Abstract
INTRODUCTION AND OBJECTIVES
Concern regarding coexisting malignant pathology in benign renal tumors deters renal biopsy and questions its validity. We examined rates of coexisting malignant and high grade pathology in resected benign solid solitary renal tumors.
METHODS
Using our prospectively maintained database we identified patients with solitary solid renal tumors who underwent surgical resection between 1994 and 2012 (n=1829). Lesions containing elements of renal oncocytoma (RO), angiomyolipoma (AML) or other benign pathology formed the basis for this analysis. Patients having an oncocytic malignancy, without presence of a classic oncocytoma, and those with known hereditary syndromes were excluded.
RESULTS
147 patients with pathologically proven elements of RO (n=96), AML (n=44), and other solid benign pathology (n=7) were identified. Median tumor size was 3.0 cm (IQR 2.2 – 4.5) and tumor anatomic complexity, as quantified by the RENAL NS, was low in 28%, moderate in 56%, and high in 16%. Only 4 patients (2.7%) were documented as having hybrid malignant pathology, all involving low grade chromophobe RCC in the setting of RO. After a median follow-up of 44 (IQR 33 – 55) months, no patients with hybrid tumors experienced regional or metastatic progression.
CONCLUSIONS
In our cohort of patients with a solitary, sporadic, solid benign renal mass, <3% of tumors exhibited coexisting hybrid malignancy. Importantly, no patients harbored coexisting high grade pathology. These data suggest that uncertainty regarding hybrid malignant pathology coexisting with benign pathologic components should not deter renal biopsy, especially in the elderly and comorbid.
Keywords: renal cell carcinoma, oncocytoma, chromophobe, solitary renal mass, benign renal mass, hybrid renal tumor
Introduction
Clinical management decisions for patients with an enhancing renal mass require a nuanced balance of risks, especially since only a minority of enhancing renal lesions prove to be biologically aggressive. Thus, accurate non-extirpative assessment of tumor histology is extremely desirable, especially in the elderly and infirmed. Percutaneous renal biopsy (PRB) has become the lynchpin strategy for non-extirpative pathologic assessment.1,2 Reliable biopsy results offer particular promise for patients who harbor benign masses. In fact, these patients comprise nearly a quarter of modern renal mass cohorts and, arguably, can avoid risks of treatment altogether.3,4 Yet, reliability of a benign renal biopsy result is called into questioned by reports of malignant histology being harbored in otherwise benign tumors.1,5 Existence of these hybrid tumors potentially deters renal biopsy and questions its validity.1 Nevertheless, hybrid histology has been largely described in patients with multifocal tumors and known genetic syndromes, with data for patients with sporadic solitary tumors being relatively sparse.6,7 As such, we set out to assess the incidence of coexisting hybrid malignancy among solitary sporadic solid benign renal masses in a large cohort of patients undergoing renal surgery at a tertiary referral center.
Materials and Methods
An IRB-approved kidney cancer database is maintained by our institution and contains prospectively-entered demographic, peri-operative, pathologic and imaging data, as well as follow-up information. A query of the kidney cancer database was performed to identify patients who underwent radical or partial nephrectomy between January 1994 and July 2012. Patients who had lesions classified as benign, or had “oncocytoma” or “oncocytic” as part of their pathologic diagnosis, were reviewed. Patients with cystic lesions or synchronous multifocal lesions were excluded, leaving only solitary solid lesions. Records were reviewed to eliminate patients with family history or diagnosis of genetic syndromes, thus excluding patients with renal oncocytosis or Birt-Hogg-Dubé syndrome. Pathology reports were reviewed in detail, and pathology slides were re-reviewed by a urologic pathologist, as needed. All hybrid tumors were identified and corresponding slides were re-reviewed. The hematoxylin and eosin (H&E) stained slides were available for all hybrid tumor cases and immunohistochemical stains, which included staining for Cytokeratin 7 (CK-7), were performed on all hybrid tumors at the time of the initial diagnosis. Karyotyping cytogenetic analyses were reviewed when available. Patients whose primary lesion was described as oncocytic and did not contain a defined area of classic RO, such as oncocytic chromophobe RCC (chRCC) or papillary RCC (pRCC) with oncocytic features, were considered as purely malignant and not hybrid, and therefore were excluded. Literature search of English language abstracts from 1990 until 2013 was performed using PubMed and Web of Science® databases with key words including “chromophobe,” “oncocytoma,” “renal” and “hybrid tumors.” Relevant articles with their respective bibliographies were reviewed, data abstracted. Descriptive statistics were generated using Microsoft Excel 2007 software.
