Summary
Acute graft-versus-host disease (aGvHD) is a major limitation to the use of allogeneic stem cell transplantation for the treatment of patients with relapsed malignant disease. Previous work using animals lacking secondary lymphoid tissue (SLT) suggested that activation of donor T cells in SLT is critically important for the pathogenesis of aGvHD. However, these studies did not determine if impaired migration into, and more importantly, out of SLT, would ameliorate aGvHD. Here, we show that T cells from mice lacking Coronin 1A (Coro 1A−/−), an actin associated protein shown to be important for thymocyte egress, do not mediate acute GvHD. The attenuation of aGvHD was associated with decreased expression of the critical trafficking proteins CCR7 and sphingosine 1 phosphate (S1P) receptor on donor T cells. This was mediated in part by impaired activation of the canonical NF-κB pathway in the absence of Coro 1A. As a result of these alterations, donor T cells from Coro 1A−/− mice were not able to initially traffic to SLT or exit SLT after bone marrow transplantation. However, this alteration did not abrogate the GvL response. Our data suggest that blocking T-cell migration into and out of SLT is a valid approach to prevent aGvHD.
Keywords: Coronin, Graft-versus-Host Disease, T Cells, Transplantation
Introduction
Acute graft-versus-host disease (aGvHD), a disease of selective epithelial damage, is a severe complication of allogeneic stem cell transplantation (SCT). aGvHD occurs when mature donor T cells recognize host alloantigen and initiate an immune response [1]. Work from our group and others has shown that prior to tissue destruction, donor T cells must migrate to secondary lymphoid tissue (SLT) where they are activated by host antigen presenting cells (APCs) [2],[3]. Upon activation, donor T cells migrate to target organs, primarily the liver, gastrointestinal tract, skin, and lung, where they cause tissue damage and destruction characteristic of aGvHD [4].
The migration of lymphocytes to target organs involves selectins, integrins, and small chemotactic proteins known as chemokines [5]. Chemokines bind G-protein coupled chemokine receptors, which direct the migration of lymphocytes to target locations. Our group has demonstrated the importance of migration of donor T cells and their interaction with APCs in GvHD pathogenesis [6]. Furthermore the importance of SLT in GvHD pathogenesis has been demonstrated, as animals lacking all SLT, including the spleen, display markedly attenuated GvHD [3],[7]. However, despite multiple attempts, we have not been successful in completely preventing aGvHD by blocking proteins important for T-cell migration.
Numerous biological processes are regulated by the actin cytoskeleton and its associated proteins. The Coronin family of actin-associated proteins has been shown to be involved in cell migration, motility, and cell survival [8]. Coronins bind F-actin and interact with the Arp2/3 complex [9], where they are critical in preventing nucleation of the branched F-actin chain. Coronin 1A (Coro 1A) was the first of the seven family members identified. Coro 1A is expressed primarily in hematopoietic cells and co-localizes with F-actin [10]. Expression of Coro 1A in T lymphocytes is important for cytoskeleton rearrangement [11]–[13]. Several groups have evaluated the function of immune cells from mice lacking Coro 1A. These studies have indicated that T cells from Coro 1A knockout mice do not function normally, although the mechanisms for this finding are still somewhat unclear and focus either on proximal signaling events after activation of the T-cell receptor, and/or the induction of apoptosis due to impaired generation of F-actin [12],[13]. In addition, a third group evaluated the migration of thymocytes using mice with a point mutation in Coro 1A that led to hypomorphic function for Coro 1A. They demonstrated impaired migration of thymocytes from these mice in response to the signaling lipid sphingosine 1 phosphate, leading to impaired thymic egress [14].
Reorganization of the actin cytoskeleton is an early response to chemokine receptor stimulation [15]. Chemokine receptors have been shown to regulate signaling molecules that are important for regulation of chemotaxis in lymphocytes and other cells [15]–[17]. Interestingly, the transcription factor nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) has been shown to be involved in the interaction between activation of T cells and cytoskeleton alterations for mobility [18]. However, the mechanisms by which these processes are linked remain unclear.
Currently, while multiple investigators have indicated that the complete absence of SLT including the spleen eliminated aGvHD, it is not clear if aGvHD would be impacted by the inability of donor T cells to egress from SLT. Previous work suggesting that the absence of Coro 1A led to impaired migration led us to investigate the biology of aGvHD in a system where T cells were impaired in their ability to enter or exit SLT. Here we show that unlike our approaches targeting specific chemokine receptors, aGvHD is completely eliminated by the inability of donor T cells to exit SLT and migrate to aGvHD target organs. These alterations in migration of Coro 1A deficient cells were mediated by decreased expression of the migratory proteins S1Pr1 and CCR7.
Results
Attenuated GvHD in multiple mouse models using Coro 1A−/− T Cells
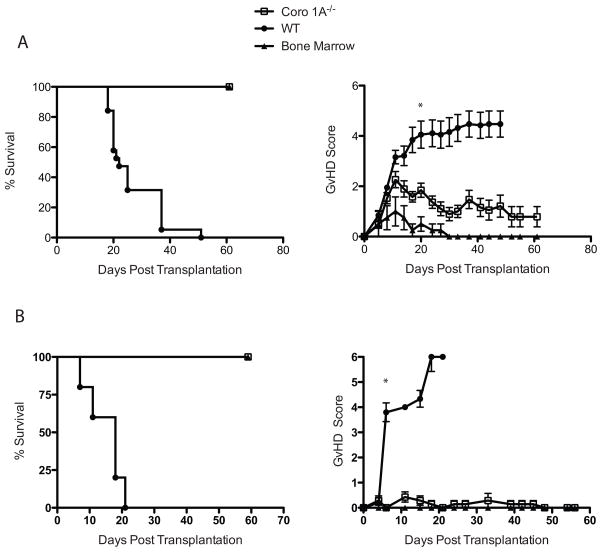
The expression of Coronin 1A (Coro 1A) has been shown to be important for T-cell trafficking [13]; however, the contribution of Coro 1A to disease pathophysiology remains unclear [14]. To address the importance of Coro 1A in aGvHD, conventional T (Tcon) cells cells from Coro 1A−/− or C57BL/6 (WT) donors supplemented with WT T cell depleted bone marrow (TCD BM) cells were transplanted into lethally irradiated B6D2 F1 recipients. As shown in Figure 1A, recipients of Coro 1A−/− Tcon cells had significantly improved survival compared to recipients of WT donor Tcon cells. The clinical GvHD scores confirmed survival data, demonstrating decreased clinical symptoms in recipients of Coro 1A−/− Tcon cells compared with WT Tcon cells recipients (Fig 1A). Recipients of Coro 1A−/− Tcon cells were fully donor by chimerism analysis by day 24 post-transplantion (data not shown).
