Abstract
Bax inhibitor-1 (BI-1) is an evolutionarily-conserved endoplasmic reticulum protein. The expression of BI-1 in mammalian cells suppresses apoptosis induced by Bax, a pro-apoptotic member of the Bcl-2 family. BI-1 has been shown to be associated with calcium (Ca2+) levels, reactive oxygen species (ROS) production, cytosolic acidification, and autophagy as well as endoplasmic reticulum stress signaling pathways. According to both in vitro and clinical studies, BI-1 promotes the characteristics of cancers. In other diseases, BI-1 has also been shown to regulate insulin resistance, adipocyte differentiation, hepatic dysfunction and depression. However, the roles of BI-1 in these disease conditions are not fully consistent among studies. Until now, the molecular mechanisms of BI-1 have not directly explained with regard to how these conditions can be regulated. Therefore, this review investigates the physiological role of BI-1 through molecular mechanism studies and its application in various diseases.
Keywords: Bax inhibitor-1, ER stress, lysosome, reactive oxygen species, unfolded protein response.
1. INTRODUCTION
Human Bax inhibitor-1 (BI-1) has been identified as a suppressor of Bax-mediated cell death in yeast [1]. BI-1 is an anti-apoptotic integral membrane protein located primarily in the intracellular membranes of the endoplasmic reticulum (ER) [2, 3]. The anti-apoptotic role of BI-1 has been clearly demonstrated in ER stress-induced cell death, but not in other stresses such as oxidative stress or death receptor-activation stress. About the issues of ER stress regulation, there are suggested mechanisms including the regulation of Ca2+ or ROS, especially ER-originated ROS. The activities of intracellular organelles such as lysosome have been also studied in relation with the BI-1 characteristics, ER stress regulation. Separately, BI-1-specific regulation of a specific arm of ER stress involving IRE-1α has been reported, in the context of secretory protein IgG and autophagy studies [4, 5].
Studies of BI-1 are now extending to include the BI-1 protein family. As such, BI-1 has been recently re-named as TMBIM6 as it is part of the transmembraneBax inhibitor-1-containing motif family [6]. Genetic and bioinformatics studies have revealed that TMBIM family members are highly conserved across species [7, 8], with a possible common ancestor in yeast [9]. TMBIM1/RECS1 (responsive to centrifugal force and shear stress gene 1 protein) has a lysosomal, Golgi, and plasma membrane cellular distribution [10, 11], and it interacts with and inhibits Fas ligand-mediated apoptosis [12]. TMBIM2/LFG (life guard) has a plasma membrane location, and attenuates Fas ligand-induced apoptosis [13]. TMBIM3/GRINA (glutamate receptor ionotropic NMDA protein 1) has been also studied with regard to a regulatory role in cell death in cellular and animal models of ER stress, with a possible link to the regulation of ER calcium homeostasis [14]. TMBIM4/GAAP (Golgi anti-apoptotic associated protein) is exclusively located at the Golgi compartment and its expression modulates the susceptibility of cells to intrinsic and extrinsic apoptotic stimuli [15, 16]. TMBIM5/MICS1 (mitochondrial morphology and cristae structure 1)/GHTIM (growth hormone-inducible transmembrane protein) is located at the mitochondria, and has been shown to prevent mitochondrial fragmentation and the release of cytochrome c induced by actinomycin D treatment [17].
The multiple helical transmembrane topology of BI-1, with short hydrophilic regions and a probable reentrant loop at the C terminus is reminiscent of the alpha-subunits of ion channels [18]. The transmembrane regions make up the main component of TMBIM family members. The C terminus residues of BI-1, EKDKKKEKK, are important for its anti-apoptotic, cell adhesion, and calcium regulatory functions [19-21]. The highly conserved hydrophobic profile of TMBIM members suggests that structure must be an important aspect of the function of these proteins. Although there has been inconsistency in the literature about whether TMBIM membranes have six or seven tranmembrane domains (TMD), and other BI-1 characteristics; a model with six TMDs and both N- and C- termini toward the cytosol has recently been more clearly documented [3, 22, 23].
The role of BI-1 has been studied in different physio/pathological models, including ischemia, diabetes, liver regeneration, and cancer. These physiological mechanisms have been applied to clinically relevant studies regarding ER stress and Ca2+ regulations [21, 24-27]. In BI-1 studies, some debate still exists about the regulatory mechanisms of ER stress and Ca2+ homeostasis. These issues are further discussed in this review. We also propose an explanation for the correlation between BI-1 physiological mechanisms and pathological roles. Therefore, this review should contribute to understanding of the basic functions of BI-1 and its role in diseases.
2. BI-1 SUPPRESSES APOPTOSIS
BI-1 was first identified by Xu and Reed [1] in a functional screen using Bax-ectopically expressing yeast. Bax stimulates mitochondria-initiated reactive oxygen species (ROS) accumulation and cell death in yeast as well as in mammalian systems [28, 29]. BI-1, which is also called as testis enhanced gene transcript (TEGT), has a protective role against ER stress-induced apoptosis [2, 30]. Ca2+ and ROS are considered initiators of ER stress and other phenomena [31]. Above a threshold, Ca2+ or ROS induce ER stress-associated cell death, and the physiological amount of Ca2+ or ROS is considered an adaptive response to ER stress [32, 33].
BI-1 regulates the amount of Ca2+ that is associated with ER stress [3, 20, 34-36] and BI-1 is also associated with the regulation of ROS [37-40]. BI-1 has been generally suggested to be an ER stress regulator, controlling ER-generated ROS accumulation, which is also related to cell death regulation. Thus, the two BI-1-associated signaling transduction pathways seem directly related to anti-apoptotic function.
BI-1 is evolutionarily conserved in other organisms including Arabidopsis thaliana, Drosophila melanogaster, Escherichia coli, and others [9]. Co-transfection of Bax with plant BI-1 homologues (oilseed rape and tobacco) in human embryonic kidney 293 cells inhibits the apoptosis induced by transfection Bax alone [41]. It was also found that a plant BI-1 homologue (rice and Arabidopsis) inhibits cell death induced by Bax in yeast [42]. Moreover, transgenic rice cells that overexpress the Arabidopsis BI-1 (AtBI-1) gene suppress fungal elicitor-induced cell death [43]. It was reported that in Arabidopsis, AtBI-1 interacts with sphingolipid fatty acid 2-hydroxylase (AtFAH1) via cytochrome b in the ER. AtBI-1 requires AtFAH1 to suppress cell death [44].
BI-1 is also involved in innate immunity in crustaceans [45]. Crayfish which were overexpressing the crayfish BI-1 homologue suppressed white spot syndrome virus-induced cell death. The Saccharomyces cerevisiae protein encoded by YNL305C, which is an ER-localized protein, has been also reported to be a bona fide member of the BI-1 superfamily, and is involved in regulation of the ER stress response with respect to resistance against heat shock, ethanol or glucose-induced programmed cell death [46]. In addition, BI-1 also seems to be linked to an ER stress-mediated unfolded protein response (UPR) and the programmed cell death response.
3. BI-1 REGULATES CA2+
BI-1 has also been described as a Ca2+ channel-like protein [14, 16]. BI-1 has been suggested to regulate intra-ER Ca2+ concentrations ([Ca2+]ER) via its interaction with IP3R [47], leading to a sensitizing effect of BI-1 on IP3R, which may contribute to a decrease in steady-state [Ca2+]ER [47, 48]. The C terminus of BI-1, which has been suggested as a binding site for its interaction with the Ca2+ channel pore of IP3R, is thought to have a key role in ER Ca2+ homeostasis [47]. Recently, the presence of BI-1 was also proposed to reduce the efficiency with which IP3Rs transfer Ca2+ from the ER into the mitochondria at mitochondria-associated microdomains (MAM) [48]. However, additional evidence needs to be gathered regarding the relation of BI-1 with IP3R.
