Abstract
The reinforcing effectiveness of a sensory stimulus such as light-onset rapidly habituates (Lloyd, Gancarz, Ashrafioun, Kausch, & Richards, 2012). According to memory-based theories, habituation occurs if a memory exists for perceived stimulation, and dishabituation occurs if a memory does not exist and the stimulation is “unexpected.” According to Redgrave and Gurney (2006), unexpected response-contingent sensory stimuli increase phasic firing of dopamine neurons, providing a sensory error signal that reflects the difference between perceived and expected stimuli. Together, memory-based theories of habituation and the sensory error signal hypothesis predict a disruption (slowing) of habituation rate by novel response-contingent sensory stimulation or by artificial increases in dopamine neurotransmission by stimulant drugs. To test these predictions, we examined the effects of stimulant drugs on both the operant level of responding (snout-poking) and operant responding for a sensory reinforcer (light-onset) presented according to a fixed ratio 1 schedule. Robust within-session decreases in responding indicating habituation were observed. The effects of stimulant drugs (saline, n = 10; nicotine, 0.40 mg/kg, n = 10; and methamphetamine, 0.75 mg/kg, n = 9) on habituation in rats were determined. Nicotine was found to decrease habituation rate and did not affect response rate, while methamphetamine decreased habituation rate and increased response rate. In addition, introduction of a novel visual stimulus reinforcer decreased habituation rate and increased responding. These findings show that habituation of reinforcer effectiveness modulates operant responding for sensory reinforcers, and that stimulant drugs may disrupt normally occurring habituation of reinforcer effectiveness by increasing dopamine neurotransmission.
Keywords: dopamine, operant conditioning, psychomotor stimulants, rats, sensory stimuli
Indifferent sensory stimuli, such as light-onset, can be reinforcers (for reviews, see Berlyne, 1969; Eisenberger, 1972; Glanzer, 1958; Kish, 1966; Lockard, 1963; Tapp, 1969). In comparison to important biological reinforcers such as food and water, the reinforcing effectiveness of sensory reinforcers is weak and transient. Responding for sensory reinforcers has often been characterized as investigatory behavior. Experiments with light reinforcement were first reported by Girdner (1953), who considered the operant responding for novel light-onset analogous to investigatory loco-motor responses that expose organisms to novel environmental change (as described by Kish, 1966, p. 111). For example, exploratory activity is high when a rat is first placed into a novel locomotor chamber and then decreases within the same session. Similarly, operant responding for novel light-onset is initially high at the start of a test session and then shows a within-session decline (Gancarz, Ashrafioun, et al., 2012; Gancarz, Robble, Kausch, Lloyd, & Richards, 2012).
Within-session declines in locomotor activity and responding for novel light-onset are often characterized as indicators of habituation involving memory processes. A rat, when placed in the novel chamber, forms an internal representation (memory) of the chamber and the objects within and around it (Antunes & Biala, 2012; Leussis & Bolivar, 2006). Locomotor activity decreases as a consistent internal representation of the test chamber is formed and perceived stimuli are recognized. This interpretation is consistent with memory-based explanations of habituation (Konorski, 1963; Sokolov, 1963; Wagner, 1979). According to memory-based explanations of habituation, perceived stimuli are compared with existing memory. If the perceived sensory stimuli do not match memory, they are novel and cause dishabituation. On the other hand, if the perceived stimuli match memory, then they are not novel or surprising and do not cause dishabituation.
McSweeney and Murphy (2009) have argued that within-session declines in operant responding are a function of habituation to the sensory properties of repeatedly presented reinforcers. In agreement with their work, we have provided evidence that within-session declines in the effectiveness of sensory reinforcers is attributable to habituation (Lloyd, Gancarz, Ashrafioun, Kausch, & Richards, 2012). Within-session decreases in light-reinforced responding can be reasonably attributed to habituation of reinforcer effectiveness because there is a programmed contingency between responding and light-onset. In contrast, locomotor activity in a novel locomotor chamber is more likely to be described as elicited or evoked by exposure to novel environmental stimuli, suggesting that locomotor activity in a novel environment is a Pavlovian process. However, as has been previously pointed out (Berlyne, 1960), exploratory behavior can be characterized as a mixture of Pavlovian and operant processes.
Novel sensory stimuli such as light-onset activate orienting or targeting reflexes that bring sensory receptors into contact with the sensory stimulus (Sokolov, 1963). Some of the sensory stimuli (e.g., localized changes in relative illumination) evoke targeting reflexes and are also reinforcers that increase the probability of the responses that produced them. It is likely that naturally occurring contingencies between responding and sensory stimulation that occur while an animal is exploring a novel environment may similarly increase locomotor activity in a novel environment. Thus, even though there are no programmed operant contingencies in a novel locomotor chamber, the habituation of the reinforcing effectiveness of inherent operant contingencies may contribute to declines in exploratory activity. This is supported by evidence from McSweeney and Swindell (1999) who showed that decreases in exploratory activity follow the same mathematical relationship as habituation in other paradigms. As was originally suggested by Girdner (1953), operant responding to produce light-onset may be an effective way to precisely measure exploratory behavior.
Habituation to the reinforcing effectiveness of sensory stimuli may be mediated by decreases in dopamine (DA) neurotransmission evoked by novel or surprising sensory stimuli. Novel sensory stimuli increase phasic firing of DA neurons. This effect rapidly habituates when sensory stimuli are repeatedly presented (Ljungberg, Apicella, & Schultz, 1992). Redgrave and Gurney (2006) have hypothesized that phasic firing of DA neurons elicited by novel sensory stimuli activate the animal to repeat responses that produced the novel sensory stimulation. According to Redgrave and Gurney, this DA-mediated mechanism allows organisms to “discover” contingencies between investigatory behaviors and novel sensory events. Following initial “discovery,” phasic firing of DA neurons is cancelled by timed inhibitory input to DA as the sensory stimulus becomes predictable (familiar) through repetition.
Redgrave and Gurney (2006) have described the phasic firing of DA neurons evoked by novel sensory stimulation as a sensory prediction error signal. The concept of phasic firing of DA neurons as a function of the difference between perceived and remembered stimuli fits well with memory-based explanations of habituation, where comparison of perceived and remembered stimuli leads to the identification of novel stimuli that produce dishabituation (arousal). However, instead of arousal, Redgrave and Gurney emphasize that an increase in DA neurotransmission evoked by novel stimuli determines the reinforcing effectiveness (or effects) of indifferent sensory stimuli. More specifically, they hypothesize that novelty-induced increases in DA cause the animal to repeat actions that precede novel stimuli, leading to the discovery of the action that produced it.
The hypothesis that the reinforcing effects of sensory stimuli rapidly habituate, in combination with the concept of phasic DA as a sensory prediction error signal that mediates the behavioral expression of habituation, makes predictions that can be tested with both behavioral and drug manipulations. To test these predictions we have developed a method for quantitatively characterizing the rate of habituation for operant responding that takes into account relative differences in response rate so that the habituation rate (HR) measure is independent of absolute response rate measures.
