Abstract
Neural stem cells (NSCs) show therapeutic potential for ischemia in young-adult animals. However, the effect of aging on NSC therapy is largely unknown. In this work, NSCs were transplanted into aged (24-month-old) and young-adult (3-month-old) rats at 1 day after stroke. Infarct volume and neurobehavioral outcomes were examined. The number of differentiated NSCs was compared in aged and young-adult ischemic rats and angiogenesis and neurogenesis were also determined. We found that aged rats developed larger infarcts than young-adult rats after ischemia (P<0.05). The neurobehavioral outcome was also worse for aged rats comparing with young-adult rats. Brain infarction and neurologic deficits were attenuated after NSC transplantation in both aged and young-adult rats. The number of survived NSCs in aged rats was similar to that of the young-adult rats (P>0.05) and most of them were differentiated into glial fibrillary acidic protein+ (GFAP+) cells. More importantly, angiogenesis and neurogenesis were greatly enhanced in both aged and young-adult rats after transplantation compared with phosphate-buffered saline (PBS) control (P<0.05), accompanied by increased expression of vascular endothelial growth factor (VEGF). Our results showed that NSC therapy reduced ischemic brain injury, along with increased angiogenesis and neurogenesis in aged rats, suggesting that aging-related microenvironment does not preclude a beneficial response to NSCs transplantation during cerebral ischemia.
Keywords: aged rats, behavioral recovery, ischemia, neural stem cell transplantation
Introduction
Ischemic stroke is the third common cause of death and the leading cause of disability in industrialized nations. Until now, the treatment of ischemic stroke remains a daunting task due to the lack of effective therapeutic strategies. Over the past decade, numerous neuroprotective drugs, which are effective for treating acute stroke in experimental animal stroke models, generally failed in clinical trials.1 One important reason for the failure is that these studies normally use young-adult animals.2 However, stroke is truly an age-related cerebrovascular disorder. Statistics from American Heart Association revealed that older adults in the United States had higher stroke prevalence including silent cerebral infarction.3 Nearly 75% of stroke patients are older than 65 years. Reports show that each successive 10 years after age 55, stroke occurrence is more than doubled in both men and women.4 More importantly, the efficacy of biologic reagents including cells are significantly varied between young-adult and aged in both human and experimental animals. Therefore, using aged animals to evaluate those potentially effective reagents are essential for stroke research and clinical translation.
Neural stem/progenitor cells (NSCs) transplantation is a potential strategy for protecting and restoring brain functions after stroke. Studies showed that NSCs from rodents,5 non-human primates,6 and humans7 could survive and differentiate into functional neurons, attenuate infarction and improve neurobehavioral recovery after stroke. Despite these exciting initial successes, most of these experiments are performed in young-adult animals. Whether these treatments would benefit aged subjects remains unknown.
Aging is a critical factor in the occurrence and development of cerebrovascular diseases especially in ischemic stroke. A series of cellular and molecular changes alter the structure and function of the adult brain during the aging process. Studies showed that aging process is associated with structural and cellular functional changes in brain,8 heart,9 and cardiovascular system,10 and metabolic alteration in brain;11 aging also affects kidney structural and morphologic changes.12, 13
Aging could also significantly change the microenvironment of the brain. For example, neuronal functions could be impaired by aging due to increased oxidative damage, reduced metabolic activity, and protein and lipid by-products accumulation. However, growth factors known to influence neural proliferation and survival, including vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor and insulin-like growth factor-1, decline with age.14 Since aging brain possesses a different environment from the young-adult brain, the receptivity to NSC transplants in aging rat brain needs to be explored.
In this study, we investigated whether the transplantation of NSCs in aged rats could have similar salutary effects as it does in young-adult rats after transient middle cerebral artery occlusion (tMCAO). In addition, the mechanisms by which NSCs facilitate their protective function in aged rats after ischemia were also explored.
Materials and methods
Cell Culture, Characterization, Proliferation, and Differentiation
Animal work in this study was performed in accordance with ARRIVE guidelines and animal protocol was approved by the Institutional Animal Care and Use Committee of University of North Texas Health Science Center at Fort Worth, Texas, USA (Permission number: 2011/12-21-A05) and Shanghai Jiao Tong University, Shanghai, China (Permission number: Bioethics 2012022). Neural stem cells were isolated from the telencephalon of the E14 green fluorescent protein (GFP) transgenic mice (Animal Research Center of Nanjing University, Nanjing, China) as previously described.15 After 2 weeks expansion of monolayer cultures on a laminin-coated dish in the DMEM/F12 (1:1) medium (Gibco, Carlsbad, CA, USA) supplemented with B27 supplement (Gibco), basic fibroblast growth factor (20 ng/ml; Gibco), and epidermal growth factor (20 ng/ml; Gibco). The primary passage of NSCs was not pure, and contains mixed cells. After passage 4, cultured NSCs start to show signs of reduced viability and differentiation capacity. Therefore, we chose NSCs from passages 2 and 4 in our study. These cells strongly maintained their proliferation and differentiation ability.
To characterize cells, cultured cells were grown on ploy-L-ornithine hydrobromide (Sigma, St Louis, MO, USA) and laminin (Sigma)-coated glass coverslips in a 24-well plate (BD Falcon, San Jose, CA, USA). Cells were then immunostained with mouse anti Nestin (Millipore, Billerica, MA, USA), mouse anti Tuj-1 (Millipore), rabbit anti-glial fibrillary acidic protein+ (GFAP+) (Millipore), and mouse anti-Galactocerebroside (Millipore). To induce differentiation, neurospheres were plated on ploy-L-ornithine hydrobromide and laminin-coated glass coverslips in a 24-well plate, cultured in the DMEM/F12 (1:1) medium supplemented with B27 supplement and 1% fetal bovine serum (Gibco). The medium was changed every 3 days and after 7 days, cells were immunostained with mouse anti-Nestin, mouse anti-Tuj-1, rabbit anti-GFAP, and mouse anti-Galactocerebroside. To assess the proliferation rate of NSCs, NSCs were cultured in proliferative medium containing 20 ng/ml basic fibroblast growth factor, 20 ng/ml epidermal growth factor, and 10 μmol/L 5-Bromo-2′-deoxyuridine (BrdU) (Sigma) for 24 hours. Then, NSCs were washed with phosphate-buffered saline (PBS) for three times and fixed with 4% paraformaldehyde. After incubation in 2 mol/L HCl at 37°C for 30 minutes, and rinsed with 0.1 mol/L boric acid (pH 8.5) at room temperature for 10 minutes, cells were blocked with 10% bovine serum albumin and stained with mouse anti-BrdU (1:100; Santa Cruz Technology, Santa Cruz, CA, USA). To test whether GFP signal was diluted in the proliferated NSCs, BrdU staining was also performed in GFP+ NSCs in the same way.
The total number of cells was counted using DAPI staining and the number of Tuj-1, GFAP, and BrdU immunoreactive cells was also counted in a random field on each coverslip using a fluorescent microscope under × 20 objective lens (Leica, Solms, Germany). Three coverslips were evaluated in each immunostaining group and six fields were randomly chosen on each coverslip. The percentage of Tuj-1, GFAP, and BrdU-positive cells was calculated.