Figure 1. Attenuated GvHD in the absence of Coro 1A.
(A) 4 × 106 Coro 1A−/− T cells (Tcon cells) or WT Tcon cells supplemented with 3 × 106 WT T cell depleted bone marrow cells (TCD BM) were injected into lethally irradiated B6D2 recipients. Recipients were then monitored for survival (left) and GvHD score (right). Data are shown as mean + SEM of n=14 for Coro 1A−/− and WT Tcon cells recipients, n=4 for bone marrow only. Data are pooled from 3 individual experiments. (B) Lethally irradiated BALB/c recipients were infused with 5 × 105 Coro 1A−/− or WT Tcon cells with 5 × 106 WT TCD BM cells. Following transplantation mice were monitored for survival (left) and clinical GvHD development (right). Data are shown as mean + SEM of n=19 for Coro 1A−/− and WT Tcon cells recipients, n=6 for bone marrow only. Data are pooled from 3 individual experiments. *p<0.001, two tailed Student’s t-test.
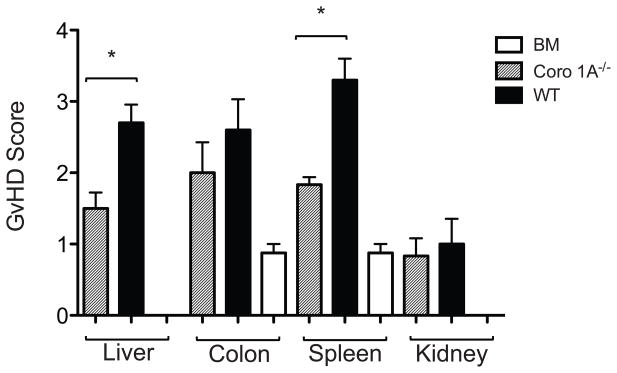
We further evaluated the importance of Coro 1A in aGvHD using a complete mismatch (B6 into BALB/c) transplant model. Previously, we were not able to completely prevent the development of aGvHD targeting chemokine ligands or receptors using this model. Lethally irradiated BALB/c recipients were administered Tcon cells from Coro 1A−/− or WT donors with WT TCD BM. Similar to the haploidentical model, recipients of Coro 1A−/− Tcon cells showed 100% survival until day 60 with minimal clinical manifestations of GvHD (Fig 1B). Histopathology analysis of aGvHD target organs revealed decreased pathology in Coro 1A−/− recipients compared to WT recipients (Fig 2) with significant differences in the liver and spleen and a trend for decreased pathology in the colon. Coro 1A−/− Tcon cells recipients also displayed decreased organ cytokine production on day 14 with the values for IFN-γ production in the liver and TNF and IFN-γ in the spleen being statistically significant (Supporting Information Fig 1). Thus, the absence of Coro 1A from donor T cells led to a profound decrease in the generation of aGvHD, even across a complete MHC mismatch.
Figure 2. Histopathology of B6D2 recipients after transplantation of Coro 1A−/− or WT Tcon cells.
Lethally irradiated B6D2 recipients were transplanted with 4 × 106 Coro 1A−/− or WT Tcon cells with 3 × 106 WT TCD BM. Fourteen days post transplantation organs were harvested for pathology analyses. Data are shown as mean + SEM of n=6 for Coro 1A−/− and WT recipients, n=4 for bone marrow controls, from a single experiment representative of 2 performed. *p<0.05, two tailed Student’s t-test.
Previous work suggested two potential mechanisms for diminished aGvHD in the absence of Coro 1A. T cells deficient in Coro 1A may be impaired in activation mediated by engagement of the T-cell receptor[12], or Coro 1A−/− T cells may be impaired in the ability to migrate in and out of lymphoid tissue[14]. Using cell proliferation dye or Brdu and cell surface markers for activation, we evaluated these potential mechanisms in the post-transplant setting at both day 3 and day 10 post-transplant. On day 3 post-transplant, there was decreased proliferation of Coro 1A−/− Tcon cells in the spleen compared to WT Tcon cells (Supporting Information Fig 2A). However, both CD4+ and CD8+ Coro 1A−/− T cells exhibited increased CD69 expression, the earliest marker of T-cell activation. Other activation markers were more varied, with increased CD62L expression by Coro 1A−/− CD4+ T cells, but decreased CD62L expression of Coro 1A−/− CD8+ T cells. No differences were found in CD25 or ICOS expression of CD4+ Tcon cells lacking Coro 1A. However, there was decreased expression of CD25 and ICOS in Coro 1A−/− CD8+ Tcon cells on day 3 post transplant. On day 10 post-transplant, there was no difference in the proliferation of donor WT versus Coro 1A−/− T cells isolated from the spleens of B6D2 recipients (Supporting Information Fig 2B). To determine if the differences in activation markers were due to intrinsic differences in T cell activation, we evaluated T-cell activation and proliferation in vitro. Proliferation, measured by loss of carboxyfluorescein succinimidyl ester (CFSE) and the generation of IFN-γ by T cells, was equivalent in WT and Coro 1A−/− Tcon cells (data not shown). Thus, intrinsically, there was no difference in the activation or proliferation of WT or Coro 1A−/− T cells.