In Phase 1 of the current experiment, we use this method to measure the HR of the operant level of snout poking in a novel environment. Memory-based theories of habituation predict that the rate of habituation will increase with repeated exposure to the novel environment as sensory consequences of investigatory behaviors become familiar. The DA as a sensory error signal hypothesis predicts that phasic firing of DA neurons will decrease with repeated exposure, resulting in decreased activation. Both theories therefore predict that repeated exposure will lead to a decrease in measured operant level responding (snout-poking).
In Phase 2, the effects of the stimulant drugs nicotine (NIC) and methamphetamine (METH) on HR are measured. The DA as a sensory error signal hypothesis predicts that stimulant drugs such as NIC and METH which artificially increase DA neurotransmission will disrupt (decrease) expression of normally occurring habituation.
In Phase 3, the effects on HR of making a novel sensory stimulus contingent upon snout poking are tested. Memory-based theories of habituation predict that introduction of a novel response-contingent sensory stimulus will decrease the rate of habituation. In addition, the DA as a sensory error signal hypothesis predicts that introduction of a novel stimulus will increase DA neurotransmission and increase activation.
The apparatus and procedures used in this experiment have previously been found to reliably produce light reinforced responding (Gancarz, Ashrafioun, et al., 2012; Gancarz, Robble, Kausch, Lloyd, & Richards, 2012, 2013; Lloyd, Gancarz, et al., 2012; Lloyd, Kausch, Gancarz, Beyley, & Richards, 2012). We selected 0.75 mg/kg METH for this experiment because it was within the range of known effective doses (Gancarz, Ashrafioun, et al., 2012), and we selected the NIC dose of 0.4 mg/kg because this dose was found to effectively increase light reinforced responding (Palmatier et al., 2007).
Materials and Methods
General Method
Subjects
Thirty naïve Holtzman Sprague-Dawley (Harlan Laboratories Inc., Indianapolis, IN) male rats weighing between 250 and 400 g at the time of testing were used. Rats were housed in pairs in plastic cages in a colony room on a 12 hour light–dark cycle. Testing took place once per day, during the light phase, 7 days a week, and each test session was 40 min in duration. The rats had ad libitum access to food and water. The study was conducted in accordance with the guidelines set up by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo.
Apparatus
Sixteen experimental chambers, previously described in detail (Richards, Mitchell, de Wit, & Seiden, 1997), were used for the experiment. These chambers have stainless steel grid floors, aluminum front and back panels, and Plexiglas sides and tops. One wall had two snout poke apertures measuring 4 cm in diameter and located 3 cm from the left and right edges of the wall. The test chambers were located inside of sound and light attenuating boxes with wall mounted fans that provided ventilation and masking noise. The visual stimulus (VS) used in the experiment was the onset of two lights: one of the lights was located above and midway between the two snout poke apertures and the other was located in the middle of the back wall of the test chamber. Onset of the VS produced an illuminance of 68 lux, as measured from the center of the test chamber. The VS was illuminated for 5 s each time it was presented. Snout pokes were monitored with infrared photo sensors located in the snout poke apertures. The chambers were connected to a computer using a MED Associates (St. Albans, VT) interface. MED-PC programming language was used for programming of the experimental contingencies.
Drug injections
All doses were calculated to form solutions resulting in a 1 ml/kg injection volume. NIC (-) nicotine ([-]-1-methyl-2-[3-pyridyl] pyrrolidine hydrogen tartrate salt) obtained from Sigma (Lot 082K1316) was dissolved in saline to form a 0.4 mg/kg dose calculated as a salt. NIC solutions were pH adjusted to 7.0 using diluted hydrochloric acid (HCl) and/or sodium hydroxide (NaOH). METH (methamphetamine d-N, α-dimethylphenethlyamine; d-desoxyephedrine hydrochloride) obtained from Sigma (Lot 054K0842) was dissolved in saline to form a 0.75 mg/kg dose calculated as a salt.
Injections were administered 15 min prior to the start of each test session. During the Operant Level phase described below, all animals were injected with SAL (saline). During the Drug Alone and the Drug & VS phases, rats were injected with SAL (veh), NIC (0.4 mg/kg), or METH (0.75 mg/kg). SAL and METH were administered intraperitoneally. NIC was administered subcutaneously.
Procedure
Operant Level phase (Sessions 1–10)
During the Operant Level phase, rats were injected with SAL and placed in dark experimental chambers. Responses to both snout poke apertures were recorded, but had no programmed consequences.
Drug Alone phase (Sessions 11–20)
The Drug Alone phase was identical to the Operant Level phase except that prior to each session rats received an injection of SAL, NIC, or METH. All rats were randomly assigned to their drug group which was unchanged for the duration of the experiment.
Drug and Visual Stimulus (Drug & VS) phase (Sessions 21–25)
During the Drug & VS phase, rats were placed in dark experimental chambers and snout pokes into the aperture designated as “active” resulted in a 5-s illumination of the two chamber lights. On Days 21–25, the VS was available according to a fixed ratio 1 (FR1) schedule of reinforcement. The “active” aperture was balanced across subjects such that half of the subjects in each group had a left “active” aperture, and the other half of the subjects had a right “active” aperture. Pokes into the other aperture, designated ‘inactive,’ were recorded but had no programmed consequence. Prior to each session, rats were injected with SAL, NIC, or METH (based on the group assignment they received during the Drug Alone phase).
Data Analysis
HR rate measure
We used a novel method to quantify HR. Habituation assumes a declining rate of responding as a function of repeated stimulation. Our HR metric estimates the rate at which responding declines during a test session. Importantly, the HR measure is calculated so that absolute differences in response rate do not affect the HR estimate. As others (Leussis & Bolivar, 2006; McSweeney, Hinson, & Cannon, 1996) have pointed out, if differences in baseline responding are not taken into consideration, differences attributed to habituation may actually be because of baseline differences in absolute response levels. Calculation of HR can be understood as a three step process.
Organize the data into epochs indicating the absolute rate of responding that occurred in each epoch, (choice of epoch length is arbitrary).
Convert the absolute responding per epoch measures to a percentage of total-session responding.
Use the normalized percentage values to calculate the difference in the percentage of responding between the first epoch and the minimum epoch (i.e., the epoch having the lowest percent of responses). This difference is then divided by the amount of time that elapsed between the first epoch and the minimum epoch to produce a rate measure.
In practical application, HR can be calculated directly from epoch data using the equation:
| (1) |
where RsFirstEpoch represents the number of responses made during the first epoch of a session, RsMinEpoch is the number of responses made during the epoch with the fewest number of responses, TotalRs is the total number of responses made during a session, and TimeBetweenEpochs is the amount of time elapsed from the midpoint of the first epoch to the midpoint of the minimum epoch.
