Abstract
Cerebral small vessel disease, mainly characterized by white matter lesions and lacunes, has a high clinical impact as it leads to vascular dementia. Recent studies have shown that this disease impairs frontoparietal networks. Here, we apply resting-state magnetic resonance imaging and data-driven whole-brain imaging analysis methods (eigenvector centrality) to investigate changes of the functional connectome in early small vessel disease. We show reduced connectivity in frontoparietal networks, whereas connectivity increases in the cerebellum. These functional changes are closely related to white matter lesions and typical neuropsychological deficits associated with small vessel disease.
Keywords: centrality, functional connectivity, microangiopathy, small vessel disease
Introduction
Cerebral small vessel disease (SVD) or cerebral microangiopathy describes a state of impaired blood circulation in the arterioles of the brain and is an important cause of cognitive impairment and vascular dementia.1, 2 Magnetic resonance imaging (MRI) is successfully employed to identify lacunar infarcts, white matter lesions (WMLs, example in Supplementary Figure S1) and alterations in white matter diffusivity in this disease.3 These indirect markers can be related to cognitive impairment.1, 3 It has been shown that white matter integrity affects functional connectivity.4 However, the effects of small WML on functional connectivity remain widely unknown.
Although patients in early SVD, a pre-stage of vascular dementia,3 show behavioral differences, the underlying alterations of large-scale functional connectivity might provide us with a more objective measure of the effects of WML. Objective and early clinical diagnostics might aid and complement therapeutical interventions.
In this study we aim to identify alterations in the functional connectome owing to SVD using resting-state functional MRI (rs-fMRI). Alterations in connectivity have been reported for the posterior cingulate cortex5, 6 and in the default mode network.7 As recent investigations have demonstrated a cerebellar role in cognitive processes,8 we also included the cerebellum in our analysis. Subsequently, we assess the behavioral relevance of the functional changes.
For analyzing the human connectome, network centrality analysis has become an important tool which captures the importance of each brain region by its connectedness.9 We use this data-driven approach to investigate the relationship between whole-brain alterations, physiologic scores and behavioral performance in patients suffering from early SVD. As our patients were mainly affected by WML in frontal and parietal areas, we hypothesized reductions of particularly frontoparietal connectivity in correlation with WML. White matter hyperintensities are consistently associated with neuropsychological impairments, namely psychomotor slowing.1, 2 We hypothesized a reduction of connectivity in areas particularly involved in attention processes such as the inferior frontal junction, and complex motoric actions such as premotor and supplementary motor area.
Materials and methods
rs-fMRI, T1- and T2-weighted anatomic data were acquired from all patients and controls. rs-fMRI data were acquired using an echo-planar image pulse sequence (300 volumes, TR of 2.3 seconds, voxel resolution of 3 × 3 × 4 mm3). T1 anatomic scans were obtained using a MPRAGE sequence (voxel resolution of 1 mm3).
Twelve patients with early SVD were recruited among former patients of the Clinic for Cognitive Neurology of the University Hospital Leipzig. Twenty-five healthy individuals matched for age, intelligence, education, and gender (Supplementary Table S1) were included from the volunteer database of the MPI CBS, Leipzig. Four control individuals were excluded owing to microangiopathic alterations (age-related white-matter changes10 (ARWMC) score >2) and one patient owing to a cerebellar infarct. Patients had been diagnosed with SVD after thorough clinical examination and all had an ARWMC score >2. Further exclusion criteria for all subjects were a history of psychiatric or neurologic disorders including stroke, craniocerebral injury, neurodegenerative disease or dementia. The research protocol was approved by the ethics committee of the University of Leipzig and was in accordance with the latest version of the Declaration of Helsinki. All participants gave informed written consent.
T2-weighted and FLAIR images were rated independently by two experienced clinicians blind to the clinical data according to the ARWMC scale.10 WML were defined as hyperintensities on both T2-weighted and FLAIR images of >5 mm in diameter. Lacunes were defined as hypointense signal alterations on both T2-weighted and FLAIR images >2 mm in diameter and rated within the same regions. Alterations >15 mm in diameter were rated as infarcts and respective subjects excluded. Interrater agreement measured with intraclass correlation coefficient κ was high (WML score κ=0.98; lacunes score κ=0.89). The two ratings were averaged for further analyses. Neuropsychological testing was performed using the Consortium to Establish a Registry for Alzheimer's disease (CERAD) test battery including tests of global functioning (Mini-Mental State Examination, MMSE), executive function and speed3 (Trail-Making-Test part A and B, phonemic and semantic fluency). In addition, executive functions were assessed by a Stroop task.2 Further subtests of the CERAD were included to control for memory abilities (word list immediate and delayed recall, recognition and figure recall), visuoconstructive abilities (figure copying) and word-finding (Boston Naming Test). A vocabulary test was administered to match groups for premorbid verbal intelligence (Supplementary Table S1). The testing was performed by two experienced psychology master students balanced across groups within 90 minutes.