Coro 1A−/− T Cells accumulate in gastrointestinal tract lymph nodes
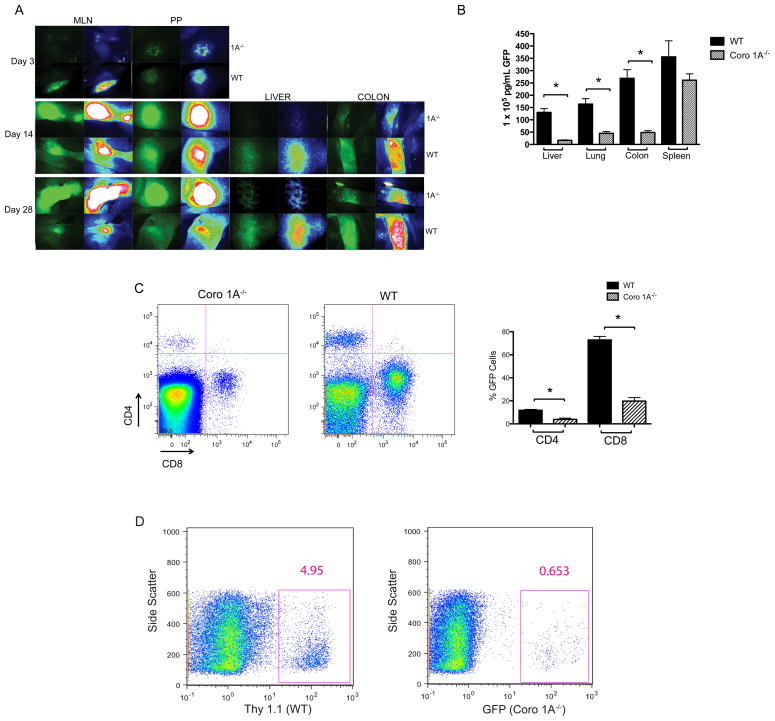
Donor T cell activation requires the migration of donor cells into the spleen and secondary lymphoid tissue of the host. One hypothesis for the inability to activate donor T cells from Coro 1A null donors is the inability of those cells to migrate to SLT. To determine if migration defects contributed to attenuated aGvHD using Coro 1A−/− donor T cells, we crossed Coro 1A−/− mice with mice that constitutively express enhanced green fluorescent protein (GFP). Using Coro 1A−/− GFP and WT GFP mice as donors, lethally irradiated B6D2 F1 recipients were administered Tcon cells with WT (non-GFP) TCD BM. Three days post-transplant, the MLN and PP were imaged by stereomicroscopy. Increased GFP expression in the MLN was seen in WT GFP recipients as compared to Coro 1A−/− GFP recipients, suggesting a delay in entry into lymph nodes by Coro 1A−/− T cells (Fig 3A). Surprisingly, accumulation of Coro 1A−/− T cells was seen in the MLN and PP of B6D2 recipients 14 days post-transplantation, which continued through 28 days post-transplantation (Fig 3A). Consistent with the accumulation seen in the MLN and PP, there was a decrease in donor T cells in the liver and colon of Coro 1A−/− T cells as measured by stereomicroscopy and GFP ELISA (Fig 3A and 3B). Migration defects displayed by microscopy were complemented by blood analysis on day 14 post-transplantation that revealed a decrease in circulating T cells in B6D2 recipients given T cells from Coro 1A−/− GFP donors compared to WT GFP donors (Fig 3C). These data were consistent with impaired entry and egress out of lymph nodes by Coro 1A−/− T cells.
Figure 3. Delayed entry and impaired egress in secondary lymphoid organs by Coro 1A−/− Tcon cells.
Coro 1A−/− GFP or WT GFP Tcon cells supplemented with WT TCD BM were infused into lethally irradiated B6D2 recipients. (A) Migration of the cells to the liver, colon, mesenteric lymph node and Peyer’s patches was determined using stereomicroscopy. Images were collected 3, 14 and 28 days post transplantation. Left panels display GFP expression while right panels reflect intensity. Data shown are representative of 6-8 B6D2 recipients given either Coro 1A−/− GFP or WT GFP Tcon cells. (B) GFP ELISA was used to quantify expression in B6D2 recipients transplanted with Coro 1A−/− GFP or WT GFP Tcon cells 14 days post transplantation. Data shown are mean + SEM of n=5 for WT GFP and Coro 1A−/− GFP recipients from a single experiment representative of 3 experiments performed. *p<0.05, two tailed Student’s t-test. (C) Peripheral blood was collected from WT GFP or Coro 1A−/− GFP Tcon cells recipients 14 days post transplantation. T cells in the blood were evaluated by flow cytometry using CD4 and CD8 surface markers. Cells were gated first by GFP positive expression then CD4 and CD8 expression. Data are shown as n=4 for Coro 1A−/− GFP recipients, n=3 for WT GFP recipients from a single experiment. (D) In vivo competitive migration using Coro 1A−/− GFP and WT (Thy 1.1+) cells was performed as detailed in the Materials and methods. Flow cytometry analysis of Coro 1A−/− GFP and WT (Thy 1.1+) Tcon cells were conducted on the spleen (data not shown) and pooled mesenteric and inguinal lymph nodes (representative sample shown) 16 hours post transplantation. Data shown are representative of n=3 for Coro 1A−/− GFP or WT (Thy 1.1+) Tcon cells recipients, from an experiment performed twice.
To further confirm a defect in entry and egress from SLT, we performed an in vivo competitive migration assay. Equal amounts of Coro 1A−/− GFP and WT Thy 1.1+ T cells were injected into lethally irradiated B6D2 F1 recipients. 16 hours post-transplantation, the MLN and inguinal lymph nodes (ILN) were harvested and analyzed by flow cytometry. As demonstrated in Figure 3D, even at this early time point, Coro 1A−/− T cells were markedly less efficient in entering the MLN and ILN, as compared to WT T cells.