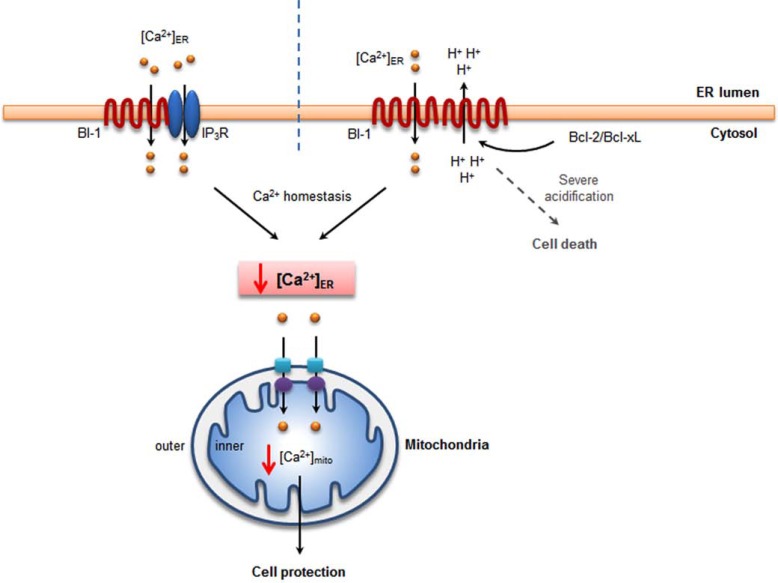
Our group reported that the effect of BI-1 on [Ca2+]ER permeability was pH-dependent [20, 21]. In a BI-1 reconstituted proteoliposome model, encapsulated Ca2+ was released from membranes when exposed to acidic pH [34, 49-51], which is consistent with the cellular model [20]. Concomitantly, proton ions were influxed into the proteoliposomes [49]. Therefore, BI-1 is thought to have Ca2+/H+ antiporter-like activity. Other studies have investigated the relationship between BI-1 and Ca2+ efflux mediated by other agents. Cardiolipin (CL) and phosphatidylserine (PS) stimulated the proton-mediated efflux of Ca2+ ions in a BI-1-encapsulated proteoliposome study [51]. The peptide corresponding to Bcl-2 homology (BH)4 domains also stimulated BI-1 activity, suggesting that CL, PS and the BH4 domains of Bcl-2 and Bcl-XL proteins are stimulating/agonistic factors for the Ca2+/H+ antiporter activity of BI-1. Consistently, BI-1 function was reported to be stimulated by anti-apoptotic Bcl-2 proteins [36], likely through the BH4 domain of Bcl-2 protein interacting with BI-1. These studies implicate the role of the BH4 domain in stimulating Ca2+/H+ antiporter activity, assuming the direct interaction of BI-1 with Bcl-2 family proteins. Studies about the effects of Bcl-2 on [Ca2+]ER have been performed [52-55], and do not show consistent results regarding the Bcl-2-associated capacitance of the intra-ER Ca2+ pool, opening some possibility about the involvement of another protein, such as BI-1, between Bcl-2 and IP3R. Endogenous expression of BI-1 might differ among tissues or cell types, which might affect the cell-type specific role of Bcl-2-associated regulation of [Ca2+]ER level. The Ca2+ channel-like activity of BI-1 is schematically shown in Fig. (1), illustrating the two theories about the BI-1 involvement in Ca2+ leak.
Fig. (1).
BI-1 reduces intra-ER and mitochondrial Ca2+, leading to cell protection. BI-1 binds to IP3R and sensitizes IP3R-induced Ca2+ release. Reduced intra-ER Ca2+ leads to decreased Ca2+ efflux into cytosol and less mitochondrial Ca2+ accumulation, resulting cell protection effect (left). BI-1 has a unique function of pH-dependent Ca2+ channel or Ca2+/H+ antiporter activity. Because of the Ca2+ leak channel activity, intra-ER and mitochondrial Ca2+ is decreased, linked to the cell protection effect (right). However, in severe acidic condition, Ca2+ is more accumulated in mitochondria because of the stimulated Ca2+ leak from ER membrane in the presence of BI-1 (right).
Recently, it was suggested that BI-1 enhances cancer metastasis by altering glucose metabolism and activating the sodium/hydrogen exchanger (Na+/H+ exchanger) [56]. In this situation, the intra-ER Ca2+ leak is likely related to the cytoplasmic ionic balances through store-operated Ca2+ channel, Na+/Ca2+ exchanger and related Na+/H+ exchanger activities [57, 58]. Thus, the BI-1 Ca2+ channel or Ca2+/H+ antiporter activity seems to be closely related to cancer progression and propagation. Two anthracycline anti-cancer drugs, doxorubicin (DXR) and daunorubicin (DNR), specifically interacted with BI-1 and inhibited its Ca2+/H+ antiporter activity, including proton-induced Ca2+ efflux and H+ uptake [34]. These results indicated that BI-1 could be a new cancer therapeutic target using anthracyclines to inhibit the Ca2+/H+ antiporter activity of BI-1.
In plants, AtBI-1 interacted with calmodulin [59-61]. AtBI-1-overexpressing cells attenuated the rise in cytosolic calcium following sarcoplasmic/endoplasmic reticulum Ca2+-ATPase inhibitor treatment, suggesting that AtBI-1 affects calcium homeostasis in plant cell death regulation [59]. It was also proposed that AtBI-1 may mediate control cellular Ca2+ homeostasis through interaction with calmodulin and Ca2+-ATPase in the ER [61]. The C terminus of AtBI-1 was capable of binding with calmodulin and a C-terminal-deleted BI-1 was associated with reduced cell death suppression activity in plants [60]. Through these studies, it has been suggested that BI-1 has a universal Ca2+ leak channel-like effect in mammalian cells and plant cells. It has also been proposed that reduced intra-ER Ca2+ leads to the regulations of mitochondrial Ca2+ accumulation and subsequent cell death.
However, in the presence of a severely acidic pH, such as pH 5.4, intra-ER Ca2+ was predominantly extruded from the ER membrane and spontaneously accumulated in the mitochondria, leading to cell death, showing that the BI-1-induced pro-apoptotic effect under severely acidic conditions is an exemption to the above mentioned BI-1-associated Ca2+ leak channel effect, which is generally interpreted as a protective mechanism. The acidic pH-dependent Ca2+ channel/Ca2+/H+ antiporter activity of BI-1 need to be more carefully studied with regard to BI-1-associated regulations of cell death.
4. BI-1 REGULATES REACTIVE OXYGEN SPECIES
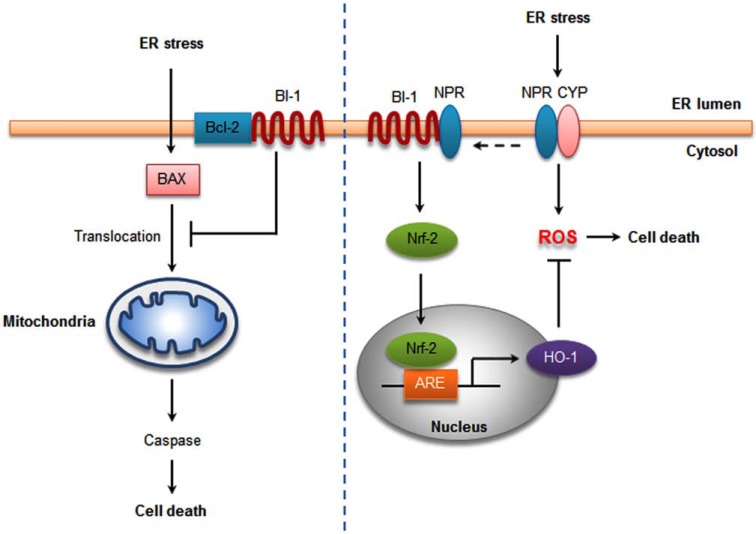
BI-1 resides in the ER membrane and protects cells from ER stress-induced apoptosis. The ER is associated with the generation of ROS through oxidative protein folding [62]. The UPR is followed by ROS accumulation during ER stress, which is regulated in BI-1 overexpressing cells [40]. BI-1 overexpression regulates UPR induction with a protective effect against ER stress, showing an inhibition of ROS accumulation under ER stress. Heme oxygenase-1 (HO-1) is the rate-limiting enzyme in the degradation of heme into biliverdin, carbon monoxide (CO), and free divalent iron and thus is a candidate to explain the reduced ROS accumulation in BI-1 overexpressing cells (Fig. 2) [40]. HO-1 may be important for the cytoprotective activity of BI-1. Inhibition of HO-1 negated BI-1-mediated protection against ER stress-induced cell death. By reducing ROS, elevated HO-1 limits the oxidative dysregulation which causes misfolding of ER proteins, thereby decreasing the unfolded protein response [40]. The modulation of HO-1 expression by BI-1 appears not to be a downstream consequence of differences in ROS. However, the HO-1 induction is still in debate. It was recently reported that no change was noted in the transcription of HO-1 in BI-1-deficient mouse embryonic fibroblast (MEF) cells [4]. This difference in HO-1 expression might vary with cell conditions and cell types.
Fig. (2).
BI-1 protects against ER stress-induced apoptosis. The BI-1-induced protection against ER stress correlates with inhibition of Bax activation and its translocation to the mitochondria, suppressing caspase activation (left). BI-1 dissociates NPR and the P450 2E1 (CYP) complex, reducing ER stress-initiated ROS generation. In addition, BI-1 elevates HO-1 expression through Nrf-2 and ARE, leading to reduced ROS production (right).
