Abstract
A review of 77 studies employing self-report measures of antiretroviral adherence published 1/1996 through 8/2004 revealed great variety in adherence assessment item content, format, and response options. Recall periods ranged from 2 to 365 days (mode = 7 days). The most common cutoff for optimal adherence was 100% (21/48 studies, or 44%). In 27 of 34 recall periods (79%), self-reported adherence was associated with adherence as assessed with other indirect measures. Data from 57 of 67 recall periods (84%) indicated self-reported adherence was significantly associated with HIV-1 RNA viral load; in 16 of 26 (62%), it was associated with CD4 count. Clearly, the field would benefit from item standardization and a priori definitions and operationalizations of adherence. We conclude that even brief self-report measures of antiretroviral adherence can be robust, and recommend items and strategies for HIV research and clinical management.
Keywords: HIV/AIDS, Antiretroviral, Medication adherence, Self-report, Viral load
Introduction
An abundance of convergent empirical evidence has confirmed that strict adherence to medication regimens is key to the successful treatment of HIV infection with antiretro-viral therapy or ART (Bangsberg et al., 2000; Hogg et al., 2002; Paterson et al., 2000). However, there is decidedly less agreement on the best strategy for assessing ART adherence. An ideal assessment instrument would be reliable, valid, and logistically practical, with low participant and staff burden.
The search for an adherence assessment “gold standard” is not unique to the field of HIV (Geletko et al., 1996; Martin et al., 2001; Rudd, 1979; Rudd, Ahmed, Zachary, Barton, & Bonduelle, 1990; Straka, Fish, Benson, & Suh, 1997; Waterhouse, Calzone, Mele, & Brenner, 1993). Across multiple clinical conditions, researchers have examined a range of methodologies for capturing medication adherence. These have been categorized as either direct or indirect methods (Liu et al., 2001; Miller & Hays, 2000; Paterson, Potoski, & Capitano, 2002; Turner, 2002; Wutoh et al., 2003). Direct methods such as biological assays of active drug, metabolite or other markers in blood, urine, or other bodily fluids confirm active drug ingestion. Indirect methods, which do not measure the presence of the drug in the individual, include self-report, clinician assessment, medical chart review, clinic attendance, behavioral observation such as directly observed therapy, pill count (PC), pharmacy refill (PR) records, electronic drug monitoring (EDM), and therapeutic impact such as HIV-1 RNA viral load (VL), CD4 lymphocyte count, Centers for Disease Control-defined stage of disease progression, and mortality. These assessment methods have advantages and disadvantages (Gao, Nau, Rosenbluth, Scott, & Woodward, 2000), with the tradeoff generally assumed to be financial and logistical cost versus psychometric and epidemiologic accuracy (Gordis, 1979).
The present study focused on the most widely used indirect method of assessing ART adherence: self-report measures. The practicality of self-report makes this approach a likely candidate for continued widespread use in clinical and research settings, including in resource-poor countries just gaining access to ART.
Patient self-report measures in the form of personal interviews or written questionnaires have many advantages, including low cost, minimal participant burden, ease and speed of administration, flexibility in terms of mode of administration and timing of assessment, and the potential to yield specific information about the timing of doses and adherence to food requirements (Wagner & Miller, 2004). Additionally, the specificity of self-report measures is high, i.e., patients’ acknowledgment of nonadherence is generally credible (Bangsberg et al., 2001). Moreover, a recent meta-analysis found that despite significant study heterogeneity, the pooled association between self-reported ART adherence and VL was statistically significant, adjusted OR = 2.31, 95% CI = 1.99–2.68 (Nieuwkerk & Oort, 2005).
On the other hand, self-report is susceptible to recall bias and inaccurate memory and potentially to social desirability bias; indeed, self-report does tend to produce estimates of adherence that are 10–20% higher than those from EDM (Arnsten et al., 2001; Wagner & Miller, 2004). Because of these limitations, some researchers have suggested that EDM or other less subjective methods may be preferable to self-report for adherence assessment in intervention trials (Miller & Hays, 2000). Others have noted practical limitations of EDM (Bova et al., 2005) and that adherence may be underestimated by EDM and overestimated by self-report and pill count, thus warranting the use of several adherence measures (Liu et al., 2001). This strategy, though, may be impractical for ongoing clinical use. Despite the perceived limitations, many clinicians and researchers alike continue to rely extensively on self-report adherence measures, probably because they continue to be the least costly and burdensome way to assess ART adherence.
For the present report, we conducted a review of the literature with the goals of identifying (a) the variety of self-report measures used in ART adherence research, (b) the pattern of associations between self-report and other adherence assessment strategies such as pill count and EDM, and (c) the relation between self-report and clinical indicators such as VL and CD4 lymphocyte count. Our aim was to determine best practices with respect to selecting self-report measures for both research purposes and clinical monitoring.
Selection of studies for review
We conducted an extensive search of PsycINFO, AIDS Line, and MEDLINE for articles published in refereed journals from January 1996 through August 2004 that contained some combination of the terms (a) HIV or human immunodeficiency virus or AIDS or acquired immunodeficiency syndrome and (b) adherence or compliance. Additionally, we scanned bibliographies of relevant articles and consulted with experts in the field for other references. From the resulting list of over 600 articles, we selected the English-language publications describing studies of individuals at least 18 years of age that utilized a self-report measure of ART adherence and reported its association with at least one other adherence assessment method (such as pill count or pharmacy refill records) or with an indicator of clinical impact (such as VL or CD4 count). We excluded the few early studies examining adherence to ART monotherapy, resulting in 77 published articles that met the a priori selection criteria.
Review strategy
From each article we extracted information on the study setting, location, and sample size; details regarding the self-report measure (including its source, number, and wording of items, and how adherence was operationalized for analysis); the recall period; and the measure’s associations with other adherence measures and clinical indicators. These are presented as a reference source in Table 1. Although not noted in the Table, we also recorded eligibility criteria, sample characteristics, and study purpose and design.
Table 1.