Decreased SLT ingress and egress receptors in Coro 1A−/− T cells
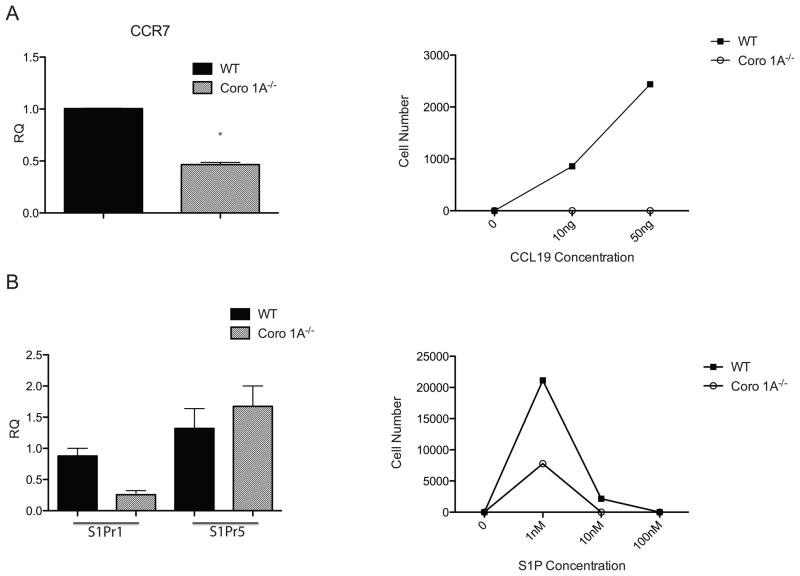
Numerous researchers have shown that the C-C chemokines receptor type 7 (CCR7) is important for entry of T cells into secondary lymphoid organs[19]. Furthermore, data from our laboratory have highlighted the importance of CCR7 in migration and GvHD pathogenesis[3]. As Coro 1A−/− T cells displayed defects in lymph node entry, we questioned whether Coro 1A−/− T cells had decreased CCR7 expression. To address this question, real time PCR analysis was performed on freshly isolated Coro 1A−/− and WT Tcon cells. Surprisingly, Coro 1A−/− Tcon cells expressed 2-fold less CCR7 than WT T cells (Fig 4A). This decrease in CCR7 was further confirmed using an in vitro chemotaxis assay to the CCR7 ligand, CCL19. Similar to the real time data results, Coro 1A−/− T cells displayed impaired migration to CCL19 (Fig 4A). Responsiveness of Coro 1A−/− T cells to a chemoattractant was confirmed using the supernatant from stimulated allogeneic dendritic cells (Supporting Information Fig 2C).
Figure 4. Decreased receptor expression and impaired chemotaxis in the absence of Coro 1A.
(A) Expression of CCR7 and chemotaxis to CCL19 of WT or Coro 1A−/− Tcon cells was determined by real-time PCR. (B) Real time expression of S1Pr1 and S1Pr5 and chemotaxis to S1P in WT and Coro 1A−/− Tcon cells is also shown. *p<0.05, two tailed Student’s t-test. Data are shown as mean + SEM of three experiments of n=9 WT and n=9 Coro 1A−/− Tcon cells.
In addition to the impaired migration into SLT, Coro 1A deficient T cells were unable to egress from lymphoid tissue compared to WT T cells. Sphingosine-1 phosphate (S1P) is a signaling sphingolipid that is produced by hematopoietic cells that has been shown to be important for immune cell egress from SLT. Of the 5 S1P receptors, S1Pr1 has been shown to be important for lymphocyte egress[20]. To evaluate S1P receptor expression in Coro 1A−/− T cells, we used quantitative real time PCR analysis. Coro 1A−/− Tcon cells displayed decreased S1Pr1 expression compared to WT Tcon cells (Fig 4B). However, no difference was found in the expression of the other S1P receptors (Fig 4B and data not shown) on Coro 1A deficient T cells. Additionally, we demonstrated a marked impairment in the migration of Coro 1A−/− T cells to S1P compared to WT T cells indicating that this difference in expression led to functional differences in response to the ligand (Fig 4B). These data indicate that decreased CCR7 and S1Pr1 expression on Coro 1A−/− T cells correlated with the decreased migration into and out of lymphoid organs.
Disruption of the NF-κB Pathway in the absence of Coro 1A
To investigate the mechanism for the diminished expression of CCR7 and S1Pr1 by Coro 1A−/− T cells, we analyzed signaling pathways in conventional T cells. Regulation of the integrity of the actin cytoskeleton is important for numerous signaling pathways including the NF-κB and the mitogen-activated protein kinase (MAPK) pathways [21],[22]. Decreased phosphorylated p65 was found under stimulating and non-stimulating conditions in Coro 1A−/− Tcon cells (Fig 5). Alterations in the NF-κB pathway were specific to the canonical pathway, as no changes in the p100 subunit were observed in WT or Coro 1A−/− Tcon cells (Fig 5). We found no difference in activation under stimulating or non-stimulating conditions of the p38 MAPK protein in Coro 1A null compared to WT T cells as evaluated by western blot (Supporting Information Fig 3). These data suggest that the expression of CCR7 and S1P1r in Coro 1A−/− Tcon cells are correlated with impaired activation of the canonical NF-κB pathway [23].
Figure 5. Decreased activation of NF-κB in Coro 1A−/− Tcon cells.
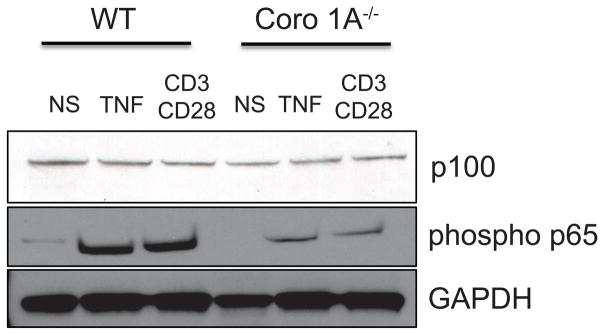
3 × 106 WT or Coro 1A−/− Tcon cells were stimulated for 30 minutes with either 20ng/mL of TNF or 20ng/mL of anti-CD3 and 10ng/mL of anti-CD28. Tcon cells were harvested and analyzed by western blot for p100 and phospho p65 expression. GAPDH was used as a loading control. Data shown are representative of 3 individual experiments.
