Abstract
Background
The anti-inflammatory properties of some flavonoids have been attributed to their ability to inhibit the production of NO by activated macrophages. Soybean cotyledons accumulate certain flavonoids following elicitation with an extract of the fungal pathogen Diaporthe phaseolorum f. sp. meridionalis (Dpm). Sodium nitroprusside (SNP), a nitric oxide donor, can substitute for Dpm in inducing flavonoid production. In this study, we investigated the effect of flavonoid-containing diffusates obtained from Dpm- and SNP-elicited soybean cotyledons on NO production by lipopolysaccharide (LPS)- and LPS plus interferon-γ (IFNγ)-activated murine macrophages.
Results
Significant inhibition of NO production, measured as nitrite formation, was observed when macrophages were activated in the presence of soybean diffusates from Dpm- or SNP-elicited cotyledons. This inhibition was dependent on the duration of exposure to the elicitor. Daidzein, genistein, luteolin and apigenin, the main flavonoids present in diffusates of elicited cotyledons, suppressed the NO production by LPS + IFNγ activated macrophages in a concentration-dependent manner, with IC50 values of 81.4 μM, 34.5 μM, 38.6 μM and 10.4 μM respectively. For macrophages activated with LPS alone, the IC50 values were 40.0 μM, 16.6 μM, 10.4 μM and 2.8 μM, respectively. Western blot analysis showed that iNOS expression was not affected by daidzein, was reduced by genistein, and was abolished by apigenin, luteolin and Dpm- and SNP-soybean diffusates at concentrations that significantly inhibited NO production by activated macrophages.
Conclusions
These results suggest that the suppressive effect of flavonoids on iNOS expression could account for the potent inhibitory effect of Dpm- and SNP-diffusates on NO production by activated macrophages. Since the physiological concentration of flavonoids in plants is normally low, the treatment of soybean tissues with SNP may provide a simple method for substantially increasing the concentration of metabolites that are beneficial for the treatment of chronic inflammatory diseases associated with NO production.
Background
Flavonoids are members of a class of natural pigments ubiquitous to plant cells and have a broad range of biological actions [1,2]. Various natural flavonoids are known to have anti-inflammatory activity in mammalian cells, and their action in inflammation has been attributed to their antioxidant activity, as well as to their ability to suppress NO production in macrophages [3-8].
NO synthesis by phagocytes depends on the expression of an NOS isoform (iNOS), which is induced by interferon-γ (IFNγ), tumor necrosis factor-α (TNFα) and bacterial endotoxins, and is crucial for eliminating intracellular pathogens internalized by these cells [9,10]. However, the continuous elevated production of NO may account for several disorders associated with chronicle inflammatory diseases [11,12]. Thus, natural flavonoids are very promising as therapeutic agents for the treatment of inflammation [13]. Several classes of natural flavonoids inhibit NO production by inflammatory cells such as activated peritoneal macrophages, RAW 264.7 cells and C6-astrocytes, in vitro [7,8,14-16]. The observation that pretreating mice with flavonoids suppressed the expression of proinflammatory molecules and reduced the lethality of LPS [17] indicates that natural flavonoids can also modulate the inflammatory process in vivo.
Many natural flavonoids have their biosynthesis activated in response to attack by pathogens [18]. The antibiotic activity of these secondary metabolites has been considered to have an important role in the protection of plants against microbe invasion [19]. Different classes of flavonoids are produced depending on the plant-microbe interaction [20]. The isoflavones daidzein and genistein, and the flavones apigenin and luteolin were recently identified as the main flavonoids accumulated in soybean cotyledons elicited with an extract of the phytopathogenic fungus, Diaporthe phaseolorum f. sp. meridionalis (Dpm), the causal agent of soybean stem canker disease [21]. An increase in L-citrulline production from L-arginine preceded Diaporthe-induced flavonoid biosynthesis in cotyledons, and pretreatment of cotyledons with NOS inhibitors reduced the flavonoid accumulation. Although the protein responsible for this activity has not yet been identified, these results suggested that soybean plants have a NOS enzymatic activity responsible for the production of NO that in turn acts as a signaling molecule for the activation of flavonoid biosynthesis. In fact, sodium nitroprusside (SNP), a nitric oxide (NO) donor, can substitute for Dpm in inducing flavonoid accumulation in soybean tissues [21].
Based on these findings, in the present study, we investigated the effects of flavonoid-containing diffusates obtained from elicited soybean cotyledons on NO production by stimulated peritoneal macrophages. The results show that flavonoids produced in response to Dpm or SNP can modulate iNOS expression and activity in macrophages to different extents.