Results
Between 1994 and 2012, we identified 2013 patients that underwent renal surgery at our institution. 147 patients with renal masses containing any proportion of benign histology were identified. Demographic and histopathologic characteristics of this cohort are summarized in Table 1. Median age at the time of surgery was 61 (IQR 53–70) years, 45.9% were male and 81.7% Caucasian. Median tumor size was 3.0 (IQR 2.2 – 4.5) cm. Tumor anatomic complexity, as quantified by the R.E.N.A.L. Nephrometry Score, was available for 96 (65.3%) patients, of which 27 (28.1%) were classified as low, 54 (56.3%) as moderate and 15 (15.6%) as high.8 One hundred and nine patients (74.1%) underwent nephron-sparing surgery. Lesions included pathologically proven elements of RO (N=96, 65.3%), AML (N=44, 29.9%), metanephric adenoma (N=2, 1.4%), renomedullary interstitial cell tumor (N=1, 0.7%), medullary fibroma (N=1, 0.7%), juxtaglomerular cell tumor (N=1, 0.7%), inflammatory pseudotumor (N=1, 0.7%) and a hemangioma (N=1, 0.7%). Adjunctive pathologic evaluation, including immunohistochemical staining and/or karyotyping cytogenetic analyses, was available in 38% of RO-containing specimens. After reviewing the pathology reports for morphological features, immunohistochemical and cytogenetic analyses, only 4 patients (2.7%) were found to have hybrid malignant pathology. In this hybrid tumor subset, all patients had tumors with features of chRCC coexisting with areas resembling RO. At a median follow-up of 9.7 (IQR 5 – 34) months (44 (IQR 33–55) months if only hybrid lesions are considered), one patient with a classic RO has evidence of a new ipsilateral lesion, away from the previous resection site, and is on active surveillance.
Table 1.
Demographic Characteristics of Benign and Hybrid Cohorts
| Benign N (%) | Hybrid* N (%) | |
|---|---|---|
| Number of patients | 143 (97.3%) | 4 (2.7%) |
| Median age (IQR) | 62 years (53 – 70) | 50.5 years (47 – 56) |
| Male | 45.5% | 50% |
| Caucasian | 116 (81%) | 4 (100%) |
| Median Tumor Size (IQR) | 3.0 cm (2.2 – 4.5) | 6.0 cm (3.5 – 9.2) |
| pT stage | ||
| pT1a | 107 (74.8%) | 2 (50%) |
| pT1b | 22 (15.4%) | - |
| pT2 | 8 (5.6%) | 2 (50%) |
| pT3 | 6 (4.2%) | - |
| Surgery type | ||
| Partial nephrectomy | 107 (74.8%) | 2 (50%) |
| Radical nephrectomy | 36 (25.2%) | 2 (50%) |
| Follow-up (IQR) | 8.7 months (5 – 31) | 43.8 months (33 – 55) |
| Histologic Subtypes: | ||
| Chromophobe RCC | - | 4 (100%) |
| Oncocytoma | 92 (64.3%) | 4 (100%) |
| Angiomyolipoma | 44 (30.7%) | - |
| Metanephric adenoma | 2 (1.4%) | - |
| Renomedullary interstitial cell tumor | 1 (0.7%) | - |
| Medullary fibroma | 1 (0.7%) | - |
| Juxtaglomerular cell tumor | 1 (0.7%) | - |
| Inflammatory pseudotumor | 1 (0.7%) | - |
| Hemangioma | 1 (0.7%) | - |
On detailed pathologic evaluation, three of four hybrid tumors primarily demonstrated the classic RO cytomorphology pattern (Figure 1A and 1B), with exception of several focal areas suggestive of chRCC (Figure 1C). Cells in these areas had irregular nuclear membranes and demonstrated perinuclear clearing. Other areas appeared pleomorphic, different from the polygonal cells with smooth, dark round nuclei of the classic oncocytoma. CK-7 staining of these sections revealed focal positivity, mainly in the areas resembling chromophobe carcinoma (Figure 1D). Conventional cytogenetic studies were performed on two of the three cases and revealed normal karyotype.