Maintenance of the GvL response using Coro 1A−/− Tcon cells
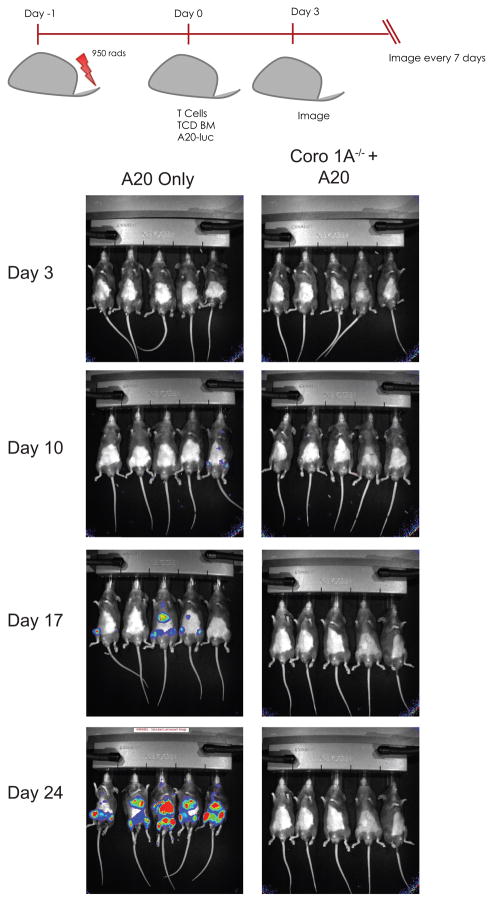
T cells responsible for the pathogenesis of aGvHD are also responsible for the graft-versus-leukemia effect (GvL) that eliminates residual tumor cells in recipients minimizing the probability of relapse. Knowingly, we investigated whether Coro 1A−/− Tcon cells maintain GvL effects. WT TCD BM with luciferase expressing A20 B cell lymphoma cells were transplanted into lethally irradiated B6D2 F1 recipients with either Coro 1A−/− Tcon cells or WT Tcon cells. B6D2 recipients of Coro 1A−/− T cells survived until completion of the experiment with no tumor infiltration, while B6D2 recipients given A20 cells with bone marrow alone succumbed to death by tumor infiltration 24 days post-transplantation (Fig 6). Mice that received WT Tcon cells succumbed to death by aGvHD, prior to infiltration of tumor cells (data not shown). These data demonstrate that in addition to attenuated aGvHD, Coro 1A−/− Tcon cells maintained the beneficial GvL response.
Figure 6. GvL response using Coro 1A−/− Tcon cells.
1 × 104 luciferase-expressing A20 B cell lymphoma cells supplemented with WT TCD BM were injected into lethally irradiated B6D2 recipients. Mice were also injected with either Coro 1A−/− or WT Tcon cells. Mice were monitored for survival and tumor infiltration via luciferase imaging using the IVIS® Kinetic Optical System. Data shown are representative of n=12 for Coro 1A−/− and n=9 for A20 + bone marrow. Data are pooled from three individual experiments.
Discussion
The contribution of T-cell migration to aGvHD pathogenesis has been well studied [3],[24]. Our group and others have shown that chemokines and their receptors, integrins, and selectins all play a critical role in T cell migration during aGvHD [6],[25],[26]. Here we extend these findings, demonstrating that cells deficient in the F-actin associating protein, Coronin 1A, are markedly impaired in their ability to mediate acute GvHD due to accumulation in secondary lymphoid organs and the inability to egress from these organs. The diminished aGvHD seen using T cells deficient in Coro 1A correlated with decreased tissue pathology in recipient mice. In vitro proliferation and cytokine production by Coro 1A−/− Tcon cells was comparable to WT Tcon cells; while decreased proliferation by Coro 1A−/− Tcon cells was seen in vivo and markers of early activation were comparable to that of WT Tcon cells. Lastly, we showed that signaling cascades downstream of the TCR are impaired in the absence of Coro 1A. The reduction in the expression and function of CCR7 and S1Pr1 correlated with impaired migration and activation of the canonical NF-κB pathway. Thus, these data suggest that one method of blocking acute GvHD is to prevent the migration of donor lymphocytes into and out of SLT.
The function of SLT in the biology of aGvHD has been studied elegantly by several different investigators predominately using genetic approaches. These data indicated that SLT was critical to the induction of acute GvHD [7],[27]. However, this activity was redundant, with all secondary lymphoid tissue and the spleen capable of initiating acute GvHD. Thus, it has not been clear if this process would be amenable to clinical intervention. Here, we show that acute GvHD can be prevented by blocking the migration of T cells into and out of secondary lymphoid tissue, which correlated with the impaired function of CCR7 and S1P1r. Clinically this is important as both CCR7 and S1Pr1 are drugable targets. S1P agonists are currently available for the treatment of patients with multiple sclerosis [28]. Our group has initiated a significant screening process to identify inhibitors of CCR7. Our data would suggest that a combination approach using these inhibitors would have significant activity in preventing acute GvHD.
Several laboratories, but most specifically the Cyster laboratory, have shown in a number of elegant manuscripts the requirement for S1Pr1 expression on T cells for migration of those cells out of lymph nodes via the efferent lymph system [29]. The function of S1Pr1 is not limited to lymphocyte migration, as S1Pr1 has also been shown to be important in inflammatory responses in other immune cells [30]. Real time PCR analysis of Coro 1A−/− T cells confirmed decreased expression of S1Pr1 but not other S1P receptors on Coro 1A−/− T cells. Interestingly, our group has previously evaluated the function of FTY720, an agonist of S1P that, in models, prevents acute GvHD pathogenesis [31]. While we were able to indicate that FTY720 administration could abrogate acute GvHD, this did not correlate with impaired egress of donor T cells from SLT. Thus, the current data are the first to indicate that egress out of SLT is important for the function of donor T cells during acute GvHD [32].
