Abstract
IMPORTANCE
Over 300,000 surgeries are performed annually in the United States for pelvic organ prolapse. Sacrospinous ligament fixation (SSLF) and uterosacral ligament suspension (ULS) are commonly performed transvaginal surgeries to correct apical prolapse. Little is known about their comparative efficacy and safety, and it is unknown whether perioperative behavioral therapy with pelvic floor muscle training (BPMT) improves outcomes of prolapse surgery.
OBJECTIVE
To compare outcomes between 1) SSLF and ULS and 2) perioperative BPMT and usual perioperative care in women undergoing surgery for vaginal prolapse and stress urinary incontinence.
DESIGN, SETTING AND PARTICIPANTS
Multi-center, 2×2 factorial randomized trial of 374 women undergoing surgery to treat both apical vaginal prolapse and stress urinary incontinence was conducted between 2008 and 2013 at 9 U.S. medical centers. Two-year follow-up rate was 84.5%.
INTERVENTIONS
Surgical intervention: Transvaginal surgery including mid-urethral sling with randomization to SSLF (n = 186) or ULS (n=188); Behavioral intervention: Randomization to perioperative BPMT (n = 186) or usual care (n=188).
MAIN OUTCOME MEASURES
The primary outcome for the surgical intervention (surgical success) was defined as: 1) no apical descent greater than one-third into vaginal canal or anterior or posterior vaginal wall beyond the hymen (anatomic success); 2) no bothersome vaginal bulge symptoms and 3) no retreatment for prolapse at 2 years. For the behavioral intervention, primary outcome at 6 months was urinary symptom scores (Urinary Distress Inventory; range 0–300, higher scores worse), and primary outcomes at 2 years were prolapse symptom scores (Pelvic Organ Prolapse Distress Inventory; range 0–300, higher scores worse) and anatomic success.
RESULTS
At 2 years, surgical group was not significantly associated with surgical success rates [ULS 59.2% (93/154) vs. SSLF 60.5% (92/152), OR 0.9 (95% CI 0.6, 1.5)] or serious adverse event rates [ULS 16.5% (31/188) vs. SSLF 16.7% (31/186), OR 0.9 (95% CI 0.5, 1.6)]. BPMT was not associated with greater improvements in urinary scores at 6 months [treatment difference −6.7 (95% CI −19.7, 6.2)], prolapse scores at 24 months [treatment difference −8.0 (95% CI −22.1, 6.1)] or anatomic success at 24 months.
CONCLUSIONS AND RELEVANCE
Two years after vaginal surgery for prolapse and stress urinary incontinence, neither ULS nor SSLF was significantly superior to the other for anatomic, functional, and or adverse event outcomes. Perioperative BPMT did not improve urinary symptoms at 6 months or prolapse outcomes at 2 years.
INTRODUCTION
Female pelvic floor disorders are a spectrum of conditions including pelvic organ prolapse and urinary incontinence. Pelvic organ prolapse occurs when the uterus descends into the lower vagina or vaginal walls protrude beyond the vaginal opening. Approximately 300,000 surgeries for prolapse are performed annually in the U.S.1 Most surgery for pelvic organ prolapse (80–90%) is performed transvaginally, with the remainder performed abdominally.1–4 Increasingly, surgeons recognize that adequate apical (upper vaginal) support is an essential component of a durable repair.5–7 The sacrospinous ligament fixation (SSLF) and the uterosacral vaginal vault suspension (ULS) are the two most widely used vaginal procedures for correcting apical prolapse, yet no comparative data exist about their relative efficacy and safety.8
Concurrent pelvic floor disorders are common in women seeking vaginal prolapse surgery. Up to 73% report urinary incontinence, including the common subtype of stress incontinence or involuntary urine loss with coughing, sneezing or physical activity.9 Following prolapse surgery, new pelvic floor symptoms may develop while pre-existing pelvic floor symptoms may improve, worsen or remain unchanged. As a stand-alone therapy, behavioral therapy with pelvic floor muscle training (BPMT) is an effective treatment for pelvic floor symptoms with incontinence cure rates as high as 78% and improved prolapse stage in up to 17%.10–14 Diminished pelvic floor muscle strength has been associated with increased risk of prolapse recurrence and reoperation.15 BPMT, therefore, may be a logical adjunct if it improves surgical outcomes. Two reviews of perioperative physiotherapy for women undergoing prolapse surgery prioritized a need for robust, well-designed trials to evaluate the efficacy of perioperative BPMT.14,16
The Operations and Pelvic Muscle Training in the Management of Apical Support Loss (OPTIMAL) trial used a 2×2 factorial design to evaluate 2 primary aims: 1) to compare surgical outcomes of SSLF to ULS 24 months after vaginal surgery for apical or uterine prolapse and stress incontinence and 2) to evaluate the impact of perioperative BPMT on urinary symptoms 6 months after surgery and on anatomic outcomes and prolapse symptoms 24 months after surgery.
METHODS
Study Design
The design of the OPTIMAL trial has been published in detail.17 This factorial randomized trial was conducted between January 2008 and May 2013 at 9 sites participating in the NIH-sponsored Pelvic Floor Disorders Network. Eligible participants included women ≥18 years undergoing vaginal surgery for Stage 2–4 prolapse (vaginal or uterine descent 1 cm proximal to the hymen or beyond)18 with a) complaints of vaginal bulge symptoms; b) descent of the uterus or vaginal apex at least half-way into the vagina; c) stress urinary incontinence symptoms; and d) objective demonstration of stress incontinence by office or urodynamic testing in the previous 12 months (See eTable 1 for detailed eligibility criteria). The institutional review boards at each site approved the protocol. All participants provided written informed consent for study participation. Race/ethnicity was obtained by self-report.
Table 1.
Demographics and Baseline Characteristics
| Variable | BPMT Group Comparisons | Surgical Group Comparisons | ||||
|---|---|---|---|---|---|---|
| Treatment Group | P value* | Treatment Group | P value* | |||
| BPMT N=186 |
Usual Care N=188 |
Uterosacral Ligament Suspension N=188 |
Sacrospinous Ligament Fixation N=186 |
|||
| Age, yrs. | N/A – Significant BPMT by Surgery interaction (P value for interaction = 0.04) | N/A – Significant BPMT by Surgery interaction (P value for interaction = 0.04) | ||||
| Mean (SD) | 57.5 (10.9) | 56.9 (10.9) | 57.3 (10.8) | 57.2 (11.0) | ||
| Min, Max | 29.0, 80.0 | 31.0, 80.0 | 29.0, 80.0 | 32.0, 80.0 | ||
| Age, n (%) | ||||||
| >=29 and <=40 | 15 (8.1%) | 10 (5.3%) | 10 (5.3%) | 15 (8.1%) | ||
| >40 and <=50 | 40 (21.5%) | 45 (23.9%) | 45 (23.9%) | 40 (21.5%) | ||
| >50 and <=60 | 59 (31.7%) | 68 (36.2%) | 66 (35.1%) | 61 (32.8%) | ||
| >60 and <=70 | 49 (26.3%) | 40 (21.3%) | 42 (22.3%) | 47 (25.3%) | ||
| >70 and <=80 | 23 (12.4%) | 25 (13.3%) | 25 (13.3%) | 23 (12.4%) | ||
| Race, n (%) | 0.45 | 0.94 | ||||
| White/Caucasian | 154 (82.8%) | 161 (85.6%) | 158 (84.0%) | 157 (84.4%) | ||
| Black/African American | 15 (8.1%) | 7 (3.7%) | 12 (6.4%) | 10 (5.4%) | ||
| Asian | 1 (0.5%) | 3 (1.6%) | 0 (0.0%) | 4 (2.2%) | ||
| American Indian/Alaskan | 1 (0.5%) | 1 (0.5%) | 0 (0.0%) | 2 (1.1%) | ||
| Other | 15 (8.1%) | 16 (8.5%) | 18 (9.6%) | 13 (7.0%) | ||
| Ethnicity, n (%) | 0.80 | 0.39 | ||||
| Hispanic | 38 (20.4%) | 37 (19.7%) | 41 (21.8%) | 34 (18.3%) | ||
| Non-Hispanic | 148 (79.6%) | 151 (80.3%) | 147 (78.2%) | 152 (81.7%) | ||
| Insurance | ||||||
| Private/HMO | 125 (67.2%) | 126 (67.0%) | 0.96 | 128 (68.1%) | 123 (66.1%) | 0.68 |
| Medicaid/Medicare | 56 (30.1%) | 53 (28.2%) | 0.68 | 56 (29.8%) | 53 (28.5%) | 0.78 |
| Self-Pay | 3 (1.6%) | 3 (1.6%) | 0.99 | 3 (1.6%) | 3 (1.6%) | 0.99 |
| Other | 33 (17.7%) | 39 (20.7%) | 0.47 | 37 (19.7%) | 35 (18.8%) | 0.89 |
| Vaginal deliveries, n | 0.18 | 0.01 | ||||
| Median | 3.0 | 3.0 | 3.0 | 2.0 | ||
| Min, Max | 0.0, 15.0 | 0.0, 12.0 | 0.0, 15.0 | 0.0, 12.0 | ||
| Cesarean deliveries, n | 0.18 | 0.01 | ||||
| Median | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Min, Max | 0.0, 4.0 | 0.0, 2.0 | 0.0, 4.0 | 0.0, 4.0 | ||
| Menstrual status, n (%) | 0.60 | 0.99 | ||||
| Pre-menopausal | 50 (26.9%) | 56 (29.8%) | 54 (28.7%) | 52 (28.0%) | ||
| Post-menopausal | 123 (66.1%) | 123 (65.4%) | 123 (65.4%) | 123 (66.1%) | ||
| Not sure | 13 (7.0%) | 9 (4.8%) | 11 (5.9%) | 11 (5.9%) | ||
| Currently using estrogen replacement therapy, n (%) | ||||||
| Oral/Patch | 22 (11.8%) | 24 (12.8%) | 0.86 | 20 (10.6%) | 26 (14.0%) | 0.34 |
| Vaginal | 42 (22.6%) | 46 (24.5%) | 0.66 | 49 (26.1%) | 39 (21.0%) | 0.24 |