Transient Middle Cerebral Artery Occlusion and NSCs Transplantation
In all, 3-month-old (young-adult; n=35) and 24-month-old (aged; n=20) male Sprague-Dawley rats (Sippr-BK Co., Shanghai, China) were anesthetized with Ketamine/Xylazine (100 mg/10 mg/kg; Sigma) intraperitoneally. Body temperature was maintained at 37±0.5°C using a heating pad (RWD Life Science, Shenzhen, China). Focal cerebral ischemia was induced by tMCAO as previously described.16 Briefly, under the surgical microscope (Leica), a 4-0 round tip and silicon coated suture (Covidien, Mansfield, MA, US) was inserted from the left external carotid artery into the internal carotid artery and reached the circle of Willis to occlude the origin of the middle cerebral artery. The success of occlusion was determined by monitoring the decrease in surface cerebral blood flow to 80% of baseline cerebral blood flow using a Laser Doppler flowmetry (Moor LAB; Moor Instruments, Devon, UK). Reperfusion was performed by withdrawing the suture 2 hours after MCAO. Six aged and two young-adult rats died 24 hours after tMCAO, no animal died after NSCs transplantation. Sham-operated rats underwent the same procedure without suture insertion. The rats in which cerebral blood flow decreased to <80% of baseline immediately after MCAO and those died during surgery were excluded from the study. pH, partial pressure of carbon dioxide (pCO2), oxygen (pO2), and glucose from venous blood were recorded using i-STAT System (Abbott Point of Care Inc., Princeton, NJ, USA), mean arterial blood pressure was measured by Softron Sphygmomanometer (Softron BP-98A; Softron Beijing Inc., Beijing, China).
Twenty-four hours after tMCAO, rats were randomly divided into four groups for NSCs or vehicle injection: (1) young-adult rats treated with PBS (vehicle, n=16); (2) young-adult rats treated with NSCs (n=17); (3) aged rats treated with PBS (n=7); (4) aged rats treated with NSCs (n=7). Neural stem cells (1 × 106 in 25 μL PBS) were stereotactically injected into the striatum of the ipsilateral hemisphere in aged rats or young-adult rats, with the following coordinates: AP −0.5 mm; L −3.0 mm; V −5.0 mm. The same amount of PBS was injected as a control. The wound was then closed and the animal was returned to the cage for follow-up experiments. 5-Bromo-2′-deoxyuridine (Sigma, two 50 mg/kg intraperitoneally doses given 8 hours apart) was administered 7 days after stroke for 7 consecutive days and cyclosporine A (CsA, 10 mg/kg; Novartis, Basel, Switzerland) for immunosuppression was daily injected intraperitoneally into rats from day 1 to day 14 after tMCAO, rats were killed after 14 days of tMCAO.
Immunohistochemistry
Histologic cryosections (20 μm in thickness) from anterior commissure to hippocampus were collected for immunohistochemical analysis. Serial frozen sections, 20 μm in thickness and 200 μm in interval from the frontal cortex, were stained with 0.1% cresyl violet (Sinopharm Chemical Reagent Co., Shanghai, China). Infarct volume was determined by subtracting the area of cresyl violet staining in the ipsilateral hemisphere from that of the contralateral hemisphere using the NIH image J software, then multiplied by the section interval thickness.
Immunohistochemistry was performed as previously described.17 After blocking with 10% BSA (Sigma), brain sections were incubated with GFP (1:100; Cell Signaling, Beverly, MA, USA), neuronal marker Tuj-1 (1:100; Millipore), neuroblast marker DCX (1:100; Santa Cruz Technology), astrocyte marker GFAP (1:100; Millipore) at 4°C overnight. Some brain sections were double immunostained using endothelial cell (EC) marker CD 31 (1:200; R&D Systems, Tustin, CA, USA) and VEGF (1:100; Santa Cruz Technology); GFAP and VEGF. Secondary antibodies were appropriate Alexa Fluor 488, 594 or 647-labeled IgG (Invitrogen, Carlsbad, CA, USA).
Double Immunostaining
To determine the proliferation of ECs and neuroblasts, brain sections were incubated in 2 mol/L HCl at 37°C for 30 minutes, and rinsed with 0.1 mol/L boric acid (pH 8.5) at room temperature for 10 minutes. After blocking with 10% bovine serum albumin, the sections were stained with CD-31 or DCX and BrdU (1:100; Santa Cruz Technology). Fluorescence signals were detected using a TCS SP5 Confocal Scanning System mounted on a TCS SP5 200 inverted microscope (Leica) equipped with LASOS laser (Lasertechnik GmbH, Jena, Germany). Images were acquired using the LAS AF Software (Leica).
Apoptosis of neurons was evaluated by terminal-deoxynucleotidyl transferase mediated nick end labeling (TUNEL) and NeuN (1:100; Millipore) double immunostaining according to the manufacturer's protocol (Roche Diagnostics, Basel, Switzerland). Every five section (200 μm apart) containing the grafted region was chosen for TUNEL staining. The sections were then stained with anti-NeuN. Slices were covered with anti-fade mounting medium (Vector Labs, Burlingame, CA, USA) and visualized under a fluorescent microscope at × 20 objective lens (Leica). The apoptotic cells were calculated from six areas per section, five sections (200 μm apart) per animal by two observers blinded to the experimental group.
Behavioral Tests
Neurobehavioral tests were performed before tMCAO and 1, 3, 7, and 14 days after tMCAO by an investigator who was blinded to the experimental groups. Rats were trained for 3 days before tMCAO with three consecutive trials to generate stable baseline values.
Rotarod test is an index of fore and hind limb motor coordination and balance.18 The duration that rats remained on the accelerating rotating rod was measured. The velocity was slowly increased from 20 to 40 r.p.m. in 5 minutes. Each animal was given three trials, and the time that the animals spent on the rungs or gripped the device and spun around for two consecutive revolutions was recorded. Data were analyzed as the average duration (three trials) on the rotarod.
Modified neurologic severity scores of the animals were graded on a scale of 0 to 14, which is a composite of motor, reflex, and balance tests.19 The higher the score, the more severe injury.
The elevated body swing test was used to test asymmetric motor behavior.20 In the test, rats were held by the base of the tail and raised 10 cm above the testing surface. The initial direction of swing, defined as the turning of the upper body by >10 degrees to either side, was recorded in 20 trials in each rat, performed over 5 minutes. The number of turns in each (left or right) direction was recorded for each rat.
Quantification of Survived Implanted NSCs and Microvessel Counting
All the GFP-positive cells were counted on nine serial coronal sections per brain (anterior-posterior: −0.8 mm to +0.8 mm, 200 μm apart). Blood vessel counting through CD31 staining was a simple and reproducible way to morphologically identify the number of blood vessels. Numerous studies of manual and computer-assisted methods confirm minimal interobserver variability in the blood vessel counting.21 Two brain coronal sections from the CD31 and BrdU double stained brain, 1 mm frontier and 1 mm posterior from the ischemic core, were chosen. Three areas of blood vessels, just in the peri-focal striatum region, were chosen at low power objective lens ( × 20) and blood vessels were counted on these pictures. Two investigators blinded to the experimental group assessed blood vessel counts separately. Only vessels with a clearly defined lumen or a well-defined linear vessels shape were taken into account. Single EC was ignored. The number of blood vessels was calculated as the mean of the blood vessel counts obtained from the six pictures. Newly formed neuroblasts were quantified in the similar ways.
Western Blot Analysis
Tissue samples were collected from the striatum of the ipsilateral hemisphere, and quantified with BCA protein assay (Pierce, Rockford, IL, USA). To analyze protein levels, equal amounts of total proteins were subjected to 10% (W/V) SDS–PAGE and transferred onto nitrocellulose membranes (Whatman, Piscataway, NJ, USA). Membranes were then blocked with 5% skim milk for 1 hour at room temperature and probed with anti-VEGF (1:500; Santa Cruz Technology) and anti-β actin (1:2,000; Santa Cruz Technology) antibodies at 4°C overnight. Subsequently, membranes were incubated with horseradish peroxidase-conjugated goat anti rabbit or rabbit anti-goat IgG for 1 hour at room temperature after three times TBST washing, and then reacted with an enhanced ECL substrate (Pierce). The result of chemiluminescence was recorded with an imaging system and semiquantified using the Quantity One software (Bio-Rad, Hercules, CA, USA).
