Abstract
Transition metal reagents and catalysts are generally effective to cleave all three bonds (one σ and two π) in a triple bond despite its high bonding energy. Recently, chemistry of single-bond cleavage by using main-group element compounds is rapidly being developed in the absence of transition metals. However, the cleavage of a triple bond using non-transition-metal compounds is less explored. Here we report that an unsymmetrical diborane(4) compound could react with carbon monoxide and tert-butyl isonitrile at room temperature. In the latter case, the carbon–nitrogen triple bond was completely cleaved in the absence of transition metal as confirmed by X-ray crystallographic analysis, 13C NMR spectroscopy with 13C labelling and DFT calculations. The DFT calculations also revealed the detailed reaction mechanism and indicated that the key for the carbon–nitrogen triple-bond cleavage could be attributed to the presence of nucleophilic nitrogen atom in one of the intermediates.
 The cleavage of triple bonds can be achieved through the use of transition metal catalysts; however, it is less well explored for metal-free systems. Here, the authors show complete cleavage of a carbon–nitrogen triple bond under mild conditions through the use of a diborane(4) reagent.
The cleavage of triple bonds can be achieved through the use of transition metal catalysts; however, it is less well explored for metal-free systems. Here, the authors show complete cleavage of a carbon–nitrogen triple bond under mild conditions through the use of a diborane(4) reagent.
A triple bond having three shared electron pairs between two atoms is known as one of the strongest chemical bonds. In spite of the large bonding energy, complete cleavage of the three (one σ and two π) bonds in a C≡C triple bond of an alkyne molecule or in a C≡N triple bond of a nitrile molecule under oxidative or acidic condition is common in general organic chemistry. In addition to the well-established chemistry of alkyne metathesis1, some stoichiometric2,3,4 and catalytic5,6,7,8,9,10,11 reactions for complete cleavage of a C≡C triple bond are also known with transition metal (TM) reagents and catalysts. However, only two examples about the cleavage of a C≡C triple bond without TMs have been reported using tandem- and multi-step reactions under harsh condition12,13. Moreover, cleavage of the C≡O triple bond in carbon monoxide (CO) is widely known as the Fischer–Tropsch process in the presence of a TM catalyst14. Recent development of TM catalysts enabled us to cleave the N≡N triple bond in dinitrogen for the formation of ammonia15,16,17. Several catalytic18 and stoichiometric19,20 cleavage reactions of a C≡N triple bond were also reported with use of TM catalyst and reagent21,22,23. Although some p-block element compounds could also react with CO 24,25,26,27,28,29,30,31,32,33,34,35,36,37,38 or isonitriles28,39,40, the strongest σ-bond among the three bonds in a triple bond remained intact in all cases.
On the other hand, chemistry containing Lewis-base adduct of sp2–sp3 diborane(4) compounds has been quickly developed recently. After the isolation of the first example of base adduct of bis(catecholato)diborane(4)41,42,43,44,45, a series of the sp2–sp3 diborane(4) compounds were applied as a boron source for copper-catalysed β-borylation of α,β-unsaturated carbonyl compounds in the absence of additional base46,47,48, as a hydrogen donor for radical reduction49 and as reactive compounds to undergo rearrangement reactions50,51,52,53. Some Lewis-base-catalysed β-borylation reactions of α,β-unsaturated carbonyl compounds were also considered to involve such sp2–sp3 diborane(4) intermediates54,55,56,57,58,59,60,61. Herein, we report a complete cleavage reaction of C≡N triple bond in isonitrile by using unsymmetrical diborane(4) compound, involving sp2–sp3 diborane(4) intermediates supported by density functional theory (DFT) calculations.
Results
Synthesis and reactivity of diborane(4) with CO and tBuNC
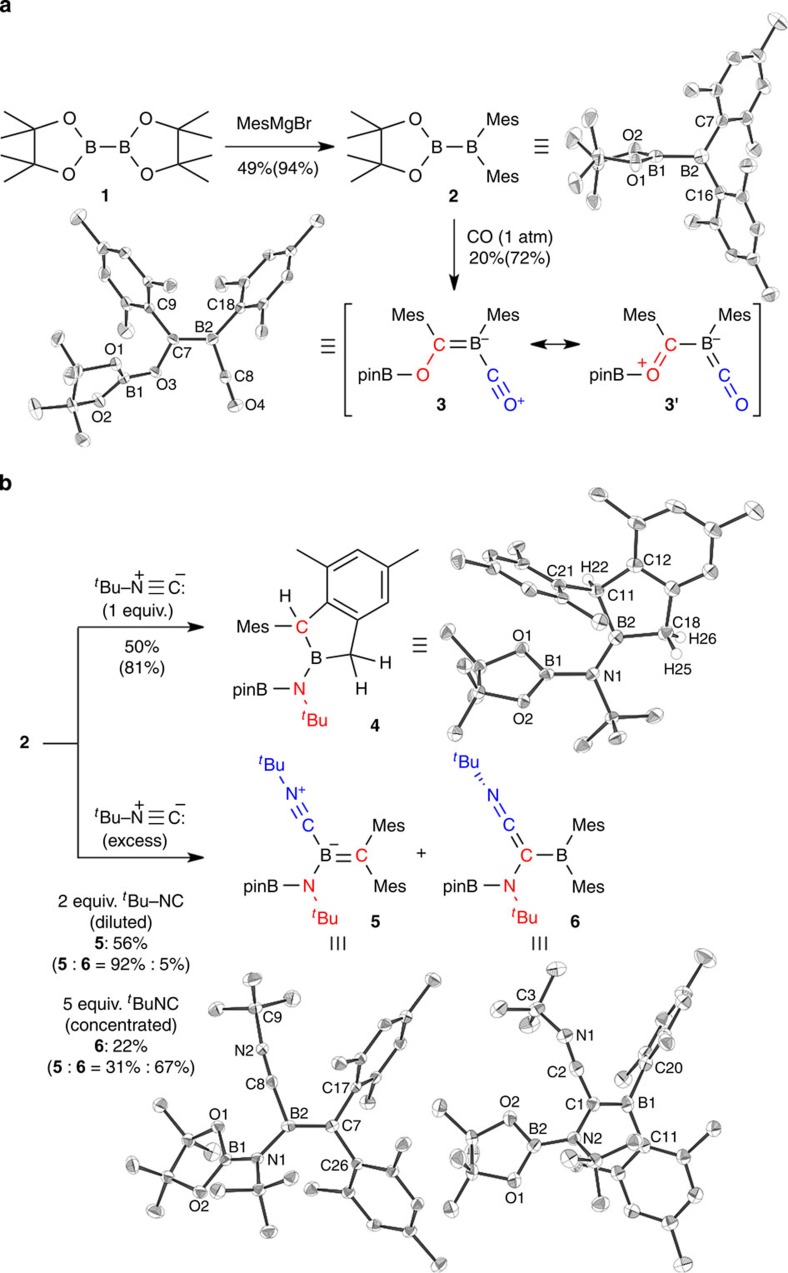
Synthesis of the unsymmetrical diborane(4) 2, its reactions and characterization of the resulting products are summarized in Fig. 1 (see also, Supplementary Figs 1–15, Supplementary Tables 1 and 2, and Supplementary Methods). Reaction of 1 with mesitylmagnesium bromide gave 2 in 49% yield. The 1H NMR spectrum of 2 showed C2v symmetrical pattern of signals. Two broad 11B NMR signals were observed at δB 34 and 89 p.p.m., indicating the selective conversion of one (pinacolato)boryl group to a dimesitylboryl group. Broadening of a 13C NMR signal at δC 144.2 p.p.m. also confirmed the connection between the mesityl groups and a quadrupolar boron nucleus. X-ray crystallographic analysis of 2 revealed twisted orientation described by the dihedral angle of O1–B1–B2–C16 in contrast to the case of 1 (Supplementary Fig. 16)62. DFT calculation showed that the vacant p-orbital of the boron atom in the Bpin moiety slightly contribute to the LUMO of 2 (Supplementary Figs 17–18), which mainly consisted of the vacant p-orbital of the boron atom in the BMes2 moiety, in spite of the twisted structure.
