Abstract
Changes of cytokines in bronchoalveolar lavage fluid (BALF) reflect immunologic reactions of the lung in pulmonary malignancies. Detection of biomarkers in BALF might serve as an important method for differential diagnosis of lung cancer. A total of 78 patients admitted into hospital with suspected lung cancer were included in our study. BALF samples were obtained from all patients, and were analyzed for TGF-β1, IL-6, and TNF-α using commercially available sandwich ELISA kits. The levels of TGF-β1 in BALF were significantly higher in patients with lung cancer compared with patients with benign diseases (P = 0.003). However, no significant difference of IL-6 (P = 0.61) or TNF-α (P = 0.72) in BALF was observed between malignant and nonmalignant groups. With a cut-off value of 10.85 pg/ml, TGF-β1 showed a sensitivity of 62.2%, and a specificity of 60.6%, in predicting the malignant nature of pulmonary disease. Our data suggest that TGF-β1 in BALF might be a valuable biomarker for lung cancer. However, measurement of IL-6 or TNF-α in BALF has poor diagnostic value in lung cancer.
A major problem in lung cancer is the lack of clinically useful tests for early diagnosis and screening patients with lung lump by noninvasive diagnostic procedures1. It is reported that approximately two-thirds of lung cancer patients is the presence of metastatic tumors at the time of diagnosis2. Lung cancer screening by chest X-ray and sputum cytology have proven ineffective in improving patient survival rate3,4, leading to the search for more sensitive and specific tests. One promising approach is the identification of lung cancer-specific biomarkers and detection of them at an early stage.
Considering that tumor biomarkers are produced directly by the tumor or by non-tumor cells as a response to the presence of tumor cells, the elevation of tumor biomarkers can be detected earlier than radiographic abnormalities5. Investigating specific molecular markers in airways might serve as an important adjunct to routine examination for lung cancer diagnosis. The utility of cytokines in bronchoalveolar lavage fluid (BALF) for differential diagnosis of lung cancer has been described in several studies6,7,8,9,10,11,12. In our previous reports, we also observed that the levels of vascular endothelial growth factor (VEGF) and neuron-specific enolase (NSE) were significantly higher in BALF of lung cancer patients than in that of patients with benign diseases13,14. Recently, transforming growth factor (TGF)-β1, interleukin (IL)-6, and tumor nectosis factor (TNF)-α were suggested in published studies as possible diagnostic biomarkers of lung cancer due to their higher concentrations in serum of lung cancer patients15,16,17. However, up to data, little study has performed to study these cytokines in BALF of lung cancer. In this study, we performed a prospective study to investigate TGF-β1, IL-6, and TNF-α expression in airways by comparing levels of them in benign diseases and lung cancer.
Results
The BAL fluid of TGF-β1 concentration was significantly higher in patients with lung cancer compared with patients with benign diseases (18.2 [8.4–46.4] pg/ml versus 8.4 [3.5–17.0] pg/ml, P = 0.003; Figure 1A). However, there was no significant difference in BAL fluid IL-6 (4.3 ± 0.4 pg/ml versus 4.1 ± 0.4 pg/ml, respectively, P = 0.61; Figure 1B) or TNF-α (1.4 ± 0.2 pg/ml versus 1.2 ± 0.3 pg/ml, respectively, P = 0.72; Figure 1C) between lung cancer patients and non-cancer controls. However, no significant difference in BALF TGF-β1, IL-6, and TNF-α levels were observed between smokers and nonsmokers in any group (data not shown).
Figure 1. Comparison of TGF-β1, IL-6, and TNF-α levels in bronchoalveolar lavage fluid between benign and malignant groups.
