Abstract
In patients with spontaneous intracerebral hemorrhage (ICH) coexisting abnormalities on brain imaging can provide clues on the etiology of the underlying small vessel disease. We examined cortical cerebral microinfarcts as a novel marker of coexistent vascular damage in ICH. Twelve patients with spontaneous ICH and 15 controls underwent 7Tesla magnetic resonance imaging (MRI). Microinfarcts were present in 9 of 12 patients with spontaneous ICH, and in 5 of 15 controls. This explorative study shows, for the first time, that microinfarcts appear to be a very common vascular comorbidity in spontaneous ICH. Future larger studies should further assess the etiological significance of these lesions.
Keywords: high-field MRI, microbleeds, microinfarcts, small vessel disease, spontaneous intracerebral hemorrhage
Introduction
Spontaneous, or primary, intracerebral hemorrhage (ICH) is the deadliest subtype of stroke with a 30-day mortality of around 40%.1 In contrast with ischemic stroke and subarachnoid hemorrhage, advances in the field have not resulted in a decrease in case fatality over the past 25 years.1 One factor that may have delayed progress is that the etiology of spontaneous ICH is heterogeneous and still poorly understood. As long as we cannot pinpoint the specific cause in an individual patient we cannot provide targeted treatment. Typically, deep ICH (i.e., located in the basal ganglia or thalamus) has been associated with hypertension, whereas lobar ICH in elderly patients is considered to be caused by cerebral amyloid angiopathy (CAA). This distinction is likely to be an oversimplification as many patients with deep ICH do not have hypertension,2 and pathologic studies found CAA in only one-third of patients with lobar ICH.3
Apart from the ICH itself, coexisting brain imaging abnormalities can provide clues on the potential cause of spontaneous ICH. Lobar cerebral microbleeds (CMBs) and superficial siderosis on T2*-weighted gradient-echo magnetic resonance imaging (MRI), for example, have been identified as helpful biomarkers of CAA. Cerebral microinfarcts (CMIs) are common manifestations of small vessel disease that have been linked to other vascular pathologies and dementia in autopsy studies.4 On pathologic examination, CMIs are characterized by microscopic regions of cellular death or tissue necrosis with gliosis, sometimes accompanied by cavitation.5 Whether the occurrence of CMIs may help to differentiate the type of small vessel diseases underlying spontaneous ICH is unclear. Recent advances in high-field MRI now allow detection of cortical CMIs in vivo.6 In this explorative study, we assessed whether cortical CMIs on 7Tesla magnetic resonance images can be observed in patients with deep or lobar spontaneous ICH and whether they are more frequent in patients than in controls.
Materials and methods
Study Population
We performed 7Tesla MRI scanning in 12 patients with spontaneous ICH under the age of 70, who presented to the UMCU (University Medical Center Utrecht), The Netherlands, who had no contraindications for 7Tesla MRI and in whom the clinical condition was stable enough to undergo MRI. In all patients, secondary causes of ICH were excluded by computed tomography angiography (5 patients), MRI/magnetic resonance angiography (all), or angiogram (7 patients). Fifteen healthy controls of similar age, without a history of neurologic or psychiatric disorders, recruited via their general practitioners as part of the PREDICT-MR study,7 served as a reference group. The study was approved by the medical ethics committee of the UMCU and all subjects gave written informed consent. The guidelines according to the Declaration of Helsinki were followed.
Magnetic Resonance Imaging Scanning Protocol
Scans were acquired on a whole-body 7Tesla MR system (Philips Healthcare, Cleveland, OH, USA) with a volume transmit and 16- or 32-channel receive head coil (Nova Medical, Wilmington, MA, USA). The standard scanning protocol included a fluid-attenuated inversion recovery (FLAIR) image (repetition time 8,000 ms; nominal echo time 300 ms; inversion time 2,250 ms; matrix size 312 × 304; voxel size 0.8 × 0.8 × 0.8 mm3; scan duration 12 minutes 48 seconds) and a T2*-weighted image (repetition time 37 ms; echo time 1 8.5 ms; echo time 2 19.1 ms; matrix size 444 × 353; voxel size 0.5 × 0.5 × 0.5 mm3; scan duration 9 minutes 15 seconds), which were used to assess small vessel pathology in these subjects.