Dependent variables
Snout pokes to the active and inactive holes were recorded for each session. A snout poke was operationally defined as interruption of the infrared photobeam. Data from each 40-min session was divided into five 8-min epochs (0–8 min, 8–16 min . . . 32–40 min). The first epoch of each session served as the initial responding rate (IR), the average number of responses made per epoch (AR) served as a measure of total session responding, and HR was calculated as describe above. These three measures AR, IR, and HR, were the dependent variables.
Statistical Analysis
One rat in the METH group had five-session AR scores greater than 19 SDs from the average for his group. He was identified as an outlier and was excluded.
The three dependent variables (AR, IR, and HR) were analyzed with separate mixed- factor analyses of variance (ANOVAs), with block and side as the within-subject variables and drug group as the between-subjects variable. Side indicated the nose poke aperture that was assigned to be active or inactive during the Drug & VS phase. Separate statistical analyses were performed for the three phases (Operant Level, Drug Alone, and Drug & VS) of the study.
As indicated, these overall ANOVAs were followed with appropriate post hoc tests to determine sources of significance. Statistical analysis was conducted using IBM SPSS 20. Unless otherwise noted, for all statistical tests an alpha criterion of p = .05 was used.
Results
Operant Level Phase
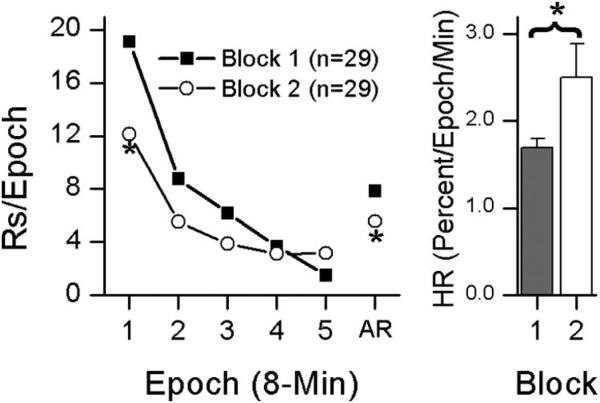
The effects of repeated testing were examined by comparing performance during Block 1 of the Operant Level phase with Block 2 of the Operant Level phase for HR, IR, and AR. The overall ANOVAs indicted that there was a significant effect of block for all three dependent measures; AR, F(1,26) = 26.993, p < .001; IR, F(1,26) = 44.714, p < .001; and HR, F(1,26) = 6.644, p = .016, but no significant effects of drug group or side nor any interactions. Because there were no effects of side or group, the data were collapsed across group and side (rates of active, inactive, and the relative frequency of active responding are shown in Table 1). As is indicated in Figure 1, the comparison of Block 1 with Block 2 of the Operant Level phase indicates that HR increased while IR and AR decreased with repeated testing.
Table 1.
Average Responding by Five-Session Block Over the Three Experimental Phases
| Operant level |
Drug alone |
Drug & VS Block 5 Sessions 21-25 | |||
|---|---|---|---|---|---|
| Drug Group | Block 1 Sessions 1-5 | Block 2 Sessions 6 -10 | Block 3 Sessions 11-15 | Block 4 Sessions 16 -20 | |
| SAL (n = 10) | |||||
| Active | 20.8 ± 1.6 | 13.2 ± 2.3 | 12.8 ± 2.3 | 11.0 ± 1.4 | 41.1 ± 7.9 |
| Inactive | 20.6 ± 2.1 | 14.9 ± 3.9 | 12.2 ± 3.2 | 12.3 ± 2.5 | 23.0 ± 7.7 |
| ActRfq | 0.51 ± 0.03 | 0.51 ± 0.06 | 0.56 ± 0.06 | 0.50 ± 0.06 | 0.66 ± 0.07 |
| NIC (n = 10) | |||||
| Active | 20.0 ± 2.7 | 15.7 ± 3.4 | 14.1 ± 5.5 | 16.8 ± 4.1 | 53.4 ± 8.4 |
| Inactive | 16.4 ± 2.4 | 12.0 ± 3.8 | 18.4 ± 12.9 | 28.5 ± 19.8 | 34.2 ± 18.7 |
| ActRfq | 0.55 ± 0.05 | 0.58 ± 0.07 | 0.56 ± 0.07 | 0.55 ± 0.06 | 0.71 ± 0.05 |
| METH (n = 9) | |||||
| Active | 16.6 ± 1.6 | 12.0 ± 1.8 | 32.0 ± 8.7 | 26.2 ± 7.2 | 73.0 ± 9.6 |
| Inactive | 21.8 ± 5.4 | 14.2 ± 4.0 | 36.6 ± 12.3 | 50.5 ± 21.1 | 40.0 ± 13.1 |
| ActRfq | 0.47 ± 0.05 | 0.52 ± 0.07 | 0.52 ± 0.05 | 0.46 ± 0.08 | 0.67 ± 0.05 |
Note. ActRFq = relative frequency of active responding. Active, inactive, and relative frequency of active responding are presented. Data are organized by drug group (saline [SAL], nicotine [NIC], methamphetamine [METH]). The relative frequency of active responding (ActRfq = active/[active + inactive]) indicates degree of preference for the active alternative. Values are the mean ± the SE of active and inactive responding emitted in each test session during the 5-session test blocks.
Figure 1.
Comparison of the changes in snout poking responding between the first 5-session block of the Operant Level phase (Block 1) and the second 5-session block of the Operant Level phase (Block 2). The left column of Figure 1 shows within-session responding during the Operant Level phase plotted in 8-min epochs. The right column shows habituation rate. Asterisks indicate significant p < .05.
Drug Alone Phase
The effects of drug treatment on the HR during the 10 session Drug Alone phase were compared with HR during the last 5 sessions of the Operant Level phase (Figure 2). Rats in three different groups were treated with SAL, NIC, or METH throughout the Drug Alone phase.
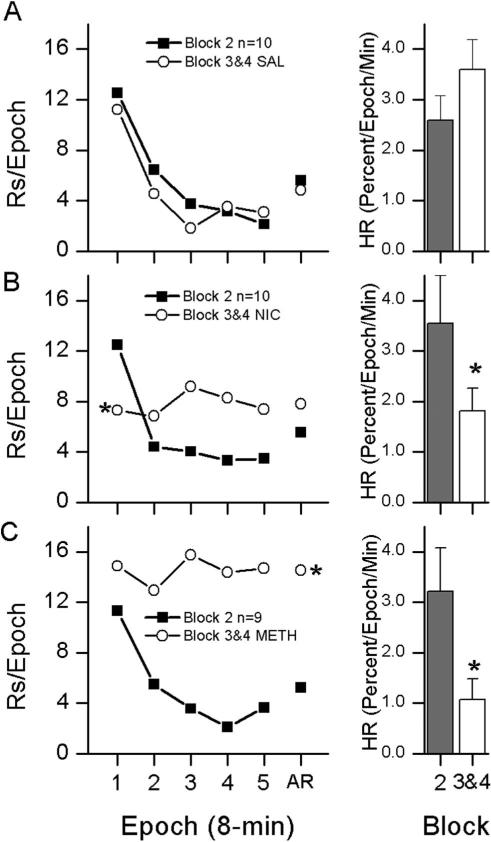
Figure 2.