| Current smoker, n (%) | 19 (10.2%) | 14 (7.4%) | 0.36 | 14 (7.4%) | 19 (10.2%) | 0.36 |
| Diabetes mellitus, n (%) | 27 (14.9%) | 17 (9.2%) | 0.10 | 24 (12.8%) | 20 (11.2%) | 0.65 |
| Connective tissue disease (SLE, Marfans, Sjogrens, Scleroderma), n (%) | 2 (1.1%) | 3 (1.6%) | N/A - LN | 4 (2.2%) | 1 (0.5%) | N/A – LN |
| Prior hysterectomy, n (%) | 45 (24.2%) | 55 (29.3%) | 0.27 | 48 (25.5%) | 52 (28.0%) | 0.62 |
| Prior stress urinary incontinence surgery, n (%) | 6 (3.2%) | 7 (3.7%) | 0.74 | 7 (3.7%) | 6 (3.2%) | 0.74 |
| Prior pelvic organ prolapse surgery, n (%) | 9 (4.8%) | 17 (9.0%) | 0.13 | 9 (4.8%) | 17 (9.1%) | 0.11 |
| Body mass index, kg/m2 | (P value for interaction = 0.048) | (P value for interaction = 0.048) | ||||
| Mean (SD) | 29.3 (5.6) | 28.4 (5.3) | 28.7 (5.2) | 29.0 (5.7) | ||
| Min, Max | 19.3, 49.8 | 19.7, 46.3 | 19.3, 46.3 | 19.6, 49.8 | ||
| Pelvic Organ Prolapse Quantification (POPQ) Stage,* n (%) | 1 | 2 | 0.93 | 0.50 | ||
| 2 | 72 (38.7%) | 71 (37.8%) | 70 (37.2%) | 73 (39.2%) | ||
| 3 | 105 (56.5%) | 108 (57.4%) | 111 (59.0%) | 102 (54.8%) | ||
| 4 | 9 (4.8%) | 9 (4.8%) | 7 (3.7%) | 11 (5.9%) | ||
| Regularly perform pelvic floor muscle exercises, N (%) | 45 (24.3%) | 35 (18.8%) | 0.21 | 38 (20.4%) | 42 (22.7%) | 0.68 |
| Prior supervised pelvic floor muscle exercise program, N (%) | 10 (5.4%) | 4 (2.2%) | 0.11 | 7 (3.8%) | 7 (3.8%) | 0.60 |
| Vaginal bulge symptoms, n, (%) | ||||||
| Any | 186 (100.0%) | 188 (100.0%) | N/A - LN | 188 (100.0%) | 186 (100.0%) | N/A - LN |
| Bothersome | 167 (93.8%) | 163 (92.6%) | 0.65 | 164 (93.7%) | 166 (92.7%) | 0.71 |
| Prolapse beyond the hymen, n (%) | ||||||
| Anterior (POPQ Aa or Ba >0) | 126 (67.7%) | 144 (76.6%) | 0.06 | 142 (75.5%) | 128 (68.8%) | 0.15 |
| Posterior (POPQ Ap or Bp>0) | 38 (20.4%) | 35 (18.6%) | 0.82 | 26 (13.8%) | 47 (25.3%) | 0.01 |
| Apical (POPQ C >0) | 56 (30.3%) | 59 (31.4%) | 0.81 | 55 (29.3%) | 60 (32.4%) | 0.51 |
| Apical descent greater than or equal to ½ of total vaginal length (n, %) (POPQ C>=−1/2*TVL) | 182 (98.4%) | 186 (98.9%) | 0.70 | 185 (98.4%) | 183 (98.9%) | 0.72 |
| Most distal point of vaginal segment, mean cm (SD)# | ||||||
| Anterior | 2.1 (2.3) | 2.2 (2.1) | 0.84 | 2.1 (2.1) | 2.2 (2.4) | 0.93 |
| Posterior | 0.6 (2.8) | 0.5 (2.7) | 0.70 | 0.2 (2.5) | 0.8 (2.9) | 0.04 |
| Apical | −0.8 (3.5) | −1.0 (3.5) | 0.58 | −1.0 (3.3) | −0.7 (3.7) | 0.38 |
| Maximum descent of any segment, mean cm, (SD)# | 2.3 (2.2) | 2.3 (2.1) | 0.90 | 2.2 (2.0) | 2.4 (2.2) | 0.53 |
| POPQ value, median cm (minimum, maximum)## | ||||||
| Aa | 1.0 (−3.0, 3.0) | 1.0 (−2.0, 3.0) | 1.0 (−2.0, 3.0) | 1.0 (−3.0, 3.0) | ||
| Ba | 2.0 (−2.0, 11.0) | 2.0 (−2.0, 11.0) | 2.0 (−2.0, 11.0) | 2.0 (−2.0, 10.0) | ||
| C | −2.0 (−6.0, 11.0) | −2.0 (−6.0, 11.0) | −2.0 (−5.0, 11.0) | −2.0 (−6.0, 10.0) | ||
| Ap | −1.3 (−3.0, 3.0) | −1.0 (−3.0, 3.0) | −2.0 (−3.0, 3.0) | −1.0 (−3.0, 3.0) | ||
| Bp | −1.0 (−3.0, 11.0) | −1.0 (−3.0, 11.0) | −1.0 (−3.0, 11.0) | −1.0 (−3.0, 10.0) | ||
| GH | 4.5 (2.0, 10.0) | 4.5 (2.0, 8.0) | 4.5 (2.0, 8.0) | 4.5 (2.0, 10.0) | ||
| PB | 3.0 (0.0, 6.0) | 3.0 (1.0, 7.0) | 3.0 (1.0, 6.0) | 3.0 (0.0, 7.0) | ||
| TVL | 9.5 (5.5, 12.0) | 9.5 (3.0, 12.0) | 9.5 (3.0, 12.0) | 10.0 (5.5, 12.0) | ||
BPMT, Behavioral and Pelvic Floor Muscle Therapy; POPQ, Pelvic Organ Prolapse Quantification system N/A – LN = The P value is not shown due to low Ns leading to low reliability of test.
Pelvic Organ Prolapse Quantification (POPQ) Stages: Stage 2 -The vagina is prolapsed between 1 cm above the hymen and 1 cm below the hymen; Stage 3 -The vagina is prolapsed more than 1 cm beyond the hymen but is less than totally everted; Stage 4 - The vagina is everted to within 2 cm of its length.
Most distal point of each compartment and overall are measured in cm relative to the hymen during maximum strain with descent to the hymen = 0, descent proximal to the hymen as a negative value and descent distal to the hymen as positive value using the POPQ system. The most distal point of the apical segment is equal to POPQ point C; Most distal point of the anterior segment = POPQ point Ba if Ba ≥C or is = C if Ba<C; most distal point of posterior segment = POPQ point Bp if Bp ≥C or is = C if Bp<C.
POPQ values provided for descriptive purposes only. In the POPQ system, the positions of C, Ba, and Bp are measured at the most dependent location (the point of greatest prolapse) of the apex, anterior vaginal wall and posterior vaginal wall respectively during a straining. Values are measured in cm and are negative if above the hymen, and positive if below the hymen. TVL (total vaginal length), GH (genital hiatus) and PB (perineal body) are measured as positive values.
NOTE: “N/A – Significant BPMT*Surgery interaction” represents an effect that was significant in the model at the .05 alpha level. In these cases, the adjusted means and test results are reported below within BPMT and Usual care groups.
Mean Age, years: ULS Group: BPMT, 58.7 vs. Usual care, 55.8, p=0.07; SSLF Group: BPMT, 56.3 vs. Usual care, 58.1, p=0.25
Mean BMI, kg/m2: ULS Group: BPMT, 28.6 vs. Usual care, 28.8, p=0.77; SSLF Group: BPMT, 30.0 vs. Usual care, 28.0, p=0.01
Mean Age, years: BPMT group, ULS, 55.8 vs. SSLF, 58.1, p=0.12; usual care group, ULS, 58.7 vs SSLF, 56.3, p=0.15
Mean BMI, kg/m2: BPMT group, ULS, 28.8 vs. SSLF, 28.0, p=0.08; usual care group, ULS 28.6 vs SSLF, 30.0, p=0.29
NOTE: The P value for race tests White vs. all other races due to low sample size in minority categories.
NOTE: P values correspond to tests between means, even for characteristics showing medians.
Using a 2×2 factorial design, each enrolled patient underwent two distinct randomizations: first, perioperative BPMT or usual care and second, SSLF or ULS. Participants were assigned with equal probability, using a random permuted block design generated by the Data Coordinating Center with the randomized treatment allocations provided in two sequentially numbered, sealed, opaque envelopes (one for randomization to BPMT or usual care, and one for randomization to ULS or SSLF). Randomization to BPMT versus usual care took place preoperatively and was stratified by clinical site. The second randomization to SSLF or ULS took place in the operating room and was stratified by surgeon and concomitant hysterectomy.
Study Interventions
Participants underwent transvaginal surgery for pelvic organ prolapse, including the assigned apical suspension procedure. The SSLF procedure, performed unilaterally, was a modification of the Michigan 4-wall technique.19,20 The ULS procedure, performed bilaterally, was a modification of the technique described by Shull.21 Both apical suspension procedures used two permanent and two delayed absorbable sutures (four sutures total).17 All patients with uterine prolapse underwent vaginal hysterectomy. A concomitant retropubic mid-urethral sling (Tension-Free Vaginal Tape [TVT®]; Ethicon Women’s Health and Urology, Somerville, NJ) was performed for stress urinary incontinence. Other concomitant surgeries were performed at the surgeon’s discretion; biologic or synthetic graft materials were not allowed for the prolapse repairs.
Usual perioperative care included routine perioperative teaching and standardized postoperative instructions. Participants randomized to perioperative BPMT received an individualized program that included one visit 2–4 weeks prior to surgery, and four post-operative visits (2, 4–6, 8, and 12 weeks after surgery).17 (eTable 2) Pelvic floor muscle training, individualized progressive pelvic floor muscle exercise, and education on behavioral strategies to reduce urinary and colorectal symptoms were performed at each visit. Self-reported adherence to BPMT was assessed at 6, 12, and 24 months. All BPMT interventionists attended centralized in-person training prior to the initiation of the study.
Data collection occurred at baseline, during surgery and hospitalization, and at regular intervals up to 24 months postoperatively with Pelvic Organ Prolapse Quantification (POPQ)18 evaluations and symptom assessments occurring at 6, 12 and 24 months. BPMT interventionists were masked to surgical randomization. All outcome assessors were masked to both perioperative BPMT and surgical intervention assignment, including research personnel who conducted vaginal examinations and trained telephone interviewers who administered patient reported outcomes from a centralized facility. Participants were masked to the surgical group assignment, and study surgeons were masked to perioperative BPMT group assignment.
