Abstract
The success of serotonin-selective reuptake inhibitors has lent support to the monoamine theory of major depressive disorder (MDD). This issue has been addressed in a number of molecular imaging studies by positron emission tomography or single-photon emission computed tomography of serotonin reuptake sites (5-HTT) in the brain of patients with MDD, with strikingly disparate conclusions. Our meta-analysis of the 18 such studies, totaling 364 MDD patients free from significant comorbidities or medication and 372 control subjects, revealed reductions in midbrain 5-HTT (Hedges' g=−0.49; 95% CI: (−0.84, −0.14)) and amygdala (Hedges' g=−0.50; 95% CI: (−0.78, −0.22)), which no individual study possessed sufficient power to detect. Only small effect sizes were found in other regions with high binding (thalamus: g=−0.24, striatum: g=−0.32, and brainstem g=−0.22), and no difference in the frontal or cingulate cortex. Age emerged as an important moderator of 5-HTT availability in MDD, with more severe reductions in striatal 5-HTT evident with greater age of the study populations (P<0.01). There was a strong relationship between severity of depression and 5-HTT reductions in the amygdala (P=0.01). Thus, molecular imaging findings indeed reveal widespread reductions of ∼10% in 5-HTT availability in MDD, which may predict altered spatial–temporal dynamics of serotonergic neurotransmission.
Keywords: 5-HT, depression, molecular imaging, PET, receptor imaging, SPECT
Introduction
An early formulation of the biogenic amine hypothesis of major depressive disorder (MDD) held that symptoms arise from a deficiency of noradrenaline and serotonin in brain, rather as motor symptoms of Parkinson's disease reflect loss of striatal dopamine. However, the evidence for a neurotransmitter abnormality in MDD remains inconclusive. Low concentrations in cerebrospinal fluid of the serotonin metabolite 5-hydroxy-indoleacetic acid have been associated with aggressiveness in males with personality disorders,1 and were predictive of suicide in previous attempters,2 and in a 5-year follow-up study of patients with melancholic depression.3 The serotonin transporter (5-HTT) in presynaptic terminals of serotonin fibers, and also in somatodendritic sites presents an important molecular target for such investigations; in vitro studies indicate a simple association between the density of 5-HTT and tissue concentration of serotonin,4 suggesting that the former can serve as a surrogate marker of the latter, at least in normal brain. Although autoradiographic analysis of cerebral cortex from suicide victims revealed no difference in the concentration of 5-HTT,5 others have reported reduced 5-HTT messenger RNA in midbrain post mortem.6 However, such findings may not be generalizable to MDD, as suicide cases may reflect a clinical subset of patients with greater aggression or disturbance of impulse control, as distinct from depressive symptoms per se. In theory, molecular imaging of 5-HTT in vivo might yield better-defined findings of the putative association between MDD and serotonergic transmission.7, 8
5-HTT ligands for single-photon emission computed tomography (SPECT) have tended to have low-specific binding, and are increasingly supplanted by tracers for positron emission tomography (PET), which offers greater specificity and sensitivity.9 As will be seen below, the composite of SPECT and PET studies to date have yielded a wide range of 5-HTT findings in MDD, which is confounded by the variety of end points used, and the potential for a variety of confounds arising from medication history, gender differences, age, among other factors. As much is perceived to be at stake in establishing the nature or extent of serotonergic abnormalities in MDD, the present lack of consensus is unsatisfactory. A similar state of affairs occurred for the case of molecular imaging markers of dopamine in schizophrenia. Recent meta-analyses have established the effect size of increased capacity for synthesis of striatal dopamine in patients with schizophrenia, as measured by PET studies with [18F]-fluoro-L-DOPA and other substrates for the enzyme DOPA decarboxylase, but failed to detect important differences in the availability of dopamine receptors or dopamine transporters.10, 11 Small sample size is generally a precondition for false-positive findings,12 so the meta-analyses have provided much-needed clarity about the extent of dopaminergic changes in schizophrenia. We determined to use similar methods to establish a better case for 5-HTT changes reported in molecular imaging studies of MDD. To this end, we searched published literature and considered the association of depression with factors such as age, gender, and medication status.
Materials and methods
Data Collection
The bibliographic databases Medline, ScienceDirect, Scopus, and PsycINFO were systematically searched in November 2013 using the terms ‘depression', ‘MDD' or ‘major depressive episode', and ‘PET', ‘SPECT' or ‘molecular imaging'. The automated search results were narrowed to studies reporting means and s.d. values of molecular imaging outcome measures reflecting cerebral 5-HTT binding in MDD patients and controls. Studies were excluded if subjects suffered from neurologic, severe somatic, psychotic, or affective disorders other than MDD and dysthymia. The remaining psychiatric comorbidities were assessed and became subject to sensitivity analyses. Current therapy with antidepressant medication was an exclusion criterion in the absence of a washout period, so as to avoid bias from residual 5-HTT occupancy, or medication-evoked changes. Next to the outcome for each region reported, demographic variables (age, sex), depression severity scores, type of tracer, type of outcome measure and psychiatric medication history (drug-free interval, number of drug-naïve subjects) were extracted from studies included in our analyses. Corresponding authors were contacted and asked if they were aware of any unpublished data on the topic.
Given the high interstudy and interrater variability in scoring depression severity, analyses involving these scores are strictly exploratory. We attempted to unify severity scores by considering the 21-item Hamilton Depression Rating Scale as equivalent to the 17-item scale with omission of the last four items.13 If available, published equations were used to translate other depression scores to Hamilton Depression Rating Scale,14 which is not possible for the Beck Depression Inventory. As some studies reported multiple scores including the Beck Depression Inventory, we ordered these studies by Beck Depression Inventory scores and assigned the two studies reporting Beck Depression Inventory exclusively an ‘equivalent' Hamilton Depression Rating Scale score.
Statistical Analysis
All statistical analyses were performed using the software package R 3.0.1. Computations specific to meta-analysis were executed as described below, and implemented in the metafor package, version 1.9-1.15 Studies providing values for both sexes were treated separately throughout the meta-analysis.