Studies reporting the association of self-reported antiretroviral adherence with adherence as measured by other indirect measures or with clinical indicators
| Source | Study | Self-report measure | Recall period |
Association with other indirect adherence measures |
Association with clinical indicators |
|---|---|---|---|---|---|
| Setting; location; sample size | Source/items/(nonadherence operationalization) (CO: continuous, CT: categorical, DI: dichotomous) |
electronic data monitoring; pharmacy refills (PR); pill count (PC); other |
HIV-1 RNA viral load (VL); CD4; other (adherent vs. nonadherent) |
||
| Alcoba et al. (2003) | 2 HIV clinics; Spain; N=106 |
N/R; N/R; DI: <90% of prescribed doses for at least one drug |
4 days | VL detectable (NS); plasma indinavir levels (NS) |
|
| Aloisi et al. (2002) | 57 ID hospital units; Italy; N = 366 |
N/R; 3 items; DI: “Yes” to all three items vs. <3 |
6 months | VL undetectable*** 68% vs. 40% @ 12 mo | |
| Altice, Mostashari, and Friedland (2001) | 4 prison HIV clinics; CT, US;N=164 |
Ickovics ‘97; N/R; DI: 80% of pills taken/prescribed |
7 days | PR r = 0.82 (significance N/R) | CD4 count (NS) |
| Ammassari et al. (2004) | 11 clinical centers; Italy; N=135 |
Murri ‘00; 1 forced-choice item on timing of last missed dose; DI: missed ≥ 1 dose over last 7 days |
7 days | CD4 higher mean (SD)* 637 (341) vs. 509 (362) |
|
| Antinori et al. (2004) | Study cohort; Italy; N = 238 | Murri ‘00; N/R; DI: missed ≥ 1 dose over last 7 days |
7 days | VL rebound > 500 copies/mL (NS) | |
| Arnsten et al. (2001) | Hospital study cohort; Bronx, NY, US; N = 67 |
N/R; N/R; CO: % of prescribed doses taken |
1 day; 7 days |
EDM; 1-day r = 0.49***; 7-day r = 0.46*** |
VL <500; 1-day r = 0.43***; 7 day r = 0.52*** |
| Bangsberg et al. (2000) | Community cohort; San Francisco, US; N = 34 |
N/R; 3 items; CO: Mean value of 3 measures of % prescribed doses |
3 days | VL r= −0.60*** | |
| Bangsberg et al. (2001) | Community cohort; San Francisco, US; N = 45 |
N/R; Day-by-day review of doses; CO: % prescribed doses, DI: >80% |
3 days | PC r = 0.85***; PC κ = 0.65***; provider estimate (NS) |
|
| Bangsberg et al. (2002) | Private clinic and county hospital; San Francisco, US;N=110 |
AACTG (Chesney ‘00); Day-by-day review of doses on computer; DI: 90 and 80% |
3 days | Provider estimate*** (test statistic NR) |
VL detectable ≥ 500; <80% OR 3.0 (95% CI 1.1–8.1) |
| Barroso et al. (2003) | HIV reference center; Rio de Janeiro, Brazil; N= 64 |
N/R; 1 item; DI: taking as prescribed >80% days |
30 days | VL<400; OR 7.2 (95% CI 1.6–31.9) in semen; ORA 8.2 (95% CI 1.2–56.7) in plasma |
|
| Brigido et al. (2001) | Public AIDS clinic; Sao Paulo, Brazil; N= 168 |
N/R; 5 items; CT: Reg: all doses taken, qReg: miss up to 4 doses or 1 full day/mo, Ireg: all other irregular |
30 days | VL median log10*; Reg 2.0 (1.6–5.6); qReg 2.0 (1.6–5.5); Ireg 3.6 (1.6–6.2); CD4 median gain*; (test statistic N/R); AIDS development or death* (test statistic N/R) |
|
| Carrieri et al. (2001) | Routine clinical sites; France; N = 436 |
AACTG; 5 items for each drug; CT: 100%, 80–99%, <80% |
4 days | VL undetectable at 4**; 12**, and 20 months** (test statistic N/R) |
|
| Carrieri et al., 2003 | Routine clinical sites; France; N = 360 |
AACTG; 5 items for each drug; CT: 100%, 80–99%, <80% |
4 days | VL suppression at 3 years: Highly adherent OR 3.4 (95% CI 1.4–7.9); Mod adherent NS; CD4 increase >200 by 3 years: highly adherent OR 2.4 (95% CI 1.0–5.5); Mod adherent NS | |
| Catz, Kelly, Bogart, Benotsch, and McAuliffe (2000) | Outpatient ID clinic; Milwaukee, US; N = 72 |
N/R; 2 items; CT: missed doses daily, weekly, monthly, or never |
3 months | VL <400* (test statistic N/R) | |
| Cederfjall, Langius-Eklof, Lidman, and Wredling (2002) | Outpatient HIV clinic; Stockholm, Sweden; N = 99 |
N/R; 1,7, 30 days =% missed in 1 month; DI: 959% |
1 month | VL <50 71% vs. 45%*; CD4 <200 8% vs. 32%*** |
|
| Cingolani et al. (2002) | Tertiary care ID department; Italy; N =127 |
Murri ‘00; 1 item, timing of last missed dose; DI: missed before 2–4 weeks (adherent) vs. yesterday, last week |
N/A | VL <500 at 3 mo* (test statistic NR); nonadherent OR 0.37 (95% CI 0.1–0.95)*; CD4 change; 3 mo + 50 vs. — 12**; 6 mo + 62 vs. −13** |
|
| Cohn, Kammann, Williams, Currier, and Chesney (2002) | AACTG sites; 29 sites in US; N = 643 |
AACTG; 2 items; DI: 100% | 48 hr | VL>500 nonadherence over 56 weeks; OR 2.3 (95% CI N/R); 70% vs. 50%*** | |
| Dorz et al. (2003) |
2 ID departments; Padua and Verona, Italy; N= 109 |
N/R; l item; CO: # pills/# prescribed, DI: 80% |
7 days | VL mean 10,854 vs. 34,149*; CD4 mean 6899 vs. 379*** | |
| Duong et al. (2001) | AIDS outpatient clinic; Dijon, France; N= 149 |
PMAQ (Paterson ‘99); 4 items; DI: 100% (nonadherent score<4) |
Combined: 4 days and 4 weeks |
VL reduction; ORA 2.9 (95% CI 1.2–7.1); Did not miss any PI last 4 days r = . 18* | |
| Duran et al. (2001) | Study cohort; France; N = 277 |
AACTG; 5 items; DI:100% | Combined: 4 days and weekend |
VL undetectable 4 months after ART initiation 59.4% vs. 41.6%** |
|
| Duran et al. (2001) | Study cohort; France; N= 57 | N/R; 1 item; CT: 100%, 99%-80%, <80% |
1 week | VL median log10** 100%: 2.3 (2.3–3.5); 80%–99%: 2.3 (2.3–3.6); <80%: 3.8 (2.6–5.04); VL undetectable** 100%: 73.1%; 80%–99%: 69.2%; <80%: 22.2%; CD4 (NS, p = 0.06); drug level*** | |
| Duran et al. (2003) | 47 hospitals; France; N = 642 | N/R; 5 items; CT: 100%, 99%-80%, <80% |
4 days | VL detectable; 100%: OR 1.0; 99%–80%: OR 1.5 (95% CI 1.0–2.3); <80%: OR 2.3 (95% CI 1.3–4.1) | |
| Eldred, Wu, Chaisson, and Moore (1998) | Hospital HIV clinic; Baltimore, MD, USA; N = 244 |
N/R; 1 item for each time frame; DI: 80% |