Results
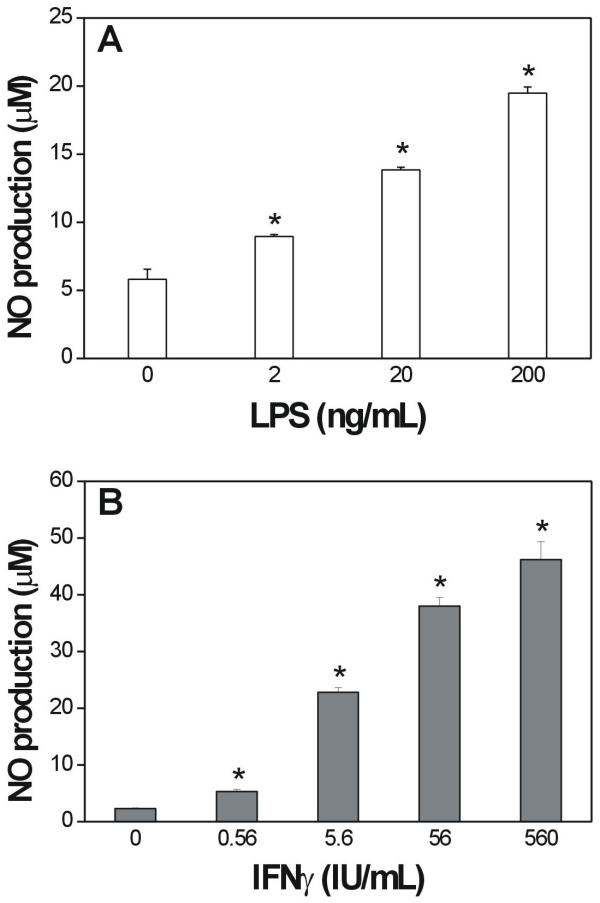
To determine the best conditions for inducing NO production by mouse macrophages, adherent peritoneal cells pre-treated with thioglycollate medium were cultured for 48 h in the presence of increasing concentrations of bacterial LPS. As shown in Fig. 1, LPS stimulated NO production by murine macrophages in a concentration-dependent manner (Panel A). The combination of LPS (20 ng/mL) with different concentrations of INFγ resulted in a synergistic effect, which enhanced the amounts of NO released by macrophages compared to the endotoxin alone (Fig. 1B). Based on these results, we chose an LPS concentration of 20 ng/mL alone or in combination with 56 IU of INFγ /mL to induce NO production in subsequent experiments.
Figure 1.
Concentration-dependent production of NO by macrophages stimulated with LPS and IFNγ. Macrophages were stimulated with LPS (A) or LPS (20 ng/mL) plus IFNγ (B) for 48 h, after which the cells were harvested and the NO released was measured as nitrite using the Griess reagent. The columns represent the means ± SE of three independent experiments, each done in quadruplicate. *P < 0.05 (by Student's t test).
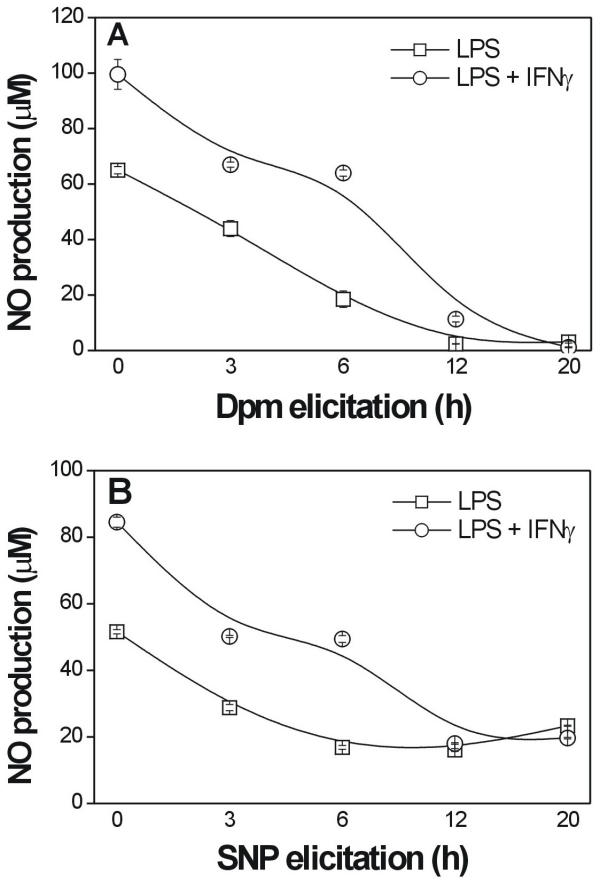
To examine the effect of soybean diffusates on NO production by LPS- or LPS + INFγ-stimulated peritoneal macrophages, the cells were treated with the inflammatory stimuli together with a 10-fold dilution of the plant diffusates, which were collected at different times after Dpm or SNP elicitation. As shown in Fig. 2, diffusates from Dpm- and SNP-elicited cotyledons (diluted 10-fold) had an inhibitory effect on NO production by LPS- or LPS + INFγ-activated macrophages that increased with the duration of elicitation. Significant inhibition of NO production was observed when murine macrophages were treated with diffusates collected as early as 6 h after exposure to the elicitors, and NO production was completely abolished by treating the cells with diffusates collected after 20 h (Fig. 2). The measurement of cell viability using the MTT assay showed that treatment of the macrophages with diffusates collected from soybean cotyledons elicited with Dpm or SNP did not cause macrophage death (data not shown).