rs-fMRI data were preprocessed using FSL, AFNI, and SPM. The steps comprised: discarding the first four volumes, slice-time correction, B0-fieldmap and motion correction, 6 mm FWHM spatial smoothing, four-dimensional mean-based intensity normalization, removing linear and quadratic trends, regressing out eight nuisance signals (white matter, cerebrospinal fluid and six motion parameters), band-pass temporal filtering (0.01 to 0.1 Hz). Spatial linear normalization to MNI space was performed using individual skull-stripped T1 as a prior. Supplementary Material contains a more detailed description.
Eigenvector centrality (EC) is a network centrality analysis9 which reflects local connectivity and weights each connection by its importance. Connections to regions which are themselves highly connected receive a higher weight and vice versa. EC is computationally efficient which enables centrality mapping on the voxel level and does not require any initial thresholding of connections. We used the EC implementation in LIPSIA.
For statistical testing we used AFNI with age, gender and micro-movements as covariates. Micro-movements were measured in average frame displacement which is the average of rotation and translation differences across time-points in mm. We performed a two-sample t-test for our group effect and whole-brain correlation analysis between the centrality of every voxel and the physiologic and behavioral scores across all individuals (patients and controls). Changes in connectivity with respect to clinical symptoms were only evaluated in those behavioral tasks where patients showed significantly poorer performance in comparison with healthy controls (Supplementary Table S2). All centrality group results were thresholded with P<0.01 on a voxel and P<0.05 at a cluster level (39 voxels) using AlphaSim (AFNI). Visualization of the cerebrum was performed using SUMA, flat maps of the cerebellum were created with CARET and SUIT.
Results
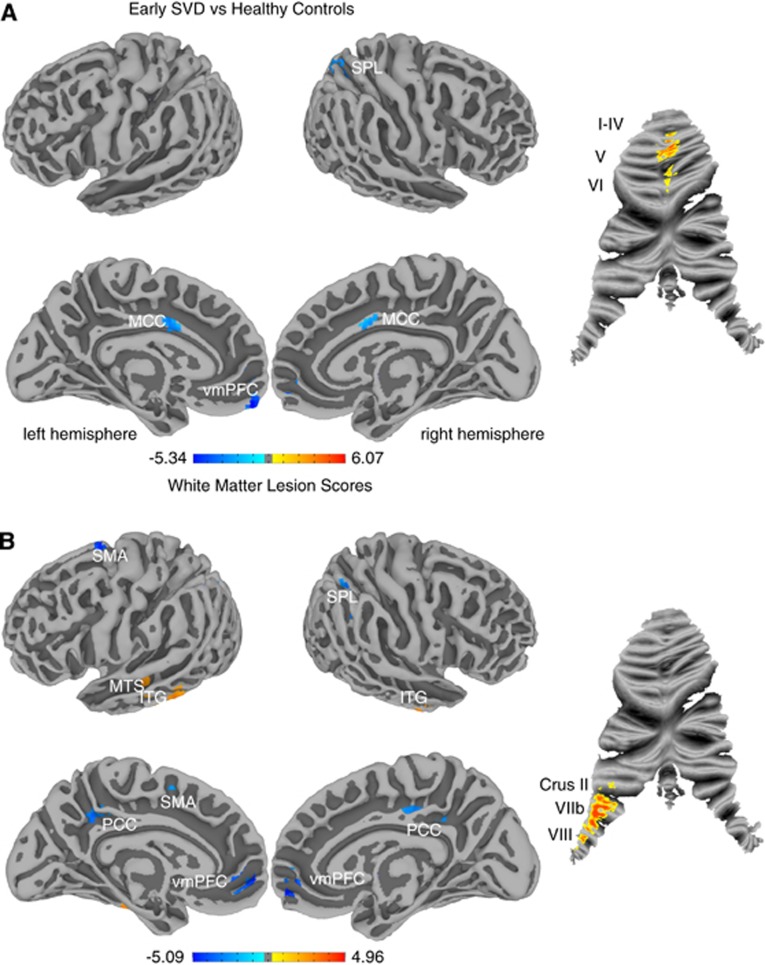
The group comparison of functional connectivity yielded significant centrality changes in SVD (Figure 1A). Patients showed decreased connectivity in left ventromedial prefrontal cortex (vmPFC, d=0.95, r=0.43), bilateral midcingulate cortex (MCC, d=1.16, r=0.50) and right superior parietal lobe (SPL, d=1.27, r=0.54) as well as increased centrality in bilateral cerebellar regions I to VI (d=1.28, r=0.54). Correlation between WML scores and centrality maps (Figure 1B) over the whole sample revealed decreased centrality in bilateral vmPFC (r=−0.59), posterior cingulate cortex (PCC, r=−0.66), left supplementary motor area (SMA, r=−0.64) and right SPL (r=−0.63) and increased centrality in bilateral inferior temporal gyrus (ITG, r=0.52) and left middle temporal sulcus (MTS, r=0.68). In the cerebellum, connectivity was increased in left lobules VIIb/VIII and Crus II in the patient group (r=0.67). Correlation of lacunar scores with centrality did not reveal significant results.