7 days; 14 days |
Medical record kappa 71%; 7 day: 60% vs. 56% (NS); 14 day: 74% vs. 67%** |
|
| Fong et al. (2003) | HIV clinic; Hong Kong; N=161 |
N/R; Number missed doses; DI: 100% |
Since last visit | VL<500; ORA 4.2 (95% CI 1.8–12.3) | |
| Gao et al. (2000) | 3 clinics; West Virginia, US; N = 72 |
Samet ‘92; N/R, assessed doses; CO: prescribed – missed/prescribed |
2 days | Disease severity* | |
| Garcia de Olalla et al. (2002) | HIV hospital unit; Barcelona, Spain; N= 1219 |
N/R; N/R; DI: 90% | 1 month | Mortality: Non adherent; Relative hazard 1.5 (95% CI 1.2–1.99) |
|
| Gifford et al. (2000) | Community practices; San Diego CA, US; N= 133 | CASQ (Berry ‘00) 4 items per drug taken; CT: 100%, 80–99%, <80% |
7 days | VL log10 Each increase in adherence category associated with 1.3 log10 decrease** |
|
| Giordano, Guzman, Clark, Charlebois, and Bangsberg (2004) | Participants’ usual place of residence; San Francisco, CA,US; N=84 |
AACTG and Visual Analog Scale - VAS (Walsh ‘98); 4 items/drug (3 day), VAS-1; CO: Mean adherence over three visits |
3 days (AACTG) 3 or 4 weeks (VAS) |
Unannounced PC and VAS: r = 0.76(95%CI 0.65–0.84); 3-day r = 0.71 (95% CI 0.59–0.80); (sig. N/R; NS diff betw VAS and 3 day) |
VL; VAS: r= − .49 (95% CI − .0.64–0.31); 3-day r= –.34 (95% CI −0.51–0.13); (sig. N/R; NS diff betw VAS and 3 day) |
| Godin, Gagne, and Naccache (2003) | 4 HIV clinics; Montreal, Quebec City; N = 256 |
Researcher-created; 9 items, # pills missed/# prescribed; DI: 95% |
1,2,7,30 days |
VL increase over 6 months; Nonadherent 1, 2, 30 day (NS); 7 days OR 1.9 (95% CI 1.0–3.6) |
|
| Golin et al. (2002) | 3 public HIV clinics; N/R; N=117 |
Composite score with EDM, PC, SR interview (Liu’01) 1 item CO: #doses taken/# prescribed |
7 days | EDM r = 0.38 (sig. N/R); PC r = 0.62 (sig. N/R) | |
| Gordillo, del Amo, Soriano, and Gonzalez-Lahoz (1999) | HIV reference center; Madrid, Spain; N = 366 |
N/R; N/R; DI: 90% | Last week | CD4 at enrollment and good adherence; >500 ORA 2.4 (95% CI 1.3–4.4); 200–499 ORA 2.8 (95% CI 1.4–5.5) |
|
| Goujard et al. (2003) | Hospital centers; France; N = 326 |
AACTG, PMAQ, and 3 items re: instructions (Metcalf ‘98) 13 items; CO: Nonadherence score 0–26 |
N/R | VL lower (test statistics N/R)*** CD4 higher (test statistics N/R)* |
|
| Guaraldi et al. (2003) | 8 tertiary centers; Northern, Central Italy; N= 175 |
MOS-HIV Health Survey N/R; 85% (>1 dose in 7 days) |
7 days | Morphologic alterations; ORA 2.36 (95% CI 1.1–5.0) | |
| Haubrich et al. (1999) | 5 university HIV clinics; CA, US; N = 164 @ 2 months, 119 @ 6 months |
N/R 24 items; assessed % prescribed doses taken; CT: 100%, 99-95%, <95-80%, <80% |
4 weeks | Provider estimate kappa = 0.02 (NS) |
VL log10 reduction (SD) @ 2 months* 100%, 99-95%, <95-80%, <80%; 0.95 (2.2); 0.79 (2.0); 0.57 (1.8); 0.04 (2.0); VL log10 increase (SD) @ 6 months* 100%: − 1.1 (2.2); <80%: 0.2 (1.2); CD4 cells @ 6 months** 100%, 99-95%, <95-80%, <80% 72 (162); + 87 (154); + 54 (162); − 19 (74) |
| Ho et al. (2002) | Clinic; Hong Kong; N= 161 | Doung ‘01 1 item, % prescribed doses taken CT: 100%, 99–95%, 94-90%, <90% |
4–6 weeks | VL detectable** ≤ 99% vs. 100% OR 4.2 (95% CI 1.8–12.3) Disease progression** |
|
| Horne et al. (2004) | Outpatient clinic; Brighton, UK; N=109 |
VAS 1 item, correct dose timing; DI: 7 pt scale 0–6: cutoff ≥ 5 |
N/R | VL>400; 18% vs. 26% (NS); CD4 (NS) | |
| Hugen et al. (2002) | University centre; Nijmegen and Arnhem Netherlands; N = 26 |
N/R; VAS; Multiple items; CT: 3 groups and range 1–10 |
N/R | EDM % taken on time*** ρ = .73; % taken** ρ = .55 |
N/R |
| Ickovics et al. (2002) | 21 AACTG sites; Multisites, US; N = 93 |
AACTG 1 item for each drug DI: 95% |
4 days | VL>50 @ 24 weeks <95% adherent OR 2.6 (95% CI 1.1–6.1) CD4 change (NS) |
|
| Ingersoll (2004) | University ID clinic; Virginia; N= 120 |
Medication Adherence Form (Ingersoll ‘99) Multiple items; DI: 95% PIs taken; DI: Adherence score 1–3, cutoff >2 |
1 week | VL undetectable 77% vs. 23%* CD4 <200 54% vs. 46%* |
|
| Kimmerling et al. (2003) | Clinics, community; Los Angeles, US; N= 58 |
N/R # doses taken q.d. of last 3; CO: score |
3 days | EDM: r = 0.47*** Subset reporting missed doses (NS) |
|
| Kleeberger et al. (2001) | Research cohort; Baltimore, Chicago, Pittsburgh, LA, US;N = 393 |
Modified AACTG; multiple items; DI: 100% |
4 days | VL undetectable <50 copies; 53.3% vs. 37.4%** CD4 ≥ 400 (NS); 64.4% vs. 58.3% |
|
| Knobel et al. (2001) | University HIV clinic; Barcelona, Spain; N = 679 |
N/R; N/R; DI: 90% | 1 month | VL <500; Adherent OR 3.1 (95% CI 2.2–4.2)***; nonadherent ORA 0.4 (95% CI 0.2–0.7)**; CD4 mean increase 171 vs. 107** |
|
| Knobel et al. (2001) | 69 hospitals; Spain; N = 2528@3,2127@6, and 1797 @ 12 months |
SMAQ (from Morisky ‘86); 6 items; DI: 95% (missed >2days in 3 mos; 2 doses 7 days or yes to 1/4 items) |
Combined 1 weekend; 1 week; 3 months |
EDM: sensitivity 72%; specificity 91%; PPV 91%; NPV 80% |
VL <500; @ 3 months OR 2.2 (95% CI 1.8–2.6)***; @ 6 months OR 2.6 (95% CI 2.2–3.1)***; @ 12 months OR 2.5 (95% CI 2.0–3.1)***; VL >500; Nonadherent ORA 1.7 (95% CI 1.4–2.1) |
| Knobel et al. (2004) | 2 hospitals; Barcelona, Spain; N= 85 |
SMAQ (from Morisky ‘86); 6 items; DI: 90% |
1 weekend; 1 week; 3 months |
VL >500 @ first year: Nonadherent OR 5.2 (95% CI 2.1–13.3); ORA 4.4 (95% CI 1.6–12.3) |
|
| Laniece et al. (2003) | 3 health clinics; Dakar, Senegal; N =158 |
N/R; N/R; # taken: #prescribed; DI: 90% |
30 days | VL mean difference nonadherent; Month 18: 1.7 log10 copies*; Month 24: 1.8 log10 copies* |
|
| Le Moing et al. (2001) | 47 clinical centers; Paris/France; N=750 |
N/R; N/R; DI: 100% | 4 days | VL <500: 84% vs. 73%***; OR 2.0 (95% CI 1.3–3.0) |