Compound 2. 2-(Dimesitylboranyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane.
Molecular Weight: 376.15
Elemental Analysis: C, 76.63; H, 9.11; B, 5.75; O, 8.51
Standard InChI: InChI=1S/C24H34B2O2/c1-15-11-17(3)21(18(4)12-15)25(22-19(5)13-16(2)14-20(22)6)26-27-23(7,8)24(9,10)28-26/h11-14H,1-10H3
Standard InChIKey: XCERFWKHKBOLQI-UHFFFAOYSA-N
SMILES: CC(C(C)(C)O1)(C)OB1B(C2=C(C)C=C(C)C=C2C)C3=C(C)C=C(C)C=C3C
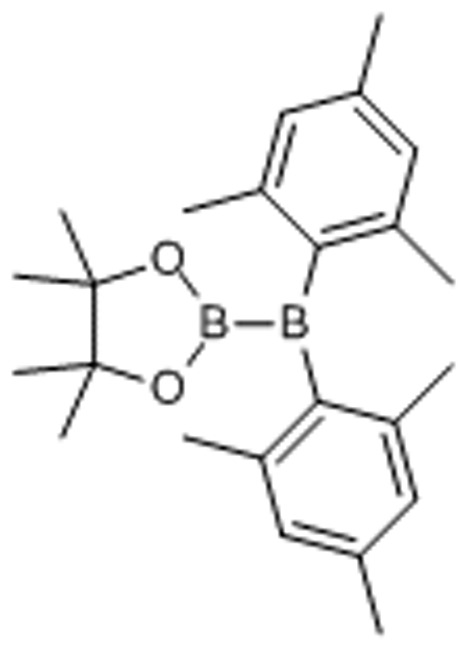
Chemical Formula: C24H34B2O2
Figure 1. Synthesis and reactions of unsymmetrical diborane(4) 2.
(a) Synthesis and reaction of 2 with CO to form 3 and their crystal structures. (b) Reaction of 2 towards tert-butylisonitrile to form 4–6 and crystal structures of the products (isolated and 1H NMR yield in parentheses, Mes=2,4,6-(CH3)3C6H2, pinB=[(CH3)2CO]2B): Selected bond lengths (Å), bond angles (°) and dihedral angles (°); 2: B1–B2=1.722(4); 3: B1–O1=1.366(3), B1–O2=1.356(3), B1–O3=1.374(3), O3–C7=1.406(3), C7–B2=1.459(4), B2–C8=1.492(4), C8–O4=1.144(3); 4: B1–N1=1.455(3), N1–B2=1.415(3), B2–C11=1.626(4), B2–C18=1.591(4); 5: B1–N1=1.413(3), N1–B2=1.495(3), B2–C7=1.456(3), B2–C8=1.569(3), C8–N2=1.152(3), B2–C8–N2=173.6(2); 6: B1–C1=1.516(7), C1–C2=1.353(6), C2–N1=1.217(5), C1–N2=1.450(5), B2–N2=1.419(6), B1–C1–C2=114.8(4), C1–C2–N1=174.5(5), C2–N1–C3=131.7(4).