Individual study effect estimates
SPECT and PET studies of 5-HTT have a number of possible outcome measures, including the simple ratio of uptake in a region of interest relative to that in a non-binding reference region. When dynamic emission sequences are available, specific binding can be calculated relative to the reference tissue as binding potential (BPND), a dimensionless quantity proportional to the ratio of the saturation binding parameters, Bmax/Kd. When the arterial input function is measured, the equilibrium distribution volume of the tracer in brain (VT; ml/g) is reported, sometimes corrected for the plasma-free fraction. This diversity of outcome measures necessitates the calculation of standardized effect size estimates amenable to combination by meta-analysis. Therefore, we computed for each report and brain region the standardized mean difference (Hedges' g), sensitive to differences in the size of the study populations,16 and corrected for small positive bias.17 Hedges' g expresses the difference in means of two groups in units of pooled s.d. The corresponding unbiased estimates of the sampling variance were supplemented by 95% confidence intervals (CI) based on a non-central t-distribution calculated using the MBESS package, version 3.3.3.18
Summary effect estimates
A separate meta-analysis was conducted for each brain region appraised in more than three studies. Given our aim of drawing an unconditional inference about effects of MDD on 5-HTT expression, we opted against a fixed-effects model.19 In a random-effects model individual study estimates are weighted inversely proportional to the sum of their sampling variance (vi) and the between-study variance (τ2). The latter result was computed using restricted maximum likelihood estimation, which unlike the classic moment-based approach20 accommodates calculation of the summary effect size and τ2 from the same data. We assessed sensitivity of summary effect sizes to changes in τ2 using τ2-sensitivity plots. Furthermore, Higgins' I2 was calculated, which constitutes an intuitive measure of the variation of study estimates that is due to between-study heterogeneity.21 Confidence intervals for summary effect sizes were based on a t-distribution.22 Further sensitivity analyses were performed using the leave-one-out approach. Publication bias was investigated by inspecting funnel plots that portray the precision of individual studies against their effect estimates.
Influence of study population characteristics and imaging procedures
Sensitivity of estimates to depression severity, tracer, outcome measure, comorbidities, antidepressant medication history (minimum duration of drug washout and percentage of drug-naïve patients), and age differences between groups was each assessed by cumulative meta-analysis, e.g., studies were added to the analysis starting with those reporting the highest depression severity scores, or those using the tracer with highest signal-to-noise ratio, thus revealing the impact of each study on the summary effect estimate. The influence of the mean age and sex ratio of subjects on individual study estimates was appraised using scatter plots. Whenever a meaningful trend for an interval scaled variable was observed in cumulative meta-analyses or in scatter plots its inclusion in an exploratory mixed-effects model was attempted.
Results
Data from 18 published studies comprising 364 depressed subjects and 372 healthy controls were included (Table 1).
Table 1. Key data of selected studies.
|
Study |
Method | Tracer | Outcome measure |
Healthy controls |
Depressed patients |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1st author | Year | n | F | Age(y) | n | F | Age(y) | |||
| Malison75 | 1998 | SPECT | [123I]β-CIT | V3'' | 15 | 8 | 45.0 | 15 | 8 | 44.0 |
| Ichimiya76 | 2002 | PET | [11C]McN5652 | BPND | 21 | 0 | 42.3 | 7 | 0 | 43.0 |
| Meyer77 | 2004 | PET | [11C]DASB | BPND | 20 | 10 | 35.0 | 20 | 11 | 35.0 |
| Reivich78 | 2004 | PET | [11C]McN5652 | DVR | 4 | 3 | 43.5 | 4 | 1 | 45.0 |
| Newberg79 | 2005 | SPECT | [123I]ADAM | V3'' | 6 | 4 | 36.7 | 7 | 4 | 38.3 |
| Catafau26 | 2006 | SPECT | [123I]ADAM | SUR | 10 | 3 | 36.2 | 10 | 6 | 36.0 |
| Herold80 | 2006 | SPECT | [123I]ADAM | V3'' | 13 | 8 | 36.0 | 21 | 6 | 42.0 |
| Parsey81 | 2006 | PET | [11C]McN5652 | BPP | 43 | 21 | 38.8 | 12 | 8 | 34.5 |
| Staley82 | 2006 | SPECT | [123I]β-CIT | V3'' | 32 | 16 | 40.1 | 32 | 16 | 40.1 |
| Cannon25 | 2007 | PET | [11C]DASB | BPND | 34 | 25 | 33.0 | 18 | 12 | 35.0 |
| Joensuu83 | 2007 | SPECT | [123I]β-CIT | DVR | 19 | 16 | 30.6 | 29 | 24 | 28.3 |
| Ruhe51 | 2009 | SPECT | [123I]β-CIT | BPND | 48 | 31 | 42.3 | 45 | 29 | 42.3 |
| Reimold84 | 2011 | PET | [11C]DASB | BPND | 20 | 8 | 44.2 | 10 | 5 | 48.3 |
| Selvaraj23 | 2011 | PET | [11C]DASB | BPP | 24 | 0 | 42.4 | 12 | 0 | 42.1 |
| Newberg85 | 2012 | SPECT | [123I]ADAM | DVR | 10 | 3 | 44.8 | 20 | 5 | 41.0 |
| Ho27 | 2013 | SPECT | [123I]ADAM | SUR | 12 | 4 | 32.0 | 40 | 27 | 36.5 |
| Miller28 | 2013 | PET | [11C]DASB | BPP | 31 | 16 | 32.6 | 51 | 28 | 41.0 |
| Nye86 | 2013 | PET | [11C]ZIENT | BPND | 10 | 4 | 21.3 | 11 | 4 | 38.5 |
| Sum: 18 | 9 PET | 372 | 364 | |||||||
F, number of female participants; PET, positron emission tomography; SPECT, single-photon emission computed tomography.
Basic demographic information for all studies included in the meta-analysis is shown. In the case of studies reporting multiple outcome measures, the one specified in this table was used for calculations.
Brainstem
Four studies comprising 91 patients and 63 controls report on 5-HTT in brainstem (Supplementary Figure 1). In spite of the low heterogeneity of effects (I2=10%), unadjusted confidence intervals did not exclude zero (summary effect: −0.22; 95% CI (unadjusted): (−0.58, 0.13)). In cumulative meta-analysis, there was a trend towards stronger 5-HTT reductions in more severely depressed patient groups.
Midbrain
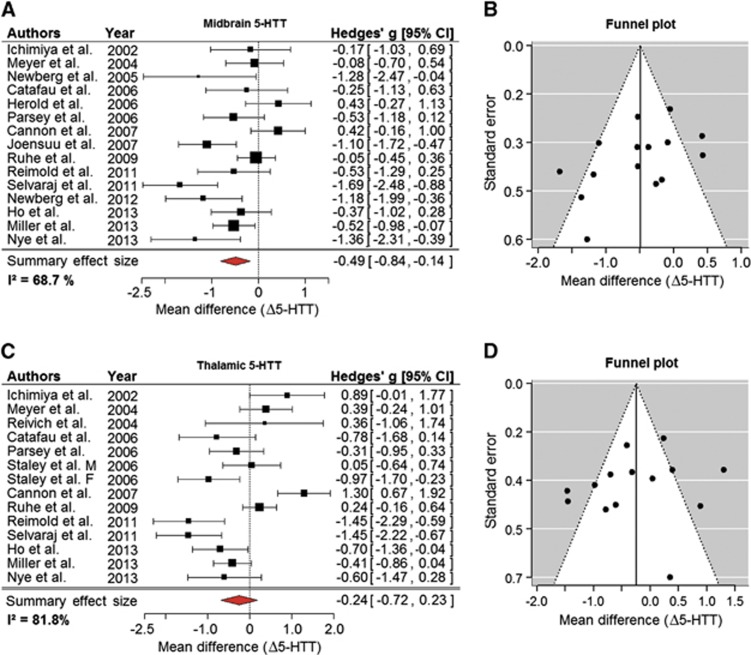
Fifteen studies characterized midbrain 5-HTT in a total of 313 patients and 321 controls (Figure 1A). Despite the considerable heterogeneity of individual study estimates (Higgins' I2=68.7%), the summary effect estimate (−0.49; 95% CI: (−0.84, −0.14)) clearly indicated lower 5-HTT binding in MDD. This result was robust to leave-one-out sensitivity analysis. The maximum shift of 95% CI toward zero was produced by excluding Selvaraj et al23 (−0.73, −0.08). Sensitivity to changes in τ2 estimates was negligible. The funnel plot for midbrain 5-HTT studies (Figure 1B) showed a slight asymmetry that was not supported by a regression test,24 testing if small studies were missing from our analysis (P=0.07). The remaining analyses concerning the population characteristics or imaging procedures did not detect any meaningful influence of these variables on effect estimates in the midbrain.