The importance of the chemokine receptor CCR7 in T lymphocyte migration has been well established. Data from our group demonstrated impaired donor T cell migration to secondary lymphoid organs of donor T cells lacking CCR7 [3]. However, in our previous work, we were unable to completely block acute GvHD in the major mismatch model by infusing T cells lacking CCR7. This indicates that the profound decrease in aGvHD found after the infusion of T cells lacking Coro 1A in BALB/c recipients is not solely due to the absence of CCR7. This would further suggest that blocking migration into and out of secondary lymphoid tissue has a more profound effect than blocking the initial interaction of donor T cells with APCs.
Blocking of GvHD that mitigates the GvL response is not a successful strategy for improving allogeneic SCT. Tumor cell elimination was seen in Coro 1A−/− Tcon cells recipients, demonstrating maintenance of GvL response by Coro 1A−/− Tcon cells in a lymphoma model. Importantly mice that received WT Tcon cells with tumor cells succumbed to aGvHD before tumor infiltration could occur. These results further highlight the benefits of Coro 1A−/− Tcon cells in allogeneic SCT. It should be noted that naïve T cells deficient in Coro 1A may have a reduced anti-leukemic response due to a decreased ability to exit SLT and traffic to the bone marrow. However previous investigations have found that antigen-specific T cells that traffic to the bone marrow can be primed by bone marrow resident DCs [33]. This priming, even in the absence of secondary lymphoid tissue, generates cytotoxic T cells that can provide an anti-tumor response and immunologic memory. Thus, we believe that blocking the migration of naïve T cells in and out of SLT may not hinder the activation of antigen-specific T cells in the bone marrow.
In summary, we have found that the absence of Coro 1A in donor T cells markedly diminished the incidence and severity of acute GvHD. We demonstrate that Coro 1A−/− T cells have impaired migration into and out of secondary lymphoid tissue, which correlated with diminished expression of CCR7 and S1P1r. These data indicate that approaches that prevent the migration of T cells into and out of secondary lymphoid tissue with maintenance of GvL response may significantly impact the occurrence of acute GvHD.
Materials and Methods
Mice
C57BL/6J (H2b) (termed WT), BALB/c, and C57BL/6J x DBA/2 F1 (termed B6D2) were purchased from The Jackson Laboratory. The generation of enhanced green fluorescent protein expressing (GFP) C57BL/6 mice has been described previously [4]. Coro 1A deficient (Coro 1A−/−) C57BL/6 mice were obtained from Niko Foger and generated as described [12],[34]. Coro 1A−/− GFP mice were generated by crossing Coro 1A−/− mice with GFP C57BL/6 mice. All experiments were performed in accordance with protocols approved by the University of North Carolina Institutional Animal Care and Use Committee.
Transplantation Models
T cell depleted bone marrow (TCD BM) was prepared as previously described [35]. CD25 depleted T cells were prepared using a total T cell isolation kit (Cedarlane Laboratories) followed by antibody depletion and magnetic cell separation as previously described [3]. The day prior to transplantation, recipient mice received either 950 cGy (B6D2) or 800 cGy (BALB/c) of total body irradiation. For B6 to B6D2 or B6 to BALB/c transplants, recipients were intravenously injected with either 4 × 106 T cells and 3 × 106 TCD BM cells, or 5 × 105 total T cells and 5 × 106 TCD BM cells, respectively, unless otherwise noted. Histopathology analyses were prepared as previously described and analyzed by one of us (A.P.M.) blinded to the genotype of the donor [36].
Stereomicroscopy
Organs from anesthetized animals were imaged with a Zeiss Stereo Lumar V12 microscope with GFP bandpass filter (Carl Zeiss MicroImaging, Inc.) at room temperature. AxioVision (Carl Zeiss) software was used to determine GFP intensities. WT GFP and Coro 1A−/− GFP recipient organs were imaged using the identical magnification (mag) and exposure (exp) times for each time point. Day +3: PP-exp 976ms, mag 32X MLN-exp 2.5s, mag 15X Day +14: PP-exp 1s, mag 30X MLN-exp 1s, mag 20X Colon-exp 4s, mag 13X Liver-exp 2s, mag 40X Lung-exp 4s, mag 18X Day +28: PP-exp 750ms, mag 30X MLN 600ms, mag 20X Colon-exp 3s, mag 13X Liver-exp 3s, mag 40X
Organ GFP Quantification
Organs from recipient animals were homogenized and absolute GFP levels determine by ELISA (Cell Biolabs). Detailed experimental procedures were conducted as described previously [3].
In Vivo Competitive Migration Assay
CD25 negative total T cells were isolated as described above from Coro 1A−/− GFP and Thy 1.1+ WT mice. Recipient B6D2 mice were injected intravenously with equal amounts of Coro 1A−/− GFP and WT Thy 1.1+ donor T cells. 16 hours post transplantation, the mesenteric lymph node, inguinal lymph node, and spleen were harvested, stained, and analyzed by flow cytometry.
Real Time PCR Analysis
Real time PCR was performed as previously described [36]. Gene expression was normalized to the housekeeping gene GusB before determining fold induction using ΔΔCt method. Taqman expression assay probes for S1Pr1, S1Pr3, S1Pr5, and CCR7 were purchased from Applied Biosystems.
Chemotaxis Analysis
Conventional T cells (Tcon cells) were isolated using Cedarlane total T cell isolation kit following by antibody depletion coupled with negative selection. Following isolation the cells were washed twice with PBS. 5 × 105 or 2 × 105 total T cells in 100μL were added to the upper chamber of a PVP treated 5μM pore polycarbonate membrane inside of a ChemoTx® chamber system (Neuroprobe). The bottom chamber was filled with the indicated concentrations of sphingosine-1 phosphate (Sigma) or C-C motif chemokine 19 (Peprotech) and incubated for 3 hours at 37°C. CyQuant cell quantification kit (Invitrogen) was used to determine cell migration from the upper chamber to the lower chamber.