Previous studies have demonstrated that yeast expressing human, Arabidopsis, Drosophila, or yeast BI-1 proteins were markedly resistant to cell death induced by oxidative stress (H2O2) [19]. Similarly, oxidative stress-induced cell death was also suppressed by the overexpression of Arabidopsis BI-1 and barley BI-1 (Fig. 2) [63-66]. Interestingly, AtBI-1 overexpression was not shown to significantly reduce ROS levels in plant cells [63, 64]. These results indicate that plant BI-1 may function downstream from
the early steps of ROS-dependent cell death pathway [61]. In contrast, BI-1 overexpression showed great suppression of mitochondria-mediated ROS production in human embryonic kidney (HEK) 293 cells and MEF cells dependent upon ERK activation [37]. Moreover, BI-1 inhibits ER stress-mediated ROS accumulation by decreasing cytochrome P450 (P450 2E1) activity and protein expression [39]. Expression of P450 2E1, a major source of ROS on the ER membrane, increases in response to ER stress. BI-1 may decrease electron uncoupling between NPR and P450 2E1 by binding to one of them, thus reducing ER stress-initiated ROS generation and cell death signaling (Fig. 2). Recently, it was reported that cells BI-1 overexpressing cells had highly enhanced lysosomal activity [67]. Lysosomal and proteasomal activity are also involved in P450 2E1-mediated degradation [68]. It was found that the BI-1 increase in lysosomal enzyme activation is related to P450 2E1 degradation, ER stress suppression and ROS reduction [38].
BI-1 was found to be cytoprotective molecule with a detoxifying effect in CCl4-treated mice [69]. CCl4 administration led to reduced BI-1 gene expression compared to that of the healthy controls’ livers. Cytochrome P450 bio-activation of the CCl4 molecule to the trichloromethyl free radical (CCl3), leads to liver injury due to lipid peroxidation [70, 71]. Recovery of CCl4-induced liver injury by mesenchymal stem cell-transplantation restored the decrease of cytoprotective genes, including the BI-1 gene [69].
In summary, the regulation of BI-1 on ER stress-associated ROS accumulation seems to occur in the intra-ER electron transfer processes including the NPR and P450 coupling system. Whether HO-1 is involved in the regulation of intra-ER ROS accumulation needs further clarification.
5. DOES BI-1 REGULATE THE ER STRESS RESPONSE SPECIFICALLY THROGH IRE-1Α?
ER stress stimulates three distinct UPR signaling pathways through sensors that include inositol-requiring enzyme 1 alpha (IRE1α), PKR-like ER kinase (PERK), and activating transcription factor 6 (ATF6) [72]. IRE1α is a serine-threonine protein kinase and endoribonuclease that, upon activation, initiates the unconventional splicing of the mRNA encoding XBP-1 [73]. Although IRE1α stimulates the adaptive up-regulation of chaperones and therefore mediates cytoprotection, prolonged activation can also trigger c-Jun N-terminal kinase (JNK) and Bax activity [74, 75].
BI-1 has been suggested to inhibit the IRE1α-dependent branch of the UPR [4]. In response to ER stress, BI-1-deficient cells exhibit exacerbated and protracted IRE1α activation. BI-1-mediated IRE1α inhibition was demonstrated in vivo in mice and flies and in vitro in cultured cells. IRE1α-deficient cells remained insulin sensitive when challenged with chemical ER stress agents, underscoring the
importance of this UPR branch in insulin resistance [25, 76]. Although BI-1 seems to show specificity for inhibiting the IRE1α branch of the UPR, its exact mechanism of action remains unclear. Functional down-regulation of the IRE1α pathway by overexpression of BI-1 results in down-regulation of key enzymes of lipid homeostasis pathways not only under conditions of increased ER stress as seen in high-fat diet (HFD) mice but also in normally fed mice [25].
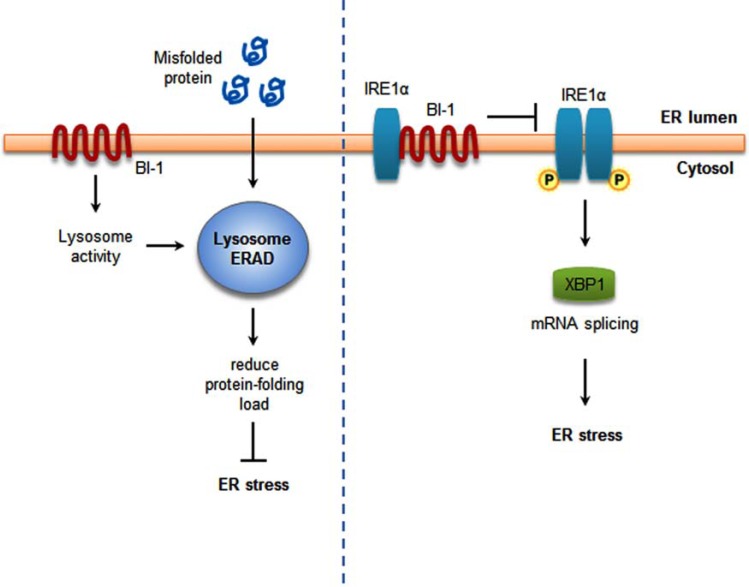
However, there is still debate surrounding the issue of whether BI-1 is an IRE1α-specific regulator or a general ER stress regulator. BI-1 deficiency enhances ATF6 processing and increases selected IRE1α activities (ribonuclease activity but not caspase-12 activation). The increases in spliced XBP-1 (sXBP-1) in the liver and kidney of BI-1-/- mice might be a reflection of both ATF6 and IRE1α activation [24]. It has also been suggested that BI-1 may inhibit ER stress-induced accumulation of ROS through regulation of the three UPR pathways including PERK, IRE1α, and ATF6 [40, 67]. It has been reported that lysosome activity is enhanced in BI-1 overexpressing cells, including lowering the lysosome pH and activating lysosome V-ATPase [38, 67]. The lysosome-associated ER-associated degradation (ERAD) pathway increases the protein folding capacity by reducing protein-folding loads, leading to regulation of the ER stress response [77]. In addition, lysosome inhibitor reversed the BI-1-associated regulation of the three main ER stress pathways, not the specific branch of ER stress, also reversing the protective effect of BI-1 against ER stress-specific cell death [67]. These studies support the role of BI-1 as a general inhibitor of ER stress, also linked to the anti-apoptotic role. The two mechanisms proposed for the BI-1-induced ER stress regulation are schematically summarized for comparison in Fig. (3).
Fig. (3).
BI-1 regulates ER stress response. BI-1 stimulates lysosome activity. The enhanced lysosomal protein degradation activity reduces protein folding requirement, leading to the reduced ER stress response (left). BI-1 inhibits IRE-1α phosphorylation and its endonuclease activity through the interaction with IRE-1α, leading to ER stress regulation (right).
In summary, whether BI-1 is a specific regulator of IRE1α or a general inhibitor of ER stress response has not yet been clarified. The specific inhibition of a specific arm of UPR-associated with IRE1α has recently been clarified in knock-out cells and animal models [4]. However, culture conditions or specific animal experiments enhancing metabolism greatly affect BI-1 physiology. In the highly enhanced metabolism conditions, BI-1 regulated all ER stress signaling pathways, not just those affecting specific IRE-1α signaling. Lysosome degradation activity may also be increased in BI-1 overexpressing cells when using a high concentration of glucose in the media. Upon exposure to ER stress, BI-1 reduced UPR through its enhanced lysosome activity. However, the issue regarding the specificity of BI-1 regulation for any single ER stress regulatory path needs to be further clarified.
6. BI-1 AND AUTOPHAGY
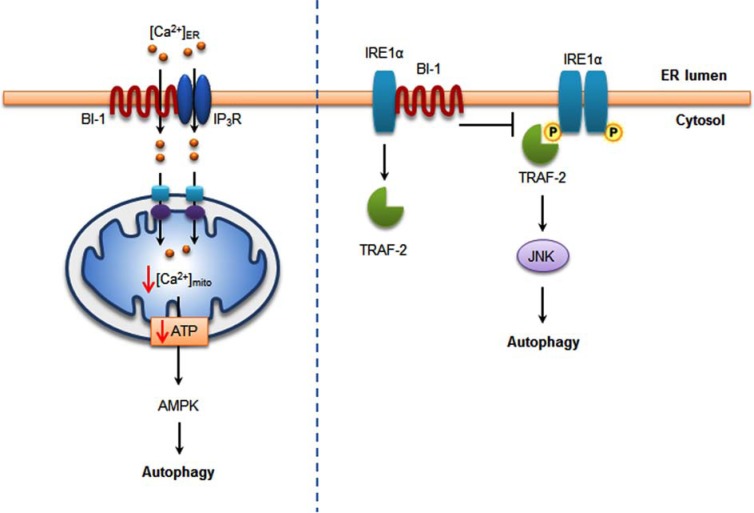
Now, it is debating about the issue of BI-1 in a field of autophagy. Castillo K et al. reported that BI-1 inhibits autophagy by suppressing IRE1-mediated JNK activation under ER stress [5]. BI-1-deficient cells presented a faster and stronger induction of autophagy showing the correlation with enhanced cell survival under nutrient deprivation. They also indicated that the repression of autophagy by BI-1 was dependent on JNK and IRE1α expression, possibly due to a displacement of TNF-receptor associated factor-2 (TRAF2) from IRE1α. They reported a novel function of BI-1 in multicellular organisms and suggested a critical role of BI-1 as a stress integrator that modulates autophagy levels and is connected to other homeostatic processes. However, in contrast to these results, Sano R et al. found that BI-1 contributes to tumorigenesis by promoting autophagy [48]. BI-1 overexpression promotes autophagy in cancer cells. BI-1-deficient mice have reduced autophagy. BI-1 reduces Ca2+/IP3R-dependent mitochondrial bioenergetics, resulting in low mitochondria adenosine triphosphate (ATP) levels, which stimulates autophagy but is an IRE1α-independent pathway. In our group’s study, BI-1-associated autophagy, probably due to the enhanced lysosomal activity, was also reported [38, 67]. These two opposing phenomena; regulation of autophagy and enhancement of autophagy might have been caused by cell-type and culturing condition-associated factors. The above mentioned signal transduction pathways explaining the opposite phenomena are summarized in Fig. (4). The role of BI-1 to positively or negatively regulate autophagy needs to be investigated along with its related molecular mechanisms.