Figure 1.
Functional connectivity changes are displayed on cerebral surface maps and cerebellar flat maps. Increases are marked in hot colors and decreases are marked in cold colors (P<0.05, cluster corrected). (A) Significant differences in eigenvector centrality between the group of small vessel disease patients and healthy controls. Increases are found in cerebellar regions I to VI, whereas decreases are found in ventromedial prefrontal cortex (vmPFC), midcingulate cortex (MCC) and superior parietal lobe (SPL). (B) Regions with a significant correlation to the white matter lesion score. Positive correlations are found in the middle temporal sulcus (MTS), inferior temporal gyrus (ITG), cerebellar lobules Crus II, VIIb, and VIII; negative correlations are found in vmPFC, supplementary motor area (SMA), posterior cingulate cortex (PCC), and SPL.
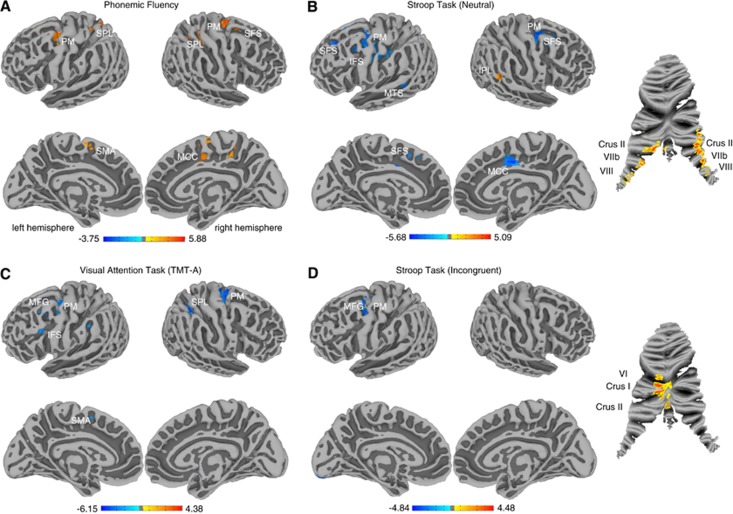
Relevance of changes in connectivity to clinical symptoms is represented as significant correlations between task performance measures and the centrality measure (Figure 2). Patients showed a slower processing speed in the Trail-Making-Test part A (TMT-A), the neutral and incongruent conditions of the Stroop task, and semantic and phonemic fluency measures (Supplementary Table 2). We found positive correlations between performance on the phonemic fluency task and centrality in bilateral SPL (r=0.63), SMA (r=0.64), premotor cortex (PM, r=0.66), MCC and posterior superior frontal sulcus (SFS, r=0.60; Figure 2A). For reaction time in the neutral condition of the Stroop task, we found negative correlations to centrality in bilateral PM (r=−0.62), SFS (r=−0.71), left inferior frontal sulcus (IFS, r=−0.71), left SMA (r=−0.71), left MTS (r=−0.73), and right MCC (r=−0.68), and positive correlations with inferior parietal lobe (r=0.65) and cerebellar lobules Crus II, VIIb and VIII (left r=0.69, right r=0.75, Figure 2B). For reaction time in the TMT-A we found negative correlation to centrality in bilateral PM (right r=−0.69, left r=−0.65), left posterior middle frontal gyrus (MFG, r=−0.65), left IFS (r=−0.69), right SPL (r=−0.56), and left SMA (r=−0.65) (Figure 2C). For reaction time in the incongruent condition of the Stroop task, we found negative correlations to centrality in left PM/posterior MFG (r=−0.73) and positive correlations with cerebellar regions VI, Crus I, and Crus II (r=0.58, Figure 2D). We found performance in the TMT-A and the phonemic fluency task to be linearly dependent (r=−0.47, P=0.007), the incongruent condition of the Stroop task was correlated with the TMT-A (r=0.59, P=0.0004) and the phonemic fluency task (r=−0.53, P=0.002). None of the other presented task parameters showed collinearity. For semantic fluency no significant association with centrality was observed. Supplementary Figures S2–S7 present scatter plots of the corresponding results in Figures 1 and 2. Supplementary Figures S8 and S9 represent the correlation between centrality and time needed in TMT-A and Stroop neutral condition without the respective slowest subject, who might be regarded as an outlier and suspected to have driven the results. Removing this subject did not change the analysis' results substantially.