Figure 2.
Effect of diffusates from elicited cotyledons on NO production by stimulated macrophages. Cells were cultured for 48 h with LPS (20 ng/mL) (square symbols) or LPS (20 ng/mL) plus IFNγ (56 IU/mL) (circle symbols) in the presence of plant diffusates collected at different times after Dpm (A) or SNP (B) inoculation. The concentrations of NO were expressed as nitrite. The points are the means ± SE of three independent experiments, each done in quadruplicate.
Previous analysis has shown that daidzein, genistein, luteolin and apigenin are the main flavonoids present in Dpm- and SNP-elicited cotyledons [21]. According to these results, time course analysis of isoflavone production using HPLC with detection at 286 nm revealed that daidzein and genistein were already detected after 6 h of elicitation with Dpm (3 mg/L and 6 mg/L, respectively) or SNP (24 mg/L and 42 mg/L, respectively). Maximal production of these compounds was reached after 12 h of elicitation with Dpm (daidzein – 60 mg/L and genistein – 39 mg/L) and SNP (daidzein – 91 mg/L and genistein – 60 mg/L). The level of these isoflavones decreased or remained unchanged after 20 h of Dpm- or SNP-elicitation, respectively. The soybean diffusates have also previously been analyzed for the presence of the flavones apigenin and luteolin since a spectral analysis showed a high absorbance at 350 nm, a wavelength typical of these compounds. Both elicitors induced a maximal accumulation of luteolin after 12 h (5.0 mg/L and 8.4 mg/L for Dpm- and SNP-elicitation, respectively), which then decreased after 20 h of incubation. Apigenin was induced mainly by Dpm and began to appear only after 12 h of elicitation (1.6 mg/L), increasing to 2.9 mg/L after 20 h, while SNP elicited the production of 1.2 mg of apigenin/L during this same period [21].
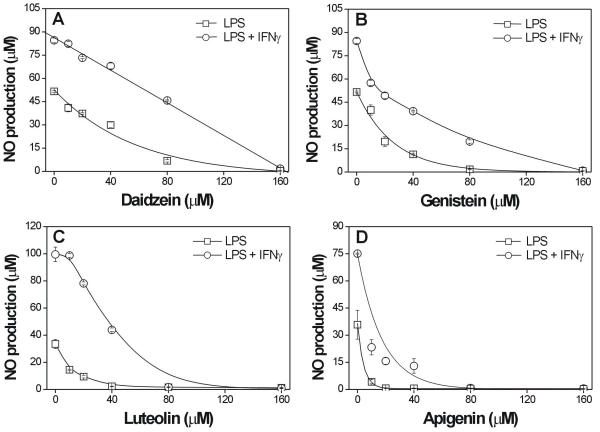
Considering that the flavonoid produced in higher amounts in soybean diffusates was daidzein (91 mg/L after 12 h of SNP elicitation) and that these diffusates were diluted ten-fold for the macrophage treatments, they would contain no more than 9.1 mg/L of each identified flavonoid since these compounds have a similar molecular weight. Thus, in the present work, we examined the effect of the main flavonoids present in soybean diffusates on NO production by macrophages, when applied at 10 μM to 160 μM, which would be equivalent to 2.5 to 40 mg/L of daidzein, and thus represent a concentration range that included the amounts of the different flavonoids found in the diffusates used to treat the macrophages. The results showed that, in the concentration range applied, all of the flavonoids inhibited NO production by macrophages, although to different extents (Fig. 3). Daidzein was the least potent, with an IC50 (the concentration causing 50% inhibition of NO production) of 40.0 μM and 81.4 μM in macrophages stimulated with LPS and LPS + INFγ, respectively. Complete inhibition was observed only at 80 μM and 160 μM of daidzein in macrophages stimulated with LPS and LPS + INFγ, respectively (Fig. 3A). Genistein inhibited NO production with an IC50 of 16.6 μM and 34.5 μM in macrophages stimulated with LPS and LPS + INFγ, respectively (Fig. 3B). The IC50 for luteolin was 10.4 μM and 38.6 μM for stimulation with LPS and LPS + INFγ, respectively (Fig. 3C). Apigenin was the most potent inhibitor and significantly reduced NO production by activated macrophages at concentrations as low as 10 μM (88% and 69% inhibition in LPS- and LPS + INFγ-activated macrophages, respectively) (Fig. 3D). The estimated IC50 for apigenin was 2.8 μM and 10.4 μM, respectively. In the concentration range used here, none of the flavonoids were toxic to the macrophages, as measured by MTT assays (data not shown).