|
| Le Moing et al. (2002) | 47 clinical centers; France; N=1129 |
N/R; 5 items; categorical: 100%, 80–99%, <80% |
4 days | VL rebound = VL>500; 27% high adher. HR = 0.4 (95% CI 0.3–0.6)***; 34% moderate adher. HR = 0.6 (95% CI 0.4–0.8)**; 53% low adher. HR= 1.0 |
|
| Liu et al. (2001) | Public HIV clinic; N/R; N=108 |
N/R; 2 items, composite adherence score; CO: mean |
1 week | EDM r = 0.38***; PR: r = 0.62*** |
VL <400 vs. VL>400 mean adherence 8 week (NS), 24 week* 0.97 (0.85–0.96) vs. 0.90(0.85 −0.96) |
| Lopez-Suarez, Fernandez-Gutierrez del Almo, Perez-Guzman, and Giron-Gonzalez (1998) |
N/R; Cadiz, Spain; N = 65 | N/R; N/R; DI: 80% | N/R | VL log10, 2 drug/3 drug regimen; 3 months: 2.9 vs. 4.4***/3.6 vs. 4.9*; 6 months: 3.1 vs. 4.5***/3.3 vs. 4.8*; CD4, 2 drug/3 drug regimen; 3 months: 550 vs. 356***/405 vs. 333*; 6 months: 567 vs. 416***/540 vs. 400* |
|
| Lucas, Cheever, Chaisson, and Moore (2001) |
Johns Hopkins AIDS Service; Baltimore, MD, US; N = 533 |
N/R; N/R; DI: missed >2 doses in 2 weeks |
2 weeks | VL log10 difference 0.4 (0.2–0.7); CD4 cell difference − .12 (−40-15) (sig. N/R) |
|
| Maggiolo et al. (2002) | Outpatient clinic; Bergamo, Italy; N= 597 |
Modified AACTG (Chesney ‘00); N/R; DI: 100% |
90 days | VL<50; 75.6 vs. 55.3%*** | |
| Mannheimer et al. (2002) | 18 CPCRA Sites; US; N=1095 |
CPCRA (Form 646, ‘02); N/R; CT: 100%, 80–99%, <80% |
7 days | VL log10 decrease***; 100%: 2.8, 80–99: 2.3, <80%: 0.7; CD4 increase***; 100%: 179, 80–99: 159, <80%: 53 |
|
| Martin et al. (2001) | Hospital HIV unit; Madrid, Spain; N =242 |
N/R; 4 items, # of pills delivered/prescribed |
6 days | SR vs. PR; 80% adherence cutoff: sens = 25%, spec = 86%, PPV = 49%, positive likelihood ratio (LR) = 1.8; 90% adherence: sens = 19%, spec = 84%, PPV = 58%, positive LR = 1.2 |
|
| Martin-Fernandez et al.(2001) | HIV unit; Madrid, Spain; N = 283 |
Tuldra ‘99; 2 items: Capable (1–5, cutoff <4); Effort (100 pt scale, cutoff < = 36); Pharmacy refill = gold standard; DI: 95% |
N/R | Area under curve of measure; Capable 0.61 (0.54–0.67); Effort 0.64 (0.57–0.70). Concordance between negative response on 2 SR to PR kappa 0.25 (0.13–0.36) |
|
| Mathews et al. (2002) | University HIV Clinic; San Diego, CA, US; N= 175 |
Modified AACTG (Chesney ‘00); 5 items; DI: Score 0–33 cutoff 5 |
30 days | EDM ρ= −0.40 (sig. N/R) | VL log10 difference*; 1 month: .04, 3 month: 1.1, 6 month: 1.3; VL undetectable*; CD4*; plasma level ρ= − .0.48 (sig. N/R) |
| Melbourne et al. (1999) | Physician offices; Providence, RI, US; N = 44 |
N/R; N/R; CO: mean % | 1 month | SR (SD) vs. EDM (SD); 1 month: 98% (3.6) vs. 90% (14)*; 2 month: 96% (5) vs. 90% (12.6)* |
|
| Moatti et al. (2000) | Hospitals; Marseilles, Avigon, Nice, Paris, France; N=164 |
N/R; N/R; DI: 80% | 7 days | VL median log10 (range)**; 2.7 (2.3–5.6) vs. 3.9 (2.3–5.8); VL undetectable or decrease >1 log10 57% vs. 40.3%*; CD4 median increase (NS); disease progression (NS) |
|
| Murri et al. (2001) | University HIV clinic; Rome, Italy; N= 140 |
Researcher-created; 16 items; DI: forgot 1 dose vs. >1 dose in 3 days |
1 day; 3 days |
VL detectable; nonadherent 3 days; OR 2.2 (95% CI 1.0–4.7); Plasma level PI 1 day: OR 15.9 (95% CI 4.9–50.7), 3 day: OR 4.4 (95% CI 1.7–11.9) |
|
| Nieuwkerk et al. (2001) | 14 hospitals; The Netherlands, Belgium; N=160 |
Researcher-created 3 items; DI: 100% |
7 days | PC measure for saquinavir*; PC measure for ritonavir (NS) |
VL>400 @ 48 weeks; nonadherent 40%, adherent 15%* |
| Nieuwkerk et al. (2001) | 22 hospitals; The Netherlands; N= 224 |
Researcher-created 4 items; DI: 100% |
7 days | VL>500; nonadherent OR 2.1 (95% CI 0.9–1.9); nonadherent ORA 4.0 (95% CI 1.4–11.6); drug level median concentration (range) 1.1 (0.6–1.4) vs. 0.8 (0.51.1)*** |
|
| Oyugi et al. (2004) | Research-affiliated clinics and hospitals; Kampala, Uganda; N= 34 |
AACTG (Chesney ‘00) and Visual Analogue Scale (VAS); N/R; CO: Mean |
3 days (AACTG): 30 day (VAS) |
EDM 3 day r = 0.87***;VAS 0.77***; PC 3 day r = 0.89***; VAS 0.86***; 3 day and VAS r = 0.82*** |
VL < 400 @ 12 weeks; 3-day r=- 0.42**; 30-day VAS r= − .036* |
| Palepu, Horton, Tibbetts, Meli, and Samet (2004) |
Medical and methadone clinics, respite facility; Boston, MA, US; N= 194 |
N/R; N/R; DI: 95%; CO: Mean |
30 days | VL log10 mean (SD); 1.8 (1.8) vs. 2.7 (1.9)***; CD4 mean (SD); 414 (254) vs. 375 (216) (NS) |
|
| Pinheiro et al. (2002) | Public clinic; Pelotas, Brazil; N=195 |
Researcher-created; N/R; DI: 95% |
2 days | VL <500; 67.5% vs. 31.5%***; CDC disease stage (NS) |
|
| Pradier et al. (2001) | Research cohort: 12 outpatient hospitals in Marseilles, Avigon, Nice, and Paris, France; N = 119 | N/R; 1 item for each medication; DI: 100% 3 groups: (1) no VL change or <0.5 decrease, (2) > 0.5 decrease but still detectable, (3) undetectable | 7 days | VL log10; G3 vs. G2 = ORA 5.8 (95% CI 1.5–22.1); G3 vs. Gl = ORA 5.6 (95% CI 1.3–24.7) |
|
| Raboud et al. (2002) | N/R; Italy, The Netherlands, Canada, and Australia; N = 311 |
INC AS, AVANTI 2 and 3 studies; N/R; DI: Adherence from 3 different studies which each dichotomized differently 92.3, 75, 75% | 28 days | PC (N/R) | Virologic failure; RR 3.0 (95% CI 1.4–6.1); Test statistics NR for the following: Virologic suppression**; Triple drug**, double drug (NS); VL undetectable * |
| Schuman et al. (2001) | Research cohort; Baltimore, Chicago, Detroit, New York, LA, Wash. DC, US; N = 371 |
N/R; 1 item; DI: 75% | 2 weeks | VL undetectable; OR 3.9 (95% CI 1.8–8.5); CD4 ≥ 200; OR 2.1 (95% CI 1.0–4.3)* |
|
| Silveira et al. (2002) | HIV/AIDS service; Pelotas, Brazil; N = 244 | N/R; 1 item (# tablets taken); CT: ≥ 95%, 94-80%, 79-60%, <60% | 48 hr | VL <80 across groups OR ≥ 95%: OR 5.5 (95% CI 2.6–11.9); 60–79%: OR 4.2 (95% CI 1.3–.3); 80%–%: OR 5.6 (95% CI 2.2–.1); <60%: 1.0 |