Figure 2.
Cerebral surface maps and cerebellar flat maps show significant correlations between task performance and centrality for tasks with significant group differences. Positive correlations are displayed in hot and negative correlations in cold colors (P<0.05, cluster corrected). (A) Correlation between centrality and number of words produced in a phonemic fluency task. Positive correlations are found in premotor (PM), posterior superior frontal sulcus (SFS), midcingulate cortex (MCC), supplementary motor area (SMA), and superior parietal lobe (SPL). (B) Correlation between centrality and reaction time in the neutral condition of the Stroop task. Negative correlations were found in PM, SFS, inferior frontal sulcus (IFS), middle temporal sulcus (MTS), and MCC. Positive correlations were found in Crus II, VIIb, and VIII of the cerebellum and the inferior parietal lobe (IPL). (C) Correlation between centrality and time needed to complete a visual attention task (TMT-A—Trail-Making-Test part A). Negative correlations were found in PM, middle frontal gyrus (MFG), supplementary motor area (SMA), SPL, and IFS. (D) Correlation between centrality and reaction time in the incongruent condition of the Stroop task. Negative correlations were found in PM/posterior MFG. Positive correlations were found in VI, Crus I and II of the cerebellum.
Discussion
In this study we investigated alterations of the functional connectome owing to early SVD and evaluated their relevance to clinical symptoms. For the group difference we have shown a breakdown of frontoparietal hubs in patients with early SVD, closely related to WML scores. Effects can be explained by the disruption of frontoparietal white matter pathways owing to WML, which in our sample were mainly located in frontal and parietal areas (Supplementary Table S1) and gives further evidence for the hypothesis that frontoparietal networks are primarily disrupted in SVD.2, 11 The reduction of centrality in the vmPFC and SPL are in line with results by Yi et al.7 We found fewer widespread group differences, which might be explained by the earlier disease state of our patient cohort (MMSE of 27.6±1.5 compared with MMSE of 25.7±2.7) or the different centrality method (degree centrality) used. Reduced connectivity to the medial frontal cortex owing to white matter hyperintensities was also found by Wu et al.6
Although our cohort was not suffering from dementia (Supplementary Table S1, S2) the subjects with more severe disease state showed reduced centrality in the PCC, an area which has been shown to have reduced metabolism and perfusion in Alzheimer's disease and its prodromal syndrome mild cognitive impairment.12 This finding is particularly interesting as Alzheimer's disease is often associated with SVD.
The relevance of our brain findings for clinical symptoms revealed that the lower the centrality in sensorimotor areas, the slower the reaction times in the TMT-A and the two conditions of the Stroop task. Together with positive correlations between centrality in secondary sensorimotor areas and phonemic fluency these findings reflect upon the concept that WML express itself in mild psychomotor slowing rather than a severe degree of specific cognitive impairments.1, 2 Although correlations between neuropsychological test scores and centrality measures identified additionally prefrontal areas, known to be related specifically to executive dysfunction,2 the group and correlation analysis with WML scores did not confirm effects in these brain regions.
Task-fMRI studies report increased and decreased activation in association with WML. Venkatraman et al13 report a reduced activation in motor and premotor areas during psychomotor performance in individuals with more WML. However, Aizenstein et al14 found that patients with late life depression show a positive correlation between activation in prefrontal and limbic areas and WML using an affective reactivity task. Although we also found premotor and higher-order regions to be affected by WML, the relationship between functional connectivity and activation is complex15 and difficult to interpret.
The consistent inverse pattern of increased centrality in the cerebellum and decreased centrality in the cerebrum has, to our knowledge, not been reported in SVD yet. We found connectivity changes in cerebellar regions which are connected to frontoparietal cognitive networks (lobules Crus II, VIIb) as well as sensorimotor regions (lobules I to V, VIII).8 As WML occur mainly in frontoparietal regions, one might hypothesize that frontoparietal hypoconnectivity might be compensated by cerebellar hyperconnectivity.