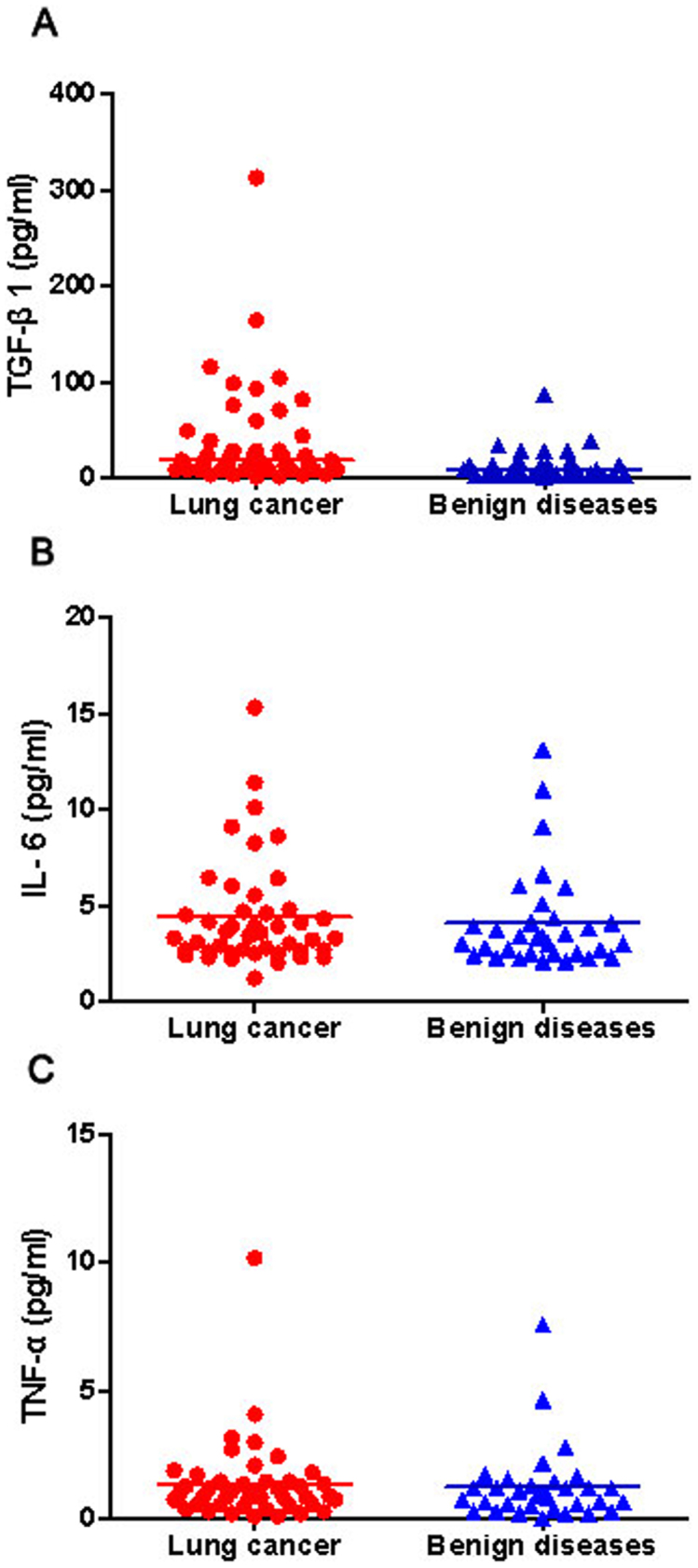
The levels of TGF-β1 were significantly higher in lung cancer patients than those in benign diseases (P = 0.003, Mann-Whitney U-test) (A); no significant difference of IL-6 (P = 0.61, Student's t-test) (B) or TNF-α (P = 0.72, Student's t-test) (C) levels was found between malignant and nonmalignant groups. Horizontal lines represent the median values for TGF-β1; Horizontal lines represent the mean values for IL-6 and TNF-α.
We evaluated the correlation of TGF-β1, IL-6, and TNF-α in BALF by the Pearson correlation analysis. A significant correlation was found between TGF-β1 and IL-6 in BALF (r = 0.337, P = 0.003; Figure 2A). Nevertheless, the levels of TGF-β1 in BALF were not relevant to that of TNF-α (r = 0.121, P = 0.290; Figure 2B). Similarly, there was no significant correlation was observed between IL-6 and TNF-α in BALF (r = −0.022, P = 0.847; Figure 2C).
Figure 2. Correlation of TGF-β1, IL-6, and TNF-α levels in bronchoalveolar lavage fluid.