The effects of drug treatment on the habituation rate during the 10-session Drug Alone phase were compared with habituation rate during the last 5-session block of the Operant Level phase. The left column of Figure 2 shows within-session responding during the Operant Level phase plotted in 8-min epochs. The right column shows habituation rate. The top row (A) shows results from saline (SAL)-treated rats, the middle row (B) shows results from nicotine (NIC, 0.4 mg/kg)-treated rats, and the bottom row (C) shows results from methamphetamine (METH, 0.75 mg/kg)-treated rats. Asterisks indicate significant p < .05.
ANOVAs conducted across the two blocks of the Drug Alone phase showed a significant main effect of group for IR, F(2,26) = 4.236, p = .026, and HR, F(2,26) = 7.593, p = .003. There were no main effects of side or block and no significant interactions for IR, HR, or AR. Because there was no effect of side or block, the data was analyzed as the sum of active and inactive responding and is presented in Figure 2 as averaged across Blocks 3&4. Rates of active, inactive and the relative frequency of active responding during Blocks 3&4 are shown separately in Table 1.
Overall ANOVAs that compared responding during Block 2 of the Operant Level phase with responding during the Drug Alone phase indicted a block by group interaction for all measures AR, F(2,26) = 4.186, p = .027; IR, F(2,26) = 6.797, p = .004; and HR, F(2,26) = 5.169, p = .013, and a main effect of block for AR, F(1,26) = 6.235, p = .019; and HR, F(2,26) = 4.718, p = .039.
Follow-up ANOVAs were carried out for each drug group to investigate the source of significant interactions. For the SAL group (Figure 2A, top row), this analysis indicated that SAL did not alter habituation or responding. For the NIC group (Figure 2B, middle row), this analysis indicated that NIC tended to decreased HR and reduced responding during the first 8-min epoch of the test session (IR). For the METH group (Figure 2C, bottom row), this analysis indicated that METH decreased HR and increased the average rate of responding (AR).
To summarize, there was no difference in HR or the measures of responding IR and AR in saline treated rats. In contrast, HR was decreased in rats treated with NIC and METH. NIC and METH had different effects on IR and AR. IR was decreased by NIC and was not significantly affected by METH while AR was increased by METH and was not significantly affected by NIC.
Drug & VS Phase
Effects of response-contingent light-onset during the Drug & VS phase (Block 5) were compared with performance during Block 4 of the Drug Alone phase in three groups of rats that were treated with SAL, NIC, or METH during both phases (Figure 3).
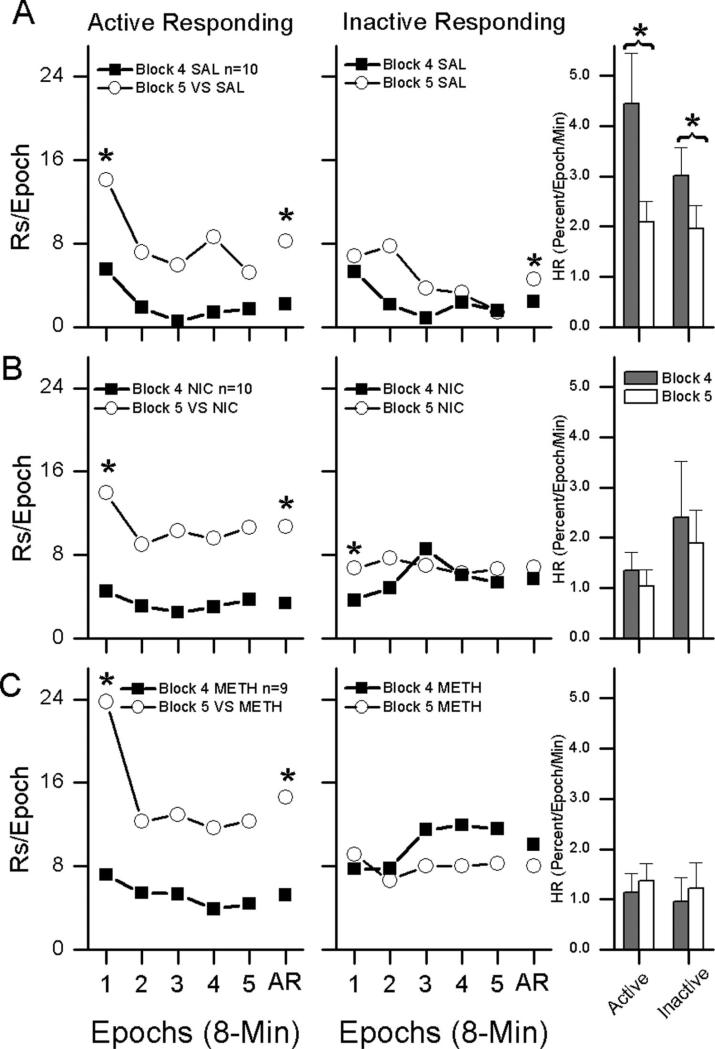
Figure 3.
Effects of response-contingent light-onset on habituation rate and responding were compared with habituation rate and responding during Block 4 of the Drug Alone phase. The left column of Figure 3 shows within-session active responding during the Operant Level phase plotted in 8-min epochs. The middle column of Figure 3 shows within-session inactive responding during the Operant Level phase plotted in 8-min epochs. The right column shows habituation rate. The top row (A) shows results from saline (SAL)-treated rats, the middle row (B) shows results from nicotine (NIC, 0.4 mg/kg)-treated rats, and the bottom row (C) shows results from methamphetamine (METH, 0.75 mg/kg)-treated rats. Asterisks indicate significant p < .05.
For the AR measure, the overall ANOVA produced a significant main effect of block, F(1,26) = 35.860, p < .001; a block by side interaction, F(1,26) = 35.652, p < .001; and a block by side by group interaction, F(2,26) = 3.385, p = .049. For the IR measure, the overall ANOVA produced significant main effects of block, F(1,26) = 66.344, p < .001; side F(1,26) = 27.404, p < .001; and group F(2,26) = 6.544, p = .005, with a block by side interaction F(1,26) = 36.288, p < .001. For the HR measure, the overall ANOVA produced a main effect of group, F(2,26) = 4.365, p = .023, and a main effect of block, F(1,26) = 6.224, p = .019, with a group by block interaction, F(2,26) = 5.243, p = .012.