|
| Spire et al. (2002) | Research cohort, 47 hospitals, France; N = 445 |
N/R; 3 items; DI: 100% | 4 days | VL log10 median decrease @ 4 months; 1.7 vs. 1.3***; VL ≤ 500 77% vs. 60%*** |
|
| Trotta et al. (2003) | Research cohorts; Rome and other sites, Italy; N= 596 |
Murri ‘00; 16 items; DI: 86% (missed ≥ 1 dose last 7 days) |
7 days |
|
VL ≤ 500; nonadherent OR 0.7 (95% CI 0.5–.9); CD4 <200/mm; OR 0.6 (95% CI 0.3–.0,p = .06) |
| Vincke and Bolton (2002) | N/R; Belgium; N= 86 | PI attitude scale (Weiss N/R) 3 items Ordinal: scale (1–, 5: excellent) |
4 weeks | Clinician: r= −0.25 (NS); Significant other: r= −0.42** |
VL; R = 0.30 (sig. N/R) |
| Wagner et al. (2001) | 3 VA Medical Centers; Cleveland, OH, Houston, TX, Manhattan, NY, US; N = 793 |
N/R; 4 items; CT: 0 (poor), 1, 2 (perfect) |
4 days | SR and provider agreement; kappa -.03, (-.09,.03) (NS) |
VL <400; 55% vs. 36% vs. 22%***; ORA 0.9 (95% CI 0.8–.3); VL median 141 vs. 393 vs. 1679***; ORA 0.04 (95% CI −0.2-0.1) |
| Wagner, 2002 | CBOs, clinics; Los Angeles, USA; N= 180 |
Modified AACTG (Chesney ‘00) N/R; CO: Mean | 3 days | 4-week EDM: r = 0.34** | |
| Wagner et al, 2003 | Mental health community; LA,CA, US; N = 47 |
N/R; N/R; CO: Means | 3 days; 2 weeks |
EDM 3 days*** r = 0.61;2 weeks*** r = 0.63 | VL log10
r = − .0.39* (recall period N/R); VL log10 mean (SD); 3 day: 2.3 log10 (1.0) vs. 3.5 log10 (1.2)**; CD4 3 days (NS); 14 days (NS) |
| Walsh etal. (2001) | Publicly funded clinic; N/R; N=178 |
Researcher-created; N/R; CO: Median |
30 day and VAS (30 days) |
PR ρ = 0.19**; Nurse rating ρ = 0.51**; MD rating ρ = 0.33**¶ |
|
| Walsh et al. (2002) | Public HIV clinic; London, England; N= 78 |
AACTG (Chesney ‘00), Hecht ‘98, Fletcher ‘79; 6 items: 3 −3day; 1–2 week, 1 last missed; VAS 30 day (0–%); CO: Mean |
3 day; 2 weeks; 30 day (VAS) |
EDM: Univariate linear regression; 3 day r = 0.32; 0.68 (95% CI 0.23–1.13)**; 2 week r = 0.62; 1.21(95% CI 0.86–.56)***; VAS r = 63; 1.09 (95% CI 0.78–1.39)*** |
VL<50; 3 day (NS); 14 day ρ = − .0.30**; VAS ρ = −0.28** |
| Weiser et al. (2003) | 3 private clinics; Gaborone, Francistown, Botswana; N = 93–109 |
Modified AACTG (Chesney ‘00); N/R; DI: 95% |
1 year | SR and provider agreement; Kappa = .35,x2 =11.13*** |
|
| Wutoh et al. (2001) | 2 large HIV clinics; Washington DC, US; N=100 |
N/R; N/R; CO: Mean | 7 days | VL mean; ρ =.−312** |
Notes. N/R: Not reported, NS: Non-significant, ID: Infectious disease, SD: Standard deviation, PI: Protease inhibitor.
Odds ratios, hazard ratios, and relative risks are unadjusted unless denoted by subscript “A”; 95% confidence intervals denote significance unless only a p-value is given.
Correlation statistics are Pearson’s r or Spearman’s ρ.
Significance level was calculated from data provided in the article using a 1 sample test of proportion.
Significance level was calculated from data provided in the article using a 1 sample t-test.***
After summarizing key descriptive information about the studies, we focused on describing the self-report adherence measures in detail and use χ2 tests to assess the association between self-report and other adherence measures. Our examination of the reported associations between self-reported adherence and clinical outcomes such as VL include a forest plot graph to visually summarize reported association effect sizes (Fig. 1). In a sub-analysis, we examined the effect of recall period length on the association between self-reported adherence and VL using χ2 tests of proportions and logistic regression.
Fig. 1.
Association is between (a) adherence and VL suppression or (b) nonadherence and VL increase or rebound. Excludes 4 studies that showed statistically significant associations due to overly-wide confidence intervals (Barroso et al., 2003) or because the association was reported differently (e.g., nonadherence as protective from VL suppression) and could not be re-calculated from published data (Cingolani et al., 2002; LeMoing et al., 2002; Trotta et al., 2003)
Findings from the review
Study description
Study date, location, and setting
The number of publications peaked in the years 2001–2002 (1997 n=1; 1998 n=1; 1999 n=3; 2000 n=6; 2001 n=22; 2002 n=22; 2003 n=14; and through August 2004 n = 8). The vast majority of studies were conducted in the United States (US, n = 26) and Europe (n = 38), mainly France (n = 12), Spain (n = 9), or Italy (n = 9). There were two from Asia, both from Hong Kong (Fong et al., 2003; Ho, Fong, and Wong, 2002), four from South America, all from Brazil (Barroso et al., 2003; Brigido et al., 2001; Pinheiro, de-Carvalho-Leite, Drachler, and Silveira, 2002) and only three recent reports from Africa, in Uganda (Oyugi et al., 2004); Botswana (Weiser et al., 2003); and Senegal (Laniece et al., 2003). Most studies (n = 61) occurred in hospital-based outpatient clinics, either offering HIV primary care or specializing in infectious diseases.
Eligibility criteria and sample characteristics
Eligibility criteria varied greatly across studies. Some studies enrolled any adult patients on ART, while others had extensive inclusion and exclusion criteria that created highly specific samples. Most studies referred to at least one of the following as part of their eligibility criteria: Disease status or clinical status as measured by CD4 count and VL; coexisting problems such as substance use; type of regimen (most required inclusion of a protease inhibitor); treatment experience (many studies required participants to be ART-na¨ıve or on ART for no more than a specified amount of time); and pregnancy status (some studies excluded pregnant women).