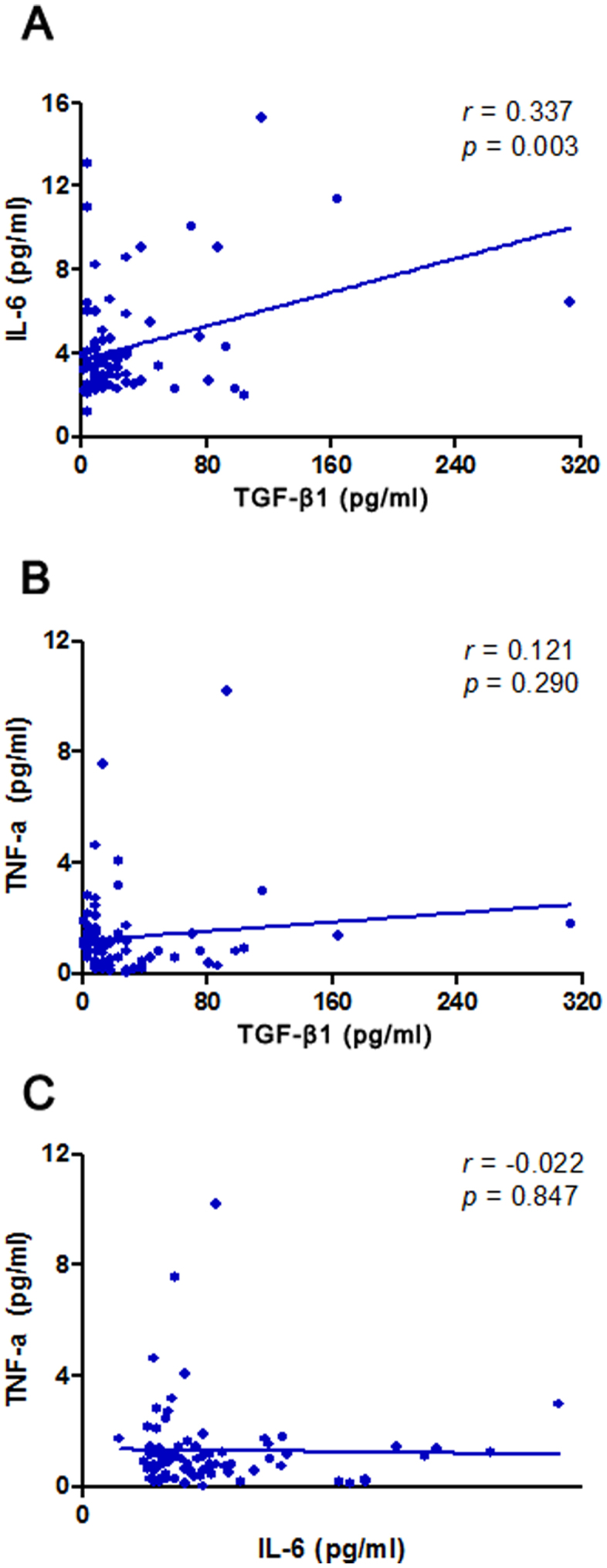
Statistically correlation was observed between TGF-β1 and IL-6 (r = 0.337, P = 0.003) (A); no significant correlation was found between TGF-β1 and TNF-α (r = 0.121, P = 0.290) (B) or between IL-6 and TNF-α (r = −0.022, P = 0.847) (C).