Follow-up ANOVAs were carried out for each drug group to investigate the source of significant interactions. Within-subject Bonferroni corrected t tests were used to determine sources of significant effects of follow-up ANOVAs that produced significant side by block interactions.
For the SAL group (Figure 3A, top row), this analysis indicated that introduction of response-contingent light decreased HR of both active and inactive responding. There were also significant increases in the average rate of both active and inactive responding. In addition, active responding during the first 8-min epoch of the test session (IR) was increased.
For the NIC group (Figure 3B, middle row), this analysis indicated that introduction of response-contingent light had no effect on HR, increased active and inactive responding during the first 8-min epoch of the test session (IR) and increased the average rate of active responding (AR).
For the METH group (Figure 3C, bottom row), this analysis indicated that introduction of response-contingent light had no effect on HR, increased active responding during the first 8-min epoch of the test session (IR) and increased the average rate of active responding (AR).
To summarize, introduction of response-contingent light-onset increased both the IR and AR measures of active responding in all three drug treatment groups. There was some evidence of increases in inactive responding in the SAL and NIC groups. However, examination of the relative frequency data in Table 1 indicates that all rats demonstrated a similar strong preference for the active alternative. It is notable that introduction of the response-contingent light-onset decreased HR in Saline rats but not in NIC or METH rats. It is likely that this is because the HR for METH and NIC rats was already reduced by the previously ongoing drug treatment.
Discussion
Operant Level Phase
During the first 10 days of testing (Operant Level phase), there was evidence of both within- and between-session habituation of snout poking (Figure 1). The overall pattern of responding showed within-session declines and spontaneous recovery at the start of each test session. Responding during the first 5 sessions of exposure to the test chamber (Block 1) was compared with responding during Sessions 6–10 of exposure to the test chamber (Block 2). Within-session HR was greater during Block 2 than Block 1, indicating that the rate of within-session habituation increased with repeated exposure to the test chamber. The IR during Block 1 was greater than the IR for Block 2, indicating between-session habituation. The AR was also greater in Block 1 than Block 2, indicating an overall decrease in response rate that reflects the effects of within- and between-session habituation. These within- and between-session declines in the operant level of responding replicate the results of previous studies (Gancarz, Kausch, Lloyd, & Richards, 2012; Gancarz, Robble, et al., 2012; Gancarz et al., 2013; Gancarz, San George, Ashrafioun, & Richards, 2011; Lloyd, Gancarz, et al., 2012) from our laboratory. These results also replicate the earliest observations made of the operant level of bar press responding, which showed within- and between-session declines in responding as well (Schoenfeld, Antonitis, & Bersh, 1950). The decrease in IR observed from Block 1 to Block 2, and the increase in HR observed from Block 1 to Block 2 represent long-term habituation of reinforcer effectiveness, and potentiation of habituation of reinforcer effectiveness, respectively. These findings have previously been reported by both our laboratory (Lloyd, Gancarz, et al., 2012) and others (McSweeney et al., 1996).
Our interpretation of these declines is that responding is reinforced by the unprogrammed inherent sensory consequences of snout poking, and that within- and between-session decreases in the operant level of snout poke responding are observed because the effectiveness of inherent sensory reinforcers produced by snout poking habituate. Support for the interpretation that the operant level of responding is maintained by inherent sensory reinforcement is provided by studies indicating that the operant level of responding is reinforced by kinesthetic feedback. Mice made more contact with a response lever that was movable than with one that was rigid, leading to the conclusion that the kinesthetic consequences of bar pressing alone are reinforcing (Kish & Barnes, 1961). In another study, mice preferred a platform that moved slightly and made clicking noises over a platform that did not move and was silent (Kish & Antonitis, 1956). Similarly, we interpret the results of the present experiment as indicating that sensory stimulation resulting from contact with the snout poke holes reinforced responding and that the effectiveness of these reinforcers habituated. The results of the Operant Level phase can be explained by both memory-based theories of habituation and the DA as a sensory error signal hypothesis. Both of these explanations predict that responding should decrease as the sensory consequences of investigatory behaviors become familiar.
Drug Alone Phase
During the Drug Alone phase there was no HR change in SAL treated rats while HR was decreased by both METH and NIC (Figure 2). HR during Block 2 of the Operant Level phase was compared with HR during the Drug Alone phase (Sessions 11–20). SAL rats showed no change in HR, IR, or AR, indicating that snout poking had reached asymptotic levels during Block 2 of the Operant Level phase. At asymptotic levels the SAL rats demonstrated spontaneous recovery of snout poking between daily test sessions followed by rapid within-session habituation. In contrast to the effects of SAL, both NIC and METH decreased HR. However, they had opposite effects on IR and AR. The NIC-induced disruption of normally occurring habituation was accompanied by decreased IR with no effect on AR, while the METH-induced disruption of normally occurring habituation increased both IR and AR. These data indicate that both NIC and METH disrupted normally occurring habituation.
In summary, while both METH and NIC have similar effects on the pattern of habituation and spontaneous recovery, they had different effects on AR. Decreased HR in NIC and METH rats is predicted by the hypothesis of DA as a sensory error signal. According to this hypothesis, phasic firing of DA neurons produces an increase in DA neurotransmission that reflects the difference between perceived and expected stimuli (Redgrave & Gurney, 2006). Thus, artificially increasing DA neurotransmission with NIC or METH should have the effect of falsely indicating a difference between perceived and expected stimuli. However, it is important to point out that Redgrave and Gurney (2006) do not propose that the sensory error signal mediates the detection of novelty; instead, they believe that it has a role in mediating the behavioral expression of habituation or dishabituation. They speculate that the phasic firing of DA neurons in response to sensory stimuli is mediated by inputs from other (perhaps memory- related) brain areas that determine the novelty of sensory inputs. More specifically Redgrave, Gurney, and Reynolds (2008), suggest that pharmacological interventions (stimulant drugs) can increase DA activity in target regions like the striatum despite the presence of inhibitory inputs that would normally prevent increased DA activity. According to these authors, the inhibitory signal may still be there but is rendered ineffective by the high levels of DA activity.
Drug & VS Phase
For the SAL rats, introduction of response-contingent light-onset during the Drug & VS phase nonspecifically decreased HR of both active and inactive responding. In contrast, introduction of the response-contingent light-onset specifically increased IR and AR metrics for active responding while having no significant effect on inactive responding (Figure 3). For NIC and METH rats, introduction of the response-contingent light had no effect on HR and increased IR and AR. No significant effects were found for inactive responding for either NIC or METH. Both memory-based theories of habituation and the DA as a sensory error signal hypothesis predict that introduction of a novel response-contingent sensory stimulus should decrease HR, as was observed in the SAL rats. The absence of an HR effect in NIC and METH rats can be attributed to the preexisting stimulant induced decreases in habituation during the Drug Alone phase. It is notable, however, that preexisting effects of drug treatment on HR appeared to have no detrimental effect on the ability of the novel response-contingent light to increase active responding. Although responding was generally higher in METH rats than in the NIC and SAL rats, the relative frequency of active responding (an indicator of preference for the active alternative) was very similar for all three drug treatments (Table 1), indicating that introduction of the response-contingent light-onset had similar effects on the propensity to make active responses in all three drug groups.