The unsymmetrical diborane(4) 2 reacted with CO or tBuNC to give a variety of products (Fig. 1). A benzene solution of 2 was exposed to CO at room temperature for 30 min to give pale yellow solids of 3 in 20% isolated yield. X-ray crystallography for 3 showed incorporation of two CO molecules (it should be noted the complete assignment of atomic order in 3, 5 and 6 would be difficult due to small difference in electron density of the second period elements; see below and Fig. 2). The O3–C7 [1.406(3) Å], C7–B2 [1.459(4) Å] and B2–C8 [1.492(4) Å] bonds are shorter than the conventional single bonds, and C8–O4 [1.144(3) Å] is slightly longer than the C≡O bond of free CO molecule (1.1283 Å)63. These data proposed resonance structures of 3 and 3' with a characteristic conjugated O–C–B–C–O linkage giving a pale yellow colour (Supplementary Fig. 19 for ultraviolet–visible spectrum and Supplementary Tables 3 and 4 for time-dependent DFT calculation). Thus, compound 3 could be described as CO-coordinated alkoxyboraalkene. Reaction of 2 with one equivalent of tBuNC gave a colourless cyclized product 4 in 50% yield through scission of the isonitrile C≡N triple bond and of a C(sp3)–H bond in one of the mesityl substituents, as the molecular structure of 4 was confirmed by X-ray crystallographic analysis. The assignment of the B2 atom in the 2-boraindane skeleton could also be supported by the relatively long B2–C11 [1.626(4) Å] and B2–C18 [1.591(4) Å] bonds. In contrast, the reaction of 2 with an excess amount of tBuNC gave a mixture of tBuNC-coordinated boraalkene 5 and borylethenylideneamine 6. The reaction with two equivalents of tBuNC in a diluted solution gave 5 as the major product, while the reaction with a large excess amount of tBuNC in a concentrated solution afforded 6 as the major product. X-ray crystallographic analysis of 5 and 6 revealed that these two compounds have similar arrangement of all atoms, except the order of the two atoms in the central B=C or C–B bond and the terminal C–N–tBu angle (5, B2–C8–N2=173.6(2)°; 6, C2–N1–C3=131.7(4)°). To form 5, the C≡N triple bond in tBuNC, one B–B bond and two B–Mes bonds were cleaved from 2, while the two mesityl groups are still attached to the boron atom in 6. In the molecular structure of 5, the boron centre has B=C double-bond character (B2–C7=1.456(3) Å) and the second equivalent of tBuNC coordinates to the boron atom in the B=C moiety. In the case of 6, the tBuNC moiety has consecutive N=C and C=C double bonds (N1=C2=1.217(5) Å, C2=C1=1.353(6) Å) with a slightly short C–B single bond (C1–B1=1.516(7) Å). All the obtained crystal structures could be reproduced by DFT calculation to support the assignment of atomic order (see below).
Compound 3. (E)-Mesityl(mesityl((4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)oxy)methylene)hydroborate.carbon monoxide.
Molecular Weight: 432.17
Elemental Analysis: C, 72.26; H, 7.93; B, 5.00; O, 14.81
Standard InChI: InChI=1S/C26H34B2O4/c1-16-11-18(3)22(19(4)12-16)24(30-28-31-25(7,8)26(9,10)32-28)27(15-29)23-20(5)13-17(2)14-21(23)6/h11-14H,1-10H3
Standard InChIKey: YIWNHEYGEMXPHS-UHFFFAOYSA-N
SMILES: CC(C(C)(C)O1)(C)OB1O/C(C2=C(C)C=C(C)C=C2C)=[B-](C#[O+])/C3=C(C)C=C(C)C=C3C

Chemical Formula: C26H34B2O4
Compound 5. N-(tert-Butyl)-N-((dimesitylmethylene)boranyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-amine tert-butyl isocyanide.
Molecular Weight: 542.41
Elemental Analysis: C, 75.29; H, 9.66; B, 3.99; N, 5.16; O, 5.90
Standard InChI: InChI=1S/C34H52B2N2O2/c1-22-17-24(3)28(25(4)18-22)30(29-26(5)19-23(2)20-27(29)6)35(21-37-31(7,8)9)38(32(10,11)12)36-39-33(13,14)34(15,16)40-36/h17-20H,1-16H3
Standard InChIKey: ARNZFXIWSKZPCY-UHFFFAOYSA-N
SMILES: CC(C=C1C)=CC(C)=C1/C(C2=C(C)C=C(C)C=C2C)=[B-](N(B3OC(C)(C)C(C)(C)O3)C(C)(C)C)/C#[N+]C(C)(C)C

Chemical Formula: C34H52B2N2O2
Compound 6. (R)-N-(tert-Butyl)-N-(2-(tert-butylimino)-1-(dimesitylboranyl)vinyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-amine.
Molecular Weight: 542.41
Elemental Analysis: C, 75.29; H, 9.66; B, 3.99; N, 5.16; O, 5.90
Standard InChI: InChI=1S/C34H52B2N2O2/c1-22-17-24(3)29(25(4)18-22)35(30-26(5)19-23(2)20-27(30)6)28(21-37-31(7,8)9)38(32(10,11)12)36-39-33(13,14)34(15,16)40-36/h17-20H,1-16H3
Standard InChIKey: PZHBEMFRMCPTCX-UHFFFAOYSA-N
SMILES: CC(C=C1C)=CC(C)=C1B(C2=C(C)C=C(C)C=C2C)C(N(B3OC(C)(C)C(C)(C)O3)C(C)(C)C)=C=NC(C)(C)C

Chemical Formula: C34H52B2N2O2
Figure 2. Assignment of atomic order in 3–6 by 13C NMR experiments with 13C labelling.
(a) Potential regioisomer 3(opp-B,C) derived from exchange of the positions of boron and carbon atoms in 3. (b) Reactions of 2 with 13C-labelled 13CO and tBuN13C to form the corresponding 13C-labelled 3-13C2, 4-13C, 5-13C2 and 6-13C2. (c) Newly appeared 13C NMR signals of 3-13C2 on 13C labelling. (d) The 4° aromatic signals of 4-13C with satellite on 13C labelling (e) enhancement of 13C NMR signal (top: 4-13C, bottom: 4). (f) The 4° aromatic signals of 5-13C2 with satellite on 13C labelling (g) newly appeared 13C NMR signals of 5-13C2 on 13C labelling (h). (i) Strengthened 13C NMR signals of 6-13C2 with satellite on 13C labelling.
Compound 4. N-(tert-Butyl)-N-(1-mesityl-5,7-dimethyl-1H-benzo[c]borol-2(3H)-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-amine.