Magnetic Resonance Imaging Rating
Rating was performed by two independent raters (SvV and WJ). Cortical CMIs were assessed on the FLAIR image and defined as hyperintense lesions, restricted to the cortex, distinct from perivascular spaces, and ⩽3 mm.6 Cerebral microbleeds were assessed on the dual echo T2* image, using the Microbleed Anatomical Rating Scale. Cerebral microbleeds directly adjacent to the ICH were not scored. Consensus was established between both raters in case of disagreement. The interrater agreement for both CMIs (intraclass correlation coefficient=0.96) and CMBs (intraclass correlation coefficient=0.96) was good. Volume of the intraparenchymal ICH was assessed on CT on admission using an in-house developed tool.8
Statistical Analysis
Between-group differences in median numbers of CMBs and CMIs between patients and controls were analyzed with Mann–Whitney U tests.
Results
Patient characteristics are provided in Table 1. The 12 patients with ICH had a mean age of 53 years (SD 7), and 8 were male. Mean age of the 15 healthy controls was 55 years (SD 7), and 6 were male. Eight out of 12 patients had hypertension versus 5 out of 15 control subjects. Three out of 12 patients and 1 out of 15 control subjects had diabetes. Two patients had hyperlipidemia compared with 6 control subjects, 1 patient was a current smoker compared with none of the control subjects, and 1 patient had a history of alcohol abuse (not known for control subjects).
Table 1. Patient characteristics and MRI findings in 12 patients with spontaneous ICH.
| Patient | Sex (M/F) | Age at time of ICH (years) | Interval between ICH and MRI (days) | ICH location (volume) | Hypertensiona | Diabetes | Current smoking | Alcohol abuse | Hyperlipidemiab | Microbleeds N (location) | Microinfarcts N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 43 | 102 | Deep (16.1 mL) | Yes | Yes | No | No | No | 0 | 4 |
| 2 | M | 48 | 6 | Deep (21.6 mL) | Yes | Yes | No | No | No | 1 (infratentorial) | 0 |
| 3 | F | 59 | 346 | Deep (0.3 mL) | Yes | No | No | No | No | 4 (deep, lobar, infratentorial) | 0 |
| 4 | M | 46 | 68 | Deep (4.3 mL) | Yes | No | No | No | No | 2 (deep, lobar) | 2 |
| 5 | M | 56 | 2 | Deep (22.0 mL) | Yes | No | No | No | No | 3 (deep) | 0 |
| 6 | M | 49 | 32 | Deep (0.2 mL) | No | No | No | No | No | 0 | 1 |
| 7 | M | 52 | 205 | Lobar (35.3 mL) | Yes | No | Yes | Yes | No | 1 (deep) | 2 |
| 8 | F | 52 | 339 | Lobar (44.5 mL) | Yes | No | No | No | Yes | 2 (deep, lobar) | >100 |
| 9 | F | 57 | 103 | Lobar (89.7 mL) | No | No | No | No | No | 0 | 5 |
| 10 | M | 57 | 265 | Lobar (19.7 mL) | Yes | No | No | No | No | 26 (deep, lobar, infratentorial) | 10 |
| 11 | F | 68 | 477 | Lobar (43.5 mL) | No | No | No | No | No | 0c | 3 |
| 12 | M | 51 | 1127 | Lobar (15.8 mL) | No | Yes | No | No | Yes | 2 (lobar)c | 4 |
Abbreviations: F, female; FLAIR, fluid-attenuated inversion recovery; ICH, intracerebral hemorrhage; M, male; MRI, magnetic resonance imaging.
Patients were considered to have hypertension, if they received drug treatment for this condition before ICH or showed left ventricle hypertrophy on their electrocardiogram.
Patients were considered to have hyperlipidemia if their cholesterol levels were >6.5 mmol/L or if they received drug treatment for this condition.
Multiple microbleeds were observed in close proximity to the ICH (6 in patient 11 and >100 in patient 12).
Cortical CMIs were found in all 6 patients with lobar ICH and in 3 of 6 patients with deep ICH. The location of CMIs was not restricted to the hemisphere of the macrohemorrhage. Cortical CMIs were found in 5 of 15 control subjects. Median number of CMIs was 2.5 (range 0 to >100) in the patient group, compared with 0 (range 0 to 2) in the reference group (P value 0.01). This difference remained significant when the patient with >100 CMIs was excluded from the analysis (P value 0.02). Cerebral microbleeds were observed in 8 of 12 patients and in 4 of 15 controls. Patients had a higher number of CMBs (median number 1.5, range 0 to 26) than the reference group (median number 0, range 0 to 4; P value 0.03). In four of the patients, CMIs were observed in the absence of CMBs.