Figure 1.
Reductions of 5-HTT sites in midbrain and thalamus of major depressive disorder (MDD) patients. (A) A forest plot of reports on 5-HTT binding in the midbrain summarizing to an effect size of −0.49 s.d. (B) The corresponding funnel plot displays study precision as a function of effect estimate. Its slightly asymmetric appearance in this instance suggests the lack of one or two data points on the lower right, which implies some publication bias favoring small studies reporting significant decreases of 5-HTT in MDD. (C) A forest plot for imaging studies appraising thalamic 5-HTT, showing a slight reduction in depressed patients. Note the deviation of data published by Cannon et al,25 the 95% confidence intervals (CI) of which are separated by a substantial gap from the 95% CI of the summary effect of all studies. (D) The corresponding funnel plot appears symmetrical except for one outlier at the right bottom. Δ5-HTT, mean difference in serotonin reuptake transporter level in units of s.d.
Thalamus
Thirteen studies in 275 patients and 310 controls appraised thalamus (Figure 1C). Effect estimates were highly heterogeneous (I2=82%). The summary effect estimate indicated a small reduction of 5-HTT in patients, while 95% confidence did not exclude zero (−0.24; 95% CI: (−0.72, 0.23)). Leave-one-out analysis revealed that omitting Cannon et al25 would result in 95% CI almost excluding zero ((−0.79, 0.06)) and the largest reduction of heterogeneity (ΔI2=−8%). Remaining sensitivity analyses did not return any significant results. None of the variables assessed showed a meaningful influence on effect size estimates.
Striatum/Putamen
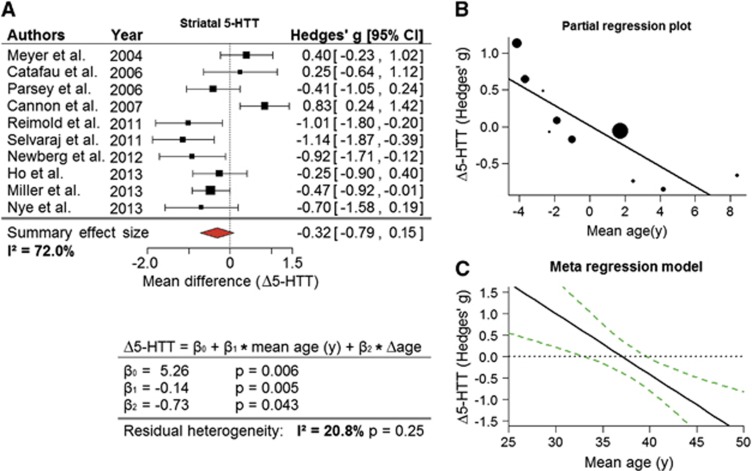
Ten studies including 204 patients and 214 controls appraised the striatum (Figure 2A). In this heterogeneous sample of studies (I2=72%), a reduction for striatal regions was detected (summary effect: −0.32; 95% CI: (−0.79, 0.15)). Leave-one-out analysis pointed to the high influence of Cannon et al25 the exclusion of which shifted 95% CI and sharply decreased heterogeneity (summary effect: −0.45; 95% CI: (−0.85, −0.05); I2=53%). When only studies investigating the putamen or its subparts were considered, i.e., with omission of Catafau et al,26 Cannon et al,25 and Ho et al,27 a clear reduction of 5-HTT was observed in MDD (summary effect: −0.56; 95% CI: (−1.05, −0.08); I2=57%). The influence of study population mean age on effect estimates became apparent during sensitivity analyses (Figure 2B), and was supported by a mixed-effects model (P<0.01; Figure 2C), especially when correction for within-study age differences was included (Δage, calculated as the standardized mean difference in age between groups), which reduced residual heterogeneity to a nonsignificant level (I2=20.8% P=0.25).
Figure 2.
Reductions in striatal 5-HTT with major depressive disorder (MDD) are moderated by age. (A) A forest plot for studies exploring the striatal 5-HTT in depressed patients, revealing a notable reduction of 5-HTT. (B) Partial regression plot showing the relationship between mean difference in 5-HTT (Δ5-HTT) and mean age corrected for standardized between-group age differences (Δage) in the current set of data. Here, residuals of effect size against age difference are plotted with residuals of mean age. (C) The definition of the meta-regression model links the mean age of study population to the changes in 5-HTT in the striatum, accounting for Δage. Uniquely in the striatum, age emerged as a factor explaining a significant part of between-study heterogeneity in effect sizes. The black line in the plot shows the predicted effect size at a given age with Δage fixed at zero. Corresponding 95% CI for predictions are indicated by the dashed lines.