Although SVD is a heterogeneous disease which makes group analysis and region-specific hypothesis testing difficult, the application of data-driven centrality analysis enabled us to identify affected network hubs.
This cross-sectional human imaging study only provides correlational but no causal associations which might be approached by longitudinal study designs. Micro-movements represent another limitation of the current study. Although we could not find a significant group difference (Supplementary Table S1), we accounted for this potential bias by using average frame displacement as a covariate in our analyses.
In conclusion, our study provides a link between the disruption of white matter pathways, behavioral impairment and functional interaction between gray matter regions in early SVD.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
KA, AV & MLS are supported by LIFE—Leipzig Research Center for Civilization Diseases at the University of Leipzig–funded by European Union, European Regional Development Fund and by Free State of Saxony within the framework of the excellence initiative. MLS is additionally supported by the German Consortium for Frontotemporal Lobar Degeneration, funded by the German Federal Ministry of Education and Research, by MaxNet Aging, and, together with KM, by the Parkinson's Disease Foundation (Grant No. PDF-IRG-1307). ER and AV are supported by IFB—Integrated Research and Treatment Center Adiposity Diseases at the University of Leipzig, funded by German Federal Ministry of Education and Research.
Supplementary Material
References
- Selnes OA, Vinters HV. Vascular cognitive impairment. Nat Clin Pract Neurol. 2006;2:538–547. doi: 10.1038/ncpneuro0294. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Cutini S, Wahl MM, Scheid R, von Cramon DY. Neurovascular coupling is impaired in cerebral microangiopathy—An event-related Stroop study. Neuroimage. 2007;34:26–34. doi: 10.1016/j.neuroimage.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Quinque EM, Arelin K, Dukart J, Roggenhofer E, Streitbuerger DP, Villringer A, et al. Identifying the neural correlates of executive functions in early cerebral microangiopathy: a combined VBM and DTI study. J Cereb Blood Flow Metab. 2012;32:1869–1878. doi: 10.1038/jcbfm.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel SJ, Bokde ALW, Meindl T, Amaro E, Soldner J, Reiser MF, et al. White matter microstructure underlying default mode network connectivity in the human brain. Neuroimage. 2010;49:2021–2032. doi: 10.1016/j.neuroimage.2009.10.067. [DOI] [PubMed] [Google Scholar]
- Sun Y, Qin L, Zhou Y, Xu Q, Qian L, Tao J, et al. Abnormal functional connectivity in patients with vascular cognitive impairment, no dementia: a resting-state functional magnetic resonance imaging study. Behav Brain Res. 2011;223:388–394. doi: 10.1016/j.bbr.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Wu M, Andreescu C, Butters M. Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Res. 2011;194:39–46. doi: 10.1016/j.pscychresns.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L, Wang J, Jia L, Zhao Z, Lu J, Li K, et al. Structural and functional changes in subcortical vascular mild cognitive impairment: a combined voxel-based morphometry and resting-state fMRI study. PLoS One. 2012;7:e44758. doi: 10.1371/journal.pone.0044758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 2012;11:352–365. doi: 10.1007/s12311-011-0260-7. [DOI] [PubMed] [Google Scholar]
- Lohmann G, Margulies DS, Horstmann A, Pleger B, Lepsien J, Goldhahn D, et al. Eigenvector centrality mapping for analyzing connectivity patterns in fMRI data of the human brain. PLoS One. 2010;5:e10232. doi: 10.1371/journal.pone.0010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjogren M, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–1322. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Anatomic and behavioral aspects of frontal-subcortical circuits. Ann NY Acad Sci. 1995;769:1–13. doi: 10.1111/j.1749-6632.1995.tb38127.x. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Stein T, Maslowski N, Neumann J. Neural correlates of Alzheimer's disease and mild cognitive impairment: a systematic and quantitative meta-analysis involving 1351 patients. Neuroimage. 2009;47:1196–1206. doi: 10.1016/j.neuroimage.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman VK, Aizenstein H, Guralnik J, Newman AB, Glynn NW, Taylor C, et al. Executive control function, brain activation and white matter hyperintensities in older adults. Neuroimage. 2010;49:3436–3442. doi: 10.1016/j.neuroimage.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenstein H, Andreescu C. fMRI correlates of white matter hyperintensities in late-life depression. Am J Psychiatry. 2011;168:1075–1082. doi: 10.1176/appi.ajp.2011.10060853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M, Kelly C, Zuo X-N, Di Martino A, Biswal BB, Castellanos FX, et al. Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. Neuroimage. 2010;50:1690–1701. doi: 10.1016/j.neuroimage.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.