Statistical Analysis
The values were given as mean±standard deviation. Parametric data in different groups were analyzed by one-way analysis of variance, followed by Tukey post hoc comparisons. For behavioral data, two-way repeated measures analysis of variance was performed to analyze the overall difference between treatment groups over time, and then Bonferroni-corrected post hoc comparisons were used to analyze the difference between treatment groups at each time point. Two-tailed P<0.05 were considered as statistically significant. All statistical analyses were performed using the SPSS software (v18.0) (SPSS Inc., Chicago, IL, USA).
Results
Characterization, Differentiation, Proliferation of NSCs, and Timeline of Experiments
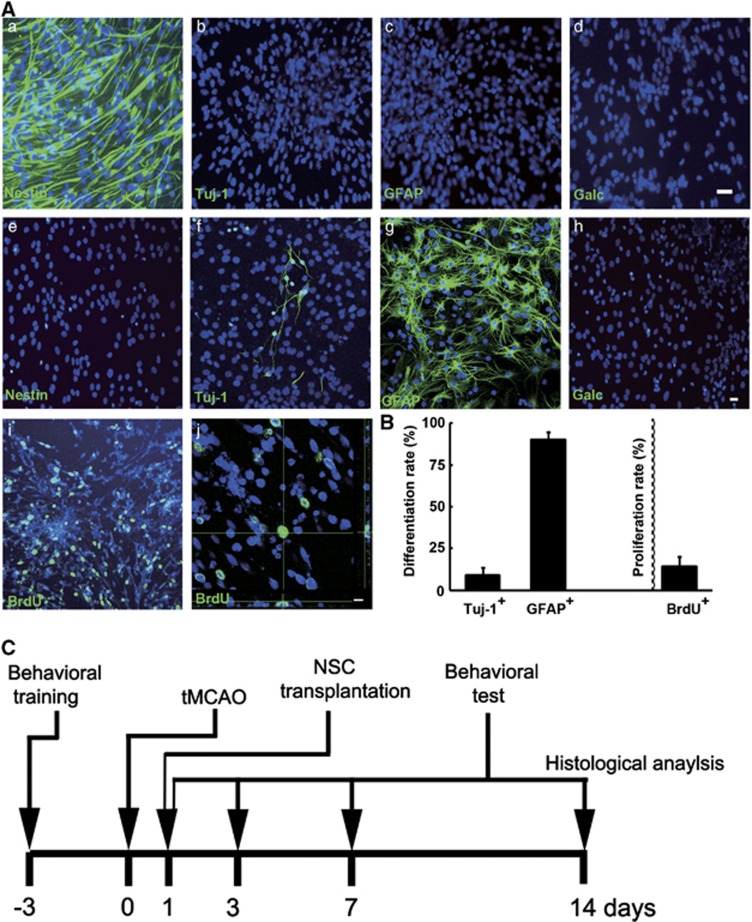
Neural stem cells were generated from the telencephalon of E14 mice and characterized by immunocytochemistry. Results showed that cells were nestin+, while Tuj-1−, GFAP− and galactocerebroside− (Galc−, Figure 1Aa–d). When cells were induced to differentiate, 9.4±4.3% of cells were Tuj-1+ and 90.3±4.2% of cells were GFAP+, while nestin− and Galc− (Figures 1Ae–h and B). In addition, the proliferation rate was evaluated by BrdU incorporation (Figure 1Ai and j). The proliferation rate of NSCs was 14.6±5.8% (Figure 1B), and the proliferated NSCs were still GFP+ (Supplementary Figure 1). Neural stem cells were then transplanted to rats' brain at 1 day after stroke (Figure 1C).
Figure 1.
Neural stem cells (NSCs) characterization, differentiation, proliferation, and timeline of experiments. (A) Fluorescent photographs showed that NSCs were immunostained positive for nestin (a), negative for Tuj-1 (b), GFAP (c), and Galactocerebroside (d). When cells were induced to differentiate, they were Tuj-1 and GFAP positive (f and g), while nestin and galactocerebroside were negative (e, h). Bar=20 μm. When cells were treated with 5-bromo-2′-deoxyuridine (BrdU) for 24 hours, confocal microscopy showed the proliferation of NSCs (green, i and j). Bar=20 μm. (B) Bar graph showed 9.41±4.34% and 90.27±4.22% of NSCs were differentiated into Tuj-1-positive neurons and GFAP-positive astrocytes. The proliferation rate of NSCs was 14.6±5.8%. (C) Behavioral training was performed at 3 days before transient middle cerebral artery occlusion (tMCAO), and NSCs were transplanted into rats' brain at 1 day after stroke. Outcomes were measured at the indicated intervals.
NSCs Treatment Attenuated Brain Infarct Volume and Improved Neurobehavioral Outcomes in Aged Rats after Transient Middle Cerebral Artery Occlusion
Mean arterial blood pressure, pH, pCO2, pO2, and glucose concentration were measured before tMCAO, cerebral blood flow was recorded before the surgery, after filament insertion, and after suture withdraw. The physiologic parameters were similar among them (Table 1).
Table 1. Summary of physiologic parameters in rats.
| Experimental group | Cerebral blood flow (% to contralateral hemisphere) Immediately after ischemia | Cerebral blood flow (% to contralateral hemisphere) Immediately after suture withdraw | Mean arterial blood pressure (MABP) mm Hg | pH | pCO2 mm Hg | pO2 mm Hg | Glucose mg/dL |
|---|---|---|---|---|---|---|---|
| PBS-young | 18.47±3.11 | 76.4±6.13 | 111.17±13.30 | 7.43±0.04 | 47.5±2.73 | 59.85±4.97 | 169.5±13.53 |
| PBS-aged | 16.8±7.11 | 75.1±5.61 | 112.5±12.29 | 7.42±0.02 | 47.7±2.43 | 58.91±3.94 | 171.3±12.01 |
| NSC-young | 15.7±4.18 | 73.64±3.08 | 115±10.45 | 7.43±0.03 | 46.79±4.69 | 59.85±4.69 | 168±19.79 |
| NSC-aged | 17. 3±6.90 | 74.88±5.78 | 113±11.90 | 7.42±0.04 | 46.8±3.93 | 60.23±3.87 | 169±11.34 |
NSC, neural stem cell; PBS, phosphate-buffered saline; tMCAO, transient middle cerebral artery occlusion.
Mean arterial blood pressure, pH, pCO2, pO2, and glucose concentration were measured before tMCAO, cerebral blood flow was recorded before the surgery, after the filament insertion, and after the suture withdraw.