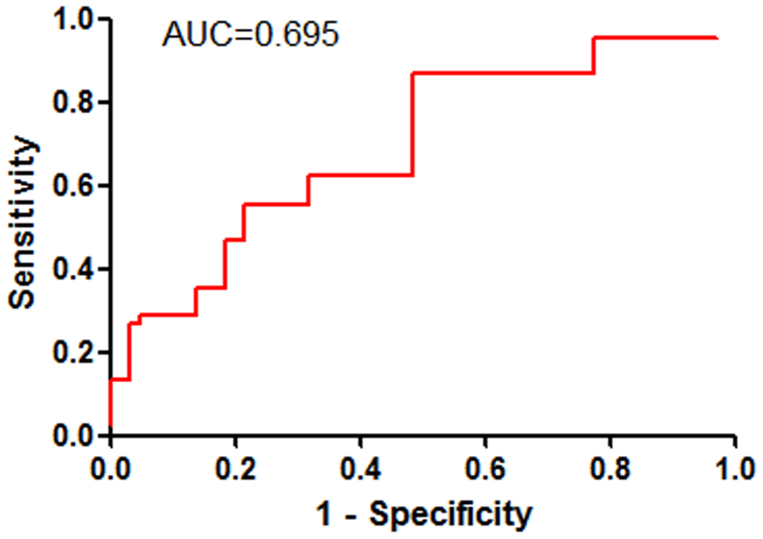
Considering TGF-β1 was statistical differences between lung cancer patients and non-cancer controls, ROC analysis was further conducted to examine the diagnostic ability of the TGF-β1 for predicting lung cancer. As shown in Figure 3, the area under the receiver operating characteristic curve (AUC) was 0.695 (P = 0.003). With a cutoff value of 10.85 pg/ml, TGF-β1 had a sensitivity of 62.2%, a specificity of 60.6%, a positive predictive value of 67.5%, and a negative predictive value of 52.6%, in predicting the malignant nature of pulmonary disease.
Figure 3. Receiver operating characteristic (ROC) curve was performed to evaluate the threshold value of TGF-β1 in differentiating malignant from benign pulmonary diseases.
TGF-β1 reached a sensitivity of 62.2%, a specificity of 60.6%, a positive predictive value of 67.5%, and a negative predictive value of 52.6% (cut-off value: 10.85 pg/ml; area under the curve: 0.695).
Disscusion
Distinguishing benign lung diseases from lung cancer through noninvasive approaches such as useful tumor markers is very important as to avoid patients undergoing surgery for a benign condition18. In our previous reports, we found levels of cancer-specific cytokines in BALF elevated much earlier and present at a higher concentration than those in peripheral blood13. Thus, detection of biomarkers in BALF can serve as an important method for lung cancer diagnosis13,14. In the present study, we conducted a prospective study to investigate whether levels of BAL fluid TGF-β1, IL-6, and TNF-α could be useful in distinguishing malignant from benign pulmonary diseases.
A total of 78 patients with suspected lung cancer were enrolled in our study. TGF-β1, IL-6, and TNF-α were determined by commercially available sandwich ELISA kits. The results showed that the levels of TGF-β1 in BALF were higher among patients with lung cancer than in that of patients with benign diseases. In addition, a significant correlation was found between TGF-β1 and IL-6 in BALF. However, no significant difference in BAL IL-6 or TNF-α was observed between lung cancer patients and non-cancer controls.
TGF-β1 expression was significantly higher in lung cancer patients compared with patients with benign diseases, indicating TGF-β1 in BALF might be a useful biomarker of lung cancer. ROC analysis was further conducted to examine the diagnostic ability of the TGF-β1 for predicting lung cancer. The result showed that the diagnostic threshold afforded by the ROC analysis was 10.85 pg/ml. The area under the ROC was 0.695. With a threshold value of 10.85 pg/ml, TGF-β1 had a sensitivity of 62.2%, a specificity of 60.6%, a positive predictive value of 67.5%, and a negative predictive value of 52.6%, in predicting the malignant nature of pulmonary disease. Therefore, the presence of low levels of TGF-β1 in BALF indicated a low probability of malignancy.
Numerous studies provided indirect evidence for a functional link between TGF-β1 and IL-6 in many human diseases. Chen and colleague demonstrated that activated TGF-β1/IL-6 pathways could be responsible for more aggressive tumor growth and resistance to treatment in oral cancer19. In another report, Yamada et al. showed that crosstalk between IL-6 and TGF-β1 was associated with key features of malignancy20. None the less, there was evidence that the levels of IL-6 in serum was good marker of lung cancer and might serve as a predictive biomarker for the efficacy of therapy21,22. However, in our study, no elevated concentrations of IL-6 in BALF were observed in lung cancer patients, although a significant correlation was found between TGF-β1 and IL-6 in BALF.