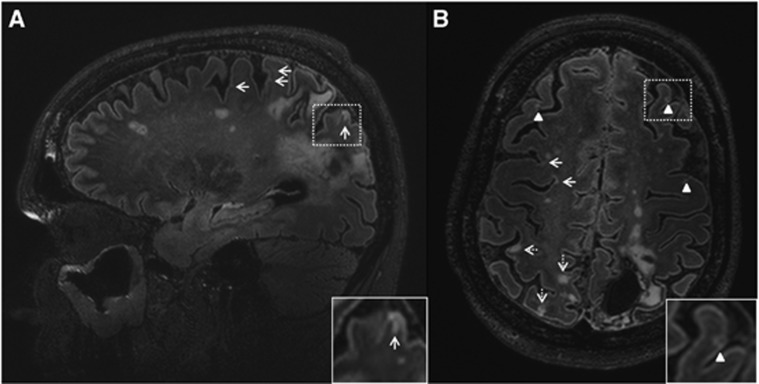
The patient with >100 CMIs not only had CMIs with gliosis, without cavitation, but also several cavitated cortical CMIs (i.e., hypointense on FLAIR with a hyperintense rim; Figure 1).
Figure 1.
Multiple cortical microinfarcts on the fluid-attenuated inversion recovery (FLAIR) scan of a 52-year-old woman (patient 8) with a left occipito-parietal intracerebral hemorrhage (ICH). A sagittal view (A) and a transversal view (B) of the brain are depicted. Numerous cortical microinfarcts (arrows, insert A) were present throughout the brain, including multiple with cavitation (arrowheads, insert B). In the cavitated cortical microinfarcts (insert B), note the interruption of the cortex and the hyperintense rim surrounding the hypointense cavity. Also many cortical infarcts (>3 mm) were present (dashed arrows).
Discussion
Multiple CMIs were found on 7Tesla MRI in the majority of patients with ICH, despite their relatively young age. Cortical CMIs occurred in patients with and without CMBs.
The occurrence of CMIs in the majority of patients with spontaneous ICH in this small series of relatively young patients is remarkable. As expected, the occurrence of CMBs and CMIs in the control group is lower than previously reported for older (mean age 72 and 68 years) healthy individuals at 7Tesla MRI.6, 9, 10 To the best of our knowledge, CMIs have never been examined before in patients with spontaneous ICH, either by neuropathology or by MRI. Neuropathologic studies reported a prevalence of CMIs of 24% in healthy elderly and 43% in patients with Alzheimer's disease.5 The few available neuropathologic studies in patients with different forms of vascular brain damage report frequencies ranging from 26% to 78%.5 Our small case series suggests that CMIs may be more frequent in patients with lobar ICH than in those with ICH in a deep location, but this observation requires further study in larger cohorts. Although 5 of the 6 patients with lobar ICH do not fulfill the Boston criteria for probable CAA, as they had deep CMBs or no CMBs, CAA cannot be excluded as a contributing factor. Neuropathologic studies have shown that severe CAA is associated with the presence of CMIs in patients with Alzheimer's disease.11
Our findings suggest that the underlying small vessel diseases in patients with spontaneous ICH not only result in macrobleeds and CMBs, but also in ischemic brain injury. In the majority of our patients, there was a substantial interval between the time of the ICH and the 7Tesla MRI. Hence, we cannot determine whether the ischemic injury was elicited by the acute event of the ICH or its treatment or an expression of the underlying vasculopathy with both hemorrhagic and ischemic insults. Recently, multiple prospective studies have shown that around one-third of patients have signs of acute ischemic brain injury on diffusion-weighted imaging on regular field strength MRI early after spontaneous ICH, and that their presence is associated with poor outcome.12, 13 A recent letter discussed whether some supposedly ischemic diffusion-weighted imaging lesions could in fact represent CMBs in evolution.14 With 7Tesla FLAIR not only acute microischemic lesions can be detected, but also persistent ones. Our study suggests that patients with spontaneous ICH have indeed a high load of ischemic brain injury in addition to the hemorrhagic burden of macrohemorrhages and microhemorrhages. Cavitation of some of the CMIs, as identified in one patient, is indicative of the chronic aspect of these ischemic lesions. It should be noted that 7Tesla FLAIR currently allows us to detect only the largest cortical CMIs, which are likely to represent only the tip of the iceberg of total CMI load.6 Also, further histopathologic validation of cortical CMIs as detected with 7Tesla FLAIR is needed.
This study has three important limitations. First, the number of patients is small. Nonetheless, we found a significant difference in number of CMIs between patients and controls. Second, we only had a FLAIR image to our disposal for the rating of CMIs, without an additional T2- or T1-weighted image. This may have affected CMI detection. Finally, it was impossible to perform rating of both CMIs and CMBs blinded to patient versus control status.
Further studies are needed to determine whether CMIs can be used as a biomarker of underlying etiology in ICH and whether they predict functional and cognitive outcome.