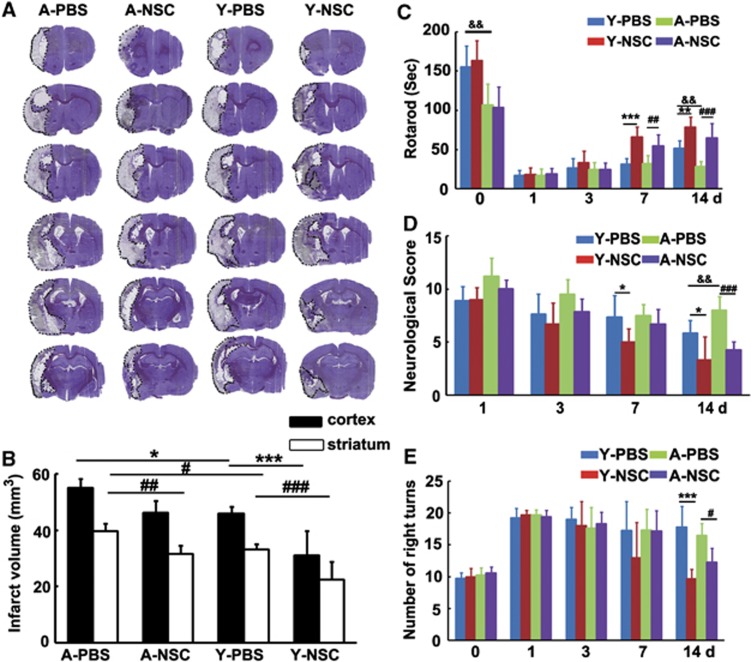
To determine whether the benefits of NSC transplantation extend to aged rats, brain sections were stained with cresyl violet after 14 days of tMCAO. Aged rats developed larger infarcts in both cortex and striatum than young-adult rats (Figures 2A and 2B), NSCs treatment reduced infarct volume of striatum in both young-adult and aged rats, compared with the PBS group (Figures 2A and 2B, P<0.05). Rotarod test showed that before tMCAO surgery, aged rats had more motor deficiency than young-adult rats, suggesting that aging affects rat motor function. While NSC transplantation significantly improved neurobehavioral outcomes in both aged and young-adult rats at 7 and 14 days after tMCAO (Figure 2C, P<0.05). Neurologic score and elevated body swing test showed that there was no significant difference among the four groups before tMCAO. However, neurobehavioral outcomes were greatly improved in the NSC-treated groups compared with the controls, in both aged and young-adult rats at 7 and 14 days after tMCAO (Figures 2D and 2E, P<0.05).
Figure 2.
Neural stem cells (NSCs) transplantation reduced infarct volume and improved neurobehavioral recovery in aged ischemic rats. (A) Representative sets of cresyl violet-stained brain sections from aged and young-adult rats, treated with PBS (vehicle) and NSCs after 14 days of transient middle cerebral artery occlusion (tMCAO). Dashed line shows the border of infarct area. (B) Bar graph showed infarct volume of cortex and striatum in aged and young-adult ischemic rats, treated with PBS and NSCs, respectively. Data are mean±s.d., n=16 for young-adult rats and n=7 for aged rats. * or #P<0.05; ##P<0.01, *** or ###P<0.001. Functional recovery was evaluated using Rotarod test (C), Neurologic score (D), and Elevated body swing test (E). The behavioral tests were performed at 1 day before MCAO and 1, 3, 7, and 14 days after tMCAO. Data are mean±s.d., n=16 for young-adult rats and n=7 for aged rats. #P<0.05, ##P<0.01, and ###P<0.001, aged-PBS vs. aged-NSC; *P<0.05, **P<0.01, ***P<0.001, young-adult-PBS vs. young-adult-NSC; &&P<0.01, aged-PBS vs. young-adult-PBS. PBS, phosphate-buffered saline.
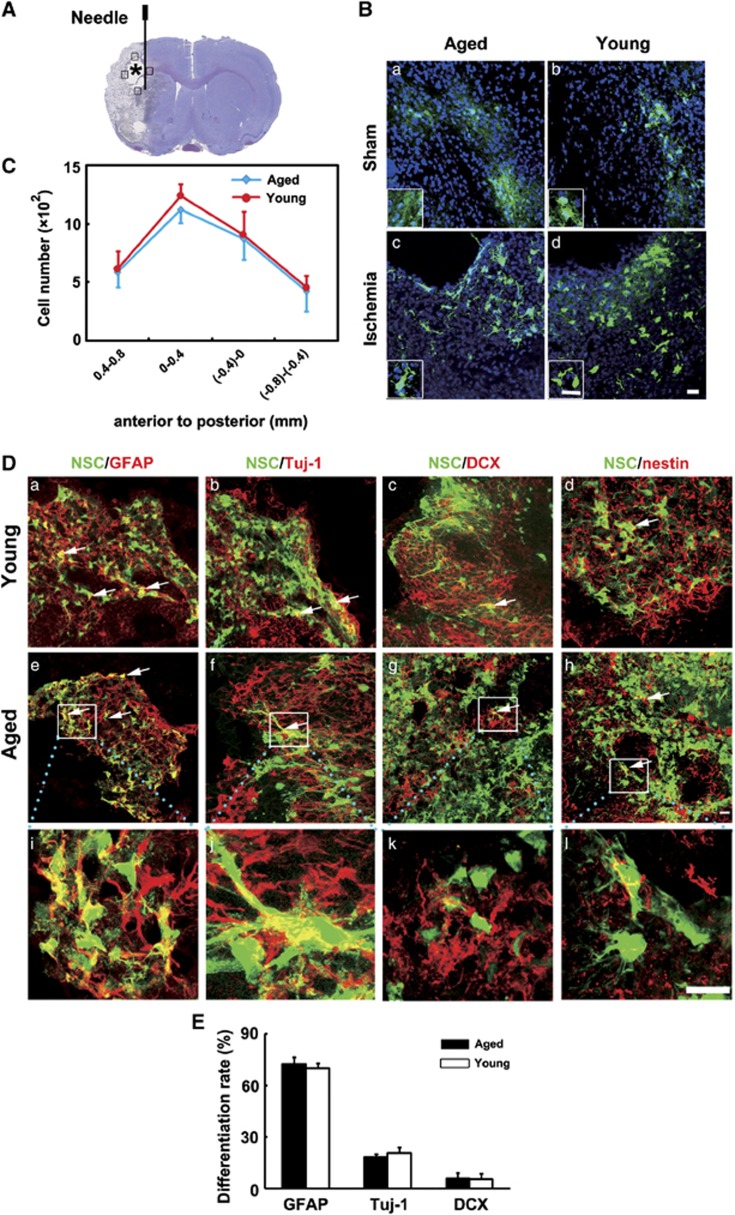
Survival and Differentiation Pattern of NSCs in Aged Rats after Transient Middle Cerebral Artery Occlusion
Neural stem cells were transplanted into the striatum in ischemic rats. Photographs and statistical results were acquired from the boundary zone of ischemic core (Figure 3A, boxed areas). Thirteen days after transplantation, GFP staining revealed an extensive migration of the grafted cells toward to the peri-infarct regions in both the aged and young-adult ischemic rats. It was noted that cavity was formed after stroke and NSCs were accumulated in the boundary of the cavity (Figure 3A, asterisk). However, only a small amount of GFP+ cells with unclear morphology were accumulated in the injection area in sham rats (Figure 3B). We found the number of GFP+ cells, which migrated into the peri-infarct area, was similar between aged and young-adult ischemic rats (Figure 3C, P>0.05). Using lineage-specific phenotype markers and GFP double staining, we showed that grafted NSCs in the peri-infarct area were differentiated into GFAP+ astrocytes, Tuj-1+ neurons, DCX+ neuroblasts, and residual cells were nestin+ cells (Figure 3D). The percentage of Tuj-1+ neurons (18.4±1.7% vs. 20.7±3.5%), GFAP+ astrocytes (72.5±4% vs. 70.3±2.6%) and DCX+ neuroblasts (5.8±3.5% vs. 5.7±3.1%) differentiated from the grafted cells was similar between aged and young-adult rats 14 days after tMCAO.