The fact that the novel response-contingent light increased responding (IR and AR) in METH and NIC groups without changing HR indicates that DA may have a dual role in the mediation of habituation and reinforcement. The nonspecific decrease in HR of both active and inactive responding in SAL rats may indicate a general “alerting” or arousal effect, while selective increases in active responding may reflect a targeted reinforcement effect. A general “alerting” process is consistent with memory-based theories of habituation which specify that only “surprising” stimuli should cause dishabituation. A reinforcement process is consistent with the DA as a sensory error signal hypothesis of Redgrave and Gurney (2006), who hypothesize that the DA error signal evoked by a novel sensory stimulus, “acts to reinforce the reselection (repetition) of actions/movements that immediately precede an unpredicted biologically salient event” (p. 970). The sensory error signal hypothesis predicts directed behavioral actions while the memory-based theory can be interpreted as predicting a more general arousal effect.
Differential Effects of NIC and METH on DA Neurotransmission
The differential effects of NIC and METH on IR and AR measures of responding rate may reflect differential effects of METH and NIC on DA neurotransmission. NIC produces impulse-dependent release of DA by activating nicotinic acetylcholine receptors. The DA increasing effects of NIC are widespread and have been observed in the nucleus accumbens, striatum and frontal cortex (Dani & De Biasi, 2001; Marshall, Redfern, & Wonnacott, 1997; Nisell, Nomikos, Hertel, Panagis, & Svensson, 1996; Wonnacott, Kaiser, Mogg, Soliakov, & Jones, 2000). METH produces impulse-independent release of DA throughout the forebrain by blocking reuptake and inducing release (Seiden, Sabol, & Ricaurte, 1993; Sharp, Zetterström, Ljungberg, & Ungerstedt, 1987). However, even though the effects of amphetamine are described as impulse-independent, fast scan cyclic voltammetry studies have shown that both amphetamine (Daberkow et al., 2013) and NIC (Rice & Cragg, 2004) augment impulse- dependent release of DA. It is possible that the 0.75 mg/kg dose of METH used in this study produced greater increases in DA neurotransmission than the 0.4 mg/kg dose of NIC and that this difference in magnitude of effect can account for differences of response rate.
A limitation of the current study is that only one dose of NIC and only one dose of METH were tested. Future studies with full dose-response determinations of METH, NIC and other stimulant drugs such as methylphenidate and cocaine are needed to confirm these results. In addition, interpretation of the effects of METH and NIC as being mediated by DA requires caution. METH does not selectively increase DA; it also increases norepinephrine (Rothman et al., 2001) and serotonin transmission (Chiu & Schenk, 2012). Similarly, the effects of NIC observed in this study could be because of nicotine- receptor mediated effects not involving DA. Mechanistic observations such as receptor mediation via drug antagonists or other neuropharmacological manipulations are needed to determine whether the effects of METH and NIC on HR are mediated by DA.
Implications
“Sensory” or “reward” error prediction signal?
The sensory error signal concept is similar to the reward error signal concept of Shultz and colleagues (Schultz, Dayan, & Montague, 1997; Schultz, Tremblay, & Hollerman, 1998). According to the reward error signal hypothesis, unexpected sensory stimuli that predict important biological reinforcers evoke phasic DA responses which reflect the expected amount of reward. Bolado-Gomez and Gurney (2013) suggest that the sensory prediction error signal is modulated by reward value. For example, the phasic firing evoked by response-contingent light- onset normally habituates, however this habituation is prevented if light-onset predicts a biologically important reinforcer. In support of this interpretation, Winterbauer and Balleine (2007) and Lloyd, Kausch et al. (2012) have reported data that indicates that the effects of psychomotor stimulants on responding for both conditioned reinforcers (that predict biologically important reinforcers) and sensory reinforcers (that do not predict biologically important reinforcers) can be explained by their effects on the immediate sensory consequences. In contrast, the effects of psychomotor stimulants on responding for conditioned and sensory reinforcers cannot both be easily explained by the reward prediction concept.
Habituation of sensory reinforcer effectiveness
The concept of sensory reinforcement is understudied. Although there is a large amount of older literature that describes research into the primary reinforcing effects of sensory stimuli, sensory reinforcement has not been an active area of research in recent years. Perhaps the primary reason that the intrinsic reinforcing properties of sensory stimuli are ignored is because they are weak reinforcers in comparison to biologically important reinforcers such as food and water. It is much easier to study the effects of powerful reinforcers that are required to maintain homeostatic balance. Nevertheless, sensory reinforcers include social stimuli and are ubiquitous in the natural environment. It is arguable that sensory reinforcers make up the majority of consequences that control daily actions.
It seems apparent that habituation plays an important role in determining the effectiveness of sensory reinforcement. In the case of biologically important reinforcers, it is arguable that reinforcer effectiveness is determined primarily by homeostatic mechanisms. However, recent data indicates that the process of habituation influences the effectiveness of biologically important reinforcers too. McSweeney and Murphy (2009) review data from a number of studies indicating that within-session declines in operant responding observed with food and water reinforcers can be best understood in terms of habituation to the sensory properties of these reinforcers. The current experiment provides additional evidence in support of McSweeney and Murphy. Light-onset reinforcers which cannot be consumed and do not serve a homeostatic function still experience within-session decreases in reinforcer effectiveness, which strongly contradicts any theory based on satiation.
There may be important differences in the regulation of behavior by sensory and biologically important reinforcers. For example, we have recently reported that the rate of sensory reinforcement and the rate of responding are inversely related (Lloyd, Gancarz, et al., 2012). In contrast, a large amount of empirical data from operant experiments with biologically important reinforcers generally indicates that response rate increases as a function of reinforcer frequency (de Villers, 1977; Herrnstein, 1974; Heyman, 1983). Rapid habituation of the effectiveness of sensory reinforcers may produce a difference in the functional relationship between reinforcer rate and response rate for sensory and biologically important reinforcers.
In another study, we found that METH increased choice of a sensory reinforcer over a concurrently presented water reinforcer. This result indicates that METH can differentially increase the reinforcing effectiveness of sensory reinforcers (Gancarz, Ashrafioun, et al., 2012). These results indicate that a better understanding of differences in how sensory and biologically reinforcers regulate behavior is needed.