Fig. (4).
BI-1 is related with autophagy. BI-1 binds to IP3R on ER and reduces basal intra ER Ca2+, transferring less Ca2+ into mitochondria. Decreased mitochondrial Ca2+ causes decrease in ATP, leading to AMPK activation and autophagy (left). In the presence of ER stress, IRE-1α binds with TRAF-2, activating JNK and autophagy. BI-1 interacts with IRE-1α, inhibiting the interaction between IRE-1α and TRAF-2 leading to the inhibition of autophagy (right).
7. DISEASES
BI-1 is closely associated with various diseases (Table 1). As mentioned above, the scenario regarding Ca2+ or ROS regulation are applied to pathological mechanisms for hepatic ischemia reperfusion injury, hepatic regeneration, CCl4-induced hepatic toxicity, neuronal diseases and the autoimmune response. With the specific interaction of BI-1 with IRE1α, hepatic I/R-induced damage and hepatic insulin resistance have been also explained as a potential mechanism. In cancer studies, the role of BI-1 has been more frequently studied including prostate, breast and pulmonary cancers. The BI-1-associated anti-apoptosis has been generally suggested as a cancer-related mechanism. The sections below describe specific studies explaining the pathological role of BI-1.
Table 1.
The role of BI-1 on different diseases.
| Disease Types | Possible Mechanisms of BI-1 | References |
|---|---|---|
| Liver Diseases | ||
| Hepatic ischemia-reperfusion (IR) injury | On ER Ca2+ -affecting Ca2+ -regulated ER chaperones that suppresses IRE1α and ATF6; direct interactions with IRE1α and ATF6 protein complexes in ER membranes | [24] |
| Chronic hepatitis | Bax-induced apoptosis inhibition | [80] |
| Liver regeneration | ER Ca2+ control; cell cycle regulation | [26] |
| CCl4-induced liver injury | ROS inhibition | [69] |
| Cancer | ||
| Tumorigenesis | Ca2+ regulation; actin interaction; down-regulation of mitochondria metabolism | [21, 48, 56, 85] |
| Prostate cancer | Up-regulation of BI-1 gene and protein expression; interaction with Bcl-2/Bcl-XL; inhibition of apoptosis | [89] |
| Pulmonary adenocarcinoma | High level BI-1 gene expression | [87] |
| Breast cancer | Up-regulation of BI-1 gene and protein expression; interaction with Bcl-2/Bcl-XL; inhibition apoptosis | [86] |
| Nasopharyngeal carcinoma | High expression of BI-1 gene and protein; stimulation of Bcl-2/Bcl-XL, Bax inhibition; apoptosis inhibition | [88] |
| Acute myeloid leukemia | BI-1 peptide as a novel immunogenic tumor-associated antigen | [94] |
| Autoimmune Response | ||
| Induction of ERK activation; reduction in intracellular ROS generation; suppression of autoimmune response-related apoptotic cell death | [37] | |
| Neuron Disease | ||
| Inhibition of ER stress; modulation of Ca2+ homeostasis; regulation of MAPK | [27, 98-100, 102] | |
| Insulin Resistance | ||
| Inhibition of IRE1α | [25] | |
7.1. Liver Diseases
Ischemia-reperfusion (IR) injury induces ER stress and cell death. During hypoxia and IR insults, ER stress-associated cell apoptosis is activated. Reperfusion also triggers oxidative stress, which produces nitric oxide and reactive oxidative species, altering cellular redox-dependent reactions, interfering with protein disulfide bonding, and resulting in protein misfolding in the ER [78, 79].
BI-1 knockout mice subjected to hepatic IR injury exhibited the following characteristics: increased histological injury, increased serum transaminases, indicative of more hepatocyte death increased percentages of TUNEL-positive hepatocytes, greater elevations in caspase activity, and greater activation of ER stress proteins IRE1α, ATF6, and XBP-1, compared to wild-type BI-1 mice [24]. Moreover, hepatic IR injury induced elevations in BI-1 mRNA in wild-type livers to limit tissue injury.
High levels of BI-1 mRNA were found in acute and chronic liver disease, representing a protective effect with respect to the virus-induced inflammatory response. The down-regulation of BI-1 observed both in hepatocellular carcinoma and in cirrhotic tissue surrounding the tumor may contribute to hepatocellular carcinogenesis and tumor progression [80].
It has been reported that BI-1 deficiency accelerates liver regeneration after partial hepatectomy [26]. Regenerating hepatocytes of BI-1-deficient mice enter the cell cycle faster and experience an earlier increase in cyclins and cyclin-dependent kinases, more rapid hyper phosphorylation of retinoblastoma proteins, and faster degradation of p27. ER Ca2+ plays an important role in cell proliferation [53]. Depletion of ER Ca2+ in response to thapsigargin causes cells to arrest in G0 [81]. The ability of BI-1 to regulate ER Ca2+ may be involved in BI-1-associated regulation of hepatocyte proliferation.
7.2. Cancer
Apoptosis evolved out of the need for tissue homeostasis depending on a delicate balance between proliferation and cell death. Dysregulation of apoptosis plays a role in numerous pathological processes. A reduced sensitivity to apoptosis may promote the survival of oncogenic cells, leading to further accumulation of mutations and a malignant progression [82, 83]. To develop innovative strategies for cancer therapy, it will be necessary to identify molecular targets in the apoptotic pathway that are differentially regulated in normal and in cancer cells [84].
BI-1 is an anti-apoptotic gene, and BI-1 overexpression inhibited apoptosis induced by growth factor and serum deprivation, as well as the intrinsic pathway stimuli, etoposide and staurosporine [2]. The aberrant overexpression of BI-1 may also lead to the acquisition of cell transformation and tumorigenicity [7, 85-89]. The overexpression of BI-1 gene in NIH3T3 cells promoted in vitro colony formation as well as in vivo tumor formation, indicating that the BI-1 gene may lead to changes in molecular mechanisms governing normal cellular growth and consequently contribute to malignant cell transformation and tumorigenesis. Similarly, in a tumor xenograft model, BI-1 shRNA-containing tumor cells regulate tumor size [48]. These results indicated that BI-1 could be important for tumor cell survival in a tumor microenvironment.
BI-1 has recently been found to be overexpressed in several human carcinomas [86, 89, 90]. The specific down-regulation of BI-1 by RNA interference (RNAi) lead to cell death in nasopharyngeal carcinoma cells [88]. Similarly, knockdown BI-1 also induces spontaneous apoptosis in 293T cells [1]. In breast cancer, levels of anti-apoptotic proteins, such as Bcl-2, Bcl-XL, IAPs, and BI-1, are elevated [86]. Expression of BI-1 was demonstrated in six human breast cancer cell lines, and specific suppression of BI-1 expression by RNAi caused a significant increase in spontaneous apoptosis in estrogen-independent MDA-MB-231 cells. In estrogen-dependent MCF7 cells, estradiol-17-β induced cell apoptosis and decreased the levels of BI-1 through Klf10 modulation. Estrogen is a potent mitogen that stimulates cell proliferation and prevents cell death in breast cancer cells through receptor binding and the subsequent activation of signaling pathways [91]. Paradoxically, estrogen is also capable of inducing tumor regression of hormone-dependent breast cancer because estrogen’s effects can be mediated by either receptor, ER-α or ER-β [92]. During embryonic development, estrogen, elicits cellular proliferation through the ER-α receptor, whereas, via ER-β, estrogen inhibits proliferation and promotes differentiation [93]. Importantly, estrogen induction of Klf10 is ER-β specific, and the activation function 1 (AF1) domain of ER-β confers this specificity. Therefore, as Klf10 induces cell apoptosis through the modulation of BI-1 expression, it regulates cell proliferation and death in breast cancers [35].