Figure 3.
Survival and differentiation pattern of transplanted neural stem cells (NSCs) in aged rat brain after 14 days of transient middle cerebral artery occlusion (tMCAO). (A) Graphic illustration indicated injection point in a rat brain coronal section. Asterisk shows the cavity that was caused by stroke. Photographs and statistics were acquired from the boundary of the cavity (boxed areas). Needle track was 5 mm under the surface of the brain. (B) Morphology of green fluorescent protein (GFP+) NSCs in sham (a, b) and ischemic (c, d) brain 13 days after transplantation. Inset box shows magnified view of the corresponded photograph. Bar=20 μm. (C) Bar graph shows the number of survived GFP+ cells in aged and young-adult rat brain after 14 days of tMCAO. Data are mean±s.d., n=6 per group. (D) Photomicrographs show fluorescent staining with GFP (green) and GFAP (red, left column), Tuj-1 (red, second column), DCX (red, third column), and nestin (red, right column) in young-adult (a, b, c, d) and aged (e, f, g, h) rats' brain after 14 days of tMCAO. (i, j, k, l) High magnifications from the box of (e, f, g, h). Arrows indicate differentiated NSCs. Bar=20 μm. (E) Bar graph showed the differentiation ratio of NSCs was similar between aged and young-adult ischemic rats.
NSC Transplantation Reduced Apoptosis of Neurons in Aged Ischemic Rats
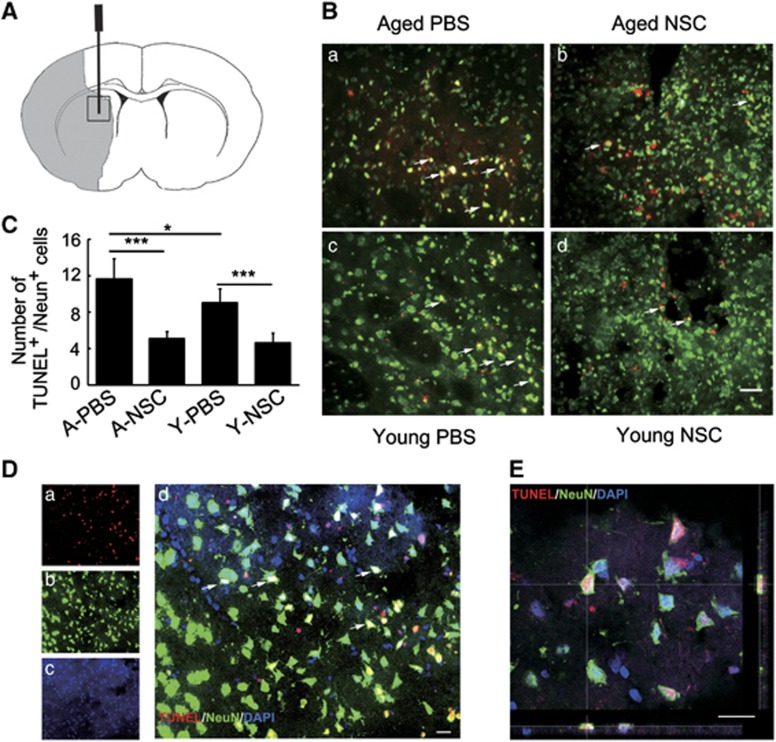
To test whether NSC could reduce neuron apoptosis in aged brain after ischemia, brain sections were double stained with TUNEL and NeuN. We found that after 14 days of tMCAO, the number of apoptotic neurons in aged rats was more than that in young-adult rats, while transplantation of NSCs greatly reduced the number of neuronal apoptosis in both aged and young-adult ischemic rats (Figures 4A–4C, P<0.05).
Figure 4.
Neural stem cells (NSCs) transplantation attenuated neuronal apoptosis in aged and young-adult rat brain after 14 days of transient middle cerebral artery occlusion (tMCAO). (A) Graphic illustration indicated injection point in a rat brain coronal section. Gray color indicates the infarct area. Black box is the area for imaging and statistical analysis. (B) Terminal-deoxynucleotidyl transferase mediated nick end labeling (TUNEL) staining showed endogenous apoptotic neurons (red) in aged-PBS (a), aged-NSC (b), young-adult-PBS (c), and young-adult-NSC rats (d). Arrows indicate apoptotic neurons. Bar=20 μm. (C) Bar graph shows the number of apoptotic neurons in each group. Data are mean±s.d., n=6 per group. *P<0.05; ***P<0.001. (D) A representative confocal microscopy showed TUNEL/NeuN-positive neurons in the peri-infarct area in aged-PBS rats (a, TUNEL; b, NeuN; c, DAPI). Arrows indicates apoptotic neurons (d). Bar=20 μm. (E). Confocal image of TUNEL and NeuN colocalization in three-dimensional space. Bar=20 μm. PBS, phosphate-buffered saline.
NSC Transplantation Promoted Endogenous Angiogenesis and Neurogenesis in Aged Ischemic Rats
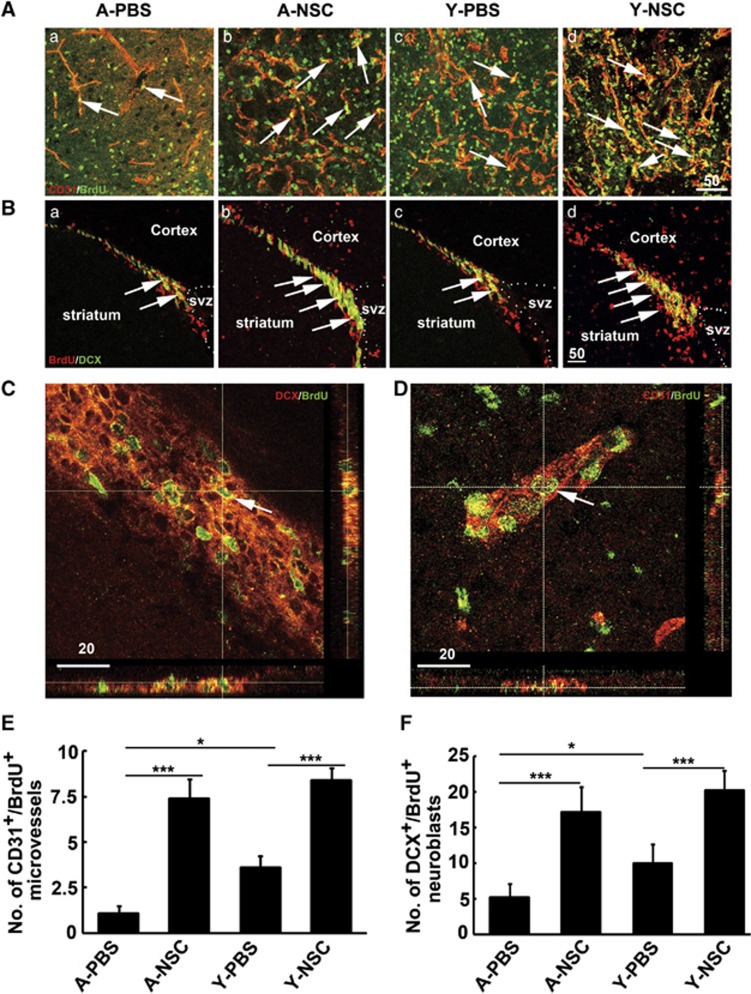
The number of newly formed microvessels and neuroblasts was analyzed in the ipsilateral striatum and subventricular zone after 14 days of tMCAO by CD31/BrdU (Figure 5A) and DCX/BrdU (Figure 5B) double immunostaining. Confocal images showed that CD-31/BrdU (Figure 5C) and DCX/BrdU (Figure 5D) were expressed in single cells. Statistical results showed that angiogenesis and neurogenesis were reduced in aged rats after 14 days of tMCAO, compared with the young-adult ischemic rats. However, angiogenesis and neurogenesis were significantly increased in both aged and young-adult rats after NSC transplantation compared with the control (Figures 5E and 5F, P<0.05).