TNF-α is a multifunctional cytokine playing a key role in apoptosis and cell survival as well as in inflammation and immunity23. Dalaveris et al. have analyzed the levels of TNF-α in exhaled breath condensate (EBC) and serum of patients with primary lung cancer24. They observed that the levels of TNF-α was higher in both serum and EBC of lung cancer patients than age and gender-matched healthy controls24. Nevertheless, we failed to find a significant difference in the levels of TNF-α in BALF between benign and malignant groups. In addition, no significant correlation between TNF-α and TGF-β1 in BALF was found. Our results demonstrated that determination of the BAL fluid TNF-α level in flexible bronchoscopy may be useless in the diagnosis of lung cancer.
Some limitations of our study are worth discussing. First, there was significant difference on smoking status between lung cancer patients and non-cancer controls. Tobacco smoking is an established risk factor for lung cancers and may have an effect on human immune response. Although no differences in cytokine levels between smokers and non-smokers were detected in our study, environmental factors should also be considered in the development of lung cancer. Second, the prognostic value of TGF-β1, IL-6, and TNF-α in BALF were not described in our study. Some cytokines may provide information about the clinical outcome of lung cancer patients25. On the other hand, biomarkers can also help clinicians in selecting the most effective anticancer treatments for each patient26. Future studies with well-matched controls are needed to detect these cytokines and their prognostic values, which might lead to better understanding biological characteristics of them in lung cancer.
In summary, our study showed that the levels of TGF-β1 were significantly higher in BALF in lung cancer patients than in that of patients with benign diseases. This work may only suggest that TGF-β1 in BALF is a valuable biomarker for lung cancer and further investigation is needed. However, measurement of TNF-α or IL-6 in BALF has poor diagnostic value in lung cancer.
Methods
Study subjects
A prospective study was conducted in Affiliated Hospital of Ningbo University in China from February 2011 to July 2013. The study protocol was approved by the Institutional Review Board for Human Studies of Affiliated Hospital, School of Medicine, Ningbo University (Ningbo, China). Written informed consent was obtained from all participants. The experiments were performed in accordance with American College of Chest Physicians Evidence-Based Clinical Practice Guidelines27. 78 patients admitted into this hospital with suspected lung cancer were included. Clinical information regarding patient characteristics was based on patient records and registries. All patients had histological confirmed and were excluded if they had received preoperative chemotherapy or radiotherapy. Basic characteristics for patients are summarized in Table 1. There were 45 lung cancer patients (60.8 ± 1.2 years) and 33 patients with noncancerous diseases (58.2 ± 1.7 years). The pathologic types included 18 squamous cell carcinomas, 11 adenocarcinomas, 10 small cell carcinomas, and 6 of other cell types. There were 31 patients with pneumonia, 2 with tuberculosis, and 1 with pulmonary sarcoidosis in the control group. Smoking habits were defined at 1 year prior to diagnosis for cases or 1 year prior to interview for controls.
Table 1. The characteristics of the patients.
| Lung cancer | Benign group | P value | |
|---|---|---|---|
| Age, years | 60.8 ± 1.2 | 58.2 ± 1.7 | 0.20 |
| Gender | 0.68 | ||
| Male | 28 | 19 | |
| Female | 17 | 14 | |
| Smoking status | 0.55 | ||
| Smokers | 20 | 12 | |
| Non-smokers | 25 | 21 | |
| Pack/years | 66.1 ± 9.3 | 29.6 ± 5.3 | 0.001 |
| Histological type | |||
| Squamous cell carcinoma | 18 | ||
| Adenocarcinoma | 11 | ||
| Small-cell lung cancer | 10 | ||
| Others | 6 |
Bronchoalveolar lavage
BALF samples were collected and the methods were referred to previous studies9,12,13,14. The lavage was done prior to brushing or biopsies to avoid contamination with blood. The bronchus on the disease side was washed with two 50-ml aliquots sterile physiological saline. The fluid was gently withdrawn into a siliconized container placed in iced water. The recovered volume of BALF > 60-ml was considered to be an acceptable level of quality. The chilled lavage fluid was filtered through a nylon filter to remove mucus and centrifuged at 3,000 rpm for 10-min. The cell pellets were separated from the supernatants and stored at −80°C.