Molecular Weight: 459.28
Elemental Analysis: C, 75.84; H, 9.44; B, 4.71; N, 3.05; O, 6.97
Standard InChI: InChI=1S/C29H43B2NO2/c1-18-13-20(3)24(21(4)14-18)26-25-22(5)15-19(2)16-23(25)17-30(26)32(27(6,7)8)31-33-28(9,10)29(11,12)34-31/h13-16,26H,17H2,1-12H3
Standard InChIKey: XYDQCWDLEXNKTR-UHFFFAOYSA-N
SMILES: CC(C=C1C)=CC2=C1C(C3=C(C)C=C(C)C=C3C)B(C2)N(C(C)(C)C)B4OC(C)(C)C(C)(C)O4

Chemical Formula: C29H43B2NO2
The NMR spectroscopic characterization of the products
The nuclear magnetic resonance (NMR) spectra of 3–5 were consistent with the crystallographically determined structures. The 1H NMR spectrum of 3 in C6D6 showed two distinct Mes groups and one pinacol moiety. Two boron nuclei resonated at δB –5 and 18 p.p.m., where the former signal could be assigned as the CO-coordinated boron atom due to the negatively charged boron atom in both the resonance structures 3 and 3′. The calculated 11B NMR chemical shift (δB –4.1, 20 p.p.m.) of 3 by the Gauge-independent atomic orbital (GIAO) method at B3LYP/6-311++G(2d,p)//B3LYP/6-31+G(d,p) level was also in good agreement with the experimental data (Supplementary Table 5). Although the two Mes groups and the pinacol moiety could be assigned in the 13C NMR spectrum of 3, no signal corresponding to a B=C unit and a coordinating CO molecule was observed. The 1H NMR spectrum of 4 showed one benzylic methine proton (δH 4.22) and two vicinally coupled methylene protons (δH 2.87 and 2.93, 2JHH=21 Hz), supporting the scission of a C(sp3)–H bond in one of the two mesityl groups to give a chiral centre on C11. Reflecting the asymmetry below and above the 2-boraindane plane in 4, all the remaining five methyl groups on the mesityl substituents were separately observed and the four methyl groups on the pinacolato moiety resonated two singlet signals. Both the boron nuclei in 4 resonated at a typical region for an sp2 boron atom (δB 27, 55 p.p.m.). The lower-field shifted signal could be assigned as the dicarbyl-substituted boron atom as supported by our GIAO calculations. Two relatively broadened 13C NMR signals at δC 30.3 and 41.2 p.p.m., compared with other signals of 4, supported their connection to quadrupolar boron nucleus. The 1H NMR spectra of 5 and 6 similarly showed six methyl signals for the Mes groups, two methyl signals for the pinacol moiety, two tBu signals and four aromatic CH signals, because they are regioisomers with the same combination of the substituents and are close in symmetry of molecule. In the 13C NMR spectrum of 5, the number of observed signals was two short of the number of 13C nuclei expected from the symmetry of the 1H NMR spectrum. Similarly, one carbon signal was missing in the 13C NMR spectrum of 6. The missing of the 13C signals was probably due to broadening of quaternary carbon bonded to quadrupolar boron nucleus. The 11B NMR signals of 5 (δB 13, 21 p.p.m.) and 6 (δB 21, 63 p.p.m.) could also be assigned by our GIAO calculations.
The 13C-labelling study to determine the atomic order
In addition to the conventional NMR spectra of 3–5, 13C-labelling experiment could confirm the structures of 3–6 including connectivity between boron and carbon atoms (Fig. 2). Complete structural characterization of 3, 5 and 6 was difficult due to the following reasons: (1) in general, X-ray crystallographic analysis has difficulty to distinguish two adjacent atoms in the same row of the periodic table. This means that positions of carbon and boron atoms in 3 versus 3(opp-B,C) (Fig. 2a) and 5 versus 6 could not be unambiguously determined by crystallography. (2) Both 10B and 11B nuclei are quadrupolar to induce significant broadening of the signal for boron-bonded nuclei, leading in difficulty for observation of quaternary carbon bonded to boron nucleus. In this context, we performed 13C-labelling experiments for 3–6 to observe the 13C–13C coupling and broadened 13C NMR signals bonded to boron atom. The unsymmetrical diborane 2 reacted with 13C-labelled 13CO (99% 13C) or tBuN13C (20% 13C) gave 13C-labelled 3-13C2, 4-13C, 5-13C2 and 6-13C2 (Fig. 2b). On 13C-labelling of 3, two broad signals at δC 197.1 and 201.8 p.p.m. appeared without apparent coupling in the 13C NMR spectrum of 3-13C2 (Fig. 2c), indicating that these two carbon atoms connected to a quadrupolar boron atom with the C–B=C skeleton in 3 (not C–C=B in 3(opp-B,C)). As described above, two broadened signals δC 30.3 and 41.2 p.p.m. may be assigned to the boron nucleus in 4. The lower-field shifted signal at δC 41.2 p.p.m. was strengthened on 13C labelling to form 4-13C (Fig. 2e), indicating this benzylic methine carbon came from tBuN13C. Concomitantly, two signals of aromatic quaternary carbons at δC 140.7 and 144.1 p.p.m. were accompanied with satellite signal with 1JCC of 40 Hz (Fig. 2d), similar to that (43 Hz) for the C(sp2)–C(sp3) linkage in strychnine64, indicating that the two ipso carbons of the two Mes groups bonded to the sp3 methine 13C are from tBuN13C. In the case of 5-13C2, two broad signals appeared at δC 132.7 and 137.1 p.p.m. on labelling (Fig. 2g), supporting the C–B=C skeleton of 5. Two split 4° aromatic signals with 1JCC of 24 Hz in 5-13C2 also showed that the two Mes groups bonds to a carbon atom (Fig. 2f). The 13C NMR spectrum of 6-13C2 showed two strengthened signals at δC 89.1 and 168.0 p.p.m. (Fig. 2h,i) with a satellite (1JCC=86 Hz), supporting the C(sp2)–C(sp) coupling (107 Hz in diphenylketene-13C2)65. One can confirm that the carbon atom with the δC 89.1 p.p.m. signal is bonded to a quadrupolar boron atom in the structure of 6, according to the broadening observed.
Proposed mechanism based on DFT calculation
The whole mechanisms for the formation of 3–6 from 2 were estimated by DFT calculations66,67,68,69 with full geometry optimization of all the available transition states (TSs) at the B3LYP/6-31G(d,p) level and single-point energy calculation at M06-2X/6-311+G(d,p) with solvent effect of benzene using conductor-like polarizable continuum model (Fig. 3: mechanism with curly arrows, Fig. 4: energy profiles with relative Gibbs free energies and Supplementary Table 6 for coordinates of all the structures). An initial coordination of CO or tBuNC to 2 gave the sp2–sp3 diborane(4) 7-O and 7-N, which would undergo two types of bond cleavage reactions (Fig. 3a): (1) B–Mes bond cleavage to give acyldiborane(4) 8-O or imidoyldiborane(4) 8-N, (2) B–Bpin bond cleavage to give diborylketone 9-O or diborylimine 9-N. In the reaction of 2 with CO, energy levels of TS8-O and TS9-O are comparable to each other and both TSs are higher than the TS7-O (Fig. 4a). The slightly lower TS8-O could be explained by the higher nucleophilicity of a Mes substituent than a Bpin substituent due to the electronegativity difference between carbon and boron atoms, as supported by natural bond orbital analysis (Supplementary Fig. 20). The subsequent reactions from 8-O and 9-O afforded the same product 3 (Fig. 3b). The former pathway through 8-O included a coordination of a second CO molecule to give 10-O and subsequent migration of the Bpin moiety by a nucleophilic attack of the acyl oxygen atom in 10-O with B–B bond cleavage to give 3. The large energy gain in this step may be attributed to the formation of B–O bond. The latter pathway through 9-O was initiated by a Bpin migration to form the borataalkene 11-O. TS11-O was the global TS, which lies 6.1 kcal mol−1 higher than TS8-O. Subsequently, one of the two Mes groups in 11-O migrated to the carbon atom to afford the boraalkene 12-O. Coordination of a second CO molecule to 12-O could form the same product 3. This step could be considered as a coordination of CO to electron-deficient boraalkene for a large energy gain. Formation of a possible C–O cleaved product 13-O would be suppressed due to the higher TS13-O (Fig. 4a).