Outcomes
Surgical Intervention
The primary outcome for surgery, assessed at 2 years, used a composite outcome measure of surgical “success” or “failure”. We defined “success” as the absence of all of the following: (1) descent of the vaginal apex more than one-third into the vaginal canal, (2) anterior or posterior vaginal wall descent beyond the hymen, (3) bothersome vaginal bulge symptoms as indicated by an affirmative response to either “Do you usually have a sensation of bulging or protrusion from the vaginal area?” or “Do you usually have a bulge or something falling out that you can see or feel in the vaginal area?” in the Pelvic Floor Distress Inventory (PFDI)22, and any response other than “not at all” to the question “How much does this bother you?”, or (4) retreatment for prolapse by either surgery or pessary. Secondary surgical outcomes included maximum prolapse of each vaginal segment (anterior, posterior and apical); urinary, bowel and prolapse symptoms (PFDI, Incontinence Severity Index23); retreatment for prolapse or urinary incontinence; and adverse events.
An independent data and safety monitoring board reviewed trial progress and safety. Investigators classified adverse events as serious/non-serious and expected/unexpected. Serious and expected adverse events were further categorized using the Dindo system.24
Behavioral Intervention
Primary outcomes for BPMT were assessed at 6 and 24 months. The primary 6-month outcome, urinary symptoms, was assessed by the Urinary Distress Inventory (UDI) score of the PFDI. The minimum important difference of the UDI is 11 points.25 The primary 24-month outcomes were 1) prolapse symptoms, assessed by the Pelvic Organ Prolapse Distress Inventory (POPDI) score of the PFDI, and 2) anatomic failure defined by: (a) descent of the vaginal apex more than one-third into the vaginal canal, or (b) anterior or posterior vaginal wall descent beyond the hymen, or (c) retreatment for prolapse. In contrast to the primary outcome of the surgical intervention, the presence of vaginal bulge symptoms was not included in the anatomic failure outcome for the BPMT analysis because it is assessed as a component of the POPDI. Secondary outcomes included maximum prolapse (anterior, posterior and apical vaginal segments); retreatment for urinary incontinence and/or prolapse; urinary, prolapse and bowel symptoms (PFDI)22; Incontinence Severity Index23; and pelvic floor muscle strength (Brink grading system).26
Sample Size
Study investigators estimated a priori that a surgical success difference of less than 15% would not change clinical practice and that the sample size should detect a difference of 10%. A sample size of 121 per surgical group would provide 80% power to detect a difference between surgical failure rates of 70% versus 85% using a two-tailed 5% level of significance. 170 women per surgical group would allow 80% power to differentiate between success rates of 70% and 83% using a two-tailed 5% level of significance and also allow 80% power to identify a group difference of 0.3 standard deviations in mean UDI score between BPMT and usual care groups using a two-tailed 5% level of significance. We increased our enrollment goal to 200 per group to allow for a 15% drop out rate. We stopped enrollment at 418 participants (374 randomized) because our early loss to follow-up was lower than expected. Our final sample size of 309 women with primary outcome data was sufficient to provide 80% power to detect a 15% treatment difference (noted a priori by the investigators to be clinically relevant) though the final number of participants with 24-month data was lower than expected. The study was not powered to detect interactions between the surgical and behavioral interventions.
Statistical Analysis
The analysis was performed on all participants who underwent randomization for both the BPMT intervention and the surgical intervention, and participants were analyzed in the groups to which they were randomized. Baseline demographic and clinical characteristics were compared between surgical treatment groups and between BPMT treatment assignments using general linear models for continuous outcomes and generalized linear models for categorical outcomes. Baseline models included variables for surgical group, BPMT treatment assignment, and their interaction. For the primary outcome, a p value (two-sided) less than 0.05 was considered statistically significant. Analyses of secondary outcomes were considered exploratory in nature, and p values and confidence intervals are provided for descriptive purposes only.
Surgical Intervention
Differences between the surgical groups in the primary outcome of surgical success at 24 months and other categorical outcomes were evaluated using generalized linear models with a logit link and terms for surgical group, BPMT treatment assignment, and their interaction, as well as stratification factors for surgeon and concomitant hysterectomy. Because of the large number of surgeons involved in the study (31 surgeons from 9 sites), surgeon was included in the outcome models as a random effect. If the interaction reached statistical significance at the p<0.05 level, surgical groups were compared within each BPMT group; otherwise the marginal differences between surgical groups were compared. Similar models were used to compare occurrence of adverse events except that a cumulative logit link was employed for multinomial outcomes. Continuous outcomes were compared using analogous general linear models. For outcomes for which data were available at multiple time points (for example, bothersome vaginal bulge symptoms at 6, 12 and 24 months), a longitudinal extension to the generalized linear model that included terms for time as a categorical variable was used, and tests comparing the surgical groups at each time point were conducted. Longitudinal models additionally included interactions between the BPMT and surgical treatments and time.
For the primary analysis, women with missing anatomic data at the 2-year time point, data from the last available physical examination were used. Women who met failure criteria based on their last exam were considered surgical failures at 2 years; however, those who did not meet failure criteria and had missing data at 2 years were considered missing at 2 years. Missing surgical failure outcomes were multiply imputed and a sensitivity analysis conducted to assess the robustness of the primary analysis results.
Behavioral Intervention
For the primary outcome of UDI score at 6 months, outcomes were imputed using Brown’s method for participants who had reported use of medication for lower urinary tract symptoms, stress incontinence surgery including urethral bulking agent injections, neuromodulation, intravesical botulinum toxin injections, or enrollment in a supervised pelvic floor therapy program to address potential biases in the UDI introduced through those added treatments.27 The imputed UDI score at 6 months was analyzed using a Mann-Whitney-Wilcoxon test. In addition, we used a common linear mixed model using non-imputed data that included terms for BPMT treatment, surgical treatment, and their interaction, as well as clinical site, and terms for time as a categorical variable and the two and three way interactions of time with the treatment variables to evaluate the effect of BPMT treatment on the change from baseline in 1) UDI at 6, 12, and 24 months, and 2) other elements of the PFDI at 6, 12, and 24 months. Two year anatomic outcomes for the behavioral intervention were analyzed using models analogous to those described above for the surgical intervention, but modified to control for site as a fixed effect rather than for hysterectomy and surgeon, reflecting the differences in the stratification factors for the BPMT and surgical randomizations. When the treatment interaction reached statistical significance at the p<0.05 level, BPMT groups were compared within each surgical group; otherwise marginal effects of the BPMT treatment groups were compared.
As with the surgical failure outcome, the anatomic failure outcome for the behavioral intervention used data from the last available physical examination for women with missing anatomic data at the 2-year time point. Women who met failure criteria based on their last examination were considered anatomic failures at 2 years; however, those who did not meet failure criteria and had missing data at 2 years were considered missing at 2 years for statistical analysis. In sensitivity analyses, missing anatomic failure outcomes were multiply imputed, as were changes from baseline in UDI scores at 6 months and POPDI subscale scores at 24 months for women who were missing the UDI or POPDI or who underwent stress incontinence or prolapse retreatment, respectively. Sensitivity analyses using the multiply imputed data were conducted to assess the robustness of the original analyses.
RESULTS
Study Populations and Treatment Assignments
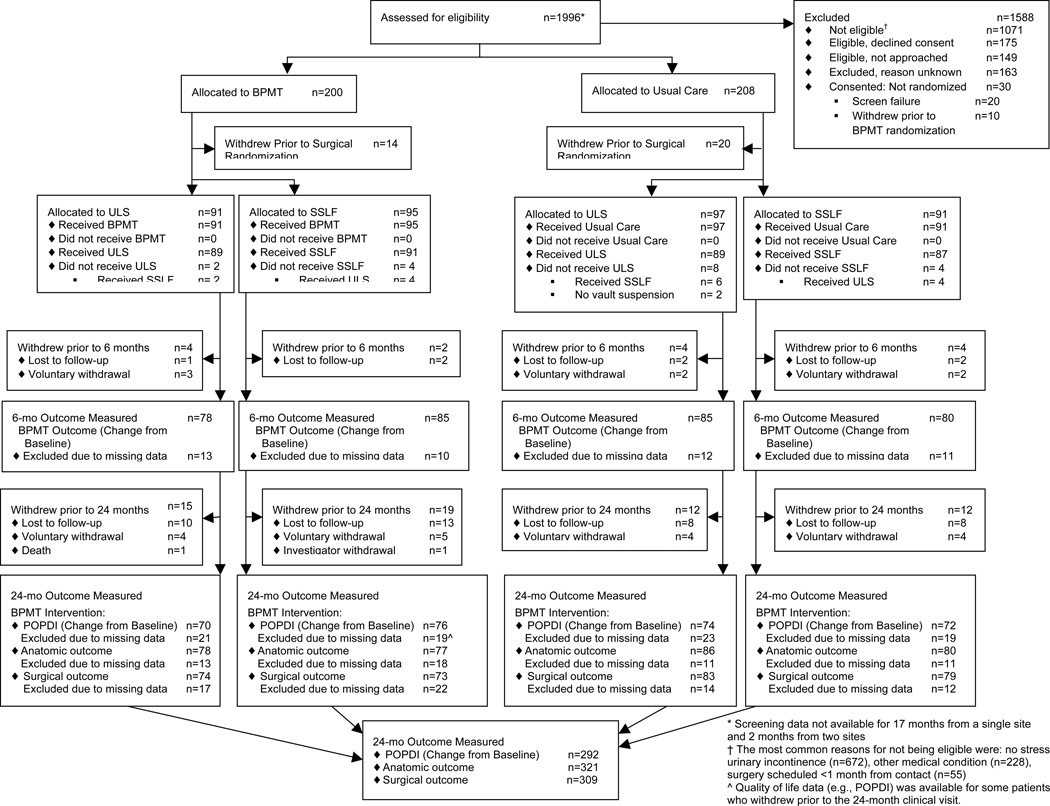
The OPTIMAL trial enrolled 418 eligible women and 408 women underwent the behavioral therapy randomization preoperatively. Thirty-four participants withdrew prior to surgery leaving 374 women who were randomized to both the surgical intervention (ULS n=188 vs. SSLF n=186) and behavioral intervention (BPMT n=186 vs. usual care n=188) and were included in this analysis (Figure 1). The groups had similar rates of post-randomization withdrawals at 24 months [ULS 27 (14.4%), SSLF 31 (16.7%), p=0.59 and BPMT 34 (18.3%), usual care 24 (12.8%), p=0.15].