Figure 3.
Dose-dependent effect of the isoflavones daidzein (A) and genistein (B) and the flavones luteolin (C) and apigenin (D) on NO production by stimulated macrophages. The cells were cultured for 48 h with LPS (20 ng/mL) (square symbols) or LPS (20 ng/mL) plus IFNγ (56 IU/mL) (circle symbols) in the presence of the indicated concentrations of isoflavones and flavones. The amount of NO released into the culture supernatants was expressed as nitrite. The points are the means ± SE of three independent experiments, each done in quadruplicate.
To determine whether the reduced NO production by macrophages exposed to soybean diffusates or to individual flavonoids was due to the inhibition of iNOS expression, crude extracts obtained from treated cells were analyzed by western blotting. In these experiments macrophages were stimulated for 12 h with LPS + INFγ alone or in combination with 80 μM apigenin or luteolin, 160 μM daidzein or genistein or 1/10 dilution of Dpm- or SNP-elicited diffusates. As shown in Figure 4, iNOS expression was prevented when macrophages were stimulated with LPS + INFγ in the presence of soybean diffusates collected from cotyledons elicited with Dpm or SNP for 20 h. Figure 4 also shows that, iNOS expression was inhibited when macrophages were activated in the presence of the flavones apigenin or luteolin. The isoflavones genistein and daidzein were less effective in preventing iNOS expression. The former slightly reduced the enzyme expression, whereas daidzein had no effect at concentrations that completely abolished NO production by activated macrophages (Fig. 3).
Figure 4.

Effect of soybean diffusates and flavonoids on iNOS expression in macrophages. Western blots were done using macrophage extracts obtained from cells activated with 20 ng/mL LPS plus 56 IU/mL IFNγ alone (Lane 1) or together with diffusates from soybean elicited with Dpm (Lane 2) or SNP (Lane 3) or with the flavonoids genistein (Lane 4), apigenin (Lane 5), luteolin (Lane 6) or daidzein (Lane 7). The arrow indicates a protein of ~130 kDa detected by the anti-mac NOS antibody.
Discussion
Flavonoids with antimicrobial activity are produced by plants in response to pathogen attack and are designed to prevent pathogen replication and spreading. Such responses can be induced either by elicitor components extracted from the pathogen or by abiotic elicitors. In soybean, an elicitor preparation of the phytopathogenic fungus Diaporthe phaseolorum f. sp. meridionalis (Dpm) induces the production of flavonoids and this response can be mimicked by the abiotic elicitor sodium nitroprusside [21].
Our results show that diffusates from soybean cotyledons elicited with a Dpm extract, as well as with SNP, adversely affected NO production induced by LPS or LPS + IFNγ in mouse peritoneal macrophages. This inhibitory activity of the soybean diffusates resulted from the inhibition of iNOS expression and/or activity. Although these stimuli did not cause macrophage death, we cannot role out the possibility that such treatments may have other adverse side effects, and should be investigated further in studies in vivo.
As shown elsewhere [21], Dpm and SNP diffusates contain mainly the phenylpropanoid derivatives daidzein, genistein, apigenin and luteolin, whose content increases with the duration of exposition of soybean cotyledons to the elicitor. To determine whether these main flavonoids present in Dpm- and SNP-elicited soybean diffusates could affect NO release by macrophages, mouse adherent peritoneal cells were treated with different concentrations of commercial preparations of these compounds during exposure to the inflammatory stimuli. The main flavonoids identified in the diffusates of elicited soybean cotyledons were able to inhibit NO production by activated macrophages in a dose-dependent manner. Based on the IC50 values obtained with moderate (LPS) and intense (LPS + INFγ) inflammatory stimuli, the order of potency for the inhibition of NO production by peritoneal macrophages was apigenin > luteolin > genistein > daidzein.
Western blot analysis showed that the inhibitory effect of the Dpm and SNP diffusates on NO production resulted mainly from the inhibition of macrophage iNOS expression by the flavonoids (Fig. 4). Accordingly, apigenin and luteolin prevented, genistein reduced and daidzein did not change iNOS expression in macrophages when tested at concentrations that significantly reduced NO production. The inhibitory effect of daidzein on NO production by macrophages may result from a direct inhibitory effect of this flavonoid in iNOS enzyme activity. The effectiveness of the inhibitory action of these compounds on NO production correlated positively with the extent to which iNOS expression was prevented in macrophages. Thus, the flavones apigenin and luteolin were more effective than the isoflavones genistein and daidzein in inhibiting NO production by activated peritoneal macrophages (Fig. 3), probably because of their ability to prevent iNOS enzyme expression. Kim and coworkers [3] showed that the inhibitory activity of natural flavonoids on NO production in LPS-activated RAW 264.7 macrophage cells was related to their chemical structure, i.e., flavonoids with a C-2,3 double bond, such as wongonin and luteolin, were able to inhibit strongly NO production through their ability to reduce iNOS expression. Liang and coworkers [22] showed that the inhibitory effect of apigenin and related flavonoids on NO production by LPS-activated RAW 264.7 cells was due to their ability to suppress the activation of nuclear factor kappa B (NF-κB), a transcription factor involved in regulating the expression of iNOS, and other inflammatory mediators. Other phytochemicals, such as resveratrol and avicins, also inhibit NO production by suppressing NF-κB activation [23,24]. Based on these studies, the potent inhibitory effect of the flavones used here could be attributed to their ability in suppress the transcriptional activation of iNOS in peritoneal macrophages.