Figure 1. Representative histopathology and immunohistochemistry.
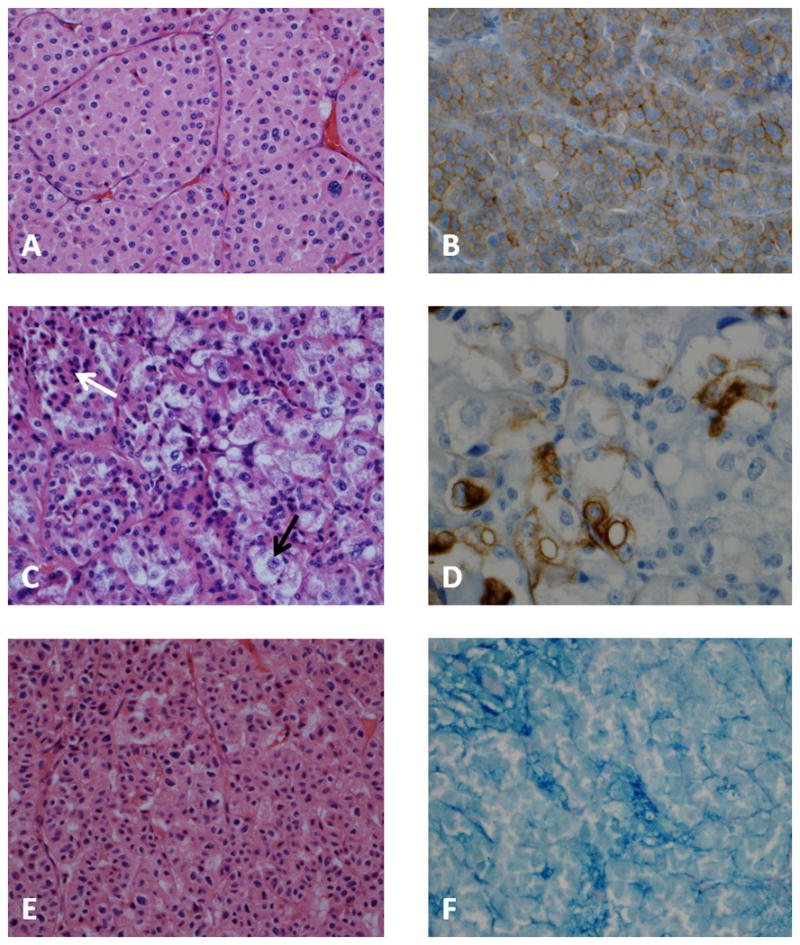
A. Classic oncocytoma, composed of round to polygonal cells with granular eosinophilic cytoplasm, round uniform nuclei, central nucleoli with smoothly dispersed chromatin; minimal intervening stroma. B. Classic oncocytoma, membranous staining with CD117. C. Hybrid tumor, focal areas show large tumor cells, some with clear cytoplasm (black arrow) and prominent nucleoli, adjacent to the classic oncocytoma (white arrow). D. Hybrid tumor, CK-7 stain demonstrates focal positivity. E. Hybrid tumor with composite morphology, immunohistochemistry and cytogenetics. Oncocytic neoplasm demonstrates a nested pattern; cells are polygonal with irregular nuclear membrane. F. Hybrid tumor demonstrating colloidal iron stained cells in clusters.
The fourth case displayed combined morphologic features of both, RO and chRCC (Figure 1E). Cytoplasm in most cells was oncocytic, lacked perinuclear halo and the nuclear membrane was irregular, raisinoid in appearance. CK-7 (performed twice, with adequate controls) stained only rare cells. Colloidal iron stained scattered foci of cells throughout the tumor section (Figure 1F). The cytogenetic analysis of this tumor revealed multiple chromosomal abnormalities (karyotype: 34,X,-X,-1,-2,-3,-6,-8,-9,-10,-13,-17,-18,-22[19]). While this tumor had a cytogenetic signature most consistent with chRCC, it was ultimately characterized as a hybrid tumor based on its composite morphology.