Figure 1.
Optimal Trial Enrollment, Randomization and Assessment.
Baseline clinical characteristics were similar between the surgical groups and the behavioral intervention groups with the exception of a greater degree of posterior vaginal prolapse in the SSLF group and a higher median number of vaginal deliveries in the ULS group (Table 1 and eTable 3). We noted significant interaction effects between surgical and BPMT groups for age and BMI; however, within BPMT groups the surgical groups were balanced. There were no BPMT or usual care group differences in surgical intervention; 50% of both groups underwent each surgical study procedure (ULS and SSLF) and all but 3 women in the study population (99%) underwent TVT (eTable 4).
Surgical Intervention Outcomes
At two years, there was no statistically significant difference in surgical success as defined by the composite primary outcome [ULS 59.2% (93/157) versus SSLF 60.5% (92/152), OR 0.9, 95% CI (0.6, 1.5)] between the surgical groups, and no clinically significant differences in any of the 4 primary outcome components (Table 2 a & b). Analysis of the multiply imputed surgical failure outcome was consistent with the primary analysis [ULS 111/188=59.1% vs. SSLF 116/186=62.4%, OR 0.9, 95% CI (0.6, 1.5)]. Overall, 18.0% of women (55/305) developed bothersome vaginal bulge symptoms, 17.5% (54/308) had anterior and/or posterior prolapse beyond the hymen and 5.1% (16/316) underwent retreatment with either a pessary or surgery by two years. An interaction effect between surgical and BPMT groups was noted for the apical descent component of surgical success (Table 2). In women receiving usual care, those in the ULS group were less likely to develop apical descent than those receiving SSLF [ULS 8.6% versus SSLF 20.8%, OR 0.3, 95% CI (0.1, 0.9)]. In women receiving BPMT there was no significant difference in apical descent [ULS 23.0% versus SSLF 12.0%, OR 2.2, 95% CI (0.9, 5.3)].
Table 2.
| a. Pelvic Organ Prolapse Outcomes at 24 months | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Surgical Group Comparisons | BPMT Group Comparison | ||||||||||||||||||||||||||
| Treatment Group | Surgical Adjusted OR (95% CI) |
P value | Treatment Group | BPMT Adjusted OR (95% CI) |
P value | |||||||||||||||||||||||
| ULS N=188 |
SSLF N=186 |
BPMT N=186 |
Usual Care N=188 |
|||||||||||||||||||||||||
| Surgical Success | 93/157 (59.2%) | 92/152 (60.5%) | 0.9 (0.6, 1.5) | 0.75 | ||||||||||||||||||||||||
| Anatomic Failure | 45/155 (29.0%) | 49/166 (29.5%) | See Table 2b | |||||||||||||||||||||||||
| Vaginal Bulge Symptoms* | ||||||||||||||||||||||||||||
| Bothersome | 25/151 (16.6%) | 30/154 (19.5%) | 0.8 (0.5, 1.5) | 0.49 | 27/151 (17.9%) | 28/154 (18.2%) | 1.0 (0.5, 1.9) | 1.00 | ||||||||||||||||||||
| Any | 29/151 (19.2%) | 32/154 (20.8%) | 0.9 (0.5, 1.6) | 0.70 | 31/151 (20.5%) | 30/154 (19.5%) | 1.1 (0.6, 2.0) | 0.75 | ||||||||||||||||||||
| Apical Descent >1/3rd of total vaginal length (POPQ point C>-2/3*TVL) | 24/155 (15.5%) | 25/152 (16.4%) | See Table 2b | 26/149 (17.4%) | 23/158 (14.6%) | See Table 2b | ||||||||||||||||||||||
| Prolapse Beyond the Hymen* | ||||||||||||||||||||||||||||
| Anterior (POPQ Aa or Ba >0) | 24/155 (15.5%) | 21/153 (13.7%) | 1.2 (0.6, 2.2) | 0.65 | 21/149 (14.1%) | 24/159 (15.1%) | 0.9 (0.5, 1.8) | 0.79 | ||||||||||||||||||||
| Posterior (POPQ Ap or Bp >0) | 7/155 (4.5%) | 11/153 (7.2%) | 0.6 (0.3, 1.3) | 0.21 | 7/149 (4.7%) | 11/159 (6.9%) | 0.6 (0.2, 1.5) | 0.24 | ||||||||||||||||||||
| Apical (POPQ C >0) | 7/155 (4.5%) | 9/152 (5.9%) | 0.9 (0.3, 2.5) | 0.86 | 6/149 (4.0%) | 10/158 (6.3%) | 0.6 (0.2, 1.8) | 0.37 | ||||||||||||||||||||
| Retreatment for POP | ||||||||||||||||||||||||||||
| Surgery | 5/161 (3.1%) | 4/155 (2.6%) | 1.4 (0.3, 6.0) | 0.66 | 4/152 (2.6%) | 5/164 (3.0%) | 0.7 (0.2, 3.3) | 0.70 | ||||||||||||||||||||
| Pessary | 5/161 (3.1%) | 5/155 (3.2%) | 1.0 (0.2, 4.7) | 0.97 | 8/152 (5.3%) | 2/164 (1.2%) | 4.4 (0.9, 21.6) | 0.07 | ||||||||||||||||||||
| Either Surgery or Pessary | 8/161 (5.0%) | 8/155 (5.2%) | 0.9 (0.3, 2.6) | 0.81 | 11/152 (7.2%) | 5/164 (3.0%) | 2.5 (0.8, 7.6) | 0.11 | ||||||||||||||||||||
| Treatment Group | Adjusted Mean Treatment Difference (95% CI) |
P value | Treatment Group | Adjusted Mean Treatment Difference (95% CI) |
P value | |||
|---|---|---|---|---|---|---|---|---|
| ULS N=188 |
SSLF N=186 |
BPMT N=186 |
Usual Care N=188 |
|||||
| Most distal point of vaginal segment adjusted mean cm (SE)#: | ||||||||
| Anterior | −0.5 (0.2) | −0.4 (0.2) | −0.1 (−0.6, 0.4) | 0.73 | −0.5 (0.2) | −0.4 (0.2) | −0.2 (−0.6, 0.3) | 0.53 |
| Posterior | −1.6 (0.2) | −1.6 (0.2) | −0.0 (−0.5, 0.5) | 0.98 | −1.6 (0.2) | −1.6 (0.2) | −0.1 (−0.5, 0.4) | 0.81 |
| Apical | See Table 2b | See Table 2b | ||||||
| Maximum descent of any segment adjusted mean cm, (SE) | −0.2 (0.2) | −0.2 (0.2) | −0.1 (−0.5, 0.4) | 0.78 | −0.3 (0.2) | −0.2 (0.2) | −0.1 (−0.6, 0.4) | 0.63 |
| b. Within-Group Comparisons for Significant Interactions Between Surgical and Behavioral Intervention Groups | ||||||||
| Variable | Comparisons within ULS Group | Comparisons within SSLF Group | ||||||
| Treatment Group | Adjusted OR (95% CI) |
P value | Treatment Group | Adjusted OR (95% CI) | P value | |||
| BPMT N=91 |
Usual Care N=97 |
BPMT N=95 |
Usual Care N=91 |
|||||
| Anatomic Failure (interaction P value 0.03) | 26/78 (33.3%) | 22/86 (25.6%) | 1.8 (0.9, 3.8) | 0.10 | 19/77 (24.7%) | 27.80 (33.8%) | 0.6 (0.3, 1.2) | 0.15 |
| Apical Descent >1/3rd of total vaginal length (POPQ point C>-2/3*TVL) (interaction P value 0.005) | 17/74 (23.0%) | 7/81 (8.6%) | 3.8 (1.4, 10.2) | 0.01 | 9/75 (12.0%) | 16/77 (20.8%) | 0.5 (0.2, 1.2) | 0.10 |
| Comparisons within ULS Group | Comparisons within SSLF Group | |||||||
| Treatment Group | Adjusted OR (95% CI) |
P value | Treatment Group | Adjusted OR (95% CI) | P value | |||
| BPMT N=91 |
Usual Care N=97 |
BPMT N=95 |
Usual Care N=91 |
|||||
| Most distal point of vaginal segment (adjusted mean, SE)# Apical (interaction P value 0.01) | −6.1 (3.3) | −6.0 (2.7) | 0.6 (−0.4, 1.6) | 0.26 | −5.6 (2.4) | −5.2 (3.7) | −1.0 (−2.0, 0.0) | 0.06 |
| Comparisons within ULS Group | Comparisons within SSLF Group | |||||||
| Treatment Group | Adjusted OR (95% CI) |
P value | Treatment Group | Adjusted OR (95% CI) | P value | |||
| BPMT N=91 |
Usual Care N=97 |
BPMT N=95 |
Usual Care N=91 |
|||||
| Apical Descent >1/3rd of total vaginal length (POPQ point C>-2/3*TVL) (interaction P value 0.005) | 17/74 (23.0%) | 9/75 (12.0%) | 2.2 (0.9, 5.3) | 0.08 | 7/81 (8.6%) | 16/77 (20.8%) | 0.3 (0.1, 0.9) | 0.03 |
| Comparisons within ULS Group | Comparisons within SSLF Group | |||||||
| Treatment Group | Adjusted OR (95% CI) |
P value | Treatment Group | Adjusted OR (95% CI) | P value | |||
| BPMT N=91 |
Usual Care N=97 |
BPMT N=95 |
Usual Care N=91 |
|||||
| Most distal point of vaginal segment (adjusted mean, SE)# Apical (interaction P value 0.03) | −5.5 (0.4) | −6.2 (0.4) | 0.7 (−0.4, 1.7) | 0.22 | −6.0 (0.4) | −5.3 (0.4) | −0.8 (−1.8, 0.3) | 0.14 |
OR=Odds Ratio; CI=Confidence Interval; POPQ=Pelvic Organ Prolapse Quantification, BPMT= Behavioral and Pelvic Floor Muscle Therapy; ULS=Uterosacral Ligament Suspension; SSLF=Sacrospinous Ligament Fixation; SE=Standard Error of Adjusted Mean.
The three-way interaction between time point and the two treatments is excluded from the models for vaginal bulge bymptoms and posterior prolapse beyond the hymen due to model convergence issues. Hysterectomy was excluded from the vaginal bulge symptoms Model due to convergence issues.