Figure 5.
Neural stem cells (NSCs) transplantation increased angiogenesis and neurogenesis in aged ischemic rats. (A) Representative photographs of CD-31 (red) and 5-Bromo-2′-deoxyuridine (BrdU) (green) double immunostaining in aged-PBS group (a), aged-NSC group (b), young-adult-PBS group (c), and young-adult-NSC group (d) in the ischemic region of striatum. Arrows indicate newly formed microvessels. (B) Photographs showed DCX (green) and BrdU (red) double immunostaining in aged-PBS group (a), aged-NSC group (b), young-adult-PBS group (c), and young-adult-NSC group (d) in SVZ region. Arrows indicate proliferating neuroblasts. Bar=50 μm. Three-dimensional confocal reconstructions of DCX +/BrdU+ (C) and CD31+/BrdU+ (D) cells are presented as viewed in the x–z (bottom) and y–z (right) planes. Arrows indicate newly formed neuroblasts and microvessels. Bar=20 μm. Quantitative analysis of angiogenesis (E) in striatum and neurogenesis (F) in SVZ region of aged and young-adult rats brain. Data are mean±s.d., n=6 per group. *P<0.05; ***P<0.001. PBS, phosphate-buffered saline; SVZ, subventricular zone.
NSCs Transplantation Increased Vascular Endothelial Growth Factor Expression in Aged Ischemic Rats
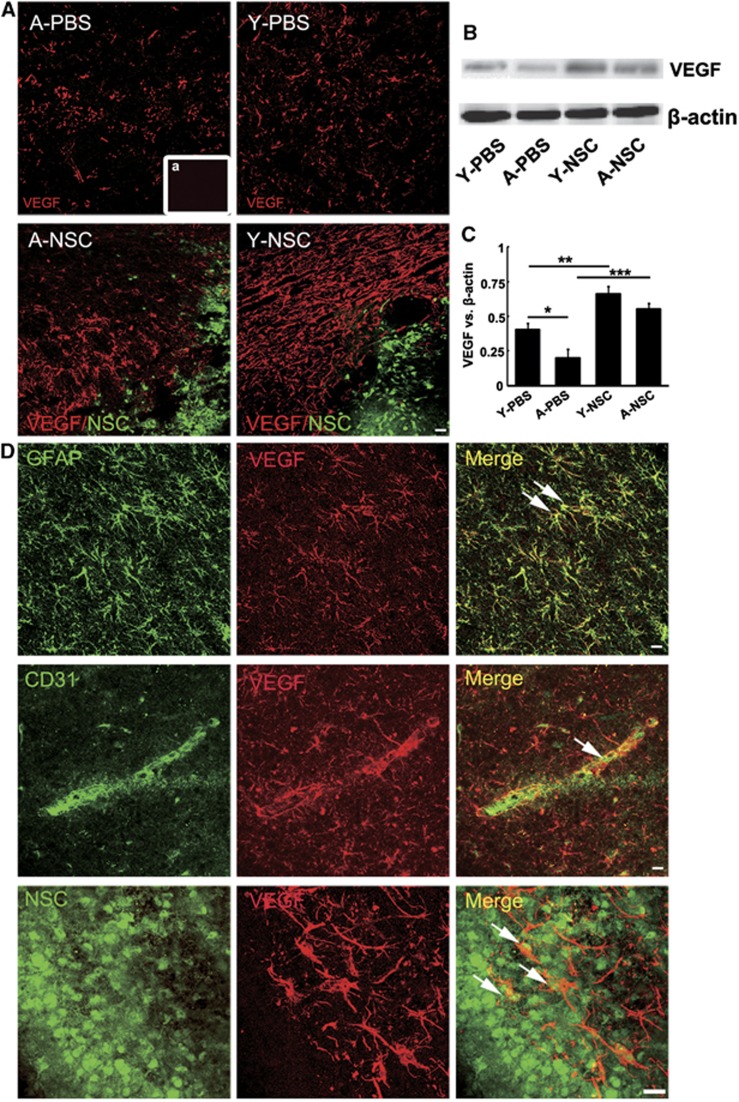
Immunostaining results showed that NSC transplantation increased VEGF expression in both aged and young-adult rats (Figure 6A), which were confirmed by western blotting (Figures 6B and 6C, P<0.05). Confocal images showed that in aged ischemic rats, VEGF was predominantly expressed in GFAP+ astrocytes and CD31+ ECs, whereas partial of implanted NSCs also expressed VEGF (Figure 6D).
Figure 6.
The expression of vascular endothelial growth factor (VEGF) was significantly increased in aged ischemic brain after neural stem cell (NSC) transplantation. (A) Photographs showed VEGF expression in aged and young-adult ischemic rats, after NSC or PBS treatment (red: VEGF; green: NSCs). NSC treatment greatly increased endogenous VEGF expression in both aged and young-adult rats, a is the negative control. Bar=20 μm. (B) Western blot analysis showed that expression of VEGF and β-actin in the ipsilateral striatum of PBS and NSC-treated aged and young-adult ischemic rats. (C) Bar graph showing the semi-quantitative data from (B). Data are mean±s.d., n=5 per group. *P<0.05, Y-PBS vs A-PBS; **P<0.01, Y-PBS vs Y-NSC; ***P<0.001, A-PBS vs A-NSC. (D) Confocal image showed localization of VEGF in aged ischemic rats. VEGF was expressed in GFAP+ astrocytes (green: GFAP; red: VEGF), CD31+ endothelial cells (ECs) (green: CD31; red: VEGF) and transplanted NSCs (green: NSCs; red: VEGF). Bar=20 μm. PBS, phosphate-buffered saline. *P<0.05, Y-PBS vs A-PBS; **P<0.01, Y-PBS vs Y-NSC; ***P<0.001, A-PBS vs A-NSC.
Discussion
Previous studies showed that transplantation of NSCs modulated inflammatory response and microglia activation. The transplanted NSCs secreted neurotrophic factors, promoted endogenous angiogenesis and neurogenesis, enhanced plasticity, even differentiated into mature neurons with electrophysiological properties and received synaptic input from host neurons after cerebral ischemia.22 These results suggest that NSC has great therapeutic potential in animal models. In contrast to these abundant studies in young-adult animals, little attention has been paid to aged rodents, which is of greater therapeutic significance since the occurrence of stroke is much higher in seniors.
Aging impairs the microenvironment of the brain. For example, aged mice suffered deficits in olfactory discrimination, most likely attributed to reduced neurogenesis.23 In terms of cerebral ischemia, our previous study showed that ischemia-induced neurogenesis was preserved but reduced in the aged rat brain.24 Angiogenesis was also reduced in the aged rodent brain after focal cerebral ischemia, compared with the young-adult animals.25 The age-dependent decline of neurogenesis and angiogenesis was attributed to decreased growth factors.14 In this study, we found that aged rats were more vulnerable than the young-adult rats to ischemic damage. Overall mortality in aged rats was significantly higher than that in the young-adult rats after tMCAO (6/20 in aged rats and 2/35 in young-adult rats). After cerebral ischemia, larger infarct volume, limited functional recovery, decreased angiogenesis and neurogenesis with decreased VEGF expression occurred in aged rats.26 Interestingly, rotarod test, but not modified neurologic severity scores and elevated body swing test, showed that aging impaired motor function in normal aged rats, suggesting that different behavioral tests could target different aspects of functional outcomes. Additionally, rotarod and modified neurologic severity score tests showed worse in aged rats 14 days after tMCAO, which was associated with an age-dependent decrease in ischemia-induced angiogenesis, neurogenesis and VEGF expression. Based on these observations, it is reasonable to speculate that aging might affect the fate of implanted NSCs in the ischemic brain and its therapeutic efficiency after tMCAO.