Figure 3. Possible reaction mechanism for the formation of 3–6 from 2 estimated by DFT calculations.
(a) Two types of possible products 8 and 9 formed by B–Mes or B–Bpin cleavage after the coordination of CO or tBuNC. (b) Two energetically comparable pathways to 3 from 8-O and 9-O. (c) Three pathways to 4-6 from 9-N.
Figure 4. Energy profiles of possible mechanism.
Energy profiles of possible mechanism for the formation of 3–6 from 2 with relative Gibbs free energies in kcal mol−1 (estimated by optimization at the B3LYP/6-31G(d,p) level and subsequent single-point energy calculation at M06-2X/6-311+G(d,p) level with consideration of entropy contribution and solvent effect of benzene (conductor-like polarizable continuum model (CPCM)), all the compound numbers are in conjunction with Fig. 3). (a) Two possible pathways for the formation of 3 by reaction of 2 with CO (red: pathway through 8-O, blue: pathway through 9-O) (b) pathway for the formation of 4-6 by reaction of 2 with tBuNC (red: main pathway to 4-6, blue: branching to each of the compounds 4-6). (Remark: before the solvation correction, TS12-O is slightly higher than 11-O and TS14-N is slightly higher than 13-N in energy.)
In the reaction of 2 with tBuNC, TS8-N was 5.8 kcal mol−1 higher than TS9-N, indicating that the pathway through 9-N would be favourable (Fig. 4a). The high energy level of TS8-N may be explained by a steric repulsion between the spectator Mes group and the tBu group (Fig. 3a and Supplementary Table 7). The intermediate 9-N would undergo Bpin migration to give 11-N followed by a Mes group migration to form the boraalkene 12-N (Fig. 3c). Coordination of tBuNC to the central carbon atom in 11-N could lead to formation of 6, but the TS to 6 was calculated to be slightly higher (by 3.8 kcal mol−1) than the TS to 12-N. Requirement of higher concentration to prepare 6 was consistent with this result. Again, the simple coordination of tBuNC to boraalkene would give a large energy gain. As a nitrogen atom in 12-N may have higher nucleophilicity than the oxygen atom in 12-O, a migration of the amino substituent (−NtBuBpin) to the Mes-bonded boron atom would take place with an assistance of electron donation from the carbon-bonded Mes group to form the amino-substituted borataalkene 13-N. This step (from 12-N to 13-N) involves a cleavage of the C–N bond originated from the C≡N triple bond in tBuNC. Electron donation from the nitrogen atom to the boron atom in 13-N induced the second Mes migration to the carbon atom to give the aminoboraalkene 14-N. The neutralization of the positively charged Mes group may contribute a large energy gain. A simple coordination of a second tBuNC molecule to 14-N affords the product 5. This result is consistent with the experimental observation that when tBuNC is in excess the product 5 was obtained. In the absence of excess tBuNC, the boron centre of 14-N would attack to one of the benzylic protons to give the cyclic hydroborate 15-N having delocalized cationic charge on the cyclized Mes ring, as a boryl anion could undergo the same deprotonation cyclization70. The energy barrier of 24.2 kcal mol−1 from 14-N to TS15-N is accessible at the room temperature (the reaction condition). Subsequent 1,2-hydride shift from 15-N and re-aromatization would form the product 4. The formation of B–N π-bond in the last step would contribute a large energy gain.
Discussion
Thus, the detailed spectroscopic and structural analysis of the obtained products and the DFT calculations revealed the complexity of the consecutive rearrangement reactions of 2. The reason why the newly synthesized diborane(4) 2 showed a remarkable reactivity towards CO and tBuNC in comparison with the conventional boron-containing compounds may be attributed to the existence of two reactive B–C bonds and one reactive B–B bond. Throughout the reactions, the boron atoms in the intermediates undergo repetitive interconversion between sp2 and sp3 states to induce the subsequent reactions. In the case of tBuNC, the intermediate 12-N, which is derived after the two π-bonds of the isonitrile moiety have been cleaved, contains a single C–N σ-bond and has a highly nucleophilic nitrogen atom. The highly nucleophilic nitrogen atom facilitates further cleavage of the remaining σ-bond through migrating to the adjacent unsaturated boron centre. Coexistence of the reactive B–B and B–C bonds, steric crowdedness in 2 and the high nucleophilicity of N in 12-N containing a single C–N σ-bond cooperatively achieved the complete cleavage of the C≡N triple bond. In conclusion, we demonstrated the first example of C≡N triple bond cleavage by using newly synthesized diborane(4) 2 in the absence of TM reagents and catalysts. The present results may inspire new idea to achieve multiple bond cleavage reactions using main group element compounds.
Author contributions
M.Y. and Z.L. designed the study. H.A. conducted all the experiments and part of DFT calculations. H.A. and M.Y. analysed crystal structures. K.H.L. and Z.L. performed mechanistic study. Z.L. and M.Y. wrote the manuscript.
Additional information
Accession codes. The X-ray crystal structure information is available at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC-985350 (1), CCDC-981112 (2), CCDC-981113 (3), CCDC-981114 (4), CCDC-981115 (5) and CCDC-985351 (6). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
How to cite this article: Asakawa, H. et al. Facile scission of isonitrile carbon–nitrogen triple bond using a diborane(4) reagent. Nat. Commun. 5:4245 doi: 10.1038/ncomms5245 (2014).