Amygdala
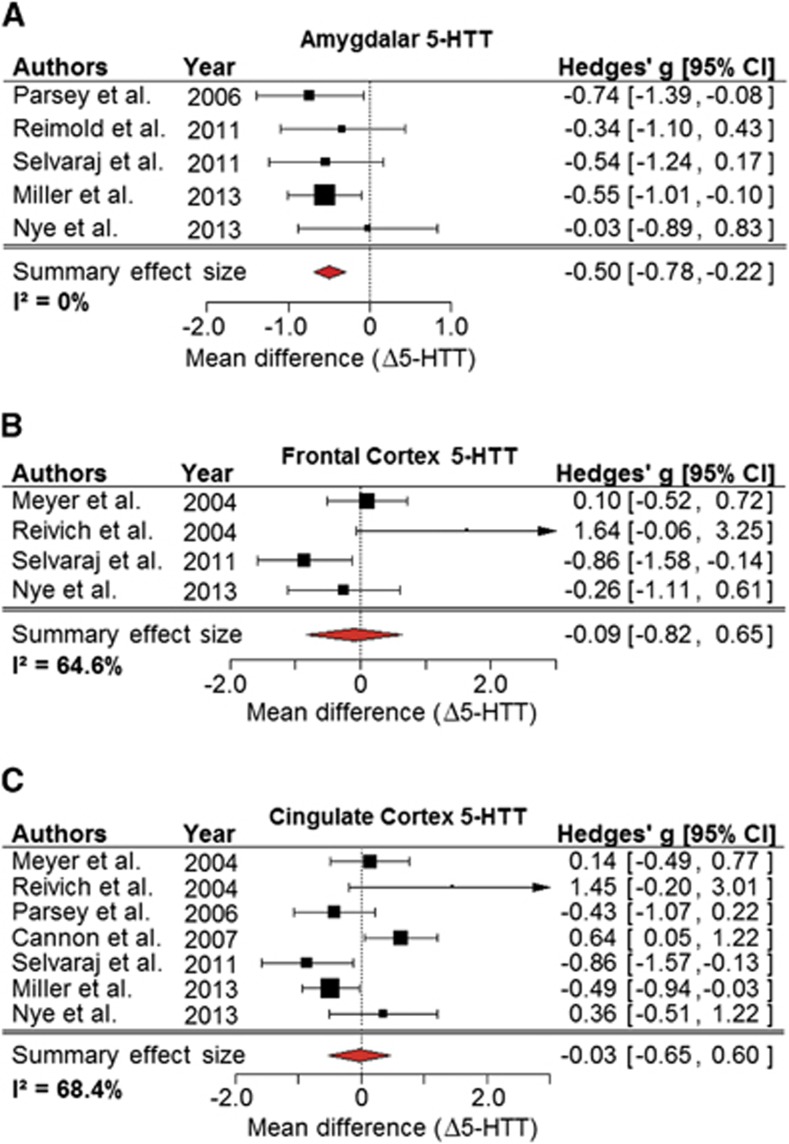
Five studies of 96 patients and 128 controls appraised amygdala (Figure 3A). Given the homogeneity of study estimates (I2=0), the weighting applied in the random-effects model was equal to that applied in a fixed-effects model, yielding identical summary effect estimates (−0.50; 95% CI: (−0.78, −0.22)). Leaving out data from Miller et al28 who investigated the largest sample, shifted the 95% CI to (−0.83, −0.09). Sensitivity to changes in τ2 estimates was negligible. A funnel plot showed that the smaller studies report smaller effects. Cumulative meta-analysis indicated that studies reporting higher depression severity found stronger reductions in 5-HTT (Supplementary Figure 2A), and that studies with MDD patients of mean age greater than controls reported weaker reductions (Supplementary Figure 2B). Therefore, depression scores were plotted against effect sizes controlling for within-study age differences (Δage, Supplementary Figure 2C). A corresponding mixed-effects model including both variables was significant (β1=−0.05, P=0.01 for the depression score component; β2=0.25, P=0.01 for Δage), with the caveat that there were only five studies on amygdala, using several severity scores. The remaining variables had little influence on effect estimates.
Figure 3.
Meta-analysis revealed significant reductions of 5-HTT in amygdala, but no effects in cortical regions. (A) A forest plot of molecular imaging studies of the amygdala in depressed patients, showing a significant reduction in 5-HTT levels. Forest plots for (B) frontal cortex and (C) cingulate cortex show no conclusive effect of major depression on 5-HTT availability. Δ5-HTT, mean difference in serotonin reuptake transporter level in units of s.d.
Frontal Cortex
Four studies with a total of 47 patients and 58 controls appraised 5-HTT in the frontal cortex (Figure 3B). In the presence of high heterogeneity (I2=65%), there was no clear effect of depression on 5-HTT (summary effect: −0.09; 95% CI (unadjusted): (−0.82, 0.65)). In cumulative meta-analysis, there was a trend toward stronger 5-HTT reductions in more severely depressed patient groups.
Cingulate Cortex
Seven studies with a total of 127 patients and 166 controls appraised 5-HTT in cingulate cortex (Figure 3C). No conclusive effect of depression on 5-HTT binding was detected (summary effect: −0.03; 95% CI: (−0.65, 0.60); I2=68.4%). There was high impact of data from Cannon et al25 on heterogeneity (ΔI2=−16.2%) and summary effect size (summary effect: −0.19, 95% CI: (−0.84, 0.45)). Sensitivity analyses revealed weak association of depression severity with 5-HTT reduction (β=−0.18, P=0.06), and a similar association with mean age (β=−0.08, P=0.06). Since mean age and depression severity correlated with each other, with the exception of one outlier, the relationships could not be evaluated separately.
Discussion
The literature search yielded groups of nearly 400 MDD patients and healthy controls in a total of 18 published studies of 5-HTT in MDD, indicating mean group sizes of 20 patients and 20 controls per imaging study. While this group size is typical of molecular imaging studies, it is unsurprising that detection of small group differences has been unreliable, given measurement error and substantial biologic variability in 5-HTT as seen (e.g.,) with season.29 For the sake of homogeneity, albeit in support of our findings, a published study on 5-HTT in seasonal affective disorder was not included in the analyses.30 Considerably more data are available for regions of high 5-HTT abundance, reflecting the lesser sensitivity of SPECT methods and the inherently lesser convenience of data acquisition by PET. Indeed, the diversity of SPECT and PET imaging methods employed in reports on 5-HTT in the high binding regions, i.e., the mesencephalon, thalamus, and striatum, may contribute to the high interstudy variability for these brain regions. Only two of 15 reports on midbrain showed any increase in 5-HTT levels among MDD patients, giving an overall effect size of −0.49, which was robust to leave-one-out analysis. This effect size is comparable in magnitude with the increase in utilization of DOPA decarboxylase substrates reported in the meta-analysis of schizophrenia noted above,10, 11 thus establishing with some considerable certainty the case for reduced 5-HTT in midbrain of several hundred patients with MDD.
Autoradiography shows that midbrain binding reflects the composite of 5-HTT in somatodendritic sites of dorsal raphe neurons31 and serotonin fibers innervating the nearby substantia nigra.32 These structures are merged in SPECT imaging, but are resolvable using the most advanced generation of PET scanners, which should enable the more precise anatomic location of reduced 5-HTT in MDD. Specificity of present results for midbrain of MDD patients is suggested by the absence of a clearly discernible effect in the adjacent brainstem, which was examined in a small number of SPECT studies with relatively large group size.
High 5-HTT availability is conspicuous in SPECT and PET studies of thalamus, where serotonin facilitates somatosensory and limbic signaling.33 We found a decline in thalamic 5-HTT in MDD patients, but of lesser effect size than in midbrain; this finding proved to be highly sensitive to exclusion of the report of Cannon et al25 in which [11C]DASB binding was notably elevated in MDD patients. Increased [11C]DASB binding was also evident in other regions examined by Cannon et al,25 suggesting a global scaling in their MDD patients.
The analysis of changes in 5-HTT availability in the striatum of MDD patients was informative in several respects. First, the aforementioned sensitivity of the summary effect size to omission or inclusion of results from Cannon et al25 was very pronounced here, substantially increasing the effect size. Available data for striatal sub-regions did not support a regional analysis, as most studies were restricted to the investigation of the putamen. However, the data did indicate increasing effect size for MDD patients of mean age increasing from 30 to 50 years. Exclusion of data from Cannon25 was without great effect on this trend, but rather served to extinguish residual heterogeneity (I2=0.0%).