Western Blot Analysis
Freshly isolated Tcon cells were lysed in RIPA (Invitrogen) buffer supplemented with protease and phosphatase inhibitors (Roche). Lysates were separated by SDS-PAGE on a 4–12% Bis-Tris gel (Life Technologies), transferred onto a nitrocellulose membrane and incubated in 5% non-fat dry milk to block non-specific binding. Membranes were incubated with the following antibodies purchased from Cell Signaling Technology: phospho NF-κB p65 (Ser536), NF-κB2 p100/p52. GAPDH antibody was purchased from Santa Cruz Biotechnology. Proteins were detected using anti-rabbit IgG HRP (Promega) and the ECL western blotting detection kit according to manufacturer’s instructions (GE Healthcare).
In vivo Proliferation
Lethally irradiated B6D2 recipients were transplanted with equal amounts of Coro 1A−/− GFP and WT Thy 1.1+ donor total T cells concurrently with WT TCD BM. Ten days post transplantation recipient mice were injected intraperitoneally with BrdU labeling reagent (Invitrogen). Four hours after injection the spleens were harvested and stained for BrdU (Invitrogen) and the following antibodies from eBioscience: CD45, CD44, CD62L, Thy 1.1.
Mixed Lymphocyte Reaction
Equal amounts of Coro 1A−/− or WT T cells and irradiated B6D2 splenocytes were cultured in RPMI complete for 24 or 48 hours. For activation the cells were stained with the following antibodies from eBioscience: CD62L, CD44, CD69. For cytokine production the cells were permeabilized using the BD Cytofix/cytoperm plus kit and stained with TNF or IFN-γ antibodies also purchased from eBioscience. Cells were analyzed by flow cytometry using FlowJo analysis software.
Proliferation Assay
Coro 1A−/− or WT T cells were labeled with 10μM carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen). Equal amounts of labeled Coro 1A−/− or WT Teffs and B6D2 irradiated splenocytes were cultured in RPMI complete for 24 or 48 hours. Following harvest the cells were stained with CD4 and CD8 antibodies (eBioscience). Proliferation was determined by flow cytometry using the FlowJo analysis software.
GvHD Scoring
Mice were observed twice weekly for clinical GvHD signs and symptoms based on a previously established clinical scoring system [37].
Statistical Analysis
Survival curves were constructed using the Kaplan Meier method. Median survival was determined using the log rank test. Continuous values including cytokine levels, total cell numbers, clinical scoring and GFP expression were determined using two-tailed Student’s t-test. P-values less than 0.05 were considered significant. Error bars represent standard error of the mean.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institutes of Health RO1 CA166794 (JSS), RO1 HL115761 (JSS), R56 AI064363 (JSS), RO1 GM083035 (JEB), 5F31AI096900-02 (LMF), and 5F31CA183556-02 (NAT). The work was also supported by the Mary Elizabeth Thomas Endowment fund (JSS) and the Medical Scientist Training Program T32 GM008719 (Eugene P. Orringer, MD). We would like to thank Amanda Rinkenbaugh for technical assistance.
List of Abbreviations
- aGvHD
Acute Graft versus Host Disease
- Coro 1A
Coronin 1A
- GvL
Graft-versus-Leukemia
- SCT
Stem Cell Tranplantation
- SLT
Secondary Lymphoid Tissue
- Tcon cell
Conventional T cell
Footnotes
Conflict of Interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Reddy P. Pathophysiology of acute graft-versus-host disease. Hematol Oncol. 2003;21:149–161. doi: 10.1002/hon.716. [DOI] [PubMed] [Google Scholar]
- 2.Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland CB, Herman EI, Baker EM, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106:1113–1122. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coghill JM, Carlson MJ, Panoskaltsis-Mortari A, West ML, Burgents JE, Blazar BR, Serody JS. Separation of graft-versus-host disease from graft-versus-leukemia responses by targeting CC-chemokine receptor 7 on donor T cells. Blood. 2010;115:4914–4922. doi: 10.1182/blood-2009-08-239848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panoskaltsis-Mortari A, Price A, Hermanson JR, Taras E, Lees C, Serody JS, Blazar BR. In vivo imaging of graft-versus-host-disease in mice. Blood. 2004;103:3590–3598. doi: 10.1182/blood-2003-08-2827. [DOI] [PubMed] [Google Scholar]
- 5.Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004;25:75–84. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Wysocki CA, Jiang Q, Panoskaltsis-Mortari A, Taylor PA, McKinnon KP, Su L, Blazar BR, et al. Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood. 2005;106:3300–3307. doi: 10.1182/blood-2005-04-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beilhack A, Schulz S, Baker J, Beilhack GF, Nishimura R, Baker EM, Landan G, et al. Prevention of acute graft-versus-host disease by blocking T-cell entry to secondary lymphoid organs. Blood. 2008;111:2919–2928. doi: 10.1182/blood-2007-09-112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pieters J. Coronin 1 in innate immunity. Subcell Biochem. 2008;48:116–123. doi: 10.1007/978-0-387-09595-0_11. [DOI] [PubMed] [Google Scholar]
- 9.Uetrecht AC, Bear JE. Coronins: the return of the crown. Trends Cell Biol. 2006;16:421–426. doi: 10.1016/j.tcb.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Nal B, Carroll P, Mohr E, Verthuy C, Da Silva M-I, Gayet O, Guo X-J, et al. Coronin-1 expression in T lymphocytes: insights into protein function during T cell development and activation. Int Immunol. 2004;16:231–240. doi: 10.1093/intimm/dxh022. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari G, Langen H, Naito M, Pieters J. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell. 1999;97:435–447. doi: 10.1016/s0092-8674(00)80754-0. [DOI] [PubMed] [Google Scholar]