Study sample size ranged from 26 (Hugen et al., 2002) to 2528 (Knobel et al., 2002); only five studies had fewer than 50 participants. The majority of participants in almost every study was male (range = 29 to 100% male). Specifically, in the 71 studies reporting sex of participants, 62 included samples that had at least 60% males; two studies had no female participants, and two studies had no male participants. Most studies did not include sufficient numbers of women to conduct analyses by sex. Where reported, these generally indicated that there were no sex differences in adherence levels and no interactions by sex among the adherence measures and other factors. Most participants in the US studies were members of racial/ethnic minority groups; in European samples, race/ethnicity was rarely reported. Some studies provided data on baseline disease stage, VL, or CD4 count.
Study design and purpose
Eighteen studies employed cross-sectional survey designs, often including chart-extracted reports of VL and CD4 counts. The earlier studies generally aimed to identify predictors of nonadherence and often were embedded within clinical trials; later studies often involved sub-analyses of intervention trials. Six studies set out specifically to evaluate adherence measures (i.e., Martin-Fernandez, Escobar-Rodriguez, Campo-Angora, & Rubio-Garcia, 2001; Martin et al., 2001; Murri et al., 2001; Vincke & Bolton, 2002; Wagner et al., 2001; Walsh, Mandalia, & Gazzard, 2002).
Self-report adherence measures
The most common self-report measure consisted of a single item querying the number of prescribed doses the participant had missed in a specified time period (n = 22). There was great heterogeneity among other assessment measures, which included items assessing missed doses on the weekends and adherence to dietary restrictions. Apart from the Adult AIDS Clinical Trials Group (AACTG) adherence measurement form and its variations, which were used in 15 studies, a visual analog scale (six studies), and the Simplified Medication Adherence Questionnaire (two studies), no other single instrument was used in more than one study.
Twenty-five studies did not provide important details about the adherence assessment strategy they employed. Those that did described measures ranging from one item to the lengthy AACTG measure that addresses each medication over each of the last 3 days in terms of number of doses taken per day, number of pills taken per dose, and adherence to any special dietary instructions (Chesney et al., 2000). Measures varied with respect to recall period (from 2 to 365 days); item response format (i.e., closed-ended, open-ended, Likert-type, visual analogue); and whether introductory statements normalizing nonadherence were included. Psychometric properties such as internal consistency of multi-item scales were reported in only three studies.
Most self-report interview modalities appeared to involve paper instruments, although this information was not always explicitly provided. Two studies employed computer-assisted self-interviews (Bangsberg, Bronstone, & Hofmann, 2002; Pinheiro et al., 2002); two were conducted over the telephone (Silveira, Draschler Mde, Leite, Pinheiro, & da Silveira, 2002; Wagner, Kanouse, Koegel, & Sullivan, 2003); and none involved the internet. Few studies reported whether providers, study staff, or the patients themselves administered the interviews.
The construct of adherence was operationalized for the data analyses in a variety of ways–sometimes multiple ways in the same study. A continuous measure of percentage of doses taken was calculated often as
Other researchers created a summary score based on some combination of multiple items. Frequently, adherence data were converted to dichotomous indicators of adherent versus nonadherent patients, with thresholds, often apparently assigned post hoc, of 80% (n = 6/48 or 13% of recall periods assessed), 90% (n = 7/48, 15%), 95% (n = 11/48, 23%), or 100% (n = 21/48, 44%) or less of prescribed doses taken.
Association of self-report and other measures of adherence
As seen in Table 2, 27 of the studies reported data on the association between self-reported adherence and adherence as assessed with another indirect measure of adherence, including EDM (n = 11); pharmacy refill records (n = 9); clinician assessments (n= 7); pill counts (n = 3, of which two were unannounced); chart review (patient report of adherence to provider; n = 1); and morphologic alterations (n = 1). In 27 of the 34, or 79%, of the recall periods examined in these studies, associations were significant or resulted in moderately strong kappa values. Sample sizes were insufficient to compare the level of association by assessment technique.
Table 2.
Association of self-reported antiretroviral adherence with adherence as measured by other indirect measures or with clinical indicators, by recall period
| Self-reported adherence recall period (days) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 7 | 14 | 28 | 30 | 30 (VAS) | 90, 180, or 365 |
Combined time periods or “last missed” |
Total | |
| Clinical impact | ||||||||||||
| HIV-1RNA viral load (VL) |
1/32–4 | 3/43,5–7 | 4/64, 8–12 | 9/1013–22 | 13/162,3,23–35 | 3/311, 12, 36 | 3/337–39 | 10/113, 10, 12, 40–47 | 2/210, 12 | 3/348, 4950 | 6/651–56 | 57/67 |
| Plasma Rx level | 1/14 | 1/14 | 0/122 | 2/224, 35 | 1/140 | 5/6 | ||||||
| CD4 count | 0/111 | 1/414, 16, 17 | 7/1123,24,26,28,29,32,34, 57–59 | 2/336,60 | 1/154 | 4/541, 42, 44, 46, 47 | 1/151 | 16/26 | ||||
| Disease progression/mortality | 1/161 | 0/16 | 0/129 | 1/139 | 2/241, 62 | 4/6 | ||||||
| Other indirect measure of adherence | ||||||||||||
| Electronic data monitoring |
1/12 | 4/411, 12, 63, 64 | 2/22, 27 | 2/211, 12 | 2/265 | 2/210, 12 | 13/13 | |||||
| Pill count | 1/166 | 1/143 | 1/230 | 2/210, 43 | 5/6 | |||||||
| Pharmacy refill | 1/127,67 | 1/168 | 2/2 | |||||||||
| Provider estimate | 0/29, 66 | 0/121 | 0/169 | 2/268 | 1/170 | 3/7 | ||||||
| Other | 1/237,71 | 1/171 | 1/237, 69 | 3/5 | ||||||||
| Significance not reported |
VL43 | PC38 VL46,69 |
PL46 EDM72 | VL43 | ||||||||
Notes. Fractions indicate the proportion of associations that were statistically significant (i.e., p < 0.05 or 95% confidence intervals excluding 1.0). Unadjusted results are reported where available. Superscripted numbers refer to citations that are marked with an asterisk in the References section. Note that some studies provided data on more than one recall period. Not represented in this table: Goujard et al., 2003; Horne et al., 2004; Hugen et at., 2002; Lopez-Suarez et al., 1998; Martin-Fernandez et al. 2001, as self-report recall period could not be determined. Results for mean VL, detectable VL, and different VL outcome categories (e.g., >500 cells, 200–) were entered individually if separate analyses were conducted. ‘Other’ = medical record, morphologic alterations, or significant other.
Association of self-reported adherence and clinical indicators
Most of the studies (60 of 77 or 78%) assessed VL, although the types of tests and their detection thresholds (e.g., Roche Amplicor, 50 copies/µL) were not uniformly described. Many were taken from a review of medical records instead of based on blood samples drawn on the same day adherence was assessed. Analyses of the relation between self-reported adherence and VL most often involved bivariate tests of association such as Pearson product moment correlations. These rarely controlled for confounders or assessed potential effect modifiers such as previous experience with ART. When they did, the association between self-reported adherence and VL usually remained statistically significant (e.g., Alcoba et al., 2003; Nieuwkerk, Gisolf, Sprangers, & Danner, 2001).
In 57 of 67 (85%) of recall periods assessed (note that some studies reported data on more than one recall period), self-reported adherence was significantly related to VL (see Table 2). The magnitude of the significant correlations ranged from 0.30 to 0.60. Across different recall periods, odds ratios and hazard ratios of the association between self-reported adherence and VL were on the order of 2.0, with 95% confidence bounds generally excluding 1.0 (see Fig. 1). Findings from analyses of the proportion of patients with good adherence (with viral suppression as the outcome) and of the proportion of patients with poor adherence (with higher VL as the outcome) were comparable.