BI-1 is also related to cancer metastasis. The overexpression of BI-1 increased cell mobility and invasiveness, showing highly elevated glucose consumption and cytosolic accumulation of lactate and pyruvate but decreased mitochondrial oxygen (O2) consumption and ATP production [56]. Glucose metabolism-associated extracellular pH also decreased as cells excreted more H+, and sodium hydrogen exchanger (NHE) activity increased, likely as a homeostatic mechanism for intracellular pH. These alterations activated MMP 2/9 and cell mobility and invasiveness, suggesting a role for NHE in cancer metastasis. C terminus- deleted BI-1-overexpressing cells showed similar results to control cells, which indicated that the C-terminal motif was required for BI-1-associated alteration of glucose metabolism, NHE activation and cancer.
BI-1 has a novel role in regulating actin polymerization to increase cell adhesion [21]. The presence of BI-1 is an enhancer for adhesion through actin polymerization. In BI-1 overexpressing cells, a high Ca2+ concentration regulates downstream actin polymerization and cell adhesion. Actin polymerization also can enhance Ca2+ release, whereas actin depolymerization decreases Ca2+ through voltage-dependent Ca2+ channels. BI-1 also induced increases in cell adhesion, which is linked to cancer metastasis mechanisms.
BI-1 has been suggested to be a novel leukemia-associated antigen [94]. A peptide derived from BI-1 was identified as an epitope recognized by cytotoxic T lymphocytes. In vitro induction of BI-1-specific cytotoxic T lymphocytes by RNA transfection or pulsing of dendritic cells with the synthetically generated peptide was an effective method to generate leukemia-specific cytotoxic T lymphocytes, suggesting that the BI-1 may represent an interesting novel tumor-associated antigen in the context of cancer vaccines.
7.3. Autoimmune Response
BI-1 may affect the autoimmune response [37]. BI-1 transgenic mice show abnormal spleen morphology, splenomegaly, and megakaryocytes. It has been suggested that BI-1-induced ERK activation reduces intracellular ROS generation, which suppresses autoimmune response-related apoptotic cell death. The exact mechanism by which BI-1 regulates ERK activation remains unknown, but it might be related to BI-1 and calcium modulation. A previous study reported that the C-terminus of plant BI-1 interacts with calmodulin which activates calcium/calmodulin-dependent protein kinase (CaMK) [59]. CaMKII regulates ERK activation as one of part of integrin signaling supporting the possible existence of cross-talk between CaMKII and ERK/Raf-1 [95]. Abnormal regulation of apoptosis has been reported to be related to autoimmune responses [96]. Inefficient apoptosis may lead to failure to delete self-reactive lymphocytes and expansion of immature and functionally defective lymphocytes. In the case of BAX deficiency or Bcl-2 overexpression, an imbalance of pro- or anti-apoptotic proteins has been reported to alter lymphocyte development [6] or lymphocytic leukemia [8]. The ERK signaling pathway is also involved in many autoimmune responses, such as lymphoproliferative disease [97]. Therefore, BI-1 might be an important target for therapeutic application in autoimmune disease.
7.4. Neuroprotection
It has been reported that BI-1 has a neuroprotective effect. Previously it was reported that neuronal expression of BI-1 afforded significant protection against oxygen-glucose deprivation [98]. More recently, it has been reported that BI-1 overexpression promotes the proliferation and neuronal differentiation of mouse embryonic stem cell in response to leukemia inhibitory factor withdrawal, which is mediated by regulating activation of ERK pathway [99]. Cortical neurons from BI-1 transgenic mice exhibited greater resistance to ER stress-induced cell death compared to non-transgenic counterparts [100], suggesting that the manipulation of BI-1 expression might produce a new therapy for stroke and acute brain trauma. In addition, the expression of BI-1 showed protection against anhedonia through calcium modulating effect [27], extending the scope of BI-1 to the psychiatric diseases including depression.
7.5. Insulin Resistance
Insulin resistance can be caused by many factors including obesity and its associated type 2 diabetes. The activations of inflammatory and stress pathways are considered as a main mechanism of insulin resistance. Recently, ER stress has been studied as a representative stress signaling transduction for diabetes and insulin resistance. When ER stress is initiated, the cytosolic domain of activated IRE1α binds tumor necrosis factor-associated factor 2 (TNF-α) and triggers the activation of the JNK signaling pathway [101]. This process increases phosphorylation of the serine end of insulin receptor substrate-1 (IRS-1), reducing its ability to undergo tyrosine phosphorylation, Subsequently, Akt/protein kinase B and glycogen synthase kinase-3 (GSK-3) are inhibited, reducing hepatic glycogen synthesis. BI-1 expression blunts the IRE1α-dependent splicing of XBP-1 mRNA by forming a complex with IRE1α, regulating the serine phosphorylation of IRS-1 and its resultant insulin resistance in a high fat diet model [25]. BI-1 causes higher GSK3 phosphorylation and increased glycogen synthesis. This improvement in glucose metabolism and insulin sensitivity was suggested to be due to the reduced gluconeogenesis caused by the reduction of glucose-6-phosphatase and phosphoenolpyruvate carboxykinase expression. This study identifies the role of BI-1 as an ER stress regulator in the development of obesity-induced insulin resistance. However, the hepatic role of BI-1 against insulin resistance warrants further future study.
8. CONCLUSION
BI-1, a generally well known anti-apoptotic protein, has been especially studied with regard to its association with the regulatory mechanism of ER stress. Its role as a negative regulator of a specific arm of ER stress signaling with the interaction of BI-1 with IRE-1α has been reported. In contrast, general ER stress regulation has also been suggested through enhanced lysosomal activity and its associated protein degradation activity. The regulation of Ca2+ and ROS has been studied as a basic characteristic of BI-1, which may contribute to its role in ER stress regulation, extrapolating to various diseases including ischemia, cancer, anhedonia and the autoimmune response. The suggested mechanisms of BI-1 Ca2+ regulation includes pH-sensitive Ca2+ channel/Ca2+/H+ antiporter activity and an IP3R-associated Ca2+ leak channel. The Bcl-2 interactive mechanism is also suggested with the BH4-domain-stimulating the BI-1 Ca2+/H+ activity. The interaction of P450 2E1 activity has been suggested as an ER stress-induced ROS accumulation. Although researchers are beginning to understand the importance of BI-1 in the cell and in human physiology, the way in which it functions still remains ambiguous. Lastly, many fundamental questions still need to be answered about how BI-1 regulates life and death, and we anticipate that an advanced understanding may pave the way for therapeutic manipulation.
ACKNOWLEDGEMENTS
The authors apologize to those investigators whose work was not cited due to an oversight or space constraints. This study was supported by a grant (2012R1A2A1A03001907) from the National Research Foundation of Korea, 2012.