- 12.Föger N, Rangell L, Danilenko DM, Chan AC. Requirement for coronin 1 in T lymphocyte trafficking and cellular homeostasis. Science. 2006;313:839–842. doi: 10.1126/science.1130563. [DOI] [PubMed] [Google Scholar]
- 13.Mueller P, Massner J, Jayachandran R, Combaluzier B, Albrecht I, Gatfield J, Blum C, et al. Regulation of T cell survival through coronin-1-mediated generation of inositol-1,4,5-trisphosphate and calcium mobilization after T cell receptor triggering. Nat Immunol. 2008;9:424–431. doi: 10.1038/ni1570. [DOI] [PubMed] [Google Scholar]
- 14.Shiow LR, Roadcap DW, Paris K, Watson SR, Grigorova IL, Lebet T, An J, et al. The actin regulator coronin 1A is mutant in a thymic egress-deficient mouse strain and in a patient with severe combined immunodeficiency. Nat Immunol. 2008;9:1307–1315. doi: 10.1038/ni.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong MM, Fish EN. Chemokines: attractive mediators of the immune response. Semin Immunol. 2003;15:5–14. doi: 10.1016/s1044-5323(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 16.Sotsios Y, Ward SG. Phosphoinositide 3-kinase: a key biochemical signal for cell migration in response to chemokines. Immunol Rev. 2000;177:217–235. doi: 10.1034/j.1600-065x.2000.17712.x. [DOI] [PubMed] [Google Scholar]
- 17.Curnock AP, Logan MK, Ward SG. Chemokine signalling: pivoting around multiple phosphoinositide 3-kinases. Immunology. 2002;105:125–136. doi: 10.1046/j.1365-2567.2002.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baldwin AS. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 19.Förster R, Schubel A, Breitfeld D, Kremmer E, Renner-Müller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 20.Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol. 2012;30:69–94. doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Doerschuk CM. The p38 mitogen-activated protein kinase mediates cytoskeletal remodeling in pulmonary microvascular endothelial cells upon intracellular adhesion molecule-1 ligation. J Immunol. 2001;166:6877–6884. doi: 10.4049/jimmunol.166.11.6877. [DOI] [PubMed] [Google Scholar]
- 22.Fazal F, Minhajuddin M, Bijli KM, McGrath JL, Rahman A. Evidence for actin cytoskeleton-dependent and -independent pathways for RelA/p65 nuclear translocation in endothelial cells. J Biol Chem. 2007;282:3940–3950. doi: 10.1074/jbc.M608074200. [DOI] [PubMed] [Google Scholar]
- 23.Kuwabara T, Tanaka Y, Ishikawa F, Kondo M, Sekiya H, Kakiuchi T. CCR7 ligands up-regulate IL-23 through PI3-kinase and NF-κB pathway in dendritic cells. J Leukoc Biol. 2012;92:309–318. doi: 10.1189/jlb.0811415. [DOI] [PubMed] [Google Scholar]
- 24.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duffner U, Lu B, Hildebrandt GC, Teshima T, Williams DL, Reddy P, Ordemann R, et al. Role of CXCR3-induced donor T-cell migration in acute GVHD. Exp Hematol. 2003;31:897–902. doi: 10.1016/s0301-472x(03)00198-x. [DOI] [PubMed] [Google Scholar]
- 26.Carlson MJ, Fulton LM, Coghill JM, West ML, Burgents JE, Wan Y, Panoskaltsis-Mortari A, et al. L-selectin is dispensable for T regulatory cell function postallogeneic bone marrow transplantation. Am J Transplant. 2010;10:2596–2603. doi: 10.1111/j.1600-6143.2010.03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson BE, Taylor PA, McNiff JM, Jain D, Demetris AJ, Panoskaltsis-Mortari A, Ager A, et al. Effects of donor T-cell trafficking and priming site on graft-versus-host disease induction by naive and memory phenotype CD4 T cells. Blood. 2008;111:5242–5251. doi: 10.1182/blood-2007-09-107953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kappos L, Radue E-W, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 29.Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- 30.Rosen H, Sanna MG, Cahalan SM, Gonzalez-Cabrera PJ. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol. 2007;28:102–107. doi: 10.1016/j.it.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Taylor PA, Ehrhardt MJ, Lees CJ, Tolar J, Weigel BJ, Panoskaltsis-Mortari A, Serody JS, et al. Insights into the mechanism of FTY720 and compatibility with regulatory T cells for the inhibition of graft-versus-host disease (GVHD) Blood. 2007;110:3480–3488. doi: 10.1182/blood-2007-05-087940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 33.Feuerer M, Beckhove P, Garbi N, Mahnke Y, Limmer A, Hommel M, Hämmerling GJ, et al. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Med. 2003;9:1151–1157. doi: 10.1038/nm914. [DOI] [PubMed] [Google Scholar]
- 34.Föger N, Jenckel A, Orinska Z, Lee K-H, Chan AC, Bulfone-Paus S. Differential regulation of mast cell degranulation versus cytokine secretion by the actin regulatory proteins Coronin1a and Coronin1b. J Exp Med. 2011;208:1777–1787. doi: 10.1084/jem.20101757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wysocki CA, Burkett SB, Panoskaltsis-Mortari A, Kirby SL, Luster AD, McKinnon K, Blazar BR, et al. Differential roles for CCR5 expression on donor T cells during graft-versus-host disease based on pretransplant conditioning. J Immunol. 2004;173:845–854. doi: 10.4049/jimmunol.173.2.845. [DOI] [PubMed] [Google Scholar]
- 36.Fulton LM, Carlson MJ, Coghill JM, Ott LE, West ML, Panoskaltsis-Mortari A, Littman DR, et al. Attenuation of Acute Graft-versus-Host Disease in the Absence of the Transcription Factor RORγt. J Immunol. 2012 doi: 10.4049/jimmunol.1200858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Den Brink MR, Moore E, Horndasch KJ, Crawford JM, Hoffman J, Murphy GF, Burakoff SJ. Fas-deficient lpr mice are more susceptible to graft-versus-host disease. J Immunol. 2000;164:469–480. doi: 10.4049/jimmunol.164.1.469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.