Clinical Significance
Clinicians and investigators studying autism spectrum disorders (ASDs) have maintained persistent interest in sensory reinforcement. Behavior that is restricted, repetitive and shows stereotyped patterns is one of the three core diagnostic criteria of ASD. There is good evidence that stereotyped behaviors in ASD are operant responses that are maintained by their sensory consequences (Cunningham & Schreibman, 2008; Lovaas, Newsom, & Hickman, 1987; Rapp & Vollmer, 2005a, 2005b). The persisted repetitive nature of stereotyped behavior in ASD suggests that the effectiveness of the perceptual consequences that automatically reinforce these stereotyped behaviors do not habituate. In the absence of normally occurring habituation to sensory consequences, the DA error signal hypothesis of Redgrave and Gurney (2006) predicts that phasic release of DA would continue to be evoked, causing repetition of movements that immediately precede the sensory event.
While stereotyped patterns of behavior in ASD may be related to the absence of habituation, impaired attention in attention-deficit/hyperactivity disorder (ADHD) may be related to very rapid habituation. Abnormally rapid habituation of the effectiveness of sensory reinforcement may cause impaired stimulus control. Disruptions (slowing) of habituation by stimulant drugs may help explain the beneficial effects of stimulant drugs for treating ADHD symptoms. If the effectiveness of sensory reinforcers habituates more rapidly in ADHD individuals, stimulant drugs may ameliorate this effect by slowing habituation of reinforcer effectiveness.
The results of these studies also have strong implications for understanding the abuse potential of stimulant drugs. The results of these experiments suggest that METH and NIC disrupt normally occurring habituation of the reinforcing effectiveness of sensory stimuli. By artificially extending the reinforcing effects of sensory stimuli, stimulant drugs may result in a more exciting and rewarding subjective experience of the world and may contribute to the abuse potential of stimulant drugs.
Summary and Conclusion
Sensory reinforcers are ubiquitous in the natural environment and the role of sensory consequences in regulating behavior may be underappreciated. The effectiveness of sensory reinforcers habituates rapidly with repetition. The effectiveness of powerful biological reinforcers habituates less rapidly, because they are regulated by both habituation and homeostatic mechanisms. Precisely defined measures of habituation are needed to study the role of habituation in operant behavior. DA may mediate habituation of sensory reinforcer effectiveness leading to the hypothesis that stimulant drugs such as METH and NIC disrupt (slow) normally occurring habituation of sensory reinforcer effectiveness by artificially increasing DA neurotransmission. Habituation of reinforcer effectiveness may play an important role in ASD, ADHD and drug abuse. Stimulant-induced decreases in normally occurring habituation of reinforcer effectiveness may mediate the effects of stimulant drugs in ADHD and drug abuse. A better understanding of how sensory reinforcers regulate behavior and the role of DA in mediating the habituation of sensory reinforcer effectiveness is needed.
Acknowledgments
This research was supported in part by NIDA Grants DA10588 and DA026600 awarded to Jerry B. Richards. NIDA had no role other than financial support. All authors contributed significantly and have approved the manuscript. No authors have any conflict of interest, real or apparent. We would like to thank Leah Militello for her assistance in preparing this article.
References
- Antunes M, Biala G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cognitive Processing. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlyne DE. Conflict, arousal, and curiosity. McGraw-Hill; New York, NY: 1960. [Google Scholar]
- Berlyne DE. The reward-value of indifferent stimulation. In: Tapp JT, editor. Reinforcement and behavior. Academic Press; New York, NY: 1969. pp. 179–214. [Google Scholar]
- Bolado-Gomez R, Gurney K. A biologically plausible embodied model of action discovery. Frontiers in Neurorobotics. 2013;7:1–24. doi: 10.3389/fnbot.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu VM, Schenk JO. Mechanism of action of methamphetamine within the catecholamine and serotonin areas of the central nervous system. Current Drug Abuse Reviews. 2012;5:227–242. doi: 10.2174/1874473711205030227. [DOI] [PubMed] [Google Scholar]
- Cunningham AB, Schreibman L. Stereotypy in autism: The importance of function. Research in Autism Spectrum Disorders. 2008;2:469–479. doi: 10.1016/j.rasd.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daberkow DP, Brown HD, Bunner KD, Kraniotis SA, Doellman MA, Ragozzino ME, Roitman MF. Amphetamine paradoxically augments exocytotic dopamine release and phasic dopamine signals. The Journal of Neuroscience. 2013;33:452–463. doi: 10.1523/JNEUROSCI.2136-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, De Biasi M. Cellular mechanisms of nicotine addiction. Pharmacology Biochemistry and Behavior. 2001;70:439–446. doi: 10.1016/s0091-3057(01)00652-9. [DOI] [PubMed] [Google Scholar]
- de Villers P. Choice in concurrent schedules a quantitative formulation of the law of effect. In: Honig WK, Staddon JER, editors. Handbook of operant behavior. Prentice Hall, Inc.; Englewood Cliffs, NJ: 1977. pp. 233–287. [Google Scholar]
- Eisenberger R. Explanation of rewards that do not reduce tissue needs. Psychological Bulletin. 1972;77:319–339. doi: 10.1037/h0032483. [DOI] [PubMed] [Google Scholar]
- Gancarz AM, Ashrafioun L, San George MA, Hausknecht KA, Hawk LW, Jr., Richards JB. Exploratory studies in sensory reinforcement in male rats: Effects of methamphetamine. Experimental and Clinical Psychopharmacology. 2012;20:16–27. doi: 10.1037/a0025701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancarz AM, Kausch MA, Lloyd DR, Richards JB. Between-session progressive ratio performance in rats responding for cocaine and water reinforcers. Psychopharmacology (Berl) 2012;222:215–223. doi: 10.1007/s00213-012-2637-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancarz AM, Robble MA, Kausch MA, Lloyd DR, Richards JB. Association between locomotor response to novelty and light reinforcement: Sensory reinforcement as an animal model of sensation seeking. Behavioural Brain Research. 2012;230:380–388. doi: 10.1016/j.bbr.2012.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancarz AM, Robble MA, Kausch MA, Lloyd DR, Richards JB. Sensory reinforcement as a predictor of cocaine and water self-administration in rats. Psychopharmacology (Berl) 2013;226:335–346. doi: 10.1007/s00213-012-2907-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancarz AM, San George MA, Ashrafioun L, Richards JB. Locomotor activity in a novel environment predicts both responding for a visual stimulus and self- administration of a low dose of methamphetamine in rats. Behavioural Processes. 2011;86:295–304. doi: 10.1016/j.beproc.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdner JB. An experimental analysis of the behavioral effects of a perceptual consequence unrelated to organic drive states. American Psychologist. 1953;8:354–355. [Google Scholar]