Interestingly, in a recent [11C]DASB PET study of healthy subjects, striatal 5-HTT expression has been associated with scores in tests of logical reasoning, executive function, and level of education.34 Furthermore, several studies report age × depression interactions with cognitive function,35 especially executive functions36, 37 and motor speed.38 Structural and functional alterations in the striatum have been consistently found in MDD,39, 40 particularly in elderly MDD patients,41, 42 and are found to correlate with cognitive performance.43, 44, 45 The presentation of marked executive dysfunction in a subgroup of elderly depressed patients has led researchers to postulate a ‘depression executive dysfunction syndrome',46 associated with lesions of the striatocortical circuits. Results of this meta-analysis may encourage imaging studies of the specific role of serotonergic pathology in the striatum in the context of geriatric depression.
The five reports on 5-HTT binding in amygdala merit special attention, as they consistently indicate a reduction in MDD patients. This may be of particular interest, given the association of the amygdala with processing of aversive or fearful stimuli. In one human fMRI study, possession of short alleles of the promoter for 5-HTT expression, which predicts low transporter expression,47 was associated with increased BOLD signal in amygdala upon exposure of fearful visual stimuli,48 whereas in a study of mice with 5-HTT overexpression, the hemodynamic response in amygdala during fear conditioning was attenuated.49 Remarkably, in our recent PET study, high baseline 5-HTT ratios between amygdala and median raphe nucleus was associated with better treatment response to selective serotonin reuptake inhibitors.50
The five molecular imaging reports on amygdala also provide important information about confounds arising from age, and the impact of severity of depression. There was a trend toward decreasing effect size in amygdala with the inclusion of studies with lesser mean depression severity (Supplementary Figure 2A), whereas there was a blunting of the difference as one proceeds to include relatively older patient groups (Supplementary Figure 2B); correcting for this age difference substantially sharpens the finding of lesser 5-HTT binding in amygdala of more depressed patients.
The literature search revealed few studies of 5-HTT quantification in cerebral cortex. Meta-analysis of four reports on frontal cortex and seven reports on cingulate cortex did not indicate any significant reduction in cortical 5-HTT sites in MDD. This may be relevant to the lack of association of cortical 5-HTT loss in MDMA users and depression, noted below. However, the sensitivity of PET is naturally less in the cerebral cortex, where transporters are less abundant, such that the risk of type II error is more pronounced. Assuming an optimistic effect size of −0.5, we calculate that a study group of at least 64 patients would be required to obtain sufficient power (β=20%) for detecting a putative reduction in MDD (α=0.05, two tailed). This requirement was met by the present meta-analysis for the cingulate cortex, but not for the frontal cortex. Needless to say, none of the 18 individual studies has come close to possessing sufficient power; the largest study (Ruhe et al51) achieved β=34%. We conclude that, barring some technical improvement imparting precision of the imaging methods, future studies must entail larger numbers of patients than have hitherto been commonplace.
An important source of variance in 5-HTT expression may arise from previous antidepressant treatment of studied patients. At least 149 out of the 364 MDD patients included in this analysis are reported to be drug-naïve, whereas the others had been drug free for washout period ranging from 5 days to over 1 year (median=7 weeks). We assessed sensitivity to the factors of percentage of drug-naïve patients and minimal drug-free intervals, which did not reveal any relevant associations with 5-HTT results. There has been a number of animal studies that have shown highly variable results for 5-HTT expression after chronic antidepressant treatment, a topic that may call for a systematic review in its own right.52, 53, 54, 55, 56, 57 These studies can be confounded by carryover of selective serotonin reuptake inhibitors into the binding assay, which may contribute to the slight preponderance of studies reporting reduced 5-HTT after several weeks of antidepressant treatment.58, 59, 60, 61, 62 The relevance of these findings to possible effects of human antidepressant treatment regimens is challenged most notably by the high drug dosages used in animals studies. Intriguing data published by Benmansour et al58 reports rapid restoration of 5-HTT binding in rodent brain within few days after discontinuation of SSRI treatment.
As polymorphisms of SLCA4, the gene coding the serotonin transporter have been associated with an increase of the risk for developing MDD63 (an effect which is not undisputed64), genetic variability likely constitutes another factor potentially contributing to heterogeneity in studies of 5-HTT in MDD. Molecular imaging findings of relationships between 5-HTT in depression and genotype have been reviewed,65 and construed as pointing to decreased 5-HTT expression in subjects possessing the short allele of the 5-HTTLPR polymorphism. Yet, later in the same year of that publication another study failed to support this conclusion,66 providing another instance of the irresolution associated with the issue. Epigenetic approaches promise new insights as evidenced by a recent twin study revealing the occurrence of increased DNA methylation of the SLCA4 promoter with depressive symptoms.67
Present findings in the amygdala and striatum stress the critical importance of proper age matching in molecular imaging studies of 5-HTT in human brain; even small differences in mean age between groups have the capacity to obscure real differences. One PET study reports a global decline in 5-HTT availability of 10% per decade of normal aging.68 However, reduced 5-HTT binding relative to age-matched controls is not pathognomonic of depression; for example, [11C]DASB binding correlated inversely with severity of OCD symptoms.69 A history of repeated MDMA use is associated with widespread reductions in 5-HTT in the human striatum and thalamus70 or throughout the cerebral cortex.71 However, extensive use of MDMA is not generally associated with depressive mood per se, but is strongly associated with increased scores for anxiety and obsessive-compulsive traits, and with impairment of specific cognitive domains, notably of verbal memory.72 These observations indicate that reduced 5-HTT binding is not a sufficient condition for MDD, if obtained by neurotoxic injury, or in the course of normal aging. That reduced 5-HTT availability in MDD may be an acquired trait is supported by a PET study of rhesus monkeys with maternal separation stress during adolescence;73 by extension, psychosocial, and environmental factors may manifest in changes in 5-HTT binding sites, which impart a risk for MDD, without simple causation.74 Disentangling the causal relationship between 5-HTT availability and mood disorders may require molecular imaging studies in individuals at risk for developing MDD.
In conclusion, it has been widely assumed that MDD must be associated with a deficit in serotonergic transmission, without consistent support for this model from molecular imaging studies. To resolve this uncertainty, we undertook a systematic search of the literature, which yielded a total of 18 molecular imaging studies of MDD suitable for our meta-analysis. Analysis of data obtained in 364 depressed subjects and a similar number of healthy controls revealed highly significant reductions in 5-HTT availability in midbrain and amygdala, and lesser reductions in the striatum, thalamus, and brainstem. No such difference was noted in the cerebral cortex, where statistical power was inadequate owing to the lower specific signal. We conclude that individual molecular imaging studies have been underpowered to detect the real deficit in serotonergic transporters in unmedicated patients with MDD, which corresponds to ∼10%.