ABBREVIATIONS
- BI-1
= Bax inhibitor-1
- ER
= Endoplasmic reticulum
- UPR
= Unfolded protein response
- IP3R
= Inositol 1,4,5-trisphosphate receptor
- ROS
= Reactive oxygen species
- HO-1
= Heme oxygenase-1
- GRP78
= Glucose response protein 78
- IRE1α
= Inositol-requiring enzyme 1
- ATF6
= Activating transcription factor 6
- XBP-1
= X-box-binding protein 1
- JNK
= c-Jun N-terminal kinase
- CHOP
= C/EBP homologous protein
- Nrf-2
= Nuclear factor erythroid 2-related factor 2
- MEF
= Mouse embryonic fibroblast
- MAM
= Mitochondria-associated microdomain
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- 1.Xu Q, Reed JC. Bax inhibitor-1. a mammalian apoptosis suppressor identified by functional screening in yeast. Mol Cell. 1998;1(3):337–46. doi: 10.1016/s1097-2765(00)80034-9. [DOI] [PubMed] [Google Scholar]
- 2.Chae HJ, Kim HR, Xu C , et al. BI-1 regulates an apoptosis pathway linked to endoplasmic reticulum stress. Mol Cell. 2004;15(3):355–66. doi: 10.1016/j.molcel.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 3.Bultynck G, Kiviluoto S, Henke N , et al. The C terminus of Bax inhibitor-1 forms a Ca2+-permeable channel pore. J Biol Chem. 2012;287(4):2544–57. doi: 10.1074/jbc.M111.275354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lisbona F, Rojas-Rivera D, Thielen P , et al. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell. 2009;33(6):679–91. doi: 10.1016/j.molcel.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castillo K, Rojas-Rivera D, Lisbona F , et al. BAX inhibitor-1 regulates autophagy by controlling the IRE1alpha branch of the unfolded protein response. EMBO J. 2011;30(21):4465–78. doi: 10.1038/emboj.2011.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou J, Zhu T, Hu C , et al. Comparative genomics and function analysis on BI1 family. Comput Biol Chem. 2008;32(3):159–62. doi: 10.1016/j.compbiolchem.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Reimers K, Choi CY, Bucan V, Vogt PM. The Bax Inhibitor-1 (BI-1) family in apoptosis and tumorigenesis. Curr Mol Med. 2008;8(2):148–56. doi: 10.2174/156652408783769562. [DOI] [PubMed] [Google Scholar]
- 8.Hu L, Smith TF, Goldberger G. LFG: a candidate apoptosis regulatory gene family. Apoptosis. 2009;14(11):1255–65. doi: 10.1007/s10495-009-0402-2. [DOI] [PubMed] [Google Scholar]
- 9.Huckelhoven R. BAX Inhibitor-1 an ancient cell death suppressor in animals and plants with prokaryotic relatives. Apoptosis. 2004;9(3):299–307. doi: 10.1023/b:appt.0000025806.71000.1c. [DOI] [PubMed] [Google Scholar]
- 10.Zhao H, Ito A, Kimura SH , et al. RECS1 deficiency in mice induces susceptibility to cystic medial degeneration. Genes Genet Syst. 2006;81(1):41–50. doi: 10.1266/ggs.81.41. [DOI] [PubMed] [Google Scholar]
- 11. Zhao H, Ito A, Sakai N, Matsuzawa Y, Yamashita S, Nojima H. RECS1 is a negative regulator of matrix metalloproteinase-9 production and aged RECS1 knockout mice are prone to aortic dilation. Circ J. 2006;70(5):615–24. doi: 10.1253/circj.70.615. [DOI] [PubMed] [Google Scholar]
- 12.Shukla S, Fujita K, Xiao Q, Liao Z, Garfield S, Srinivasula SM. A shear stress responsive gene product PP1201 protects against Fas-mediated apoptosis by reducing Fas expression on the cell surface. Apoptosis. 2011;16(2):162–73. doi: 10.1007/s10495-010-0556-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Somia NV, Schmitt MJ, Vetter DE, Van Antwerp D, Heinemann SF, Verma IM. LFG: an anti-apoptotic gene that provides protection from Fas-mediated cell death. Proc Natl Acad Sci U S A. 1999;96(22):12667–72. doi: 10.1073/pnas.96.22.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rojas-Rivera D, Armisen R, Colombo A , et al. TMBIM3/GRINA is a novel unfolded protein response (UPR) target gene that controls apoptosis through the modulation of ER calcium homeostasis. Cell Death Differ. 2012;19(6):1013–26. doi: 10.1038/cdd.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gubser C, Bergamaschi D, Hollinshead M, Lu X, van Kuppeveld FJ, Smith GL. A new inhibitor of apoptosis from vaccinia virus and eukaryotes. PLoS Pathog. 2007;3(2):e17. doi: 10.1371/journal.ppat.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Mattia F, Gubser C, van Dommelen MM , et al. Human Golgi antiapoptotic protein modulates intracellular calcium fluxes. Mol Biol Cell. 2009;20(16):3638–45. doi: 10.1091/mbc.E09-05-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oka T, Sayano T, Tamai S , et al. Identification of a novel protein MICS1 that is involved in maintenance of mitochondrial morphology and apoptotic release of cytochrome c. Mol Biol Cell. 2008;19(6):2597–608. doi: 10.1091/mbc.E07-12-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol Rev. 2005;57(4):387–95. doi: 10.1124/pr.57.4.13. [DOI] [PubMed] [Google Scholar]
- 19.Chae HJ, Ke N, Kim HR , et al. Evolutionarily conserved cytoprotection provided by Bax Inhibitor-1 homologs from animals. plnts.and yeast. Gene . 2003;323:101–13. doi: 10.1016/j.gene.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Kim HR, Lee GH, Ha KC , et al. Bax Inhibitor-1 Is a pH-dependent regulator of Ca2+ channel activity in the endoplasmic reticulum. J Biol Chem. 2008;283(23):15946–55. doi: 10.1074/jbc.M800075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee GH, Ahn T, Kim DS , et al. Bax inhibitor 1 increases cell adhesion through actin polymerization: involvement of calcium and actin binding. Mol Cell Biol. 2010;30(7):1800–13. doi: 10.1128/MCB.01357-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrara G, Saraiva N, Gubser C, Johnson BF, Smith GL. Six-transmembrane topology for Golgi anti-apoptotic protein (GAAP) and Bax inhibitor 1 (BI-1) provides model for the transmembrane Bax inhibitor-containing motif (TMBIM) family. J Biol Chem. 2012;287(19):15896–905. doi: 10.1074/jbc.M111.336149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henke N, Lisak DA, Schneider L, Habicht J, Pergande M, Methner A. The ancient cell death suppressor BAX inhibitor-1. Cell Calcium. 2011;50(3):251–60. doi: 10.1016/j.ceca.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Bailly-Maitre B, Fondevila C, Kaldas F , et al. Cytoprotective gene bi-1 is required for intrinsic protection from endoplasmic reticulum stress and ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2006;103(8):2809–14. doi: 10.1073/pnas.0506854103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailly-Maitre B, Belgardt BF, Jordan SD , et al. Hepatic Bax inhibitor-1 inhibits IRE1alpha and protects from obesity-associated insulin resistance and glucose intolerance. J Biol Chem. 2010;285(9):6198–207. doi: 10.1074/jbc.M109.056648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailly-Maitre B, Bard-Chapeau E, Luciano F , et al. Mice lacking bi-1 gene show accelerated liver regeneration. Cancer Res. 2007;67(4):1442–50. doi: 10.1158/0008-5472.CAN-06-0850. [DOI] [PubMed] [Google Scholar]
- 27.Hunsberger JG, Machado-Vieira R, Austin DR , et al. Bax inhibitor 1. a modulator of calcium homeostsis.confers affective resilience. Brain Res. 2011; 1403:19–27. doi: 10.1016/j.brainres.2011.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Priault M, Bessoule JJ, Grelaud-Coq A, Camougrand N, Manon S. Bax-induced cell death in yeast depends on mitochondrial lipid oxidation. Eur J Biochem. 2002;269(22):5440–50. doi: 10.1046/j.1432-1033.2002.03234.x. [DOI] [PubMed] [Google Scholar]
- 29.Kirkland RA, Saavedra GM, Cummings BS, Franklin JL. Bax regulates production of superoxide in both apoptotic and nonapoptotic neurons: role of caspases. J Neurosci. 2010;30(48):16114–27. doi: 10.1523/JNEUROSCI.2862-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe N, Lam E. BAX inhibitor-1 modulates endoplasmic reticulum stress-mediated programmed cell death in Arabidopsis. J Biol Chem. 2008;283(6):3200–10. doi: 10.1074/jbc.M706659200. [DOI] [PubMed] [Google Scholar]
- 31.Zhang K. Integration of ER stress. oxidative stress and the inflammatory response in health and disease. Int J Clin Exp Med. 2010;3(1):33–40. [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Soboloff J, Zhu Z, Berger SA. Inhibition of Ca2+ influx is required for mitochondrial reactive oxygen species-induced endoplasmic reticulum Ca2+ depletion and cell death in leukemia cells. Mol Pharmacol. 2006;70(4):1424–34. doi: 10.1124/mol.106.024323. [DOI] [PubMed] [Google Scholar]