As seen in Table 2, fewer studies found a positive correlation between self-reported adherence and CD4 count (16/26 or 62%) of recall periods. Five studies (Brigido et al., 2001; Gao et al., 2000; Ho et al., 2002; Moatti et al., 2000; Pinheiro et al., 2002) reported associations of self-reported adherence with disease progression as defined by development of a new opportunistic infection or disease staging; three were significant. Two studies assessed mortality as the outcome; in both, the association with self-report was significant (Brigido et al., 2001; Garcia de Olalla et al., 2002).
Association of length of recall period and VL
As seen in Table 2, there was some suggestion of an effect of the length of the self-report adherence assessment recall period on the relation with VL: Adherence was associated with VL in 88% of recall periods that were greater than 3 days and in 64% of those that were 3 days or less, χ2 (N = 63) = 4.16, p= 0.04. However, an unadjusted bivariate logistic regression included 1.0 (crude odds ratio 0.25, 95% confidence interval 0.06–1.0, p = 0.05).
Conclusions and implications
A review of the literature on self-report measures of ART adherence identified 77 published articles meeting eligibility criteria. Most were published in 2000–2001 and were based on data from hospital-based clinic samples of predominantly men from the US and Europe. The most common assessment strategy involved asking patients about the number of missed doses over a specified recall period; otherwise, there was great variability in the content of the items, the response format, and the recall period. The lack of widespread use of standardized measures made it difficult to evaluate any particular measure or to compare measures across studies.
Nonetheless, self-reported adherence was significantly related to adherence as assessed by other indirect measures such as EDM and pill count in 79% of studies comparing measurement approaches. Although we were not able to statistically examine these issues in this review, it would be helpful to know which techniques are most closely associated with VL and whether any socio-demographic indicators moderate these relationships. Self-report measures may not be feasible with some individuals (such as the cognitively impaired); therefore, data on which other methods are appropriate options would be useful.
We observed a robust pattern of association between self-reported adherence and VL: In 84% of recall periods, self-reported adherence was associated with VL based on odds ratios or simple measures of correlation. The association was statistically significant across a variety of self-report measures, administration modalities, and recall periods. These findings are consistent with the conclusions of a recent metaanalysis of adherence studies (Nieuwkerk & Oort, 2005). These results may provide some reassurance to practitioners and researchers employing self-reported adherence strategies.
There was some suggestion that longer recall periods may be more likely than shorter ones to yield estimates of adherence that are significantly correlated with VL, although this was not statistically conclusive in our review or in the previously published meta-analysis (P. Nieuwkerk, personal communication April 21, 2005). The association between self-report and CD4 was less consistent, a finding that is not entirely unexpected, as viral load and CD4 count generally correlate but discordant results are common. Furthermore, CD4 response can be somewhat delayed following initial ART initiation. For this reason, many experts believe that VL is the best measure of therapeutic response to ART, though CD4 remains the best clinical prognostic indicator (Bartlett & Gallant, 2004).
These findings are limited by several factors. Because most of the studies were conducted in the West, results may not be generalizable to resource-poor settings. The lack of data on refusal rates and the preponderance of non-probability samples of patients who were largely in care, participants in cohort studies, or volunteers receiving monetary incentives further limit the generalizability of these findings to other HIV populations. Relatedly, we were not able to determine whether self-report measures have differential validity for groups varying in socio-demographic or disease factors, because these variables, if assessed and reported, were not usually included in the analyses and small sample sizes limited the ability to conduct subgroup analyses. The possibility of publication bias—that studies with non-significant associations between adherence and VL are less likely to be published—also cannot be definitively ruled out.
Lack of information about the interviewer’s relationship to the participant and mode of interview administration (Di-Matteo, 2004; Rudd et al., 1990), as well as the lack of any systematic manipulation of these two variables in the studies we reviewed, limits the extent to which we can comment on their relevance to our findings. It is worth exploring whether audio computer-assisted self-interviews (ACASI) can contribute to the quality and validity of ART self-reporting, as has been seen with respect to sex and other sensitive behaviors (Schroder, Carey, & Vanable, 2003). An example of the visual analog scale as presented in a handheld computer can be viewed at http://faculty.washington.edu/wcurioso/emulator/emulator.htm.
Finally, the timing of the adherence assessment may affect the strength of its association with clinical outcome. We would not expect perfect agreement between assessment of self-reported adherence over a brief, recent recall period and current VL, given all the other potential effect modifiers such as co-morbidity and earlier periods of nonadherence that may have resulted in resistance (Bangsberg et al., 2003). Most studies examined the association of adherence and VL cross-sectionally, but adherence over time (serial measurements within patients) may better predict VL prospectively. Longitudinal HIV studies increasingly include tests for genotypic or phenotypic resistance, parameters that may be useful in future ART adherence evaluations.
Obtaining accurate data on the association between assessed ART adherence and relevant outcomes requires methodologically precise studies. Future research in this area should report baseline characteristics that may confound or modify (Raboud, Harris, Rae, & Montaner, 2002) the association between self-reported adherence and health outcomes, including CD4 count nadir, baseline VL, class and duration of previous ART experience, and possibly, evidence of specific ART viral resistance. This precision will enable more accurate estimations of the quality of assessment methods, although given the complex and dynamic nature of HIV disease, no single adherence assessment measure can be expected to correlate perfectly with clinical indicators or clinical outcomes.
Recommendations for best practices in HIV research and clinical management
Our findings suggest that both researchers and clinicians may proceed with the use of self-report measures of ART adherence with some confidence in their validity at least in terms of their associations with other indirect measures of adherence and VL, a reliable surrogate marker of clinical impact. Some experts have advocated the use of multiple adherence measures (Caplan, Harrison, Wellons, & Frech, 1980; Ickovics, 1997; Konkle-Parker, 2000; Samet, Sullivan, Traphagen, & Ickovics, 2001). Our findings suggest this may not be routinely required in clinical arenas, where VL and other biological markers are often readily available and funds for additional assessments are limited. However, there are at least two situations in which further assessment may be warranted.
First, in intervention trials, the use of less subjective methods such as EDM or unannounced pill counts may be worthwhile because of the potential reporting bias with self-report strategies in the intervention conditions. Second, although patient reports of nonadherence can generally be believed, clinicians may be at a loss to interpret individual patient reports of perfect (100%) adherence. Pharmacy refill data, where accessible, may be useful in validating self-reported “perfect” adherence. In one study, adherence as measured by time-to-pharmacy refill was able to distinguish VL impact among self-reportedly perfect adherers (Grossberg, Zhang, & Gross, 2004). Other strategies to mitigate the ceiling effect of reportedly perfect adherence include calculating the proportion of times across multiple interviews that 100% adherence was reported and supplementing the standard 3-day missed dose item with another item assessing the timing of the last missed a dose or whether any doses were missed in the last 30 days (Mannheimer, Friedland, Matts, Child, & Chesney, 2002). These approaches may assist clinicians in identifying patients claiming to be adherent who, in fact, need ART adherence support.
When employing self-report strategies, researchers and clinicians alike should capitalize on the flexibility of self-report methodologies and inquire beyond the assessment of missed doses, gathering information on other aspects of adherence such as knowledge of medication names and prescribed dosing regimens, attention to special dietary instructions, and patterns of nonadherence on weekends, mid-day, or when daily schedules change. Barriers to adherence and facilitators are also important factors that are inaccessible with other adherence assessment methodologies.