This analysis was partly funded by a grant from the Austrian funding agency, FWF. The authors have no conflicts of interest related to this paper to declare. Without any relevance to this work, R Lanzenberger received travel grants and conference speaker honoraria from AstraZeneca, Lundbeck A/S and Roche Austria GmbH.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This research was supported by a grant from the Austrian Science Fund (P22981) to R Lanzenberger.
Supplementary Material
References
- Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF. Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res. 1979;1:131–139. doi: 10.1016/0165-1781(79)90053-2. [DOI] [PubMed] [Google Scholar]
- Traskman L, Asberg M, Bertilsson L, Sjostrand L. Monoamine metabolites in CSF and suicidal behavior. Arch Gen Psychiatry. 1981;38:631–636. doi: 10.1001/archpsyc.1981.01780310031002. [DOI] [PubMed] [Google Scholar]
- Roy A, De Jong J, Linnoila M. Cerebrospinal fluid monoamine metabolites and suicidal behavior in depressed patients. A 5-year follow-up study. Arch Gen Psychiatry. 1989;46:609–612. doi: 10.1001/archpsyc.1989.01810070035005. [DOI] [PubMed] [Google Scholar]
- Dewar KM, Reader TA, Grondin L, Descarries L. [3H]paroxetine binding and serotonin content of rat and rabbit cortical areas, hippocampus, neostriatum, ventral mesencephalic tegmentum, and midbrain raphe nuclei region. Synapse. 1991;9:14–26. doi: 10.1002/syn.890090104. [DOI] [PubMed] [Google Scholar]
- Andersson A, Eriksson A, Marcusson J. Unaltered number of brain serotonin uptake sites in suicide victims. J Psychopharmacol. 1992;6:509–513. doi: 10.1177/026988119200600406. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, et al. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- Baldinger P, Kranz GS, Haeusler D, Savli M, Spies M, Philippe C, et al. Regional differences in SERT occupancy after acute and prolonged SSRI intake investigated by brain PET. Neuroimage. 2013;88:252–262. doi: 10.1016/j.neuroimage.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Hahn A, Haeusler D, Kraus C, Höflich A, Kranz G, Baldinger P, et al. Attenuated serotonin transporter association between dorsal raphe and ventral striatum in major depression Hum Brain Mapp 2014. in press. [DOI] [PMC free article] [PubMed]
- Saulin A, Savli M, Lanzenberger R. Serotonin and molecular neuroimaging in humans using PET. Amino Acids. 2012;42:2039–2057. doi: 10.1007/s00726-011-1078-9. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FusarPoli P, Meyer-Lindenberg A. Striatal presynaptic dopamine in schizophrenia, Part I: meta-analysis of dopamine active transporter (DAT) density. Schizophr Bull. 2013;39:22–32. doi: 10.1093/schbul/sbr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo M, Murphy CF, Meyers BS. Relationship between the Hamilton Depression Rating Scale and the Montgomery-Asberg Depression Rating Scale in depressed elderly: a meta-analysis. Am J Geriatr Psychiatry. 2007;15:899–905. doi: 10.1097/JGP.0b013e318098614e. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- Hedges LV. Distribution theory for glass's estimator of effect size and related estimators. J Educ Behav Stat. 1981;6:107–128. [Google Scholar]
- Hedges L, Olkin I.(1st edn). Statistical Methods for Meta-Analysis Academic Press: San Diego, CA, USA1985 [Google Scholar]
- Kelley K. Confidence intervals for standardized effect sizes: theory, application, and implementation. J Stat Softw. 2007;20:1–24. [Google Scholar]
- Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis. Psychol Methods. 1998;3:486–504. [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- Selvaraj S, Murthy NV, Bhagwagar Z, Bose SK, Hinz R, Grasby PM, et al. Diminished brain 5-HT transporter binding in major depression: a positron emission tomography study with [11C]DASB. Psychopharmacology (Berl) 2011;213:555–562. doi: 10.1007/s00213-009-1660-y. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon DM, Ichise M, Rollis D, Klaver JM, Gandhi SK, Charney DS, et al. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11C]DASB; comparison with bipolar disorder. Biol Psychiatry. 2007;62:870–877. doi: 10.1016/j.biopsych.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Catafau AM, Perez V, Plaza P, Pascual JC, Bullich S, Suarez M, et al. Serotonin transporter occupancy induced by paroxetine in patients with major depression disorder: a 123I-ADAM SPECT study. Psychopharmacology (Berl) 2006;189:145–153. doi: 10.1007/s00213-006-0540-y. [DOI] [PubMed] [Google Scholar]
- Ho PS, Ho KK, Huang WS, Yen CH, Shih MC, Shen LH, et al. Association study of serotonin transporter availability and SLC6A4 gene polymorphisms in patients with major depression. Psychiatry Res. 2013;212:216–222. doi: 10.1016/j.pscychresns.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Miller JM, Hesselgrave N, Ogden RT, Sullivan GM, Oquendo MA, Mann JJ, et al. Positron emission tomography quantification of serotonin transporter in suicide attempters with major depressive disorder. Biol Psychiatry. 2013;74:287–295. doi: 10.1016/j.biopsych.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praschak-Rieder N, Willeit M, Wilson AA, Houle S, Meyer JH. Seasonal variation in human brain serotonin transporter binding. Arch Gen Psychiatry. 2008;65:1072–1078. doi: 10.1001/archpsyc.65.9.1072. [DOI] [PubMed] [Google Scholar]
- Willeit M, Praschak-Rieder N, Neumeister A, Pirker W, Asenbaum S, Vitouch O, et al. [123I]-beta-CIT SPECT imaging shows reduced brain serotonin transporter availability in drug-free depressed patients with seasonal affective disorder. Biol Psychiatry. 2000;47:482–489. doi: 10.1016/s0006-3223(99)00293-0. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Haycock JW, Thompson PA, Lowy MT. Quantitative subregional distribution of serotonin1A receptors and serotonin transporters in the human dorsal raphe. Brain Res. 1996;727:1–12. doi: 10.1016/0006-8993(96)00239-9. [DOI] [PubMed] [Google Scholar]
- Parent M, Wallman MJ, Gagnon D, Parent A. Serotonin innervation of basal ganglia in monkeys and humans. J Chem Neuroanat. 2011;41:256–265. doi: 10.1016/j.jchemneu.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Cudennec A, Duverger D, MacKenzie ET, Scatton B, Serrano A. Serotonergic neuron stimulation modulates thalamocortical glucose use in the conscious rat. J Cereb Blood Flow Metab. 1987;7:502–506. doi: 10.1038/jcbfm.1987.94. [DOI] [PubMed] [Google Scholar]
- Madsen K, Erritzoe D, Mortensen EL, Gade A, Madsen J, Baare W, et al. Cognitive function is related to fronto-striatal serotonin transporter levels—a brain PET study in young healthy subjects. Psychopharmacology (Berl) 2011;213:573–581. doi: 10.1007/s00213-010-1926-4. [DOI] [PubMed] [Google Scholar]