Discussion
In a cohort of patients who underwent extirpative renal surgery at a large U.S. tertiary referral center, we found that < 3% of patients harbored coexisting malignant pathology in a solitary sporadic renal tumor that contained any component of benign histology. Importantly, no benign tumor coexisted with high grade malignancy.
These findings are clinically relevant in an era of frequent cross-sectional imaging, when rates of incidental diagnosis for the small renal mass (SRM) are on the rise and quantifiable reduction of mortality from RCC is yet to be demonstrated.9 In fact, treatment trade-off decisions are especially challenging in the elderly and comorbid for whom SRM surgery presents a non-trivial risk.10,11 As such, use of PRB has gained significant clinical traction in order to better risk stratify patients prior to intervention and to minimize overtreatment of benign lesions, which are found in up to 33% of patients undergoing renal surgery.1–3,12 Yet, concern for a coexisting malignancy presents a significant barrier to routine use of PRB, undermining the validly of PRB and likely deterring its routine use.1
Consistent with existing data, we demonstrated that coexisting malignant pathology is found in lesions harboring oncocytoma. 5,13–19 Neither AMLs, nor other rare benign renal tumors in our cohort contained coexisting hybrid malignancy. Although isolated case reports of AMLs coexisting with a malignancy do exist, such lesions appear to be exceedingly rare.20
Historically, hybrid tumors containing distinct areas with features resembling RO and chRCC were largely described in the setting of renal oncocytosis or Birt-Hogg-Dubé syndrome.6,7 Indeed, recognition of sporadic solitary hybrid tumors is fairly recent, with the first case-report published in 1995 and a first case-series reported in 2005.14,19 To our knowledge, less than 50 cases of sporadic hybrid tumors exist in the English literature (Table 2), yet risk estimates of an oncocytoma harboring a chRCC have been reported to be between 3.4 and 27%. For instance, Petersson et al, in a multicenter report from international pathology departments, documented 14 (3.4%) hybrid tumors in a cohort of 412 oncocytoma-containing neoplasms (398 pure oncocytomas).17 In contrast, a recent single-center report by Waldert et al from Austria identified 59 tumors containing oncocytoma, 16 of which were hybrid tumors (27.1%). In our cohort, 4.2% (4 of 96) of oncocytoma-containing tumors revealed evidence of malignancy. The dramatic difference in the rate of coexisting malignancy between our cohort and that of Waldert et al likely stems from the pathologic criteria employed to classify the malignant component. Waldert et al. defined >10% CK7-positive staining, regardless of the tumor’s histological architecture, as being diagnostic for chRCC, thus categorizing a lesion as a hybrid tumor.5 Meanwhile, experienced academic genitourinary pathologists at our Center believe that immunohistochemistry and cytogenetics should preferentially be employed in equivocal cases, and therefore were adjunctively utilized in assessing only 38% of our RO-containing specimens. This is similar to the approach described in the multi-institutional report by Petersson et al.17 As such, our data identify an opportunity to arrive at a universal set of standards for differentiating RO from chRCC, since in the era of increased utilization of PRB critical clinical decision-making may pivot on high-fidelity diagnosis of oncocytoma.
Table 2.