Supplementary Material
Supplementary Figures 1-20, Supplementary Tables 1-7, Supplementary Methods and Supplementary References
Combined CIF file for crystallographic data
Acknowledgments
We thank Professor Hiyama T. of Research and Development Initiative, Chuo University, for providing X-ray diffractometer and Professor Sasamori T. of Kyoto University for fruitful discussion about analysing crystal structure. This research was supported by Grants-in-Aid for Scientific Research on Innovative Areas (‘Stimulus-responsible Chemical Species for Creation of Functional Molecules’ (24109012 to M.Y.)) from MEXT, The Science Research Promotion Fund from The Promotion and Mutual Aid Corporation for Private Schools of Japan (M.Y.) and the Research Grants Council of Hong Kong (HKUST 603313 and CUHK7/CRF/12G) (Z.L.). Part of the computations was performed using Research Center for Computational Science, Okazaki, Japan.
References
- Fürstner A. Alkyne Metathesis on the Rise. Angew. Chem. Int. Ed. 52, 2794–2819 (2013). [DOI] [PubMed] [Google Scholar]
- Sullivan B. P., Smythe R. S., Kober E. M. & Meyer T. J. Cleavage of the triple bond in phenylacetylene by monomeric ruthenium(II) and osmium(II) complexes. Formation of stable ruthenium(II) alkyls from terminal alkynes. J. Am. Chem. Soc. 104, 4701–4703 (1982). [Google Scholar]
- O'Connor J. M. & Pu L. Surreptitious involvement of a metallacycle substituent in metal-mediated alkyne cleavage chemistry. J. Am. Chem. Soc. 112, 9013–9015 (1990). [Google Scholar]
- Cairns G. A., Carr N., Green M. & Mahon M. F. Reaction of [W(η2-PhC2Ph)3(NCMe)] with o-diphenylphosphino-styrene and -allylbenzene; evidence for novel carbon-carbon double and triple bond cleavage and alkyne insertion reactions. Chem. Commun. 2431–2432 (1996). [Google Scholar]
- Hayashi N., Ho D. M. & Pascal R. A. Jr An unusual cobalt-mediated cleavage of a hindered alkyne. Tetrahedron Lett. 41, 4261–4264 (2000). [Google Scholar]
- Jun C. -H., Lee H., Moon C. W. & Hong H. -S. Cleavage of carbon–carbon triple bond of alkyne via hydroiminoacylation by Rh(I) catalyst. J. Am. Chem. Soc. 123, 8600–8601 (2001). [DOI] [PubMed] [Google Scholar]
- Lee D. -Y., Hong B. -S., Cho E. -G., Lee H. & Jun C. -H. A hydroacylation-triggered carbon–carbon triple bond cleavage in alkynes via retro-Mannich type fragmentation. J. Am. Chem. Soc. 125, 6372–6373 (2003). [DOI] [PubMed] [Google Scholar]
- Datta S., Chang C.-L., Yeh K.-L. & Liu R.-S. A new ruthenium-catalyzed cleavage of a carbon–carbon triple bond: efficient transformation of ethynyl alcohol into alkene and carbon monoxide. J. Am. Chem. Soc. 125, 9294–9295 (2003). [DOI] [PubMed] [Google Scholar]
- Liu Y., Song F. & Guo S. Cleavage of a carbon–carbon triple bond via gold-catalyzed cascade cyclization/oxidative cleavage reactions of (Z)-enynols with molecular oxygen. J. Am. Chem. Soc. 128, 11332–11333 (2006). [DOI] [PubMed] [Google Scholar]
- Wang A. & Jiang H. Palladium-catalyzed cleavage reaction of carbon–carbon triple bond with molecular oxygen promoted by Lewis acid. J. Am. Chem. Soc. 130, 5030–5031 (2008). [DOI] [PubMed] [Google Scholar]
- Shen T., Wang T., Qin C. & Jiao N. Silver-catalyzed nitrogenation of alkynes: a direct approach to nitriles through C≡C bond cleavage. Angew. Chem. Int. Ed. 52, 6677–6680 (2013). [DOI] [PubMed] [Google Scholar]
- Roy S. et al. Dissecting alkynes: full cleavage of polarized C≡C moiety via sequential bis-michael addition/retro-Mannich cascade. J. Org. Chem. 76, 7482–7490 (2011). [DOI] [PubMed] [Google Scholar]
- Okamoto N., Ishikura M. & Yanada R. Cleavage of carbon–carbon triple bond: direct transformation of alkynes to nitriles. Org. Lett. 15, 2571–2573 (2013). [DOI] [PubMed] [Google Scholar]
- Griesbaum K. et al. in:Ullmann’s Encyclopedia of Industrial Chemistry Wiley-VCH Verlag GmbH & Co. KGaA (2000). [Google Scholar]
- Yandulov D. V. & Schrock R. R. Catalytic reduction of dinitrogen to ammonia at a single molybdenum center. Science 301, 76–78 (2003). [DOI] [PubMed] [Google Scholar]
- Arashiba K., Miyake Y. & Nishibayashi Y. A molybdenum complex bearing PNP-type pincer ligands leads to the catalytic reduction of dinitrogen into ammonia. Nat. Chem. 3, 120–125 (2011). [DOI] [PubMed] [Google Scholar]
- Anderson J. S., Rittle J. & Peters J. C. Catalytic conversion of nitrogen to ammonia by an iron model complex. Nature 501, 84–87 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Wang C., Li Z., Zhang S. & Xi Z. Zirconocene-mediated intermolecular coupling of one molecule of si-tethered diyne with three molecules of organonitriles: one-pot formation of pyrrolo[3,2-c]pyridine derivatives via cleavage of C≡N triple bonds of organonitriles. J. Am. Chem. Soc. 126, 7172–7173 (2004). [DOI] [PubMed] [Google Scholar]
- Tanabe Y., Seino H., Ishii Y. & Hidai M. Reaction mechanism of the C≡N triple bond cleavage of β-ketonitriles on a molybdenum(0) center. J. Am. Chem. Soc. 122, 1690–1699 (2000). [Google Scholar]
- Kawashima T., Takao T. & Suzuki H. Cleavage of the C≡N bond on a triruthenium cluster: synthesis and structure of a triruthenium complex containing a μ3-nitrido ligand. Angew. Chem. Int. Ed. 45, 485–488 (2006). [DOI] [PubMed] [Google Scholar]