- Christensen H, Griffiths K, Mackinnon A, Jacomb P. A quantitative review of cognitive deficits in depression and Alzheimer-type dementia. J Int Neuropsychol Soc. 1997;3:631–651. [PubMed] [Google Scholar]
- Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159:1119–1126. doi: 10.1176/appi.ajp.159.7.1119. [DOI] [PubMed] [Google Scholar]
- King DA, Caine ED, Cox C. Influence of depression and age on selected cognitive functions. Clin Neuropsychol. 1993;7:443–453. [Google Scholar]
- Thomas AJ, Gallagher P, Robinson LJ, Porter RJ, Young AH, Ferrier IN, et al. A comparison of neurocognitive impairment in younger and older adults with major depression. Psychol Med. 2009;39:725–733. doi: 10.1017/S0033291708004042. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Bradshaw JL, Pantelis C, Phillips JG. Frontostriatal deficits in unipolar major depression. Brain Res Bull. 1998;47:297–310. doi: 10.1016/s0361-9230(98)00126-9. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Khundakar A, Morris C, Oakley A, Thomas AJ. Morphometric analysis of neuronal and glial cell pathology in the caudate nucleus in late-life depression. Am J Geriatr Psychiatry. 2011;19:132–141. doi: 10.1097/JGP.0b013e3181df4642. [DOI] [PubMed] [Google Scholar]
- Sexton CE, Mackay CE, Ebmeier KP. A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. Am J Geriatr Psychiatry. 2013;21:184–195. doi: 10.1016/j.jagp.2012.10.019. [DOI] [PubMed] [Google Scholar]
- Hickie IB, Naismith SL, Ward PB, Little CL, Pearson M, Scott EM, et al. Psychomotor slowing in older patients with major depression: Relationships with blood flow in the caudate nucleus and white matter lesions. Psychiatry Res. 2007;155:211–220. doi: 10.1016/j.pscychresns.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Hickie I, Ward P, Scott E, Haindl W, Walker B, Dixon J, et al. Neo-striatal rCBF correlates of psychomotor slowing in patients with major depression. Psychiatry Res. 1999;92:75–81. doi: 10.1016/s0925-4927(99)00038-4. [DOI] [PubMed] [Google Scholar]
- Naismith S, Hickie I, Ward PB, Turner K, Scott E, Little C, et al. Caudate nucleus volumes and genetic determinants of homocysteine metabolism in the prediction of psychomotor speed in older persons with depression. Am J Psychiatry. 2002;159:2096–2098. doi: 10.1176/appi.ajp.159.12.2096. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS. ‘The depression-executive dysfunction syndrome of late life': a specific target for D3 agonists. Am J Geriatr Psychiatry. 2001;9:22–29. [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Barkus C, Line SJ, Huber A, Capitao L, Lima J, Jennings K, et al. Variation in serotonin transporter expression modulates fear-evoked hemodynamic responses and theta-frequency neuronal oscillations in the amygdala Biol Psychiatry 2013(in press). [DOI] [PMC free article] [PubMed]
- Lanzenberger R, Kranz GS, Haeusler D, Akimova E, Savli M, Hahn A, et al. Prediction of SSRI treatment response in major depression based on serotonin transporter interplay between median raphe nucleus and projection areas. Neuroimage. 2012;63:874–881. doi: 10.1016/j.neuroimage.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Ruhe HG, Booij J, Reitsma JB, Schene AH. Serotonin transporter binding with [123I]beta-CIT SPECT in major depressive disorder versus controls: effect of season and gender. Eur J Nucl Med Mol Imaging. 2009;36:841–849. doi: 10.1007/s00259-008-1057-x. [DOI] [PubMed] [Google Scholar]
- Gould GG, Altamirano AV, Javors MA, Frazer A. A comparison of the chronic treatment effects of venlafaxine and other antidepressants on serotonin and norepinephrine transporters. Biol Psychiatry. 2006;59:408–414. doi: 10.1016/j.biopsych.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Hebert C, Habimana A, Elie R, Reader TA. Effects of chronic antidepressant treatments on 5-HT and NA transporters in rat brain: an autoradiographic study. Neurochem Int. 2001;38:63–74. doi: 10.1016/s0197-0186(00)00043-7. [DOI] [PubMed] [Google Scholar]
- Hrdina PD, Vu TB. Chronic fluoxetine treatment upregulates 5-HT uptake sites and 5-HT2 receptors in rat brain: an autoradiographic study. Synapse. 1993;14:324–331. doi: 10.1002/syn.890140410. [DOI] [PubMed] [Google Scholar]
- Kovachich GB, Aronson CE, Brunswick DJ. Effect of repeated administration of antidepressants on serotonin uptake sites in limbic and neocortical structures of rat brain determined by quantitative autoradiography. Neuropsychopharmacology. 1992;7:317–324. [PubMed] [Google Scholar]
- Cheetham SC, Viggers JA, Slater NA, Heal DJ, Buckett WR. [3H]paroxetine binding in rat frontal cortex strongly correlates with [3H]5-HT uptake: effect of administration of various antidepressant treatments. Neuropharmacology. 1993;32:737–743. doi: 10.1016/0028-3908(93)90181-2. [DOI] [PubMed] [Google Scholar]
- Dewar KM, Grondin L, Nenonene EK, Ohayon M, Reader TA. [3H]paroxetine binding and serotonin content of rat brain: absence of changes following antidepressant treatments. Eur J Pharmacol. 1993;235:137–142. doi: 10.1016/0014-2999(93)90833-4. [DOI] [PubMed] [Google Scholar]
- Benmansour S, Owens WA, Cecchi M, Morilak DA, Frazer A. Serotonin clearance in vivo is altered to a greater extent by antidepressant-induced downregulation of the serotonin transporter than by acute blockade of this transporter. J Neurosci. 2002;22:6766–6772. doi: 10.1523/JNEUROSCI.22-15-06766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmansour S, Cecchi M, Morilak DA, Gerhardt GA, Javors MA, Gould GG, et al. Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J Neurosci. 1999;19:10494–10501. doi: 10.1523/JNEUROSCI.19-23-10494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DA, Ingram CD, Grant EJ, Craighead M, Gartside SE. Glucocorticoid receptor antagonism augments fluoxetine-induced downregulation of the 5-HT transporter. Neuropsychopharmacology. 2009;34:399–409. doi: 10.1038/npp.2008.70. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Zhang HT, Bootzin E, Millan MJ, O'Donnell JM. Association of changes in norepinephrine and serotonin transporter expression with the long-term behavioral effects of antidepressant drugs. Neuropsychopharmacology. 2009;34:1467–1481. doi: 10.1038/npp.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineyro G, Blier P, Dennis T, de Montigny C. Desensitization of the neuronal 5-HT carrier following its long-term blockade. J Neurosci. 1994;14 (5 Pt 2:3036–3047. doi: 10.1523/JNEUROSCI.14-05-03036.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke H, Flint J, Attwood AS, Munafo MR. Association of the 5- HTTLPR genotype and unipolar depression: a meta-analysis. Psychol Med. 2010;40:1767–1778. doi: 10.1017/S0033291710000516. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, et al. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry. 2000;57:729–738. doi: 10.1001/archpsyc.57.8.729. [DOI] [PubMed] [Google Scholar]