We stereotactically injected NSCs into the brain at 1 day after tMCAO. Up to date, various stem cell delivery routes such as intravenous, intracerebral, intraartery, and intraventricular have been applied to explore their therapeutic benefits and the mechanism involved in stroke.27 Intracerebral injection was chosen in our experimental design because the cells could be precisely transplanted into the striatum of ischemic brain without cell loss. Vascular injection could trap stem cells into systemic organs, with few stem cells could be homed to the ischemic brain hemisphere.28 Previous studies showed that both early and delayed transplantation of NSCs reduced infarct volume, improved neurobehavioral outcomes, increased angiogenesis and neurogenesis. It is noted that cell survival was higher in early transplantation after stroke.29 We transplanted NSC after 1 day of MCAO, which was practically difficulty for clinical application. Further work is needed to test whether transplantation of NSCs in later phases would also benefit for cerebral ischemia.
Our present study showed that GFP-labeled NSCs could survive in both aged and young-adult brain for at least 14 days after MCAO, and most of the transplanted NSCs were differentiated into GFAP+ astrocytes. However, when NSCs were injected into the sham surgery rats, unclear and atypical cell morphology could be observed in both aged and young-adult rats. No GFP signal could be found at 35 days after GFP+ NSC transplantation into sham rats (data not shown), indicating that normal rat brain could not provide suitable environment, which are critical for exogenous cell survival and differentiation. In addition, we showed that grafted NSCs' differentiation was similar between aged and young-adult rats after 14 days of tMCAO, suggesting that microenvironment in aged rat brain did not affect NSC differentiation.
Neuronal apoptosis occurs after brain ischemia is well documented.30 Neural stem cells transplantation into young-adult ischemic rodents greatly reduced TUNEL+ apoptotic cells and inflammatory infiltration, further improved neurobehavioral outcomes.31 We found that brain ischemia induced more neuronal apoptosis in aged rats compared with the young-adult rats, while NSC transplantation greatly attenuated neuronal apoptosis in both young-adult and aged rats. Furthermore, we also found that angiogenesis and neurogenesis were increased after NSC transplantation. Angiogenesis and neurogenesis are fundamental events during development and some disease processes. Cerebral ischemia induced angiogenesis and neurogenesis have been shown in a variety of rodent models and in humans.32 Newly formed capillaries could supply oxygen and nutrition to the ischemic region, which is extremely important for the tissue repairing and remodeling.33 Newly formed neuroblasts could migrate to the damaged area, where they become matured and functional cells to replace the dead neurons.34 Thus, angiogenesis and neurogenesis are two important processes responsible for restoring brain functions after stroke.
It was reported that implantation of NSCs at 24 or 72 hours after stroke protected blood–brain barrier, reduced neuron apoptosis, facilitated neuroprotection, and attenuated infarct volume through multiple mechanisms.35 Our present study further showed that transplanted NSCs reduced neuronal apoptosis, promoted focal angiogenesis and neurogenesis, increased VEGF expression in the lesion area, thus attenuated infarct volume at 14 days after stroke. This could be due to neuroprotection or neurorestoration.36
Vascular endothelial growth factor is an important neurotrophic factor for angiogenesis and neurogenesis. Numerous studies show that brain injury upregulates VEGF, which is critical for angiogenesis and neurogenesis.37 In addition, NSCs secreted VEGF promoted the density of capillary, enhanced axonal sprouting, and regulated microglia response.38 We found that VEGF was mainly expressed in GFAP+ astrocytes, CD31+ ECs, and implanted NSCs, even in astrocytes derived from transplanted NSCs. In short, VEGF was greatly upregulated in both NSC-treated aged and young-adult ischemic rats. Upregulated VEGF subsequently increased focal angiogenesis and neurogenesis, and in the meantime reduced neuron apoptosis in aged and young-adult ischemic rats.
Astrocytes have a dual role after injury. Focal cerebral ischemia induces astrocytes swelling and leads to blood–brain barrier breakdown, reduces the uptake and release of glutamate.39 Enhancing the survival of astrocytes restores blood–brain barrier and improves neuron survival in the acute phase of cerebral ischemia while glial scar formation during the subacute phase significantly inhibits functional recovery and process of regeneration.40 In our study, we showed that limited transplanted NSCs were differentiated into neurons, and no direct evidence showed that dead neurons could be replaced by new neurons that were derived from transplanted NSCs. Neural stem cells differentiated into astrocytes and expressed VEGF (Supplementary Figure 2), which were defined as cytokine-activated astrocytes. These cytokine-stimulated astrocytes then promoted the recovery of central nervous system function. In addition, transplanted NSCs not only enhanced endogenous secretion of VEGF by astrocytes and ECs, but also protected endogenous neurons from apoptosis, consequently promoted angiogenesis and neurogenesis, thus reduced infarct volume and improved neurobehavioral recovery.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by research grants from the National Natural Science Foundation of China (81070939 and 81100868), National Basic Research Program of China (973 Program 2011CB504405), the Science and Technology Commission of Shanghai Municipality (10JC1408100) and KC Wong Foundation (GYY), and by National Institute of Health (NIH) grants AG21980 and NS057186 (KJ).