Solitary Sporadic Hybrid Tumors Reported in the Urologic Literature
| Reference: | N | Malignant histology | Approach to differentiating RO from chRCC | Criteria for HT classification | Size [cm] | % Male | % high grade | Length of f/u [months] | Progression/metastases |
|---|---|---|---|---|---|---|---|---|---|
| Noguchi{Noguchi, 1995 #77} | 1 | chRCC | Histomorphology | - | 3 | 100 | 0 | 9 | none |
| Mai{Mai, 2005 #54} | 5 | chRCC | Histomorphology | chRCC in >20% of tumor cells | 3.0 | 80 | 0 | 84 | none |
| Neuzillet{Neuzillet, 2005 #59} | 1 | chRCC | n/a | - | 7.0 | - | 100 | 28 | none |
| Schmidbauer{Schmidba uer, 2008 #76} | 2 | chRCC | Immunohistochemistry | - | 2.3 | - | - | - | none |
| Delongchamps{Delong champs, 2009 #16} | 7 | chRCC | Histomorphology | 5.5 | 29 | - | 20 | none | |
| Aslam{Aslam, 2009 #63} | 1 | EVchRCC | Immunohistochemistry | - | - | 100 | 100 | 20 | liver metastasis |
| Waldert{Waldert, 2010 #50} | 16 | chRCC | Immunohistochemistry | CK-7 positivity in >10% of tumor cells | 4.2 | 63 | 12.5 | 50 | none |
| Petersson{Petersson, 2010 #49} | 14 | chRCC | Histomorphology | - | 5.2 | 64 | 0 | 36 | n/a |
| Present series | 4 | chRCC | Histomorphology | - | 6.0 | 50 | 0 | 44 | none |
| Combined | 51 | 4.6 | 60.6 | 9.5 | 42.8 | 1 patient (2%) |
Values reported as means
chRCC – chromophobe RCC
EVchRCC – eosinophilic variant of chromophobe RCC
We did not identify any high grade pathology coexisting with benign lesions in our cohort. Although case reports of coexisting benign pathology and high grade malignancy exist in the literature, these clinical entities appear to be extremely rare.5,15,16 Furthermore, these data must be interpreted in the context of the recommendation that that Fuhrman grading for chRCC is no longer recommended, as it does not contribute additional prognostic information to TNM staging and to presence of sarcomatoid differentiation.21,22
When discussing treatment of benign renal neoplasms, it is important to note the lack of robust literature on the natural history of RO.23 Nevertheless, these lesions are uniformly described as benign.24–26 While chRCC is classified as a malignant lesion, it is of low metastatic potential with <17% of patients presenting with advanced disease, 88% – 100% 5-year survival and 4% risk of metastatic spread.27–29 Natural history of hybrid tumors available in the literature is even more limited, since definitive diagnosis is facilitated by tumor excision which alters its clinical course. Generally, hybrid tumors are believed to be non-aggressive, with no reports of local recurrence and only one case report of distant metastasis (Table 2).15,17,30 Because all lesions in our cohort were resected, the current study does not meaningfully contribute to better understanding of natural history and biology of pure oncocytomas nor hybrid tumors. Nevertheless, following resection, no lesions in this study demonstrated distant or local recurrence.
We acknowledge that this is a single institution retrospective analysis, with its inherent biases and limitations. Importantly, we did not reexamine lesions that may have contained a benign component but were reported as uniformly malignant (e.g. reportedly pure chRCC or lesions described as oncocytic neoplasms). Furthermore, the cohort spans two decades with pathological expertise and judgment potentially evolving over this time. Our series underscores that finding of a tumor that harbors co-existing benign and malignant components is a rare event at a tertiary referral center with high volumes of renal malignancy. When contrasted with recent European literature, the study also emphasizes the need for a consensus among the pathologic community on how to best differentiate chRCC from RO.
Conclusions
Incidence of solitary sporadic hybrid renal tumors may be lower than that recently reported in the literature and was less than 3% in our cohort. None of the hybrid tumors contained coexisting high grade pathology. These data suggest that uncertainty regarding hybrid malignant pathology coexisting with benign pathologic components should not deter renal biopsy in efforts to minimize overtreatment of the renal mass, especially in the frail and the comorbid.