Measurements of TGF-β1, IL-6, and TNF-α
The levels of TGF-β1 (pg/ml), IL-6 (pg/ml), and TNF-α (pg/ml) were measured using sandwich enzyme linked immunosorbent assays (ELISA). All reagents used for the experiments were standard high-quality chemicals from international companies (TGF-β1: R&D systems, Minneapolis, MN, USA; IL-6 and TNF-α: eBioscience, San Diego, CA, USA). The assays were conducted according to the manufacturer's guidelines. The samples were analyzed in batches to minimize interassay variability.
Statistical analysis
Data was presented as means ± standard error of the mean (SEM). TGF-β1 levels were not normally distributed and were expressed as medians and interquartile ranges (IQR). If the data distribution was normal, comparison between different groups was done using the Student's t-test; otherwise, the nonparametric Mann-Whitney U-test was applied. The relationships between different markers were determined using Pearson correlation. Sensitivity, specificity, positive predictive value, and negative predictive value were calculated to evaluate diagnostic performance for TGF-β1. Receiver operating characteristic (ROC) analysis was conducted to examine the diagnostic ability of the TGF-β1 for predicting lung cancer. All hypothesis tests were 2-sided, with statistical significance defined as having a P value of less than 0.05. Statistical analyses were conducted using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA) or SPSS for windows (version 13; SPSS, Chicago, IL).
Author Contributions
C.C., Z.D., Z.C. and Z.X. designed the experiments. C.C., Z.D., Z.C., Z.X., S.S., Y.Y. and D.L. carried out the experiments and calculations. C.C., Z.D., Z.C. and Z.X. wrote and edited the paper.
Acknowledgments
This work was supported by the grant of Social Development of Science and Technology Project of Ningbo (No. 2011C50025, C Cao), grant of Social Development of Science and Technology Project of Ningbo (No. 2012C50006, Z Chen), and grant of Natural Science Foundation of Ningbo (No. 2012A610257, ZC Deng).
References
- Sozzi G. et al. Detection of microsatellite alterations in plasma DNA of lung cancer patients: a prospect for early diagnosis. Clinical. Cancer. Research. 5, 2689–92 (1999). [PubMed] [Google Scholar]
- Wingo P. A. et al. Annual report to the nation on the status of cancer, 1973–1996, with a special section on lung cancer and tobacco smoking. J. Natl. Cancer. Inst. 9, 675–90 (1999). [DOI] [PubMed] [Google Scholar]
- Ellis J. R. & Gleeson F. V. Lung cancer screening. J. Br. J. Radiol. 74, 478–85 (2001). [DOI] [PubMed] [Google Scholar]
- Marcus P. M. Lung cancer screening: an update. J. Clin. Oncol. 19, 83S–6S (2001). [PubMed] [Google Scholar]
- Wang P., Piao Y., Zhang X., Li W. & Hao X. The concentration of CYFRA 21-1, NSE and CEA in cerebro-spinal fluid can be useful indicators for diagnosis of meningeal carcinomatosis of lung cancer. Cancer. Biomark. 13, 123–30 (2013). [DOI] [PubMed] [Google Scholar]
- Bugdayci G. et al. Matrix metalloproteinase-9 in broncho-alveolar lavage fluid of patients with non-small cell lung cancer. Exp. Oncol. 28, 169–71 (2006). [PubMed] [Google Scholar]
- Domaga∤a-Kulawik J., Hoser G., Safianowska A., Grubek-Jaworska H. & Chazan R. Elevated TGF-beta1 concentration in bronchoalveolar lavage fluid from patients with primary lung cancer. Arch. Immunol. Ther. Exp. 54, 143–7 (2006). [DOI] [PubMed] [Google Scholar]
- Cremades M. J., Menéndez R., Rubio V. & Sanchis J. Fibronectin in bronchoalveolar lavage fluid in lung cancer: tumor or inflammatory marker? Respiration. 65, 178–82 (1998). [DOI] [PubMed] [Google Scholar]
- Cremades M. J., Menéndez R., Pastor A., Llopis R. & Aznar J. Diagnostic value of cytokeratin fragment 19 (CYFRA 21-1) in bronchoalveolar lavage fluid in lung cancer. Respir. Med. 92, 766–71 (1998). [DOI] [PubMed] [Google Scholar]