The observation that iNOS expression in macrophages was also prevented when the cells were treated with diffusates elicited with Dpm or SNP suggests that the flavones, mainly apigenin, present in such diffusates could account for most of the inhibitory action of the diffusates on NO production in these cells. In agreement with this, time-course analyses of the accumulation of flavonoids showed that daidzein, genistein and luteolin began to appear after 6 h and reached peak levels after 12 h of Dpm-elicitation, whereas apigenin appeared only after 12 h and increased for up to 20 h after elicitation [21]. Indeed, the amount of flavonoids present in each diffusate correlated well with their inhibitory effect on NO production by macrophages. Thus, diffusates (10-fold diluted) collected 6 h after exposure to Dpm extract caused only partial inhibition (35.7%) of NO production by macrophages in the presence of strong inflammatory stimuli (LPS + INFγ), and such diffusates contained 0.3 mg/L daidzein, 0.6 mg/L genistein; 0.08 mg/L luteolin and 0.001 mg/L apigenin, concentrations that were considerably below the IC50 for the inhibition of NO production by these flavonoids (20.7 mg/L, 9.3 mg/L, 11 mg/L and 2.8 mg/L, respectively). Increased amounts of these flavonoids were present in 10-fold diluted 12 h Dpm diffusates (5.9 mg/L daidzein, 3.9 mg/L genistein, 0.5 mg/L luteolin and 0.16 mg/L apigenin) and, although the concentration of each flavonoid was below the corresponding IC50, the Dpm diffusates still showed a very potent inhibitory activity (88.6%) on NO production, suggesting a synergistic interaction between the flavonoids. NO production was abolished with 20 h Dpm diffusates. Although this extract contains lower amounts of daidzein (2.14 mg/L), genistein (0.9 mg/L) and luteolin (0.14 mg/L) its apigenin content (0.3 mg/L) is considerably increased compared with that of 12 h diffusates. These results suggest that apigenin could account for the potent inhibitory effect of 20 h Dpm-diffusates on NO production by activated macrophages, through its ability in suppress iNOS expression (Fig. 4).
SNP instead of Dpm could also substantially increase the inhibitory effect of soybean diffusates on iNOS expression and activity in macrophages. Although the 10-fold diluted 20 h SNP diffusates contained lower levels of apigenin (0.12 mg/L) than the Dpm diffusates (0.3 mg/L), the contents of daidzein, genistein and luteolin (10.2 mg/L, 4.2 mg/L and 0.4 mg/L, respectively) was much higher than that of the Dpm diffusates (2.1 mg/L, 0.9 mg/L and 0.14 mg/L, respectively). Thus, the inhibitory effect of SNP diffusates on NO production by murine macrophages may result from a synergistic interaction of the different flavonoids in inhibiting iNOS activity and expression. Since the physiological concentration of flavonoids in plants is usually low [2], these results suggest that SNP may be used to increase the production of metabolites that could serve as therapeutic agents for the treatment of several types of inflammatory diseases.
Although the results obtained here may not explain the control of NO production by flavonoids during the elicitation of soybean cotyledons, they do provide information for future experiments that may help in understanding the molecular interactions between flavonoids and NO during plant responses to microbial invasion. NO is an upstream element in the signal cascade that leads to the synthesis of soybean flavonoids in response to Dpm-elicitation [21]. This finding supports previous observations that NO can activate defense genes associated with initial enzymes of the phenylpropanoid pathway [25,26]. Once produced, flavonoids with antimicrobial activity would protect the plant against invading pathogens. However, an excess of NO can lead to tissue damage so that flavones produced later could prevent the deleterious effects of excessive NO through their excellent ability to scavenge radicals [2]. In animal cells, flavonoids can modulate signaling pathway components such as NF-κB involved in NO production prior to affecting iNOS expression [27]. Although no counterparts for these factors have been described in plants, the control of the transcriptional activation of an NOS-like enzyme by flavonoids could down regulate NO synthesis, thereby preventing an excess of nitrogen radical generation during plant-microbe interactions.