- Glanzer M. Curiosity, exploratory drive, and stimulus satiation. Psychological Bulletin. 1958;55:302–315. doi: 10.1037/h0044731. [DOI] [PubMed] [Google Scholar]
- Herrnstein RJ. Formal properties of the matching law. Journal of the Experimental Analysis of Behavior. 1974;21:159–164. doi: 10.1901/jeab.1974.21-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman GM. A parametric evaluation of the hedonic and motoric effects of drugs: Pimozide and amphetamine. Journal of the Experimental Analysis of Behavior. 1983;40:113–122. doi: 10.1901/jeab.1983.40-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish GB. Studies of sensory reinforcement. In: Honig W, editor. Operant behavior: Areas of research and application. Appletone-Century-Crofts; New York, NY: 1966. pp. 100–159. [Google Scholar]
- Kish GB, Antonitis JJ. Unconditioned operant behavior in two homozygous strains of mice. The Journal of Genetic Psychology: Research and Theory on Human Development. 1956;88:121–129. doi: 10.1080/00221325.1956.10532960. [DOI] [PubMed] [Google Scholar]
- Kish GB, Barnes GW. Reinforcing effects of manipulation in mice. Journal of Comparative and Physiological Psychology. 1961;54:713–715. doi: 10.1037/h0046683. [DOI] [PubMed] [Google Scholar]
- Konorski JJ. Integrative activity of the brain. University of Chicago Press; Chicago, IL: 1963. [Google Scholar]
- Leussis MP, Bolivar VJ. Habituation in rodents: A review of behavior, neurobiology, and genetics. Neuroscience and Biobehavioral Reviews. 2006;30:1045–1064. doi: 10.1016/j.neubiorev.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. Journal of Neurophysiology. 1992;67:145–163. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- Lloyd DR, Gancarz AM, Ashrafioun L, Kausch MA, Richards JB. Habituation and the reinforcing effectiveness of visual stimuli. Behavioural Processes. 2012;91:184–191. doi: 10.1016/j.beproc.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DR, Kausch MA, Gancarz AM, Beyley LJ, Richards JB. Effects of novelty and methamphetamine on conditioned and sensory reinforcement. Behavioural Brain Research. 2012;234:312–322. doi: 10.1016/j.bbr.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockard RB. Some effects of light upon the behavior of rodents. Psychological Bulletin. 1963;60:509–529. doi: 10.1037/h0046113. [DOI] [PubMed] [Google Scholar]
- Lovaas I, Newsom C, Hickman C. Self-stimulatory behavior and perceptual reinforcement. Journal of Applied Behavior Analysis. 1987;20:45–68. doi: 10.1901/jaba.1987.20-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall DL, Redfern PH, Wonnacott S. Presynaptic nicotinic modulation of dopamine release in the three ascending pathways studied by in vivo microdialysis: Comparison of naive and chronic nicotine-treated rats. Journal of Neurochemistry. 1997;68:1511–1519. doi: 10.1046/j.1471-4159.1997.68041511.x. [DOI] [PubMed] [Google Scholar]
- McSweeney FK, Hinson JM, Cannon CB. Sensitization–habituation may occur during operant conditioning. Psychological Bulletin. 1996;120:256–271. [Google Scholar]
- McSweeney FK, Murphy ES. Sensitization and habituation regulate reinforcer effectiveness. Neurobiology of Learning and Memory. 2009;92:189–198. doi: 10.1016/j.nlm.2008.07.002. [DOI] [PubMed] [Google Scholar]
- McSweeney FK, Swindell S. General-process theories of motivation revisited: The role of habituation. Psychological Bulletin. 1999;125:437–457. [Google Scholar]
- Nisell M, Nomikos GG, Hertel P, Panagis G, Svensson TH. Condition- independent sensitization of locomotor stimulation and mesocortical dopamine release following chronic nicotine treatment in the rat. Synapse. 1996;22:369–381. doi: 10.1002/(SICI)1098-2396(199604)22:4<369::AID-SYN8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, Sved AF. The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections. Drug and Alcohol Dependence. 2007;89:52–59. doi: 10.1016/j.drugalcdep.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp JT, Vollmer TR. Stereotypy I: A review of behavioral assessment and treatment. Research in Developmental Disabilities. 2005a;26:527–547. doi: 10.1016/j.ridd.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Rapp JT, Vollmer TR. Stereotypy II: A review of neurobiological interpretations and suggestions for an integration with behavioral methods. Research in Developmental Disabilities. 2005b;26:548–564. doi: 10.1016/j.ridd.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Gurney K. The short-latency dopamine signal: A role in discovering novel actions? Nature Reviews Neuroscience. 2006;7:967–975. doi: 10.1038/nrn2022. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Gurney K, Reynolds J. What is reinforced by phasic dopamine signals? Brain Research Reviews. 2008;58:322–339. doi: 10.1016/j.brainresrev.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nature Neuroscience. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Richards JB, Mitchell SH, de Wit H, Seiden LS. Determination of discount functions in rats with an adjusting-amount procedure. Journal of the Experimental Analysis of Behavior. 1997;67:353–366. doi: 10.1901/jeab.1997.67-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Schoenfeld WN, Antonitis JJ, Bersh PJ. Unconditioned response rate of the white rat in a bar-pressing apparatus. Journal of Comparative and Physiological Psychology. 1950;43:41–48. doi: 10.1037/h0059309. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward prediction in primate basal ganglia and frontal cortex. Neuropharmacology. 1998;37:421–429. doi: 10.1016/s0028-3908(98)00071-9. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: Effects on catecholamine systems and behavior. Annual Review of Pharmacology and Toxicology. 1993;33:639–677. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- Sharp T, Zetterström T, Ljungberg T, Ungerstedt U. A direct comparison of amphetamine-induced behaviours and regional brain dopamine release in the rat using intracerebral dialysis. Brain Research. 1987;401:322–330. doi: 10.1016/0006-8993(87)91416-8. [DOI] [PubMed] [Google Scholar]
- Sokolov YN. Perception and the conditioned reflex. Pergamon Press; Oxford, United Kingdom: 1963. [Google Scholar]
- Tapp JT. Activity, reactivity, and the behavior-directing properties of stimuli. In: Tapp JT, editor. Reinforcement and behavior. Academic Press; New York, NY: 1969. pp. 148–178. [Google Scholar]
- Wagner AR. Habituation and memory. In: Dickenson A, Boakes RA, editors. Mechanisms of learning and memory: A memorial volume to Jerzy Konorski. Erlbaum; Hillsdale, NJ: 1979. [Google Scholar]
- Winterbauer NE, Balleine BW. The influence of amphetamine on sensory and conditioned reinforcement: Evidence for the re-selection hypothesis of dopamine function. Frontiers in Integrative Neuroscience. 2007;1:9. doi: 10.3389/neuro.07.009.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S, Kaiser S, Mogg A, Soliakov L, Jones IW. Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. European Journal of Pharmacology. 2000;393:51–58. doi: 10.1016/s0014-2999(00)00005-4. [DOI] [PubMed] [Google Scholar]