58-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris J, Lee H, Vahidi B, Tu C, Cribbs D, Cotman C, et al. , Non-plasma bonding of PDMS for inexpensive fabrication of microfluidic devices. J Vis Exp 2007;9:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlotto S, Fortunati I, Ferrante C, Schwille P, Polimeno A. Time correlated fluorescence characterization of an asymmetrically focused flow in a microfluidic device. Microfluid Nanofluid 2011;10:551-61 [Google Scholar]
- 16.Albertin G, Guidolin D, Sorato E, Spinazzi R, Mascarin A, Oselladore B, et al. , Proangiogenic activity of Urotensin-II on different human vascular endothelial cell populations. Regul Pept 2009;157:64-71 [DOI] [PubMed] [Google Scholar]
- 17.Son Y. Determination of shear viscosity and shear rate from pressure drop and flow-rate relationship in a rectangular channel. Polymer 2007;48:632-7 [Google Scholar]
- 18.Papaioannou TG, Stefanadis C. Vascular wall shear stress: basic principles and methods. Hellenic J Cardiol 2005;46:9-15 [PubMed] [Google Scholar]
- 19.Samuel SP, Jain N, O’Dowd F, Paul T, Kashanin D, Gerard VA, et al. , Multifactorial determinants that govern nanoparticle uptake by human endothelial cells under flow. Int J Nanomedicine 2012;7: 2943-56 [DOI] [PMC free article] [PubMed] [Google Scholar]