Most distal point of each compartment and overall are measured in cm relative to the hymen during maximum strain with descent to the hymen = 0, descent proximal to the hymen as a negative value and descent distal to the hymen as positive value using the POPQ system. The most distal point of the apical segment is equal to POPQ point C; Most distal point of the anterior segment = POPQ point Ba if Ba ≥C or is = C if Ba<C; most distal point of posterior segment = POPQ point Bp if Bp ≥C or is = C if Bp<C.
Surgical groups were not significantly different for most secondary outcome measures including operative variables such as estimated blood loss, time of surgery and duration of hospitalization (eTable 4) and postoperative treatments for prolapse and incontinence (eTable 5). A greater proportion of women in the SSLF group had “any” or “bothersome” vaginal bulge symptoms at 6 and 12 months as compared to the women in the ULS group (eTable 6a). By 24 months, these proportions were similar without clinically relevant differences.
The most common perioperative adverse event was bladder perforation associated with TVT placement; the most common long-term complication was presence of vaginal granulation tissue (Table 3). The proportion of women who experienced serious adverse events during the study was not significantly different between surgical groups [ULS 16.5% vs. SSLF 16.7%, OR 0.9, 95% CI (0.5, 1.6)]. The rate of neurologic pain requiring intervention was higher in the SSLF group [ULS 6.9% vs. SSLF 12.4%, OR 0.5, 95% CI (0.2, 1.0), p=0.049] and persisted to the 4–6 week postoperative visit in more participants [ULS n=1, (0.5%) versus SSLF, n=8 (4.3%)]. Ureteral obstruction was recognized and successfully managed intraoperatively in 5 (3.2%) in the ULS group. One patient’s ureteral injury was detected post-operatively after ULS (0.5%). Ureteral obstruction was not seen in the SSLF group.
Table 3.
Adverse Events Related to the Surgical Outcome
| Variable | Treatment Group | Adjusted OR* (95% CI) | P value* | |
|---|---|---|---|---|
| Uterosacral Ligament Suspension N=188 |
Sacrospinous Ligament Fixation N=186 |
|||
| Participants with any Adverse Event (AE), n (%) | 140 (74.5%) | 142 (76.3%) | 0.9 (0.6, 1.4) | 0.65 |
| Serious AE, n (%) | 31 (16.5%) | 31 (16.7%) | 0.9 (0.5, 1.6) | 0.83 |
| Expected AE, n (%) | 130 (69.1%) | 130 (69.9%) | 0.9 (0.6, 1.5) | 0.80 |
| Perioperative Adverse Events (Surgery through 4–6 week postoperatively) – n (%) | ||||
| Participants with: | ||||
| Bladder injury | 22 (11.7%) | 18 (9.7%) | 1.2 (0.6, 2.4) | 0.60 |
| During mid-urethral sling | 18 (9.6%) | 18 (9.7%) | 1.0 (0.5, 2.0) | 1.00 |
| Other | 4 (2.1%) | 0 (0.0%) | N/A - LR | |
| Intraoperative ureteral obstruction | 6 (3.2%) | 0 (0.0%) | N/A - LR | |
| Treatment: | ||||
| Suture removed intra-operatively | 5 (2.7%) | 0 (0.0%) | N/A - LR | |
| Stent placement | 1 (0.5%) | 0 (0.0%) | N/A - LR | |
| Additional Procedure | 0 (0.0%) | 0 (0.0%) | N/A - LR | |
| Ureteral injury – delayed recognition* | 1 (0.5%) | 0 (0.0%) | N/A - LR | |
| Urethral injury | 0 (0.0%) | 0 (0.0%) | N/A - LR | |
| Rectal injury | 0 (0.0%) | 1 (0.5%) | N/A - LR | |
| Major vascular injury | 0 (0.0%) | 0 (0.0%) | N/A - LR | |
| Blood transfusion | 7 (3.7%) | 4 (2.2%) | 1.9 (0.5, 7.8) | 0.38 |
| Neurologic pain requiring treatment** | 13 (6.9%) | 23 (12.4%) | 0.5 (0.2, 1.0) | 0.049 |
| Treatment: | ||||
| Narcotic pain medication | 10 (5.3%) | 18 (9.7%) | ||
| Nerve block | 0 (0.0%) | 2 (1.1%) | ||
| Physical therapy | 2 (1.1%) | 3 (1.6%) | ||
| Other medication | 8 (4.3%) | 13 (7.0%) | ||
| Surgical (return to operating for suture removal) | 0 (0.0%) | 3 (1.6%) | ||
| Long-term complications n (%) | ||||
| Participants with: | ||||
| Vaginal granulation tissue at 6 to 24 months*** | 36 (19.1%) | 26 (14.0%) | 1.5 (0.8, 2.6) | 0.18 |
| Mesh erosion/exposure at 4 weeks to 24 months*** | 3 (1.6%) | 1 (0.5%) | N/A - LR | |
| Suture exposure at 6 to 24 months*** | 29 (15.4%) | 32 (17.2%) | 0.9 (0.5, 1.5) | 0.60 |
| Severity (Dindo Scores) for Expected Adverse Events n (%) | ||||
| Participants with Expected AE severity (most severe per participant), n (%) | ||||
| No such events | 30 (16.0%) | 22 (11.8%) | N/A | 0.58 |
| I | 29 (15.4%) | 38 (20.4%) | ||
| II | 74 (39.4%) | 74 (39.8%) | ||
| III | 33 (17.6%) | 24 (12.9%) | ||
| IV | 1 (0.5%) | 0 (0.0%) | ||
| V | 0 (0.0%) | 0 (0.0%) | ||
| Summary of Serious Adverse Events | ||||
| Participants with any SAE | 31 (16.5%) | 31 (16.7%) | 0.9 (0.5, 1.6) | 0.83 |
| Number of SAEs | 40 | 44 | ||
| Dindo Classification: | N/A | 0.75 | ||
| No such events | 157 (83.5%) | 155 (83.3%) | ||
| I | 3 (1.6%) | 2 (1.1%) | ||
| II | 11 (5.9%) | 11 (5.9%) | ||
| III | 15 (8.0%) | 17 (9.1%) | ||
| IV | 1 (0.5%) | 1 (0.5%) | ||
| V | 1 (0.5%)# | 0 (0.0%) | ||
| Participants with SAE by relationship to study | ||||
| No such events | 157 (83.5%) | 155 (83.3%) | N/A | 0.74 |
| Not assessable | 2 (1.1%) | 0 (0.0%) | ||
| Unlikely | 24 (12.8%) | 22 (11.8%) | ||
| Likely | 5 (2.7%) | 9 (4.8%) | ||
N/A = Not applicable; OR = Odds Ratio; CI = Confidence Interval.
N/A – LR = The Adjusted OR and P value isn’t shown due to reliability of test.
Not identified during surgical procedure.
Defined a priori as acute-onset pain involving the buttock, groin and/or lower extremity, usually unilateral, occurring on the side or sides where vault suspension stitches have been placed and within one week of the index surgery requiring an alteration of routine postoperative care (e.g., nerve block, physical therapy, return to OR for suture removal, addition of medications used to treat neuropathic pain such as anticonvulsants or tricyclic anti-depressants, or the increase or persistence of narcotic pain medication use beyond 14 days after surgery).
Not mutually exclusive. Location and need for or type of treatment not collected. Mesh erosion excludes from the denominator patients who did not receive TVT at surgery.
Patient death not attributable to study surgery.
| Grade | Definition | |
|---|---|---|
| I | Any deviation from the normal intraoperative or postoperative course without the need for pharmacological treatment or surgical, endoscopic, and radiological interventions | |
| Allowed therapeutic regimens are: drugs as antiemetics, antipyretics, analgesics, diuretics, electrolytes, and physiotherapy. This grade also includes wound infections opened at the bedside | ||
| II | Requiring pharmacological treatment with drugs other than such allowed for grade I complications. | |
| IIa | Oral administration of drugs other than such allowed for grade I, including antibiotics for wound or bladder infections | |
| IIb | IV administration of drugs other than such allowed for grade I, including antibiotics; blood transfusions and total parenteral nutrition are also included | |
| III | Requiring surgical, endoscopic or radiological intervention | |
| IIIo | Additional surgical measures required during OPTIMAL procedure | |
| IIIa | Intervention not under general anesthesia | |
| IIIb | Intervention under general anesthesia | |
| IV | Life-threatening complication (including CNS complications)* requiring IC/ICU management | |
| IVa | Single organ dysfunction (including dialysis) | |
| IVb | Multiorgan dysfunction | |
| V | Death of a patient | |
| Suffix “d” | If the patient suffers from a complication at the time of discharge, the suffix “d” (for “disability”) is added to the respective grade of complication. This label indicates the need for a follow-up to fully evaluate the complication. |
Behavioral Intervention Outcomes
There were no significant differences between BPMT and usual perioperative care in the 6-month and 24-month patient-reported primary outcomes (Table 4). After imputation by Brown’s method, the median UDI score at 6 months was 12.7 in both the BPMT and usual care groups, and a test for differences in distributions between groups was not significant (p=0.61). Using non-imputed data, the adjusted mean change from baseline UDI total score at 6 months was −94.6 in the BPMT group versus −87.9 in the usual care group (95% CI for difference −19.7 to 6.2; p=0.31); these findings remained stable through 24 months. Results based on the multiply imputed change from baseline UDI at 6 months were similar [−94.5 in BPMT versus −87.0 in usual care, 95% CI for difference (−19.7, 4.9), p=0.24]. Compared to baseline, the decrease in POPDI score at 24 months was not significantly different between groups [−73.3 in BPMT versus −65.2 in usual care, 95% CI for difference (−22.1, 6.1), p=0.26]. Results from the multiply imputed POPDI scores were consistent [−74.6 in BPMT versus −65.5 in usual care, 95% CI for difference (−24.6, 6.6), p=0.25]. There were no significant group differences at other time points for PFDI subscales (Table 4). To examine potential effect modification or confounding of outcomes by baseline pelvic floor muscle strength, these analyses were repeated including terms for baseline Brink score and Brink score by BPMT treatment assignment interaction with no substantive change in results.
Table 4.