- Geyer A. M. et al. Synthetic, mechanistic, and computational investigations of nitrile-alkyne cross-metathesis. J. Am. Chem. Soc. 130, 8984–8999 (2008). [DOI] [PubMed] [Google Scholar]
- Boyd J. P., Schlangen M., Grohmann A. & Schwarz H. Room-temperature alkyne–nitrile metathesis and unambiguous proof for the existence of a high-valent iron-nitrido dication in the gas phase. Helv. Chim. Acta 91, 1430–1434 (2008). [Google Scholar]
- Wiedner E. S., Gallagher K. J., Johnson M. J. A. & Kampf J. W. Synthesis of molybdenum nitrido complexes for triple-bond metathesis of alkynes and nitriles. Inorg. Chem. 50, 5936–5945 (2011). [DOI] [PubMed] [Google Scholar]
- Sprangers W. J. J. M. & Louw R. Carbonylation of organomagnesium compounds: catalysis by hexamethylphosphoric triamide. J. Chem. Soc. Parkin Trans. 2, 1895–1901 (1976). [Google Scholar]
- Seyferth D. & Weinstein R. M. High-yield acyl-anion trapping reactions: a synthesis of acyltrimethylsilanes. J. Am. Chem. Soc. 104, 5534–5535 (1982). [Google Scholar]
- Brown H. C. Organoborane-carbon monoxide reactions. Synthesis of carbon structures. Acc. Chem. Res. 2, 65–72 (1969). [Google Scholar]
- Paetzold P., Redenz-Stormanns B. & Boese R. Boroboration of CO with Tri-tert-butylazadiboriridine. Angew. Chem. Int. Ed. Engl. 29, 900–902 (1990). [Google Scholar]
- Teichmann J., Stock H., Pritzkow H. & Siebert W. Carbon monoxide and isonitrile insertion into the B−B bond of five-membered cyclic organo-1,2-diboranes. Eur. J. Inorg. Chem. 1998, 459–463 (1998). [Google Scholar]
- Mason M. R., Song B. & Kirschbaum K. Remarkable room-temperature insertion of carbon monoxide into an aluminum−carbon bond of tri-tert-butylaluminum. J. Am. Chem. Soc. 126, 11812–11813 (2004). [DOI] [PubMed] [Google Scholar]
- Li X., Ni C., Song H. & Cui C. Formation of aluminacyclobutenes via carbon monoxide and isocyanide insertion. Chem. Commun. 1763–1765 (2006). [DOI] [PubMed] [Google Scholar]
- Mason M. R., Song B., Han Y. & Hu X. Reaction of carbon monoxide with tri-tert-butylgallium:the first example of CO insertion into a gallium–carbon bond. Inorg. Chim. Acta 361, 3332–3337 (2008). [Google Scholar]
- Lavallo V., Canac Y., Donnadieu B., Schoeller W. W. & Bertrand G. CO fixation to stable acyclic and cyclic alkyl amino carbenes: stable amino ketenes with a small HOMO–LUMO gap. Angew. Chem. Int. Ed. 45, 3488–3491 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajid M. et al. Facile carbon monoxide reduction at intramolecular frustrated phosphane/borane lewis pair templates. Angew. Chem. Int. Ed. 52, 2243–2246 (2013). [DOI] [PubMed] [Google Scholar]
- Dobrovetsky R. & Stephan D. W. Stoichiometric metal-free reduction of CO in syn-gas. J. Am. Chem. Soc. 135, 4974–4977 (2013). [DOI] [PubMed] [Google Scholar]
- Braunschweig H. et al. Metal-free binding and coupling of carbon monoxide at a boron–boron triple bond. Nat. Chem. 5, 1025–1028 (2013). [DOI] [PubMed] [Google Scholar]
- Nakata N., Oikawa T., Matsumoto T., Kabe Y. & Sekiguchi A. Silyl-substituted 1,4-disila(dewar benzene): new synthesis and unexpected insertion of CO into the Si−Si bond to form a disilyl ketone. Organometallics 24, 3368–3370 (2005). [Google Scholar]
- Wang X. et al. Room-temperature reaction of carbon monoxide with a stable diarylgermylene. J. Am. Chem. Soc. 131, 6912–6913 (2009). [DOI] [PubMed] [Google Scholar]
- Brown Z. D. & Power P. P. Mechanisms of reactions of open-shell, heavier group 14 derivatives with small molecules: n−π* back-bonding in isocyanide complexes, C–H activation under ambient conditions, CO coupling, and ancillary molecular interactions. Inorg. Chem. 52, 6248–6259 (2013). [DOI] [PubMed] [Google Scholar]
- Luckert S. et al. Tri-tert-butylazadiboriridin: ringerweiterung mit isonitrilen, α-bromlithioalkanen und aziden. Chem. Ber. 128, 1029–1035 (1995). [Google Scholar]
- Bauer F., Braunschweig H. & Schwab K. 1,1-diboration of isocyanides with [2]borametalloarenophanes. Organometallics 29, 934–938 (2010). [Google Scholar]
- Nguyen P. et al. Lewis base adducts of diboron compounds: molecular structures of [B2(cat)2(4-picoline)] and [B2(cat)2(4-picoline)2] (cat=1,2-O2C6H4). Inorg. Chem. 34, 4290–4291 (1995). [Google Scholar]
- Clegg W. et al. Lewis-base adducts of the diborane(4) compounds B2(1,2-E2C6H4)2 (E=O or S). J. Chem. Soc. Dalton 839–846 (1997). [Google Scholar]
- Grigsby W. J. & Power P. P. One-electron reductions of organodiborane(4) compounds: Singly reduced anions and rearrangement reactions. Chem. Eur. J. 3, 368–375 (1997). [Google Scholar]
- Neu A. et al. Novel tetraalkyltetraboranes of the type B4R4, B4H2R4 and B4H4R4. Inorg. Chim. Acta 289, 58–69 (1999). [Google Scholar]
- Clegg W., Marder T. B., Nlate S. & Scott A. J. 2,3,5,6-Tetrakis[3,5-bis(trifluoromethyl)phenoxy]-2,5-bis(dimethylamino)2,3,5,6-tetrabora-1,4-dioxane diethyl ether 0.667-solvate. Act. Cryst. Sec. C 63, O603–O605 (2007). [DOI] [PubMed] [Google Scholar]