- 33.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7(12):1013–30. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 34.Yun CH, Chae HJ, Kim HR, Ahn T. Doxorubicin- and daunorubicin-induced regulation of Ca2+ and H+ fluxes through human bax inhibitor-1 reconstituted into membranes. J Pharm Sci. 2012;101(3):1314–26. doi: 10.1002/jps.23007. [DOI] [PubMed] [Google Scholar]
- 35.Hsu CF, Sui CL, Wu WC , et al. Klf10 induces cell apoptosis through modulation of BI-1 expression and Ca2+ homeostasis in estrogen-responding adenocarcinoma cells. Int J Biochem Cell Biol. 2011;43(4):666–73. doi: 10.1016/j.biocel.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Xu C, Xu W, Palmer AE, Reed JC. BI-1 regulates endoplasmic reticulum Ca2+ homeostasis downstream of Bcl-2 family proteins. J Biol Chem. 2008;283(17):11477–84. doi: 10.1074/jbc.M708385200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JH, Lee ER, Jeon K , et al. Role of BI-1 (TEGT)-mediated ERK1/2 activation in mitochondria-mediated apoptosis and splenomegaly in BI-1 transgenic mice. Biochim Biophys Acta. 2012;1823(4):876–88. doi: 10.1016/j.bbamcr.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Lee GH, Kim HR, Chae HJ. Bax inhibitor-1 regulates the expression of P450 2E1 through enhanced lysosome activity. Int J Biochem Cell Biol. 2012;44(4):600–11. doi: 10.1016/j.biocel.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 39.Kim HR, Lee GH, Cho EY, Chae SW, Ahn T, Chae HJ. Bax inhibitor 1 regulates ER-stress-induced ROS accumulation through the regulation of cytochrome P450 2E1. J Cell Sci. 2009;122(Pt 8):1126–33. doi: 10.1242/jcs.038430. [DOI] [PubMed] [Google Scholar]
- 40.Lee GH, Kim HK, Chae SW , et al. Bax inhibitor-1 regulates endoplasmic reticulum stress-associated reactive oxygen species and heme oxygenase-1 expression. J Biol Chem. 2007;282(30):21618–28. doi: 10.1074/jbc.M700053200. [DOI] [PubMed] [Google Scholar]
- 41.Bolduc N, Ouellet M, Pitre F, Brisson LF. Molecular characterization of two plant BI-1 homologues which suppress Bax-induced apoptosis in human 293 cells. Planta. 2003;216(3):377–86. doi: 10.1007/s00425-002-0879-1. [DOI] [PubMed] [Google Scholar]
- 42.Kawai M, Pan L, Reed JC, Uchimiya H. Evolutionally conserved plant homologue of the Bax inhibitor-1 (BI-1) gene capable of suppressing Bax-induced cell death in yeast(1). FEBS Lett. 1999;464(3):143–7. doi: 10.1016/s0014-5793(99)01695-6. [DOI] [PubMed] [Google Scholar]
- 43.Matsumura H, Nirasawa S, Kiba A , et al. Overexpression of Bax inhibitor suppresses the fungal elicitor-induced cell death in rice (Oryza sativa L) cells. Plant J. 2003;33(3):425–34. doi: 10.1046/j.1365-313x.2003.01639.x. [DOI] [PubMed] [Google Scholar]
- 44.Nagano M, Takahara K, Fujimoto M, Tsutsumi N, Uchimiya H, Kawai-Yamada M. Arabidopsis sphingolipid fatty acid 2-hydroxylases (AtFAH1 and AtFAH2) are functionally differentiated in fatty acid 2-hydroxylation and stress responses. Plant Physiol. 2012;159(3):1138–48. doi: 10.1104/pp.112.199547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du ZQ, Lan JF, Weng YD, Zhao XF, Wang JX. BAX inhibitor-1 silencing suppresses white spot syndrome virus replication in red swamp crayfish. Procambarus clarkii. Fish Shellfish Immunol. 2013 doi: 10.1016/j.fsi.2013.03.376. [DOI] [PubMed] [Google Scholar]
- 46.Cebulski J, Malouin J, Pinches N, Cascio V, Austriaco N. Yeast Bax inhibitor. Bi1p.is an ER-localized protein that links the unfolded protein response and programmed cell death in Saccharomyces cerevisiae. PLoS One . 2011; 6(6):e20882. doi: 10.1371/journal.pone.0020882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kiviluoto S, Schneider L, Luyten T , et al. Bax inhibitor-1 is a novel IP(3) receptor-interacting and -sensitizing protein. Cell Death Dis. 2012;3:e367. doi: 10.1038/cddis.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sano R, Hou YC, Hedvat M , et al. Endoplasmic reticulum protein BI-1 regulates Ca(2)(+)-mediated bioenergetics to promote autophagy. Genes Dev. 2012;26(10):1041–54. doi: 10.1101/gad.184325.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahn T, Yun CH, Chae HZ, Kim HR, Chae HJ. Ca2+/H+ antiporter-like activity of human recombinant Bax inhibitor-1 reconstituted into liposomes. FEBS J. 2009;276(8):2285–91. doi: 10.1111/j.1742-4658.2009.06956.x. [DOI] [PubMed] [Google Scholar]
- 50.Ahn T, Yun CH, Chae HZ, Kim HR, Chae HJ. Refolding and reconstitution of human recombinant Bax inhibitor-1 into liposomes from inclusion bodies expressed in Escherichia coli. Protein Expr Purif. 2009;66(1):35–8. doi: 10.1016/j.pep.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 51.Ahn T, Yun CH, Kim HR, Chae HJ. Cardiolipin. phosphatidylseine.and BH4 domain of Bcl-2 family regulate Ca2+/H+ antiporter activity of human Bax inhibitor-1. Cell Calcium . 2010;47(4):387–96. doi: 10.1016/j.ceca.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Xu L, Kong D, Zhu L, Zhu W, Andrews DW, Kuo TH. Suppression of IP3-mediated calcium release and apoptosis by Bcl-2 involves the participation of protein phosphatase 1. Mol Cell Biochem. 2007;295(1-2):153–65. doi: 10.1007/s11010-006-9285-5. [DOI] [PubMed] [Google Scholar]
- 53.Lam M, Dubyak G, Chen L, Nunez G, Miesfeld RL, Distelhorst CW. Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc Natl Acad Sci U S A. 1994;91(14):6569–73. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pinton P, Ferrari D, Magalhaes P , et al. Reduced loading of intracellular Ca(2+) stores and downregulation of capacitative Ca(2+) influx in Bcl-2-overexpressing cells. J Cell Biol. 2000;148(5):857–62. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Foyouzi-Youssefi R, Arnaudeau S, Borner C , et al. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2000;97(11):5723–8. doi: 10.1073/pnas.97.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee GH, Yan C, Shin SJ , et al. BAX inhibitor-1 enhances cancer metastasis by altering glucose metabolism and activating the sodium-hydrogen exchanger: the alteration of mitochondrial function. Oncogene. 2010;29(14):2130–41. doi: 10.1038/onc.2009.491. [DOI] [PubMed] [Google Scholar]
- 57.Parekh AB, Putney JWJr. Store-operated calcium channels. Physiol Rev. 2005;85(2):757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 58.Peters SC, Piper HM. Reoxygenation-induced Ca2+ rise is mediated via Ca2+ influx and Ca2+ release from the endoplasmic reticulum in cardiac endothelial cells. Cardiovasc Res. 2007;73(1):164–71. doi: 10.1016/j.cardiores.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 59.Ihara-Ohori Y, Nagano M, Muto S, Uchimiya H, Kawai-Yamada M. Cell death suppressor Arabidopsis bax inhibitor-1 is associated with calmodulin binding and ion homeostasis. Plant Physiol. 2007;143(2):650–60. doi: 10.1104/pp.106.090878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kawai-Yamada M, Hori Z, Ogawa T , et al. Loss of calmodulin binding to Bax inhibitor-1 affects Pseudomonas-mediated hypersensitive response-associated cell death in Arabidopsis thaliana. J Biol Chem. 2009;284(41):27998–8003. doi: 10.1074/jbc.M109.037234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ishikawa T, Watanabe N, Nagano M, Kawai-Yamada M, Lam E. Bax inhibitor-1: a highly conserved endoplasmic reticulum-resident cell death suppressor. Cell Death Differ. 2011;18(8):1271–8. doi: 10.1038/cdd.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases. mitochondrial electron transort.and NADPH oxidase. Antioxid Redox Signal. 2009; 11(10):2409–27. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 63.Kawai-Yamada M, Ohori Y, Uchimiya H. Dissection of Arabidopsis Bax inhibitor-1 suppressing Bax-. hydrogen peroxde-.and salicylic acid-induced cell death. . Plant Cell. 2004; 16(1):21–32. doi: 10.1105/tpc.014613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baek D, Nam J, Koo YD , et al. Bax-induced cell death of Arabidopsis is meditated through reactive oxygen-dependent and -independent processes. Plant Mol Biol. 2004;56(1):15–27. doi: 10.1007/s11103-004-3096-4. [DOI] [PubMed] [Google Scholar]
- 65.Eichmann R, Dechert C, Kogel KH, Huckelhoven R. Transient over-expression of barley BAX Inhibitor-1 weakens oxidative defence and MLA12-mediated resistance to Blumeria graminis f.p. hordei. Mol Plant Pathol. 2006;7(6):543–52. doi: 10.1111/j.1364-3703.2006.00359.x. [DOI] [PubMed] [Google Scholar]
- 66.Ishikawa T, Takahara K, Hirabayashi T , et al. Metabolome analysis of response to oxidative stress in rice suspension cells overexpressing cell death suppressor Bax inhibitor-1. Plant Cell Physiol. 2010;51(1):9–20. doi: 10.1093/pcp/pcp162. [DOI] [PubMed] [Google Scholar]