Pelvic Floor Distress Inventory Results
| Variable | Time Frame |
Surgical Treatment Comparison | BPMT Treatment Comparison | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ULS N=188 |
SSLF N=186 |
Adjusted Mean Treatment Difference (95% CI) |
P value |
BPMT N=186 |
Usual Care N=188 |
Adjusted Mean Treatment Difference (95% CI) |
P value |
||
| Urinary Distress Inventory (UDI) Score N Adjusted mean (SE) [Min, Max] |
Baseline | 175 | 179 | 178 | 176 | ||||
| 123.0 (63.2) | 130.0 (57.4) | 128.1 (60.4) | 124.9 (60.4) | ||||||
| [9.6, 292.3] | [28.2, 280.0] | [15.2, 280.0] | [9.6, 292.3] | ||||||
| Baseline to 6 Mo Change | 163 | 165 | 3.6 (−9.6, 16.7) | 0.60 | 163 | 165 | −6.7 (−19.7, 6.2) | 0.31 | |
| −96.2 (5.9) | −99.7 (5.8) | −94.6 (4.9) | −87.9 (4.9) | ||||||
| [−276.5, 49.0] | [−256.0, 95.1] | [−276.5, 49.0] | [−264.0, 95.1] | ||||||
| Baseline to 12 Mo Change | 153 | 159 | −0.3 (−13.5, 12.9) | 0.96 | 156 | 156 | 0.1 (−12.9, 13.2) | 0.98 | |
| −98.6 (5.9) | −98.3 (5.8) | −91.7 (5.0) | −91.8 (4.9) | ||||||
| [−276.5, 65.6] | [−244.0, 73.1] | [−276.5, 65.6] | [−259.2, 73.1] | ||||||
| Baseline to 24 Mo Change | 144 | 148 | 1.8 (−11.5, 15.0) | 0.79 | 146 | 146 | −1.3 (−14.4, 11.8) | 0.84 | |
| −86.6 (5.9) | −88.4 (5.8) | −81.4 (5.0) | −80.1 (5.0) | ||||||
| [−274.6, 107.5] | [−261.5, 77.6] | [−274.6, 107.5] | [−242.4, 77.6] | ||||||
| Pelvic Organ Prolapse Distress Inventory (POPDI) Score N Adjusted mean (SE) [Min, Max] |
Baseline | 175 | 179 | 178 | 176 | ||||
| 120.2 (70.4) | 127.4 (66.8) | 126.0 (67.8) | 121.6 (69.5) | ||||||
| [0.0, 300.0] | [0.0, 296.4] | [0.0, 300.0] | [7.1, 292.3] | ||||||
| Baseline to 6 Mo Change | 163 | 165 | 6.5 (−7.5, 20.4) | 0.36 | 163 | 165 | −13.6 (−27.4, 0.2) | 0.054 | |
| −85.3 (6.2) | −91.7 (6.0) | −86.8 (5.3) | −73.2 (5.2) | ||||||
| [−267.3, 121.4] | [−278.0, 54.2] | [−267.3, 98.2] | [−278.0, 121.4] | ||||||
| Baseline to 12 Mo Change | 153 | 159 | 5.3 (−8.8, 19.4) | 0.46 | 156 | 156 | −3.7 (−17.6, 10.3) | 0.61 | |
| −87.7 (6.2) | −93.0 (6.1) | −83.7 (5.3) | −80.0 (5.3) | ||||||
| [−241.7, 130.4] | [−273.2, 42.9] | [−263.7, 130.4] | [−273.2, 33.9] | ||||||
| Baseline to 24 Mo Change | 144 | 148 | 1.5 (−12.7, 15.7) | 0.84 | 146 | 146 | −8.0 (−22.1, 6.1) | 0.26 | |
| −77.1 (6.2) | −78.6 (6.1) | −73.3 (5.4) | −65.2 (5.3) | ||||||
| [−252.4, 131.5] | [−288.7, 131.5] | [−252.4, 131.5] | [−288.7, 131.5] | ||||||
| Colorectal-anal Distress Inventory (CRADI) Score N Adjusted mean (SE) [Min, Max] |
Baseline | 175 | 179 | 178 | 176 | ||||
| 107.7 (85.6) | 113.4 (82.2) | 111.8 (85.5) | 109.3 (82.3) | ||||||
| [0.0, 380.4] | [0.0, 357.1] | [0.0, 357.1] | [0.0, 380.4] | ||||||
| Baseline to 6 Mo Change | 163 | 165 | 6.7 (−9.2, 22.7) | 0.41 | 163 | 165 | −7.7 (−23.6, 8.2) | 0.34 | |
| −67.7 (6.5) | −74.4 (6.4) | −67.9 (6.1) | −60.2 (6.0) | ||||||
| [−283.6, 84.5] | [−295.7, 89.5] | [−276.4, 64.3] | [−295.7, 89.5] | ||||||
| Baseline to 12 Mo Change | 153 | 159 | −2.9 (−19.0, 13.2) | 0.73 | 156 | 156 | −5.1 (−21.2, 10.9) | 0.53 | |
| −73.0 (6.6) | −70.1 (6.4) | −66.9 (6.1) | −61.7 (6.0) | ||||||
| [−363.3, 126.2] | [−295.7, 115.1] | [−269.3, 126.2] | [−363.3, 115.1] | ||||||
| Baseline to 24 Mo Change | 144 | 148 | −10.9 (−27.2, 5.3) | 0.19 | 146 | 146 | −6.3 (−22.5, 10.0) | 0.45 | |
| −62.1 (6.6) | −51.1 (6.5) | −52.5 (6.2) | −46.2 (6.1) | ||||||
| [−363.3, 96.7] | [−283.6, 206.2] | [−280.0, 151.5] | [−363.3, 206.2] | ||||||
CI=Confidence Interval; SE=Standard Error of Adjusted Mean.
NOTE: Patient reported outcome scale ranges: Pelvic Floor Distress Inventory (PFDI) scales, Urinary Distress Inventory (UDI) (0–300), Pelvic Organ Prolapse Distress Inventory (POPDI) (0–300), and Colorectal-anal Distress Inventory (CRADI) (0–400) with higher scores indicting greater symptom bother.
There was a significant interaction (effect modification) between the behavioral and surgical intervention groups for the primary anatomic failure outcome at 24 months; however, failure was not significantly different between behavioral groups within each surgical treatment [ULS group: BPMT 26/78 (33.3%) versus usual care 22/86 (25.6%), OR 1.8, 95% CI (0.9, 3.8); SSLF group: BPMT 19/77 (24.7%) versus usual care 27/80 (33.8%), OR 0.6, 95% CI (0.3, 1.2)] (Table 2 a & b). Results based on the multiply-imputed anatomic failure outcome were consistent [ULS group: BPMT 32/91 (34.9%) versus usual care 24/97 (24.5%), OR 1.9, 95% CI (0.9, 3.9); SSLF group: BPMT 23/95 (24.2%) versus usual care 29/91 (32.1%), OR 0.6, 95% CI (0.3, 1.2)]. We did not detect group differences in the proportion of women with an anatomic failure due to anterior or posterior prolapse, or retreatment (Table 2 & eTables 5, 6b, 6c). The anterior vagina was the most likely vaginal compartment to prolapse beyond the hymen; proportions were not significantly different between groups [BPMT 14.1% versus usual care 15.1%, OR 0.9, 95% CI 0.5, 1.8] (Table 2). As described previously, there was a significant interaction between the BPMT and surgical groups such that women in the ULS group randomized to BPMT were more likely to have apical descent more than one-third of total vaginal length (Table 2). Apical descent in women in women randomized to SSLF was not different between behavioral groups.
No differences were noted between BPMT and usual care groups for secondary patient reported outcome measures including retreatment for incontinence [OR 1.4; 95% CI (0.8, 2.3)] or prolapse [OR 2.5; 95% CI (0.8, 7.6)] (eTables 5 and 7). Baseline pelvic floor muscle strength was moderately strong, with a mean score of 8 on the Brink score (Brink range 3–12). No group differences in change in pelvic floor strength were noted from baseline at 6 or 24 months. At 24 months, mean Brink scores were 8.2 and 8.0 in BPMT and usual care groups respectively (p=0.27). Self-reported performance of pelvic muscle exercises in the BPMT group was 93.4% at 6 months and 81.4% at 24 months. In the usual care group, 8 of 186 women (4.3%) received supervised BPMT outside of the study by 24 months.
DISCUSSION
In women with apical vaginal prolapse (uterine or post-hysterectomy vault) and stress urinary incontinence, the OPTIMAL trial found that neither of two common apical transvaginal prolapse repair procedures, ULS and SSLF, was superior to the other. In addition, a multi-component, perioperative BPMT program did not improve urinary or prolapse outcomes and is likely unnecessary as a routine aspect of perioperative care.
Our success rates for the surgical intervention, defined by a rigorous composite definition for treatment success that included anatomic results, patient reported symptoms, and retreatment, were lower than the 70–90% success rates generally reported in the literature for these procedures. This is consistent with other multi-center surgical trials where treatment success rates were typically lower when defined by composite outcomes.28–30 However, retreatment rates remained low at 5%. Additionally, the use of masked POPQ examiners31 and strict anatomic criteria for apical descent likely contributed to these lower success rates. In the absence of high quality clinical trials, surgeons have relied primarily on case series reporting rates of anterior vaginal wall prolapse after SSLF as high as 40%,32,33 and have attributed these high rates to posterior deviation of the vaginal apex. Our findings showed that the proportion of women with recurrent anterior (ULS 15.5% vs. SSLF 13.7%) or posterior prolapse (ULS 4.5% vs. SSLF 7.2%) beyond the hymen were not significantly different between treatment groups, highlighting the importance of these Level 1 data. One unexpected finding was that women randomized to ULS had greater apical descent if they received perioperative BPMT rather than usual care; this was not seen in those randomized to SSLF. It is unclear why BPMT would have this differential effect but one possible explanation is the difference in orientation of the vagina after the two suspension procedures.
The low rates of serious adverse events seen in both groups are consistent with the clinical experience of the safety of native tissue vaginal reconstructive surgery. Fewer than one in five women experienced a serious adverse event over the two-year follow-up, with <5% directly related to the index surgery. As expected based on anatomical differences in surgical approach, we observed more ureteral obstructions occurring in ULS (3.7%) than SSLF (0%). These rates are within previously reported ureteral injury rates, which range from 1 to 11% following ULS.34 Notably, all intra-operatively identified ureteral obstructions were adequately treated during the index surgery by removing the obstructing sutures or stent placement. This study confirms the findings of previous case series suggesting that SSLF is more likely to result in acute neurologic pain, particularly buttock pain thought due to gluteal nerve entrapment.32,35 While the majority had resolution of the pain by 4–6 weeks postoperatively, persistent pain occurred in 4.3% after SSLF highlighting the need to counsel patients about this risk preoperatively.