- Ohta Y. et al. Vascular endothelial growth factor expression in airways of patients with lung cancer: a possible diagnostic tool of responsive angiogenic status on the host side. Chest. 121, 1624–27 (2002). [DOI] [PubMed] [Google Scholar]
- Charalabopoulos K. et al. CEA levels in serum and BAL in patients suffering from lung cancer: correlation with individuals presenting benign lung lesions and healthy volunteers. Med. Oncol. 24, 219–25 (2007). [DOI] [PubMed] [Google Scholar]
- Emad A. & Emad V. The value of BAL fluid LDH level in differentiating benign from malignant solitary pulmonary nodules. J. Cancer. Res. Clin. Oncol. 134, 489–93 (2008). [DOI] [PubMed] [Google Scholar]
- Cao C. et al. Utility of VEGF and sVEGFR-1 in bronchoalveolar lavage fluid for differential diagnosis of primary lung cancer. Asian. Pac. J. Cancer. Prev. 14, 2443–6 (2013). [DOI] [PubMed] [Google Scholar]
- Cao C. et al. Evaluation of VEGF-C and tumor markers in bronchoalveolar lavage fluid for lung cancer diagnosis. Sci. Rep. 3, 3473 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Santiago A. E. et al. TGF-β1 serum concentration as a complementary diagnostic biomarker of lung cancer: establishment of a cut-point value. J Clin Lab Anal. 25, 238–43 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilonidis G. et al. Interleukin -1beta (IL-1 beta), interleukin 6 (IL-6) and tumor necrosis factor (TNF) in plasma and pleural fluid of pneumonia, lung cancer and tuberculous pleuritis. J Biol Regul Homeost Agents. 20, 41–6 (2006). [PubMed] [Google Scholar]
- Dalaveris E. et al. VEGF, TNF-alpha and 8-isoprostane levels in exhaled breath condensate and serum of patients with lung cancer. Lung Cancer. 64, 219–25 (2009). [DOI] [PubMed] [Google Scholar]
- Crohns M. et al. Exhaled pentane as a possible marker for survival and lipid peroxidation during radiotherapy for lung cancer--a pilot study. Free Radic Res. 43, 965–74 (2009). [DOI] [PubMed] [Google Scholar]
- Chen M. F., Wang W. H., Lin P. Y., Lee K. D. & Chen W. C. Significance of the TGF-β1/IL-6 axis in oral cancer. Clin. Sci. (Lond) 122, 459–72 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada D. et al. Role of crosstalk between interleukin-6 and transforming growth factor-beta 1 in epithelial-mesenchymal transition and chemoresistance in biliary tract cancer. Eur. J. Cancer. 49, 1725–40 (2013). [DOI] [PubMed] [Google Scholar]
- Wojciechowska-Lacka A., Matecka-Nowak M., Adamiak E., Lacki J. K. & Cerkaska-Gluszak B. Serum levels of interleukin-10 and interleukin-6 in patients with lung cancer. Neoplasma. 43, 155–8 (1996). [PubMed] [Google Scholar]
- Wang Y. S. et al. Serum cytokine levels in patients with advanced non-small cell lung cancer: correlation with clinical outcome of erlotinib treatment. Chin. Med. J. (Engl) 126, 3931–5 (2013). [PubMed] [Google Scholar]
- van Horssen R., Ten Hagen T. L. & Eggermont A. M. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 11, 397–408 (2006). [DOI] [PubMed] [Google Scholar]
- Dalaveris E. et al. VEGF, TNF-alpha and 8-isoprostane levels in exhaled breath condensate and serum of patients with lung cancer. Lung. Cancer. 64, 219–25 (2009). [DOI] [PubMed] [Google Scholar]
- Fleitas T. et al. VEGF and TSP1 levels correlate with prognosis in advanced non-small cell lung cancer. Clin Transl Oncol. 15, 897–902 (2013). [DOI] [PubMed] [Google Scholar]
- Wang Y. S. et al. Serum cytokine levels in patients with advanced non-small cell lung cancer: correlation with clinical outcome of erlotinib treatment. Chin Med J (Engl). 126, 3931–5 (2013). [PubMed] [Google Scholar]
- Kozower B. D., Larner J. M., Detterbeck F. C. & Jones D. R. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 143, e369S–99S (2013). [DOI] [PubMed] [Google Scholar]