- Gao M., Thorpe S. B. & Santos W. L. sp2−sp3 hybridized mixed diboron: synthesis, characterization, and copper-catalyzed β-boration of α,β-unsaturated conjugated compounds. Org. Lett. 11, 3478–3481 (2009). [DOI] [PubMed] [Google Scholar]
- Gao M. et al. Structure and reactivity of a preactivated sp2–sp3 diboron reagent: catalytic regioselective boration of α,β-unsaturated conjugated compounds. J. Org. Chem. 76, 3997–4007 (2011). [DOI] [PubMed] [Google Scholar]
- Thorpe S. B., Guo X. & Santos W. L. Regio- and stereoselective copper-catalyzed β-borylation of allenoates by a preactivated diboron. Chem. Commun. 47, 424–426 (2011). [DOI] [PubMed] [Google Scholar]
- Nozaki K. et al. Boryltrihydroborate: synthesis, structure, and reactivity as a reductant in ionic, organometallic, and radical reactions. J. Am. Chem. Soc. 132, 11449–11451 (2010). [DOI] [PubMed] [Google Scholar]
- Bissinger P. et al. Generation of a carbene-stabilized bora-borylene and its insertion into a C–H bond. J. Am. Chem. Soc. 133, 19044–19047 (2011). [DOI] [PubMed] [Google Scholar]
- Braunschweig H., Damme A., Jimenez-Halla J. O. C., Kupfer T. & Radacki K. Phosphine adducts of 1,2-dibromo-1,2-dimesityldiborane(4): between bridging halides and rearrangement processes. Angew. Chem. Int. Ed. 51, 6267–6271 (2012). [DOI] [PubMed] [Google Scholar]
- Braunschweig H. et al. Quaternizing diboranes(4): highly divergent outcomes and an inorganic Wagner–Meerwein rearrangement. J. Am. Chem. Soc. 135, 8702–8707 (2013). [DOI] [PubMed] [Google Scholar]
- Braunschweig H., Damme A. & Kupfer T. Synthesis of a bicyclic diborane by selective boron carbon bond formation. Chem. Commun. 49, 2774–2776 (2013). [DOI] [PubMed] [Google Scholar]
- Bonet A., Gulyás H. & Fernández E. Metal-free catalytic boration at the β-position of α,β-unsaturated compounds: a challenging asymmetric induction. Angew. Chem. Int. Ed. 49, 5130–5134 (2010). [DOI] [PubMed] [Google Scholar]
- Bonet A., Pubill-Ulldemolins C., Bo C., Gulyás H. & Fernández E. Transition-metal-free diboration reaction by activation of diboron compounds with simple Lewis bases. Angew. Chem. Int. Ed. 50, 7158–7161 (2011). [DOI] [PubMed] [Google Scholar]
- Pubill-Ulldemolins C., Bonet A., Bo C., Gulyás H. & Fernández E. Activation of diboron reagents with Brønsted bases and alcohols: an experimental and theoretical perspective of the organocatalytic boron conjugate addition reaction. Chem. Eur. J. 18, 1121–1126 (2012). [DOI] [PubMed] [Google Scholar]
- Pubill-Ulldemolins C., Bonet A., Gulyas H., Bo C. & Fernandez E. Essential role of phosphines in organocatalytic β-boration reaction. Org. Biomol. Chem. 10, 9677–9682 (2012). [DOI] [PubMed] [Google Scholar]
- Sanz X. et al. Metal-free borylative ring-opening of vinyl epoxides and aziridines. Org. Biomol. Chem. 11, 7004–7010 (2013). [DOI] [PubMed] [Google Scholar]
- Lee K.-s., Zhugralin A. R. & Hoveyda A. H. Efficient C−B bond formation promoted by N-heterocyclic carbenes: synthesis of tertiary and quaternary B-substituted carbons through metal-free catalytic boron conjugate additions to cyclic and acyclic α,β-unsaturated carbonyls. J. Am. Chem. Soc. 131, 7253–7255 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Radomkit S., O’Brien J. M. & Hoveyda A. H. Metal-free catalytic enantioselective C–B bond formation: (pinacolato)boron conjugate additions to α,β-unsaturated ketones, esters, Weinreb amides, and aldehydes promoted by chiral N-heterocyclic carbenes. J. Am. Chem. Soc. 134, 8277–8285 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid J., Carbó J. J. & Fernández E. A clear-cut example of selective Bpin-Bdan activation and precise Bdan transfer on boron conjugate addition. Chem. Eur. J. 20, 3616–3620 (2014). [DOI] [PubMed] [Google Scholar]
- Nöth H. Contributions to the chemistry of boron, 153. The crystal and molecular-structure of a 1.3.2-dioxaborolan-2-yl-1',3',2'-dioxaborolane. Z. Naturforsch. B Chem. Sci. 39, 1463–1466 (1984). [Google Scholar]
- Lide D. R. (ed)CRC Handbook of Chemistry and Physics CRC Press (2012). [Google Scholar]
- Williamson R. T., Buevich A. V. & Martin G. E. Experimental and theoretical investigation of 1JCC and nJCC coupling constants in strychnine. Org. Lett. 14, 5098–5101 (2012). [DOI] [PubMed] [Google Scholar]
- Anderson J. C. & Broughton S. Efficient synthesis of diphenylketene-13C2. Synthesis 2001, 2379–2380 (2001). [Google Scholar]
- Becke A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098–3100 (1988). [DOI] [PubMed] [Google Scholar]
- Lee C., Yang W. & Parr R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988). [DOI] [PubMed] [Google Scholar]
- Zhao Y. & Truhlar D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2008). [Google Scholar]
- Cossi M., Barone V., Mennucci B. & Tomasi J. Ab initio study of ionic solutions by a polarizable continuum dielectric model. Chem. Phys. Lett. 286, 253–260 (1998). [Google Scholar]
- Segawa Y., Suzuki Y., Yamashita M. & Nozaki K. Chemistry of boryllithium: synthesis, structure, and reactivity. J. Am. Chem. Soc. 130, 16069–16079 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-20, Supplementary Tables 1-7, Supplementary Methods and Supplementary References
Combined CIF file for crystallographic data