The findings of our perioperative BPMT intervention are consistent with a pilot study (n=51) by Frawley and colleagues, who found no significant effects for perioperative physiotherapist-supervised pelvic floor muscle training for women undergoing vaginal surgery for prolapse or hysterectomy.36 Although their intervention included more sessions (8) over a longer period of time (12 months), their study also did not detect significant group differences 12 months post-surgery on urinary questionnaires, bladder diary or pad test.
In contrast, Jarvis and colleagues reported that perioperative physiotherapy improved outcomes 3 months after surgery for prolapse and/or stress incontinence with significant group differences in urinary symptoms, quality of life, pelvic floor muscle strength, and voiding frequency. Despite the similarities of the OPTIMAL trial to that trial, several relevant differences include a smaller sample (n=60) and different outcomes at an earlier time point (3 months).37 The training of BPMT interventionists also differed. Similar to Frawley, the Jarvis study intervention was implemented by established interventional physiotherapists, whereas our trial provided centralized training for clinicians with varying degrees of BPMT experience. Nonetheless, only those certified based on rigorous in-person testing were allowed to participate as BPMT interventionists in the OPTIMAL trial.17 Additionally, the broad range of experience of the OPTIMAL interventionists increases the generalizability our findings.
The outcomes in this study should not be extrapolated to women who do not match our eligibility criteria, including women who do not undergo concomitant mid-urethral sling for treatment of stress incontinence. Our participants underwent both prolapse and stress incontinence procedures with high efficacy rates, which may have overshadowed any additional improvement provided by a perioperative BPMT intervention. These findings should also not be extrapolated to women undergoing transvaginal mesh or abdominal mesh augmented prolapse repairs.
Our conclusions benefit from a robust study design, standardized anatomic and functional outcomes with validated patient reported outcomes, and patients and outcome assessors masked to the surgical intervention assignment. Our findings are further strengthened by the multi-center, multi-surgeon, randomized design, with standardization of surgical techniques and the high rate of participant retention. In addition, perioperative BPMT program intervention was individualized using a standard protocol and consisted of multiple components, including strategies for stress and urgency incontinence, recommendations for normal voiding and defecation techniques, and ongoing reinforcement of functional bracing (pelvic floor contraction during lifting and physical activity) thought to protect the surgical repair and the pelvic floor long-term.
This study provides evidence for patients and their surgeons about the benefits, risks and complications of two widely used native tissue vaginal approaches for apical prolapse, as well as the role of perioperative BPMT. While our results do not support routinely offering perioperative BPMT to women undergoing vaginal surgery for prolapse and stress urinary incontinence, previous evidence supports offering individualized treatment, including behavioral or physical therapy, to those who report new or unresolved pelvic floor symptoms. Our surgical outcomes inform preoperative discussions that include a patient’s preferences for anatomic and subjective outcomes as well as consideration of likely and possible adverse events. While variability in surgical recommendations for vaginal prolapse repair are likely to persist due to individualized patient characteristics, our data provide a metric against which other vaginal procedures, including those which use synthetic or biologic mesh, can be assessed.
Conclusions
In women undergoing vaginal surgery for pelvic organ prolapse and stress urinary incontinence, neither ULS nor SSLF was significantly superior to the other for anatomic, functional, or adverse event outcomes two years after surgery. Perioperative BPMT in these women did not improve urinary symptoms at 6 months or prolapse outcomes two years after surgery.
Supplementary Material
ACKNOWLEDGEMENTS
Funding/Support: This research as supported by grants U01 HD041249, U10 HD041250, U10 HD041261, U10 HD041267, U10 HD054136, U10 HD054214, U10 HD054215, U01 HD069031, and U10 HD054241 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the NIH Office of Research on Women’s Health.
Pelvic Floor Disorders Network (PFDN) Members
In addition to the authors, the following members of the Pelvic Floor Disorders Network participated in the Operations and Pelvic Muscle Training in the Management of Apical Support Loss (OPTIMAL) trial:
RTI International, Research Triangle Park, NC (PFDN Data Coordinating Center, 7/1/2011 to present): Dennis Wallace, Kevin A. Wilson, Daryl Matthews, Tamara L. Terry, Jutta Thornberry, Amanda Youmans-Weisbuch, Ryan E. Whitworth, Michael P. Hieronymus
University of Michigan, Ann Arbor, MI (PFDN Data Coordinating Center, until 7/1/2011): Morton Brown, Nancy Janz, John Wei, Xiao Xu, Beverley Marchant, Donna DiFranco, Yang Casher, Kristina Slusser, Zhen Chen
Cleveland Clinic, Cleveland OH: Mark D. Walters, J. Eric Jelovsek, Marie F.R. Paraiso, Beri M. Ridgeway, Ly Pung, Cheryl Williams, Linda McElrath, Betsy O’Dougherty, Megan Edgehouse, Gouri Diwadkar, Anna Frick
Loyola University Chicago, Chicago IL: Mary Tulke, Elizabeth Mueller, Kimberly Kenton, Kathleen Jesse
University of California-San Diego Health Systems, San Diego CA: Charles. W. Nager, Michael E. Albo, Cara Grimes, Heidi W. Brown, Anna C. Kirby, Leah Merrin, JoAnn Columbo, Nehal Mehta
Southern California Kaiser Permanente, Downey CA: Mercedes Cardona, Eudocia Zapata
Southern California Kaiser Permanente, San Diego CA: Emily L. Whitcomb, Keisha Y. Dyer, Karl M. Luber, Jasmine Tan-Kim, Gouri B. Diwadkar, Lynn M. Hall, Linda M. Mackinnon, Gisselle Zazueta-Damian
University of Utah, Salt Lake City UT: Yvonne Hsu, Jan Baker, Linda Freedman, Linda Griffin, Maria Masters, Amy Orr, Kristina Heintz
University of Alabama at Birmingham, Birmingham AL: R. Edward Varner, Robert Holley, William J. Greer, L. Keith Lloyd, Tracy S. Wilson, Alayne Markland, Jonathan L. Gleason, Alicia Ballard, Candace Parker-Autry, Lisa Pair, Velria Willis, Nancy Saxon, Lachele Ward, Kathy Carter, Julie Burge
Duke University Medical Center, Durham NC: Anthony G. Visco, Cindy L. Amundsen, Nazema Y. Siddiqui, Jennifer M. Wu, Mary Raynor, Mary McGuire, Ingrid Harm-Ernandes
University of Texas-Southwestern, Dallas TX: – David Rahn, Marlene Corton, Clifford Wai, Kelly Moore, Shanna Atnip, Pam Martinez, Deborah Lawson
Formerly NICHD: Anne Weber
PFDN Steering Committee Chair: Katherine Hartmann
Footnotes
Role of the Sponsor: The Eunice Kennedy Shriver National Institute of Child Health and Human Development, Project Scientist for the PFDN, Anne Weber, MD played a role in the design. Dr. Meikle became the project scientist just as the study was initiated, and played a role in conduct of the study; the collection, analysis, and interpretation of the data; and in the preparation, review, and approval of the manuscript.
Data Access, Responsibility and Analysis: Dr. M. Gantz, and Ms. L. Warren had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author Contributions:
Study concept and design: Barber, Brubaker, Burgio, Richter, Nygaard, Menefee, Weidner, Norton, Schaffer, Borello-France, Goode, Spino, Meikle
Acquisition of data: Barber, Brubaker, Richter, Nygaard, Menefee, Weidner, Norton, Lukacz, Schaffer, Nguyen, Jakus-Waldman
Analysis and interpretation of data: Barber, Burgio, Brubaker, Richter, Nygaard, Menefee, Weidner, Lukacz, Norton, Schaffer, Nguyen, Goode, Borello-France, Spino, Warren, Gantz, Meikle
Drafting of manuscript: Barber, Brubaker, Richter, Nygaard, Burgio, Borello-France, Menefee, Weidner, Norton, Lukacz, Goode, Schaffer, Nguyen, Jakus-Waldman, Spino, Warren, Gantz
Critical revision of the manuscript for important intellectual content: Barber, Brubaker, Richter, Nygaard, Burgio, Borello-France, Menefee, Weidner, Norton, Lukacz, Goode, Schaffer, Nguyen, Jakus-Waldman, Spino, Warren, Gantz
Statistical analysis: Warren, Gantz, Spino, Barber
Obtaining funding: Barber, Brubaker, Richter, Burgio, Nygaard, Schaffer, Spino, Meikle
Administrative, technical or material support: Brubaker, Weidner, Richter, Spino, Warren
Study supervision: Barber, Spino, Gantz, Meikle, Menefee
Conflict of Interest Disclosures – The authors report the following potential conflicts of interest and financial disclosures consistent with the ICMJE Form for Disclosure of Potential Conflicts of Interest and JAMA policy:
Barber: Research Grant: Foundation for Female Health Awareness; Royalty: UptoDate, Elsevier
Burgio: Research Grant: Pfizer; Consultant: Pfizer, Astellas
Brubaker: Royalty: UptoDate
Lukacz: Research Grant: Renew Medical; Educational Grant: Ethicon/Johnson & Johnson; Consultant: Pfizer, Renew Medical, American Medical Systems; Payment for development of educational content: Sharp Chula Vista
Goode: Research Grant: Pfizer; Consultant: Astellas
Richter: Research Grant: Astellas, Univ. of CA/Pfizer, Pfizer; Consultant: Astellas Advisory Board, GlaxoSmithKline, Uromedica, IDEO, Xanodyne; Education Grant: Warner Chilcott
Schaffer: Research Support: Boston Scientific; Consultant: Ferring Pharmaceuticals; Advisory Board: Astellas, Cadence Pharmaceuticals; Speakers Bureau: Astellas, Cadence Pharmaceuticals; Royalty: McGraw-Hill.
The following authors report no such conflicts: Nygaard, Menefee, Norton, Weidner, Nguyen, Jakus-Waldman, Borello-France, Spino, Gantz, Warren, Wallace, Meikle
Contributor Information
Matthew D. Barber, Gynecology and Women’s Health Institute, Cleveland Clinic, Cleveland, OH.
Linda Brubaker, Departments of Obstetrics & Gynecology and Urology, Loyola University Chicago Stritch School of Medicine, Chicago, IL.