Supplementary Material
References
- Fisher M. New approaches to neuroprotective drug development. Stroke. 2011;42:S24–S27. doi: 10.1161/STROKEAHA.110.592394. [DOI] [PubMed] [Google Scholar]
- Harris NR, Rumbaut RE. Age-related responses of the microcirculation to ischemia-reperfusion and inflammation. Pathophysiology. 2001;8:1–10. doi: 10.1016/s0928-4680(01)00064-5. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini C, Triggiani L, Cimini N, Ciancarelli I, De Santis F, Russo T, et al. Proportion of older people in the community as a predictor of increasing stroke incidence. Neuroepidemiology. 2001;20:91–95. doi: 10.1159/000054766. [DOI] [PubMed] [Google Scholar]
- Zhu W, Mao Y, Zhao Y, Zhou LF, Wang Y, Zhu JH, et al. Transplantation of vascular endothelial growth factor-transfected neural stem cells into the rat brain provides neuroprotection after transient focal cerebral ischemia Neurosurgery 200557325–333.discussion 325-333. [DOI] [PubMed] [Google Scholar]
- Borlongan CV. Cell therapy for stroke: remaining issues to address before embarking on clinical trials. Stroke. 2009;40:S146–S148. doi: 10.1161/STROKEAHA.108.533091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani Z, O'Reilly J, Pearse Y, Stroemer P, Tang E, Sinden J, et al. Human neural progenitor cell engraftment increases neurogenesis and microglial recruitment in the brain of rats with stroke. PLoS ONE. 2012;7:e50444. doi: 10.1371/journal.pone.0050444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury MH, Nagai A, Bokura H, Nakamura E, Kobayashi S, Yamaguchi S. Age-related changes in white matter lesions, hippocampal atrophy, and cerebral microbleeds in healthy subjects without major cerebrovascular risk factors. J Stroke Cerebrovasc Dis. 2011;20:302–309. doi: 10.1016/j.jstrokecerebrovasdis.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Gazoti Debessa CR, Mesiano Maifrino LB, Rodrigues de Souza R. Age related changes of the collagen network of the human heart. Mech Ageing Dev. 2001;122:1049–1058. doi: 10.1016/s0047-6374(01)00238-x. [DOI] [PubMed] [Google Scholar]
- Venkataraman K, Khurana S, Tai TC. Oxidative stress in aging–matters of the heart and mind. Int J Mol Sci. 2013;14:17897–17925. doi: 10.3390/ijms140917897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent S, Tremblay S, Chen KW, Ayutyanont N, Roontiva A, Castellano CA, et al. Brain glucose and acetoacetate metabolism: a comparison of young and older adults. Neurobiol Aging. 2013;35:1386–1395. doi: 10.1016/j.neurobiolaging.2013.11.027. [DOI] [PubMed] [Google Scholar]
- Lindeman RD, Goldman R. Anatomic and physiologic age changes in the kidney. Exp Gerontol. 1986;21:379–406. doi: 10.1016/0531-5565(86)90044-6. [DOI] [PubMed] [Google Scholar]
- Epstein M. Aging and the kidney. J Am Soc Nephrol. 1996;7:1106–1122. doi: 10.1681/ASN.V781106. [DOI] [PubMed] [Google Scholar]
- Tripathy D, Sanchez A, Yin X, Martinez J, Grammas P. Age-related decrease in cerebrovascular-derived neuroprotective proteins: effect of acetaminophen. Microvasc Res. 2012;84:278–285. doi: 10.1016/j.mvr.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azari H, Sharififar S, Rahman M, Ansari S, Reynolds BA. Establishing embryonic mouse neural stem cell culture using the neurosphere assay. J Vis Exp. 2011;47:2457. doi: 10.3791/2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YF, Lu CZ, Xie J, Zhao YX, Yang GY. Apoptosis inhibition in ischemic brain by intraperitoneal PTD-BIR3-RING (XIAP) Neurochem Int. 2006;48:50–59. doi: 10.1016/j.neuint.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Hao Q, Chen Y, Zhu Y, Fan Y, Palmer D, Su H, et al. Neutrophil depletion decreases VEGF-induced focal angiogenesis in the mature mouse brain. J Cereb Blood Flow Metab. 2007;27:1853–1860. doi: 10.1038/sj.jcbfm.9600485. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Pike BR, O'Dell DM, Lyeth BG, Jenkins LW. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma. 1994;11:187–196. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- Li Y, Chopp M, Chen J, Wang L, Gautam SC, Xu YX, et al. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20:1311–1319. doi: 10.1097/00004647-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Sanberg PR. Elevated body swing test: a new behavioral parameter for rats with 6-hydroxydopamine-induced hemiparkinsonism. J Neurosci. 1995;15:5372–5378. doi: 10.1523/JNEUROSCI.15-07-05372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkerth RD. Descriptive analysis and quantification of angiogenesis in human brain tumors. J Neurooncol. 2000;50:165–172. doi: 10.1023/a:1006499824379. [DOI] [PubMed] [Google Scholar]
- Weick JP, Liu Y, Zhang SC. Human embryonic stem cell-derived neurons adopt and regulate the activity of an established neural network. Proc Natl Acad Sci USA. 2011;108:20189–20194. doi: 10.1073/pnas.1108487108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Xie L, Sun Y, Mao XO, Wang Y, et al. Ischemia-induced neurogenesis is preserved but reduced in the aged rodent brain. Aging Cell. 2004;3:373–377. doi: 10.1111/j.1474-9728.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- Kurotobi T, Sato H, Kinjo K, Nakatani D, Mizuno H, Shimizu M, et al. Reduced collateral circulation to the infarct-related artery in elderly patients with acute myocardial infarction. J Am Coll Cardiol. 2004;44:28–34. doi: 10.1016/j.jacc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- Jin K, Mao X, Xie L, Greenberg RB, Peng B, Moore A, et al. Delayed transplantation of human neural precursor cells improves outcome from focal cerebral ischemia in aged rats. Aging Cell. 2010;9:1076–1083. doi: 10.1111/j.1474-9726.2010.00638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Chen X, Hu C, Li J, Yu Z, Cai W. Transplantation of neural stem cells expressing hypoxia-inducible factor-1alpha (HIF-1alpha) improves behavioral recovery in a rat stroke model. J Clin Neurosci. 2010;17:92–95. doi: 10.1016/j.jocn.2009.03.039. [DOI] [PubMed] [Google Scholar]
- Li L, Jiang Q, Ding G, Zhang L, Zhang ZG, Li Q, et al. Effects of administration route on migration and distribution of neural progenitor cells transplanted into rats with focal cerebral ischemia, an MRI study. J Cereb Blood Flow Metab. 2010;30:653–662. doi: 10.1038/jcbfm.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsalia V, Allison SJ, Cusulin C, Monni E, Kuzdas D, Kallur T, et al. Cell number and timing of transplantation determine survival of human neural stem cell grafts in stroke-damaged rat brain. J Cereb Blood Flow Metab. 2011;31:235–242. doi: 10.1038/jcbfm.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruma K, Nakagawa T, Morimoto N, Minami M, Hara H, Uehara T, et al. Glucocorticoid modulatory element-binding protein 1 binds to initiator procaspases and inhibits ischemia-induced apoptosis and neuronal injury. J Biol Chem. 2006;281:11397–11404. doi: 10.1074/jbc.M510597200. [DOI] [PubMed] [Google Scholar]
- Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131:616–629. doi: 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- Greenberg DA. Angiogenesis and stroke. Drug News Perspect. 1998;11:265–270. doi: 10.1358/dnp.1998.11.5.657287. [DOI] [PubMed] [Google Scholar]
- Zhu W, Fan Y, Frenzel T, Gasmi M, Bartus RT, Young WL, et al. Insulin growth factor-1 gene transfer enhances neurovascular remodeling and improves long-term stroke outcome in mice. Stroke. 2008;39:1254–1261. doi: 10.1161/STROKEAHA.107.500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dihne M, Hartung HP, Seitz RJ. Restoring neuronal function after stroke by cell replacement: anatomic and functional considerations. Stroke. 2011;42:2342–2350. doi: 10.1161/STROKEAHA.111.613422. [DOI] [PubMed] [Google Scholar]
- Bacigaluppi M, Pluchino S, Peruzzotti-Jametti L, Kilic E, Kilic U, Salani G, et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132:2239–2251. doi: 10.1093/brain/awp174. [DOI] [PubMed] [Google Scholar]
- Bonnamain V, Neveu I, Naveilhan P. Neural stem/progenitor cells as a promising candidate for regenerative therapy of the central nervous system. Front Cell Neurosci. 2012;6:17. doi: 10.3389/fncel.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DA, Jin K. Vascular endothelial growth factors (VEGFs) and stroke. Cell Mol Life Sci. 2013;70:1753–1761. doi: 10.1007/s00018-013-1282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher KI, Andres RH, Fukuhara T, Bieri G, Hasegawa-Moriyama M, He Y, et al. Neural progenitor cells regulate microglia functions and activity. Nat Neurosci. 2012;15:1485–1487. doi: 10.1038/nn.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Takano T, Oberheim N, Cotrina ML, Nedergaard M. Astrocytes and ischemic injury. Stroke. 2009;40:S8–S12. doi: 10.1161/STROKEAHA.108.533166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.