Acknowledgments
This publication was supported in part by grant number P30 CA006927 from the National Cancer Institute and by the Department of Defense, Physician Research Training Award (AK). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute, the National Institutes of Health, nor the Department of Defense. Additional funds were provided by Fox Chase Cancer via institutional support of the Kidney Cancer Keystone Program
Abbreviations
- SRM
small renal mass
- PRB
percutaneous renal biopsy
- RO
renal oncocytoma
- chRCC
chromophobe renal cell carcinoma
- AML
angiomyolipoma
- H&E
hematoxylin and eosin
- CK-7
Cytokeratin 7
- pRCC
papillary renal cell carcinoma
References
- 1.Lane BR, Samplaski MK, Herts BR, Zhou M, Novick AC, Campbell SC. Renal mass biopsy--a renaissance? The Journal of urology. 2008 Jan;179(1):20–27. doi: 10.1016/j.juro.2007.08.124. [DOI] [PubMed] [Google Scholar]
- 2.Leveridge MJ, Finelli A, Kachura JR, et al. Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. European urology. 2011 Sep;60(3):578–584. doi: 10.1016/j.eururo.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Halverson SJ, Kunju LP, Bhalla R, et al. Accuracy of determining small renal mass management with risk stratified biopsies: confirmation by final pathology. The Journal of urology. 2013 Feb;189(2):441–446. doi: 10.1016/j.juro.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 4.Remzi M, Marberger M. Renal tumor biopsies for evaluation of small renal tumors: why, in whom, and how? European urology. 2009 Feb;55(2):359–367. doi: 10.1016/j.eururo.2008.09.053. [DOI] [PubMed] [Google Scholar]
- 5.Waldert M, Klatte T, Haitel A, et al. Hybrid renal cell carcinomas containing histopathologic features of chromophobe renal cell carcinomas and oncocytomas have excellent oncologic outcomes. European urology. 2010 Apr;57(4):661–665. doi: 10.1016/j.eururo.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Pavlovich CP, Walther MM, Eyler RA, et al. Renal tumors in the Birt-Hogg-Dube syndrome. The American journal of surgical pathology. 2002 Dec;26(12):1542–1552. doi: 10.1097/00000478-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Tickoo SK, Reuter VE, Amin MB, et al. Renal oncocytosis: a morphologic study of fourteen cases. The American journal of surgical pathology. 1999 Sep;23(9):1094–1101. doi: 10.1097/00000478-199909000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. The Journal of urology. 2009 Sep;182(3):844–853. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 9.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. Journal of the National Cancer Institute. 2006 Sep 20;98(18):1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 10.Kutikov A, Egleston BL, Canter D, Smaldone MC, Wong YN, Uzzo RG. Competing risks of death in patients with localized renal cell carcinoma: a comorbidity based model. The Journal of urology. 2012 Dec;188(6):2077–2083. doi: 10.1016/j.juro.2012.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kutikov A, Egleston BL, Wong YN, Uzzo RG. Evaluating overall survival and competing risks of death in patients with localized renal cell carcinoma using a comprehensive nomogram. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010 Jan 10;28(2):311–317. doi: 10.1200/JCO.2009.22.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corcoran AT, Russo P, Lowrance WT, et al. A review of contemporary data on surgically resected renal masses-benign or malignant? Urology. 2013 Apr;81(4):707–713. doi: 10.1016/j.urology.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Delongchamps NB, Galmiche L, Eiss D, et al. Hybrid tumour ‘oncocytoma-chromophobe renal cell carcinoma’ of the kidney: a report of seven sporadic cases. BJU international. 2009 May;103(10):1381–1384. doi: 10.1111/j.1464-410X.2008.08263.x. [DOI] [PubMed] [Google Scholar]
- 14.Mai KT, Dhamanaskar P, Belanger E, Stinson WA. Hybrid chromophobe renal cell neoplasm. Pathology, research and practice. 2005;201(5):385–389. doi: 10.1016/j.prp.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Aslam MI, Spencer L, Garcea G, et al. A case of liver metastasis from an oncocytoma with a focal area of chromophobe renal cell carcinoma: a wolf in sheep’s clothing. International journal of surgical pathology. 2009 Apr;17(2):158–162. doi: 10.1177/1066896908318741. [DOI] [PubMed] [Google Scholar]