Kathryn L. Burgio, Division of Gerontology, Geriatrics, and Palliative Care, University of Alabama at Birmingham and Department of Veterans Affairs, Birmingham, AL.
Holly E. Richter, Department of Obstetrics and Gynecology, University of Alabama at Birmingham, Birmingham, AL.
Ingrid Nygaard, Department of Obstetrics and Gynecology, University of Utah Medical Center, Salt Lake City UT.
Alison C. Weidner, Department of Obstetrics and Gynecology, Duke University Medical Center, Durham, NC.
Shawn A. Menefee, Department of Obstetrics and Gynecology, Southern California Kaiser Permanente, San Diego CA
Emily S. Lukacz, Department of Reproductive Medicine, University of California-San Diego Health Systems, San Diego, CA.
Peggy Norton, Department of Obstetrics and Gynecology, University of Utah Medical Center, Salt Lake City, UT.
Joseph Schaffer, Department of Obstetrics and Gynecology, University of Texas-Southwestern, Dallas, TX.
John N. Nguyen, Department of Obstetrics and Gynecology, Southern California Kaiser Permanente, Downey, CA.
Diane Borello-France, Department of Physical Therapy, Duquesne University, Pittsburgh PA.
Patricia S. Goode, Division of Gerontology, Geriatrics, and Palliative Care, University of Alabama at Birmingham and Department of Veterans Affairs, Birmingham, AL.
Sharon Jakus-Waldman, Department of Obstetrics and Gynecology, Southern California Kaiser Permanente, Downey CA.
Cathie Spino, Department of Biostatistics, University of Michigan, Ann Arbor, MI.
Lauren Klein Warren, Social, Statistical & Environmental Sciences, RTI International, Research Triangle Park, NC.
Marie G. Gantz, Social, Statistical & Environmental Sciences, RTI International, Research Triangle Park, NC.
Susan F. Meikle, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD.
REFERENCES
- 1.FDA. Urogynecologic Surgical Mesh: Update on the Safety and Effectiveness of Transvaginal Mesh Placement for Pelvic Organ Prolapse: US Food and Drug Administration. 2011 http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM262760.pdf.
- 2.Brown JS, Waetjen LE, Subak LL, Thom DH, Van den Eeden S, Vittinghoff E. Pelvic organ prolapse surgery in the United States, 1997. Am J Obstet Gynecol. 2002;186(4):712–716. doi: 10.1067/mob.2002.121897. [DOI] [PubMed] [Google Scholar]
- 3.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 4.Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States, 1979–1997. Am J Obstet Gynecol. 2003;188(1):108–115. doi: 10.1067/mob.2003.101. [DOI] [PubMed] [Google Scholar]
- 5.Shull BL. Pelvic organ prolapse: anterior, superior, and posterior vaginal segment defects. Am J Obstet Gynecol. 1999;181(1):6–11. doi: 10.1016/s0002-9378(99)70427-8. [DOI] [PubMed] [Google Scholar]
- 6.Toozs-Hobson P, Boos K, Cardozo L. Management of vaginal vault prolapse. Br J Obstet Gynaecol. 1998;105(1):13–17. doi: 10.1111/j.1471-0528.1998.tb09343.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Ashton-Miller JA, Hsu Y, DeLancey JO. Interaction among apical support, levator ani impairment, and anterior vaginal wall prolapse. Obstet Gynecol. 2006;108(2):324–332. doi: 10.1097/01.AOG.0000227786.69257.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maher C, Feiner B, Baessler K, Schmid C. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;4:CD004014. doi: 10.1002/14651858.CD004014.pub5. [DOI] [PubMed] [Google Scholar]
- 9.Ellerkmann RM, Cundiff GW, Melick CF, Nihira MA, Leffler K, Bent AE. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185(6):1332–1337. doi: 10.1067/mob.2001.119078. [DOI] [PubMed] [Google Scholar]
- 10.Berghmans LC, Hendriks HJ, Bo K, Hay-Smith EJ, de Bie RA, van Waalwijk van Doorn ES. Conservative treatment of stress urinary incontinence in women: a systematic review of randomized clinical trials. Br J Urol. 1998;82(2):181–191. doi: 10.1046/j.1464-410x.1998.00730.x. [DOI] [PubMed] [Google Scholar]
- 11.Berghmans LC, Hendriks HJ, De Bie RA, van Waalwijk van Doorn ES, Bo K, van Kerrebroeck PE. Conservative treatment of urge urinary incontinence in women: a systematic review of randomized clinical trials. BJU Int. 2000;85(3):254–263. doi: 10.1046/j.1464-410x.2000.00434.x. [DOI] [PubMed] [Google Scholar]
- 12.Burgio KL, Locher JL, Goode PS, et al. Behavioral vs drug treatment for urge urinary incontinence in older women: a randomized controlled trial. JAMA. 1998;280(23):1995–2000. doi: 10.1001/jama.280.23.1995. [DOI] [PubMed] [Google Scholar]
- 13.Dumoulin C, Hay-Smith J. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2010;(1):CD005654. doi: 10.1002/14651858.CD005654.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Hagen S, Stark D. Conservative prevention and management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2011;(12):CD003882. doi: 10.1002/14651858.CD003882.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vakili B, Zheng YT, Loesch H, Echols KT, Franco N, Chesson RR. Levator contraction strength and genital hiatus as risk factors for recurrent pelvic organ prolapse. Am J Obstet Gynecol. 2005;192(5):1592–1598. doi: 10.1016/j.ajog.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Lakeman MM, Koops SE, Berghmans BC, Roovers JP. Peri-operative physiotherapy to prevent recurrent symptoms and treatment following prolapse surgery: supported by evidence or not? Int Urogynecol J. 2013;24(3):371–375. doi: 10.1007/s00192-012-1973-y. [DOI] [PubMed] [Google Scholar]
- 17.Barber MD, Brubaker L, Menefee S, et al. Operations and pelvic muscle training in the management of apical support loss (OPTIMAL) trial: design and methods. Contemp Clin Trials. 2009;30(2):178–189. doi: 10.1016/j.cct.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175(1):10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 19.Morley GW, DeLancey JO. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol. 1988;158(4):872–881. doi: 10.1016/0002-9378(88)90088-9. [DOI] [PubMed] [Google Scholar]
- 20.DeLancey JO, Morley GW, Howard D. Sacrospinous suspension: Michigan 4-wall offers better support. OBG Management. 2001 Mar;:18–29. [Google Scholar]
- 21.Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365–1373. doi: 10.1067/mob.2000.110910. [DOI] [PubMed] [Google Scholar]
- 22.Barber MD, Kuchibhatla MN, Pieper CF, Bump RC. Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. Am J Obstet Gynecol. 2001;185(6):1388–1395. doi: 10.1067/mob.2001.118659. [DOI] [PubMed] [Google Scholar]
- 23.Sandvik H, Hunskaar S, Seim A, Hermstad R, Vanvik A, Bratt H. Validation of a severity index in female urinary incontinence and its implementation in an epidemiological survey. J Epidemiol Community Health. 1993;47(6):497–499. doi: 10.1136/jech.47.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barber MD, Spino C, Janz NK, et al. The minimum important differences for the urinary scales of the Pelvic Floor Distress Inventory and Pelvic Floor Impact Questionnaire. Am J Obstet Gynecol. 2009;200(5):580, e581–e587. doi: 10.1016/j.ajog.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.FitzGerald MP, Burgio KL, Borello-France DF, et al. Pelvic-floor strength in women with incontinence as assessed by the brink scale. Physical therapy. 2007;87(10):1316–1324. doi: 10.2522/ptj.20060073. [DOI] [PubMed] [Google Scholar]
- 27.Brown MB. A test for the difference between two treatments in a continuous measure of outcome when there are dropouts. Control Clin Trials. 1992;13(3):213–225. doi: 10.1016/0197-2456(92)90004-j. [DOI] [PubMed] [Google Scholar]
- 28.Richter HE, Albo ME, Zyczynski HM, et al. Retropubic versus transobturator midurethral slings for stress incontinence. New Engl J Med. 2010;362(22):2066–2076. doi: 10.1056/NEJMoa0912658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albo ME, Richter HE, Brubaker L, et al. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. New Engl J Med. 2007;356(21):2143–2155. doi: 10.1056/NEJMoa070416. [DOI] [PubMed] [Google Scholar]
- 30.Nygaard I, Brubaker L, Zyczynski HM, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA. 2013;309(19):2016–2024. doi: 10.1001/jama.2013.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antosh DD, Iglesia CB, Vora S, Sokol AI. Outcome assessment with blinded versus unblinded POP-Q exams. Am J Obstet Gynecol. 2011;205(5):489, e481–e484. doi: 10.1016/j.ajog.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Sze EH, Karram MM. Transvaginal repair of vault prolapse: a review. Obstet Gynecol. 1997;89(3):466–475. doi: 10.1016/S0029-7844(96)00337-7. [DOI] [PubMed] [Google Scholar]
- 33.Morgan DM, Rogers MA, Huebner M, Wei JT, Delancey JO. Heterogeneity in anatomic outcome of sacrospinous ligament fixation for prolapse: a systematic review. Obstet Gynecol. 2007;109(6):1424–1433. doi: 10.1097/01.AOG.0000264066.89094.21. [DOI] [PubMed] [Google Scholar]
- 34.Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183(6):1402–1410. doi: 10.1067/mob.2000.111298. [DOI] [PubMed] [Google Scholar]
- 35.Roshanravan SM, Wieslander CK, Schaffer JI, Corton MM. Neurovascular anatomy of the sacrospinous ligament region in female cadavers: Implications in sacrospinous ligament fixation. Am J Obstet Gynecol. 2007;197(6):660, e661–e666. doi: 10.1016/j.ajog.2007.08.061. [DOI] [PubMed] [Google Scholar]
- 36.Frawley HC, Phillips BA, Bo K, Galea MP. Physiotherapy as an adjunct to prolapse surgery: an assessor-blinded randomized controlled trial. Neurourol Urodyn. 2010;29(5):719–725. doi: 10.1002/nau.20828. [DOI] [PubMed] [Google Scholar]
- 37.Jarvis SK, Hallam TK, Lujic S, Abbott JA, Vancaillie TG. Peri-operative physiotherapy improves outcomes for women undergoing incontinence and or prolapse surgery: results of a randomised controlled trial. Aust N Z J Obstet Gynaecol. 2005;45(4):300–303. doi: 10.1111/j.1479-828X.2005.00415.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.