Conclusions
Flavonoid-containing diffusates obtained from Dpm- and SNP-elicited soybean cotyledons inhibited NO production induced by LPS or LPS + IFNγ in mouse peritoneal macrophages. The inhibitory activity of the soybean diffusates on NO production by macrophages resulted from the suppressive effect of its flavonoids content on iNOS activity and expression. SNP-elicitation could be used to increase the production of soybean flavonoids of medical interest for the treatment of inflammatory diseases associated with NO production.
Methods
Animals
Female BALB/c mice (1–4 weeks old) were obtained from the Central Animal House Service (CEMIB) at UNICAMP. The mice were maintained in a temperature-controlled room in the Department of Microbiology and Immunology and received water and food ad libitum throughout the study.
Reagents
The standard flavonoids luteolin, apigenin, daidzein, genistein and other reagents used were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The reagents used in electrophoresis were purchased from Bio-Rad (Hercules, CA, USA).
Plant material
Soybean (Glycine max [L.] Merr.) seeds of the cultivar IAC-18, resistant to the fungus Diaporthe phaseolorum (Cooke & Ellis) Sacc. f. sp. meridionalis (Dpm), were grown in vermiculite at room temperature in a green house. Cotyledons detached from 7–8-day-old seedlings were used for the elicitation assays.
Preparation of the elicitor
Elicitor from Dpm (strain 8498) was obtained by autoclaving aqueous spore suspensions of 30–40-day-old cultures grown in PDA media in the dark at room temperature. The autoclaved suspension was centrifuged at 10,000 g for 6 min and the pellet was discarded. Oligossacharides extracted from the cell wall of Dpm spores, which are compounds known to elicit defence response in plant tissues [28], were quantified in the supernatant as total carbohydrates by the phenol-sulfuric procedure using glucose as standard. Oligossacharides from Dpm (0.4 mg/mL) and an aqueous solution of sodium nitroprusside (SNP, 10 mM) were used for soybean cotyledon elicitation.
Elicitation assay
The plant diffusates were prepared using the soybean cotyledon assay in which a small section (i.d. = 1.0 cm) was removed from the adaxial surface of each cotyledon and the wounded surface was then treated with 50 μL of elicitor (Dpm or SNP). The cotyledons were kept in the dark at 26°C for different periods of time, after which the diffusates were collected and diluted ten fold for use in the activation of macrophages. The efficiency in eliciting flavonoid production was evaluated by HPLC analysis, as previously described [21].
Macrophage culture
Macrophages were harvested with 3 mL of RPMI medium from peritoneal cavities of naive or stimulated (1–3 mL of 3% thioglycollate medium; Gibco, Gaithersburg, MD; 4 days before) mice. The cells were centrifuged and adjusted to 1 × 106/mL in RPMI 1640 medium supplemented with 12.5 mM HEPES, 2 g of sodium bicarbonate/L, 2 mM L-glutamine, 50 μg of gentamicin/mL and 10% fetal bovine serum (complete medium). The cell suspensions were seeded onto 96-well flat-bottomed plates (Corning Corporation; Cambridge, MA, USA) at a density of 2 × 105/200 μL/well and then incubated for 2 h in a humidified atmosphere with 5% CO2 at 37°C to allow the cells to adhere. Adherent cells (95% macrophages) were washed three times with PBS (pH 7.2), and incubated for 48 h with either RPMI medium alone or medium containing the indicated concentrations of LPS and/or IFNγ. Different amounts of standard flavonoids or soybean diffusates were added to the cultures simultaneously with the stimuli.
Measurement of nitrite concentration
NO released into the supernatants of mouse macrophages was determined by the standard Griess reaction by adding 50 μL of test solution to 96-well flat-bottomed plates containing 50 μL of Griess reagent [1% sulfanilamide/0.1% N-(1-naphthyl)ethylenediamine dihydrochloride/2.5% H3PO4]. The samples were assayed in quadruplicate. After 15 min at room temperature, the absorbance of each well was measured in a Multiskan MS microplate reader (Labsystems Oy, Helsink, Finland) at 540 nm and the nitrite concentration was determined from a standard curve of sodium nitrite. The results were expressed as μM nitrite/106 cells. Statistical analysis was done using Student's t test, with a value of P < 0.05 indicating significance.
iNOS detection by western blotting
Peritoneal macrophage monolayers were prepared as described above, except the cells were seeded onto plastic Petri dishes at a density of 1 × 107 cells/5 mL/dish (Corning). After a 2 h incubation, the non-adherent cells were removed by washing and the macrophages were then treated with LPS + IFNγ in the absence or presence of soybean diffusates or flavonoids, as indicated in the Results. The plates were incubated for an additional 12 h, under the same conditions as described above. To remove the stimuli, the plastic dishes were washed three times with PBS, and the cells then lysed with 1 mL of sample buffer (125 mM Tris-HCl pH 6.8, 2% SDS, 5% glycerol, 0.003% bromophenol blue and 1% β-mercaptoethanol). The lysates were boiled for 3 min and the debris was removed by centrifugation at 10,000 × g (Eppendorf Micro Centrifuge 5415 C, Brinkmann Instruments, Inc., NY, USA) and the supernatant was used for western blotting.