- Willeit M, Praschak-Rieder N. Imaging the effects of genetic polymorphisms on radioligand binding in the living human brain: a review on genetic neuroreceptor imaging of monoaminergic systems in psychiatry. Neuroimage. 2010;53:878–892. doi: 10.1016/j.neuroimage.2010.04.030. [DOI] [PubMed] [Google Scholar]
- Murthy NV, Selvaraj S, Cowen PJ, Bhagwagar Z, Riedel WJ, Peers P, et al. Serotonin transporter polymorphisms (SLC6A4 insertion/deletion and rs25531) do not affect the availability of 5-HTT to [11C] DASB binding in the living human brain. Neuroimage. 2010;52:50–54. doi: 10.1016/j.neuroimage.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Zhao J, Goldberg J, Bremner JD, Vaccarino V. Association between promoter methylation of serotonin transporter gene and depressive symptoms: a monozygotic twin study. Psychosom Med. 2013;75:523–529. doi: 10.1097/PSY.0b013e3182924cf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Suhara T, Okubo Y, Ichimiya T, Sudo Y, Inoue M, et al. Age-related decline of serotonin transporters in living human brain of healthy males. Life Sci. 2002;71:751–757. doi: 10.1016/s0024-3205(02)01745-9. [DOI] [PubMed] [Google Scholar]
- Reimold M, Smolka MN, Zimmer A, Batra A, Knobel A, Solbach C, et al. Reduced availability of serotonin transporters in obsessive-compulsive disorder correlates with symptom severity—a [11C]DASB PET study. J Neural Transm. 2007;114:1603–1609. doi: 10.1007/s00702-007-0785-6. [DOI] [PubMed] [Google Scholar]
- Buchert R, Thiele F, Thomasius R, Wilke F, Petersen K, Brenner W, et al. Ecstasy-induced reduction of the availability of the brain serotonin transporter as revealed by [11C](+)McN5652-PET and the multi-linear reference tissue model: loss of transporters or artifact of tracer kinetic modelling. J Psychopharmacol. 2007;21:628–634. doi: 10.1177/0269881106071975. [DOI] [PubMed] [Google Scholar]
- Urban NB, Girgis RR, Talbot PS, Kegeles LS, Xu X, Frankle WG, et al. Sustained recreational use of ecstasy is associated with altered pre and postsynaptic markers of serotonin transmission in neocortical areas: a PET study with [(1)(1)C]DASB and [(1)(1)C]MDL 100907. Neuropsychopharmacology. 2012;37:1465–1473. doi: 10.1038/npp.2011.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomasius R, Zapletalova P, Petersen K, Buchert R, Andresen B, Wartberg L, et al. Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users: the longitudinal perspective. J Psychopharmacol. 2006;20:211–225. doi: 10.1177/0269881106059486. [DOI] [PubMed] [Google Scholar]
- Ichise M, Vines DC, Gura T, Anderson GM, Suomi SJ, Higley JD, et al. Effects of early life stress on [11C]DASB positron emission tomography imaging of serotonin transporters in adolescent peer- and mother-reared rhesus monkeys. J Neurosci. 2006;26:4638–4643. doi: 10.1523/JNEUROSCI.5199-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbitzer J, Kalbitzer U, Knudsen GM, Cumming P, Heinz A. How the cerebral serotonin homeostasis predicts environmental changes: a model to explain seasonal changes of brain 5-HTT as intermediate phenotype of the 5-HTTLPR. Psychopharmacology (Berl) 2013;230:333–343. doi: 10.1007/s00213-013-3308-1. [DOI] [PubMed] [Google Scholar]
- Malison RT, Price LH, Berman R, van Dyck CH, Pelton GH, Carpenter L, et al. Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry. 1998;44:1090–1098. doi: 10.1016/s0006-3223(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Ichimiya T, Suhara T, Sudo Y, Okubo Y, Nakayama K, Nankai M, et al. Serotonin transporter binding in patients with mood disorders: a PET study with [11C](+)McN5652. Biol Psychiatry. 2002;51:715–722. doi: 10.1016/s0006-3223(01)01351-8. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Houle S, Sagrati S, Carella A, Hussey DF, Ginovart N, et al. Brain serotonin transporter binding potential measured with carbon 11-labeled DASB positron emission tomography: effects of major depressive episodes and severity of dysfunctional attitudes. Arch Gen Psychiatry. 2004;61:1271–1279. doi: 10.1001/archpsyc.61.12.1271. [DOI] [PubMed] [Google Scholar]
- Reivich M, Amsterdam JD, Brunswick DJ, Shiue CY. PET brain imaging with [11C](+)McN5652 shows increased serotonin transporter availability in major depression. J Affect Disord. 2004;82:321–327. doi: 10.1016/j.jad.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Amsterdam JD, Wintering N, Ploessl K, Swanson RL, Shults J, et al. 123I-ADAM binding to serotonin transporters in patients with major depression and healthy controls: a preliminary study. J Nucl Med. 2005;46:973–977. [PubMed] [Google Scholar]
- Herold N, Uebelhack K, Franke L, Amthauer H, Luedemann L, Bruhn H, et al. Imaging of serotonin transporters and its blockade by citalopram in patients with major depression using a novel SPECT ligand [123I]-ADAM. J Neural Transm. 2006;113:659–670. doi: 10.1007/s00702-005-0429-7. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Huang YY, Simpson N, Arcement J, et al. Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am J Psychiatry. 2006;163:52–58. doi: 10.1176/appi.ajp.163.1.52. [DOI] [PubMed] [Google Scholar]
- Staley JK, Sanacora G, Tamagnan G, Maciejewski PK, Malison RT, Berman RM, et al. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry. 2006;59:40–47. doi: 10.1016/j.biopsych.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Joensuu M, Tolmunen T, Saarinen PI, Tiihonen J, Kuikka J, Ahola P, et al. Reduced midbrain serotonin transporter availability in drug-naive patients with depression measured by SERT-specific [(123)I] nor-beta-CIT SPECT imaging. Psychiatry Res. 2007;154:125–131. doi: 10.1016/j.pscychresns.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Reimold M, Knobel A, Rapp MA, Batra A, Wiedemann K, Strohle A, et al. Central serotonin transporter levels are associated with stress hormone response and anxiety. Psychopharmacology (Berl) 2011;213:563–572. doi: 10.1007/s00213-010-1903-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberg AB, Amsterdam JD, Wintering N, Shults J. Low brain serotonin transporter binding in major depressive disorder. Psychiatry Res. 2012;202:161–167. doi: 10.1016/j.pscychresns.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye JA, Purselle D, Plisson C, Voll RJ, Stehouwer JS, Votaw JR, et al. Decreased brainstem and putamen sert binding potential in depressed suicide attempters using [C]-Zient pet imaging. Depress Anxiety. 2013;30:902–907. doi: 10.1002/da.22049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.