Acknowledgments
The authors would like to thank Willem Bouvy MD for his assistance acquiring the data and Hugo Kuijf PhD for providing us with the tool to calculate the volumes of the ICH.
The authors declare no conflict of interest.
Footnotes
Dr CJM Klijn is supported by a clinical established investigator grant of The Netherlands Heart Foundation (grant number 2012 T077) and an ASPASIA grant from ZonMw (grant number 015008048). Professor GJ Biessels is supported by a VIDI grant from ZonMw, The Netherlands Organisation for Health Research and Development (grant number 91711384), and a clinical established investigator grant from The Netherlands Heart Foundation (grant number 2010 T073).
References
- Van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- Jackson CA, Sudlow CL. Is hypertension a more frequent risk factor for deep than for lobar supratentorial intracerebral haemorrhage. J Neurol Neurosurg Psychiatry. 2006;77:1244–1252. doi: 10.1136/jnnp.2006.089292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Yamada M, Hayakawa M, Otomo E, Miyatake T. Cerebral amyloid angiopathy: a significant cause of cerebellar as well as lobar cerebral haemorrhage in the elderly. J Neurol Sci. 1993;116:135–141. doi: 10.1016/0022-510x(93)90317-r. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Hughes TM, White LR. Microinfarcts, brain atrophy, and cognitive function: the Honolulu Asia Aging Study Autopsy Study. Ann Neurol. 2011;70:774–780. doi: 10.1002/ana.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundel M, de Bresser J, van Dillen JJ, Kappelle LJ, Biessels GJ. Cerebral microinfarcts: a systematic review of neuropathological studies. J Cereb Blood Flow Metab. 2012;32:425–436. doi: 10.1038/jcbfm.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veluw SJ, Zwanenburg JJ, Engelen-Lee J, Spliet WG, Hendrikse J, Luijten PR, et al. In vivo detection of cerebral cortical microinfarcts with high-resolution 7T MRI. J Cereb Blood Flow Metab. 2013;33:322–329. doi: 10.1038/jcbfm.2012.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse LE, Gerritsen L, Zwanenburg JJ, Kuijf HJ, Luijten PR, Biessels GJ, et al. Subfields of the hippocampal formation at 7 T MRI: in vivo volumetric assessment. Neuroimage. 2012;61:1043–1049. doi: 10.1016/j.neuroimage.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Kuijf HJ.2013Image Processing Techniques for Quantification and Assessment of Brain MRI. (Doctoral dissertation) Utrecht University: Utrecht; ISBN: 978-90-393-6037-8; http://www.isi.uu.nl/Research/Publications/publicationview.php?id=2545 . [Google Scholar]
- Brundel M, Heringa SM, de Bresser J, Koek HL, Zwanenburg JJ, Kappelle JL, et al. High prevalence of cerebral microbleeds at 7Tesla MRI in patients with early Alzheimer's disease. J Alzheimers Dis. 2012;31:259–263. doi: 10.3233/JAD-2012-120364. [DOI] [PubMed] [Google Scholar]
- Van Veluw SJ, Heringa SM, Kuijf HJ, Koek HL, Luijten PR, Biessels GJ. Cerebral cortical microinfarcts at 7Tesla MRI in patients with early Alzheimer's disease. J Alzheimers Dis. 2013;39:163–167. doi: 10.3233/JAD-131040. [DOI] [PubMed] [Google Scholar]
- Soontornniyomkij V, Lynch MD, Mermash S, Pomakian J, Badkoobehi H, Clare R, et al. Cerebral microinfarcts associated with severe cerebral beta-amyloid angiopathy. Brain Pathol. 2010;20:459–467. doi: 10.1111/j.1750-3639.2009.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran S, Naidech AM. Ischemic brain injury after intracerebral hemorrhage: a critical review. Stroke. 2012;43:2258–2263. doi: 10.1161/STROKEAHA.112.655910. [DOI] [PubMed] [Google Scholar]
- Auriel E, Gurol ME, Ayres A, Dumas AP, Schwab KM, Vashkevich A, et al. Characteristic distributions of intracerebral hemorrhage-associated diffusion-weighted lesions. Neurology. 2012;79:2335–2341. doi: 10.1212/WNL.0b013e318278b66f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoamanesh A, Catanese L, Sakai O, Pikula A, Kase CS. Diffusion-weighted imaging hyperintensities in intracerebral haemorrhage: microinfarcts or microbleeds. Ann Neurol. 2013;73:795–796. doi: 10.1002/ana.23853. [DOI] [PubMed] [Google Scholar]