The polypeptides in macrophage samples were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) according to [29], using a 7% acrylamide slab gel with a 3% acrylamide stacking gel, in a Mini-Protean® II Dual Slab Cell (Bio-Rad). The current was set at 25 mA and the gels were run for 2 h. After electrophoresis, the gels were blotted onto 0.45 μm nitrocellulose membranes (Schleicher & Schüll, Inc., Keene, NH, USA) for 2 h at 180 mA [30]. The membranes were then blocked by incubation for 1 h with 5% non-fat powdered milk in Tris-buffered saline (TBS: 20 mM Tris-HCl, pH 7.6, 137 mM NaCl) containing 0.05% Tween 20 (TBS-Tween), and then rinsed three times with TBS-Tween. The protein blots were probed with mouse IgG2a anti-macNOS at 0.5 μg.mL-1 (Transduction Laboratories, Lexington, HY, USA). After overnight incubation at 4°C, the blots were washed six times with TBS-Tween (10 min each) at room temperature followed by treatment overnight at 4°C with an anti-mouse rabbit IgG-horseradish peroxidase conjugate in TBS-Tween. The blots were then washed six times with TBS (10 min each) at room temperature and the colour reaction was developed using 0.6 mg 3,3'-diaminobenzidine/mL (Sigma) in 50 mM Tris-HCl, pH 7.4, and 0.03% H2O2.
Authors' contributions
LSS, PUS and DLG carried out the experiments with macrophage cells, EES carried out the western blotting, WMSCT supervised the work of LSS, PUS and DLG and participated in drafting the manuscript, LVM prepared the soybean diffusates and participated in drafting the manuscript, and IS conceived the study, participated in its design and coordination, and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgments
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). We thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for research fellowships.
Contributor Information
Loren S Scuro, Email: jubarte100@hotmail.com.
PU Simioni, Email: psimioni@unicamp.br.
DL Grabriel, Email: gabriel@unicamp.br.
Elzira E Saviani, Email: esaviani@unicamp.br.
Luzia V Modolo, Email: modolo@unicamp.br.
Wirla MSC Tamashiro, Email: wirlatam@unicamp.br.
Ione Salgado, Email: ione@unicamp.br.
References
- McClure JW. Physiology and functions of flavonoids. In: Harborne JB and Mabry IJ, editor. In The Flavonoids. New York: Academic Press; 1975. pp. 970–1055. [Google Scholar]
- Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002;96:67–202. doi: 10.1016/S0163-7258(02)00298-X. [DOI] [PubMed] [Google Scholar]
- Kim HK, Cheon BS, Kim YH, Kim SY, Kim HP. Effects of naturally occurring flavonoids on nitric oxide production in macrophage cell line RAW 264.7 and their structure – activity relationships. Biochem Pharmacol. 1999;58:759–765. doi: 10.1016/S0006-2952(99)00160-4. [DOI] [PubMed] [Google Scholar]
- Bastianetto S, Zheng WH, Quirion R. Neuroprotective abilities of resveratrol and other red wine constituents against nitric oxide-related toxicity in cultured hippocampal neurons. Br J Pharmacol. 2000;131:711–720. doi: 10.1038/sj.bjp.0703626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor L, Pezzato E, Dell'Aica I, Caniato R, Biggin S, Garbisa S. Inhibition of matrix-proteases by polyphenols: chemical insights for anti-inflammatory and anti-invasion drug design. Biochem Pharmacol. 2002;64:229–37. doi: 10.1016/S0006-2952(02)01069-9. [DOI] [PubMed] [Google Scholar]
- Bito T, Roy S, Sen CK, Shirakawa T, Gotoh A, Ueda M, Ichihashi M, Packer L. Flavonoids differentially regulate IFN gamma-induced ICAM-1 expression in human keratinocytes: molecular mechanisms of action. FEBS Lett. 2002;520:145–152. doi: 10.1016/S0014-5793(02)02810-7. [DOI] [PubMed] [Google Scholar]
- Wang J, Mazza G. Inhibitory effects of anthocyanins and other phenolic compounds on nitric oxide production in LPS/IFN-gamma-activated RAW 264.7 macrophages. J Agric Food Chem. 2002;50:850–857. doi: 10.1021/jf010976a. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Morikawa T, Ando S, Toguchida I, Yoshikawa M. Structural requirements of flavonoids for nitric oxide production inhibitory activity and mechanism of action. Bioorg Med Chem. 2003;11:1995–2000. doi: 10.1016/S0968-0896(03)00067-1. [DOI] [PubMed] [Google Scholar]
- Green SJ, Nacy CA, Meltzer MS. Cytokine-induced synthesis of nitrogen oxides in macrophages: a protective host response to Leishmania and other intracellular pathogens. J Leukoc Biol. 1991;50:93–103. doi: 10.1002/jlb.50.1.93. [DOI] [PubMed] [Google Scholar]