- 16.Neuzillet Y, Lechevallier E, Andre M, Daniel L, Nahon O, Coulange C. Follow-up of renal oncocytoma diagnosed by percutaneous tumor biopsy. Urology. 2005 Dec;66(6):1181–1185. doi: 10.1016/j.urology.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Petersson F, Gatalica Z, Grossmann P, et al. Sporadic hybrid oncocytic/chromophobe tumor of the kidney: a clinicopathologic, histomorphologic, immunohistochemical, ultrastructural, and molecular cytogenetic study of 14 cases. Virchows Archiv : an international journal of pathology. 2010 Apr;456(4):355–365. doi: 10.1007/s00428-010-0898-4. [DOI] [PubMed] [Google Scholar]
- 18.Schmidbauer J, Remzi M, Memarsadeghi M, et al. Diagnostic accuracy of computed tomography-guided percutaneous biopsy of renal masses. European urology. 2008 May;53(5):1003–1011. doi: 10.1016/j.eururo.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 19.Noguchi S, Nagashima Y, Shuin T, et al. Renal oncocytoma containing “chromophobe” cells. International journal of urology : official journal of the Japanese Urological Association. 1995 Sep;2(4):279–280. doi: 10.1111/j.1442-2042.1995.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 20.Inomoto C, Umemura S, Sasaki Y, Yasuda M, Terachi T, Osamura RY. Renal cell carcinoma arising in a long pre-existing angiomyolipoma. Pathology international. 2007 Mar;57(3):162–166. doi: 10.1111/j.1440-1827.2006.02075.x. [DOI] [PubMed] [Google Scholar]
- 21.Delahunt B, Sika-Paotonu D, Bethwaite PB, et al. Fuhrman grading is not appropriate for chromophobe renal cell carcinoma. The American journal of surgical pathology. 2007 Jun;31(6):957–960. doi: 10.1097/01.pas.0000249446.28713.53. [DOI] [PubMed] [Google Scholar]
- 22.Cheville JC, Lohse CM, Sukov WR, Thompson RH, Leibovich BC. Chromophobe renal cell carcinoma: the impact of tumor grade on outcome. The American journal of surgical pathology. 2012 Jun;36(6):851–856. doi: 10.1097/PAS.0b013e3182496895. [DOI] [PubMed] [Google Scholar]
- 23.Kawaguchi S, Fernandes KA, Finelli A, Robinette M, Fleshner N, Jewett MA. Most renal oncocytomas appear to grow: observations of tumor kinetics with active surveillance. The Journal of urology. 2011 Oct;186(4):1218–1222. doi: 10.1016/j.juro.2011.05.080. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Ordonez B, Hamed G, Campbell S, et al. Renal oncocytoma: a clinicopathologic study of 70 cases. The American journal of surgical pathology. 1997 Aug;21(8):871–883. doi: 10.1097/00000478-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Haifler M, Copel L, Sandbank J, et al. Renal oncocytoma--are there sufficient grounds to consider surveillance following prenephrectomy histologic diagnosis. Urologic oncology. 2012 Jul-Aug;30(4):362–368. doi: 10.1016/j.urolonc.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 26.Trpkov K, Yilmaz A, Uzer D, et al. Renal oncocytoma revisited: a clinicopathological study of 109 cases with emphasis on problematic diagnostic features. Histopathology. 2010 Dec;57(6):893–906. doi: 10.1111/j.1365-2559.2010.03726.x. [DOI] [PubMed] [Google Scholar]
- 27.Keegan KA, Schupp CW, Chamie K, Hellenthal NJ, Evans CP, Koppie TM. Histopathology of surgically treated renal cell carcinoma: survival differences by subtype and stage. The Journal of urology. 2012 Aug;188(2):391–397. doi: 10.1016/j.juro.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patard JJ, Leray E, Rioux-Leclercq N, et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005 Apr 20;23(12):2763–2771. doi: 10.1200/JCO.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 29.Amin MB, Amin MB, Tamboli P, et al. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. The American journal of surgical pathology. 2002 Mar;26(3):281–291. doi: 10.1097/00000478-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Shannon BA, Cohen RJ, de Bruto H, Davies RJ. The value of preoperative needle core biopsy for diagnosing benign lesions among small, incidentally detected renal masses. The Journal of urology. 2008 Oct;180(4):1257–1261. doi: 10.1016/j.juro.2008.06.030. discussion 1261. [DOI] [PubMed] [Google Scholar]