- 67.Lee GH, Kim DS, Kim HT , et al. Enhanced lysosomal activity is involved in Bax inhibitor-1-induced regulation of the endoplasmic reticulum (ER) stress response and cell death against ER stress: involvement of vacuolar H+-ATPase (V-ATPase). J Biol Chem. 2011;286(28):24743–53. doi: 10.1074/jbc.M110.167734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Guan S, Acharya P , et al. Ubiquitin-dependent proteasomal degradation of human liver cytochrome P450 2E1: identification of sites targeted for phosphorylation and ubiquitination. J Biol Chem. 2011;286(11):9443–56. doi: 10.1074/jbc.M110.176685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cho KA, Woo SY, Seoh JY, Han HS, Ryu KH. Mesenchymal stem cells restore CCl4-induced liver injury by an antioxidative process. Cell Biol Int. 2012;36(12):1267–74. doi: 10.1042/CBI20110634. [DOI] [PubMed] [Google Scholar]
- 70.Recknagel RO, Glende EA Jr, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43(1):139–54. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 71.Bruckner JV, Ramanathan R, Lee KM, Muralidhara S. Mechanisms of circadian rhythmicity of carbon tetrachloride hepatotoxicity. J Pharmacol Exp Ther. 2002;300(1):273–81. doi: 10.1124/jpet.300.1.273. [DOI] [PubMed] [Google Scholar]
- 72.Lai E, Teodoro T, Volchuk A. Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology (Bethesda) 2007;22:193–201. doi: 10.1152/physiol.00050.2006. [DOI] [PubMed] [Google Scholar]
- 73.Hetz C, Martinon F, Rodriguez D, Glimcher LH. The unfolded protein response: integrating stress signals through the stress sensor IRE1alpha. Physiol Rev. 2011;91(4):1219–43. doi: 10.1152/physrev.00001.2011. [DOI] [PubMed] [Google Scholar]
- 74.Urano F, Wang X, Bertolotti A , et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287(5453):664–6. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 75.Hetz C, Bernasconi P, Fisher J , et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312(5773):572–6. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- 76.Ozcan U, Cao Q, Yilmaz E , et al. Endoplasmic reticulum stress links obesity. insulin acion.and type 2 diabetes. Science. 2004; 306(5695):457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 77.Fujita E, Kouroku Y, Isoai A , et al. Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II). Hum Mol Genet. 2007;16(6):618–29. doi: 10.1093/hmg/ddm002. [DOI] [PubMed] [Google Scholar]
- 78.Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res. 2010;107(9):1071–82. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- 79.Yung HW, Korolchuk S, Tolkovsky AM, Charnock-Jones DS, Burton GJ. Endoplasmic reticulum stress exacerbates ischemia-reperfusion-induced apoptosis through attenuation of Akt protein synthesis in human choriocarcinoma cells. FASEB J. 2007;21(3):872–84. doi: 10.1096/fj.06-6054com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kotsafti A, Farinati F, Cardin R, Burra P, Bortolami M. Bax inhibitor-1 down-regulation in the progression of chronic liver diseases. BMC Gastroenterol. 2010;10:35. doi: 10.1186/1471-230X-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Short AD, Bian J, Ghosh TK, Waldron RT, Rybak SL, Gill DL. Intracellular Ca2+ pool content is linked to control of cell growth. Proc Natl Acad Sci U S A. 1993;90(11):4986–90. doi: 10.1073/pnas.90.11.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.LaCasse EC, Baird S, Korneluk RG, MacKenzie AE. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998;17(25):3247–59. doi: 10.1038/sj.onc.1202569. [DOI] [PubMed] [Google Scholar]
- 83.Reed JC. Mechanisms of apoptosis avoidance in cancer. Curr Opin Oncol. 1999;11(1):68–75. doi: 10.1097/00001622-199901000-00014. [DOI] [PubMed] [Google Scholar]
- 84.Yang L, Cao Z, Yan H, Wood WC. Coexistence of high levels of apoptotic signaling and inhibitor of apoptosis proteins in human tumor cells: implication for cancer specific therapy. Cancer Res. 2003;63(20):6815–24. [PubMed] [Google Scholar]
- 85.Xiang-yong L, Yang-chao C, Ke-yuan Z , et al. Overexpression of Bax inhibitor-1 (BI-1) induces cell transformation in NIH3T3 cells. Cell Biol Int. 2010;34(11):1099–104. doi: 10.1042/CBI20090400. [DOI] [PubMed] [Google Scholar]
- 86.Grzmil M, Kaulfuss S, Thelen P , et al. Expression and functional analysis of Bax inhibitor-1 in human breast cancer cells. J Pathol. 2006;208(3):340–9. doi: 10.1002/path.1902. [DOI] [PubMed] [Google Scholar]
- 87.Tanaka R, Ishiyama T, Uchihara T , et al. Expression of the Bax inhibitor-1 gene in pulmonary adenocarcinoma. Cancer. 2006;106(3):648–53. doi: 10.1002/cncr.21639. [DOI] [PubMed] [Google Scholar]
- 88.Zhang M, Li X, Zhang Y, Zhou K. Bax inhibitor-1 mediates apoptosis-resistance in human nasopharyngeal carcinoma cells. Mol Cell Biochem. 2010;333(1-2):1–7. doi: 10.1007/s11010-009-0198-y. [DOI] [PubMed] [Google Scholar]
- 89.Grzmil M, Thelen P, Hemmerlein B , et al. Bax inhibitor-1 is overexpressed in prostate cancer and its specific down-regulation by RNA interference leads to cell death in human prostate carcinoma cells. Am J Pathol. 2003;163(2):543–52. doi: 10.1016/S0002-9440(10)63682-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schmits R, Cochlovius B, Treitz G , et al. Analysis of the antibody repertoire of astrocytoma patients against antigens expressed by gliomas. Int J Cancer. 2002;98(1):73–7. doi: 10.1002/ijc.10170. [DOI] [PubMed] [Google Scholar]
- 91.Lewis-Wambi JS, Jordan VC. Estrogen regulation of apoptosis: how can one hormone stimulate and inhibit?. Breast Cancer Res. 2009;11(3):206. doi: 10.1186/bcr2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Toufexis DJ, Myers KM, Bowser ME, Davis M. Estrogen disrupts the inhibition of fear in female rats. possibly through the antagonistic effects of estrogen receptor alpha (ERalpha) and ERbeta. J Neurosci. 2007;27(36):9729–35. doi: 10.1523/JNEUROSCI.2529-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004;64(1):423–8. doi: 10.1158/0008-5472.can-03-2446. [DOI] [PubMed] [Google Scholar]
- 94.Schmidt SM, Konig T, Bringmann A , et al. Characterization of BAX inhibitor-1 as a novel leukemia-associated antigen. Leukemia. 2009;23(10):1818–24. doi: 10.1038/leu.2009.138. [DOI] [PubMed] [Google Scholar]
- 95.Illario M, Cavallo AL, Bayer KU , et al. Calcium/calmodulin-dependent protein kinase II binds to Raf-1 and modulates integrin-stimulated ERK activation. J Biol Chem. 2003;278(46):45101–8. doi: 10.1074/jbc.M305355200. [DOI] [PubMed] [Google Scholar]
- 96.Oliveira JB, Gupta S. Disorders of apoptosis: mechanisms for autoimmunity in primary immunodeficiency diseases. J Clin Immunol. 2008;28(Suppl 1 ):S20–8. doi: 10.1007/s10875-007-9161-4. [DOI] [PubMed] [Google Scholar]
- 97.Miyaji M, Kortum RL, Surana R , et al. Genetic evidence for the role of Erk activation in a lymphoproliferative disease of mice. Proc Natl Acad Sci U S A. 2009;106(34):14502–7. doi: 10.1073/pnas.0903894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dohm CP, Siedenberg S, Liman J , et al. Bax inhibitor-1 protects neurons from oxygen-glucose deprivation. J Mol Neurosci. 2006;29(1):1–8. doi: 10.1385/JMN:29:1:1. [DOI] [PubMed] [Google Scholar]
- 99.Jeon K, Lim H, Kim JH , et al. Bax inhibitor-1 enhances survival and neuronal differentiation of embryonic stem cells via differential regulation of mitogen-activated protein kinases activities. Biochim Biophys Acta. 2012;1823(12):2190–200. doi: 10.1016/j.bbamcr.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 100.Krajewska M, Xu L, Xu W , et al. Endoplasmic reticulum protein BI-1 modulates unfolded protein response signaling and protects against stroke and traumatic brain injury. Brain Res. 2011;1370:227–37. doi: 10.1016/j.brainres.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gao D, Nong S, Huang X , et al. The effects of palmitate on hepatic insulin resistance are mediated by NADPH Oxidase 3-derived reactive oxygen species through JNK and p38MAPK pathways. J Biol Chem. 2010;285(39):29965–73. doi: 10.1074/jbc.M110.128694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sui ZY, Chae HJ, Huang GB, Zhao T, Shrestha Muna S, Chung YC. Effects of chronic mild stress in female bax inhibitor-1-gene knockout mice. Clin Psychopharmacol Neurosci. 2012;10(3):155–62. doi: 10.9758/cpn.2012.10.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]