- Nathan CF, Hibbs JB. Role of nitric-oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3:65–70. doi: 10.1016/0952-7915(91)90079-G. [DOI] [PubMed] [Google Scholar]
- Lamas S, Pérez-Sala D, Moncada S. Nitric oxide: from discovery to the clinic. Trends Pharmacol Sci. 1998;19:436–438. doi: 10.1016/S0165-6147(98)01265-6. [DOI] [PubMed] [Google Scholar]
- Grisham MB, Pavlick KP, Laroux FS, Hoffman J, Bharwani S, Wolf RE. Nitric oxide and chronic gut inflammation: controversies in inflammatory bowel disease. J Investig Med. 2002;50:272–283. doi: 10.2310/6650.2002.33281. [DOI] [PubMed] [Google Scholar]
- Lai HH, Yen GC. Inhibitory effect of isoflavones on peroxynitrite-mediated low-density lipoprotein oxidation. Biosci Biotechnol Biochem. 2002;66:22–28. doi: 10.1271/bbb.66.22. [DOI] [PubMed] [Google Scholar]
- Krol W, Czuba ZP, Treadgill MD, Cunningham BD, Pietse G. Inhibition of nitric oxide (NO) production in murine macrophages by flavones. Biochem Pharmacol. 1995;50:1031–1035. doi: 10.1016/0006-2952(95)00237-T. [DOI] [PubMed] [Google Scholar]
- Sadowska-Krowicka H, Mannick EE, Oliver PD, Sandoval M, Zhang XJ, Eloby-Chiless S, Clark DA, Miller MJS. Genistein and gut inflammation: role of nitric oxide. Proc Soc Exp Biol Med. 1998;217:351–357. doi: 10.3181/00379727-217-44244. [DOI] [PubMed] [Google Scholar]
- Soliman KF, Mazzio EA. In vitro attenuation of nitric oxide production in C6 astrocyte cell culture by various dietary compounds. Proc Soc Exp Biol Med. 1998;218:390–397. doi: 10.3181/00379727-218-44309. [DOI] [PubMed] [Google Scholar]
- Kotanidou A, Xagorari A, Bagli E, Kitsanta P, Fotisis T, Papapetropoulos A, Roussos C. Luteolin reduces lipopolysaccharide-induced lethal toxicity and expression proinflammatory molecules in mice. Am J Respir Crit Care Med. 2002;165:818–823. doi: 10.1164/ajrccm.165.6.2101049. [DOI] [PubMed] [Google Scholar]
- Paxton JD. Biosynthesis and accumulation of legume phytoalexins. In: Sharma RP and Shalunke DK, editor. In Mycotoxins and Phytoalexins. Boca Raton: CRC Press Inc; 1991. pp. 485–499. [Google Scholar]
- Hahlbrock K, Scheel D. Physiology and molecular-biology of phenylpropanoid metabolism. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:347–369. doi: 10.1146/annurev.pp.40.060189.002023. [DOI] [Google Scholar]
- Dixon RA, Paiva NL. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995;7:1085–1097. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modolo LV, Cunha FQ, Braga MR, Salgado I. Nitric oxide synthase-mediated phytoalexin accumulation in soybean cotyledons in response to the Diaporthe phaseolorum f. sp. meridionalis elicitor. Plant Physiol. 2002;130:1288–1297. doi: 10.1104/pp.005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YC, Huang YT, Tsai SH, Lin-Shiau SY, Chen CF, Lin JK. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. Carcinogenesis. 1999;20:1945–1952. doi: 10.1093/carcin/20.10.1945. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis. 2001;480:243–268. doi: 10.1016/S0027-5107(01)00183-X. [DOI] [PubMed] [Google Scholar]
- Haridas V, Arntzen CJ, Gutterman JU. Avicins, a family of triterpenoid saponins from Acacia victoriae (Bentham), inhibit activation of nuclear factor-kappa B by inhibiting both its nuclear localization and ability to bind DNA. Proc Natl Acad Sci USA. 2001;98:11557–11562. doi: 10.1073/pnas.191363498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delledonne M, Xia Y, Dixon RA, Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394:585–588. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- Durner J, Wendehenne D, Klessig DF. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Natl Acad Sci USA. 1998;95:10328–10333. doi: 10.1073/pnas.95.17.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kim YS, Kim SY, Suk K. The plant flavonoid wogonin suppresses death of activated C6 rat glial cells by inhibiting nitric oxide production. Neurosci Lett. 2001;309:67–71. doi: 10.1016/S0304-3940(01)02028-6. [DOI] [PubMed] [Google Scholar]
- Bailey JA, Mansfield JW. Phytoalexins. Blackie & Son Limited: Bishopbriggs; 1982. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets – procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]