Adherence experts have developed guidelines for assessment that are geared toward minimizing social desirability. These include using self-administered measures with open-ended and forced choice items; broaching the topic with a preamble acknowledging the low prevalence and difficulty of perfect adherence; wording items in such a way that non-adherence is presented as expected and accepted; querying reasons for nonadherence; focusing on recent behavior; specifying a time frame; aiding recall when possible using medication lists and diagrams of pills; anchoring reports to salient events; embedding threatening with non-threatening items; using authority to justify and normalize the behavior; and ending with a reliability check of the accuracy of responses (Miller & Hays, 2000).
Researchers designing statistical analyses and clinicians seeking guidance for advising patients could benefit from recommendations regarding an appropriate threshold of adherence necessary for favorable clinical outcomes. In the studies we reviewed, thresholds appeared to be often determined post hoc, increasing the probability of Type I error. In some instances, a threshold was predetermined but analyses were conducted with a continuous measure of adherence. Generally speaking, parametric tests of continuous variables will have more power than nonparametric analyses of di-chotomous variables but will not define a clinically relevant cutoff. Given that continuous measures of self-report are highly skewed and non-normal, it may be most valid to dichotomize at 100% for statistical analyses. However, as a clinical goal, this level may be unreasonable for patients in the long term. Optimal virologic success declines rapidly in patients taking fewer than 95% of their prescribed doses (Paterson et al., 2000). Nonetheless, one study using pharmacy refill data among 923 HIV-positive patients showed that there was no difference in the risk of disease progression between those with moderate (70–90%) and high (>90%) levels of adherence compared to those with low (<70%) adherence (Kitahata et al., 2004). It is worth exploring whether patients can reliably make fine distinctions about their adherence behavior, such as judging it as either less than 80% or less than 85% (Bangsberg, Moss, & Deeks, 2004).
Which recall period is best to use is an open question. Patients do report more accurately over briefer time periods, with accuracy dropping off as rapidly as beyond 24 hr (Turner & Hecht, 2001; Wagner & Miller, 2004; Walsh, Horne, Dalton, Burgess, & Gazzard, 2001). It is worth considering, however, whether somewhat longer recall periods may yield more useful data as the increasing use of once-daily ART dosing may now result in too few dosing times in a very brief (i.e., 1–3 day) recall period to provide sufficient variability in adherence (Paterson et al., 2000). A very short interval may not allow for differentiation between patients whose good adherence is consistent and those who report good adherence over a recent brief time period but who are generally less adherent. A particular advantage of a 7-day recall period is that it will always include a weekend, during which adherence is often problematic.
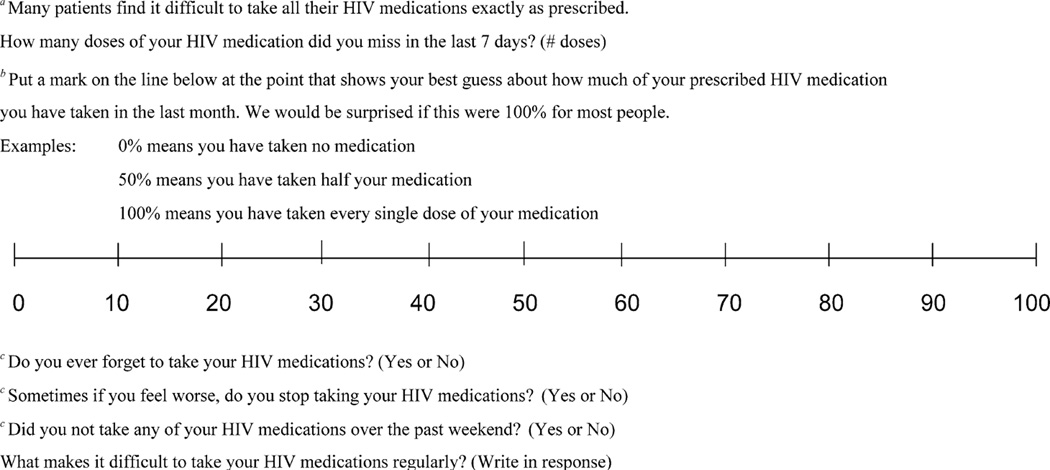
Recommended self-report measures are presented in Fig. 2. These items are drawn both from the literature and from clinical experience and incorporate use of normalizing language, 7-day recall, and exploration of barriers to adherence (Morisky, Green, & Levine, 1986). Many different self-report measures appear to have an association with VL. Researchers and clinicians may choose single or multiple items based on their needs, weighing the need to assess inaccurate dosing or dietary adherence with the desire to reduce respondent burden. Longitudinal use of the increasingly utilized visual analog scale may be enhanced by measuring the exact distance from zero to the patient’s mark. We suggest use of the term “dose” over “pills” as patients generally do not take partial doses (G. Wagner, personal communication March 2, 2005) and it is easier to calculate the number of missed doses than the exact number of pills missed across missed doses. Exploring the reasons why patients “forget” to take their medications may uncover important issues that can be addressed with subsequent potential problem-solving (Bartlett, 2002). More consistent use of items such as these would allow comparison of self-report measure psychometric and clinical performance across populations.
Fig. 2.
Recommended items for assessing self-reported antiretroviral adherence
Notes: aBased on Golin et al. (2002); bBased on Wash, Mandalia, and Gazzard (2002). An exact percentage can be calculated by measuring the distance from 0 to mark in cm or inches; cBased on Knobel et al. (2002).
The ability to make more definitive recommendations regarding precise measurement strategies will be enhanced with further research that explicitly addresses some of the issues we have raised. In the meantime, results from this extensive literature review offer some direction for HIV researchers and clinicians in their critically important work attempting to address and enhance ART adherence.
Acknowledgments
We are grateful to researchers who shared their materials with us and provided feedback regarding this review, especially Pythia Nieuwkerk and Glenn Wagner. This work was supported by University of Washington Center for AIDS Research So-ciobehavioral and Prevention Research Core (P30 AI 27757) funding to Dr. Kurth, 2 R01 MH58986 to Dr. Simoni, and F31 MH71179 to Mr. Pantalone.
Contributor Information
Jane M. Simoni, Department of Psychology, University of Washington, Seattle, Washington 98195-1525 Box 351525, jsimoni@u.washington.edu
Ann E. Kurth, School of Nursing/CFAR, University of Washington, Seattle, Washington
Cynthia R. Pearson, School of Public Health & Community Medicine, University of Washington, Seattle, Washington
David W. Pantalone, Department of Psychology, University of Washington, Seattle, Washington 98195-1525 Box 351525
Joseph O. Merrill, Department of Medicine, University of Washington, Seattle, Washington
Pamela A. Frick, Department of Pharmacy, University of Washington, Seattle, Washington
References
(Numbered asterisks indicate studies cited in Fig. 1)
- *22.Alcoba M, Cuevas MJ, Perez-Simon MR, Mostaza JL, Ortega L, Ortiz de Urbina J, et al. Assessment of adherence to triple antiretroviral treatment including indinavir: Role of the determination of plasma levels of indinavir. Journal of Acquired Immune Deficiency Syndromes. 2003;33(2):253–258. doi: 10.1097/00126334-200306010-00022. [DOI] [PubMed] [Google Scholar]
- *50.Aloisi MS, Arici C, Balzano R, Noto P, Piscopo R, Filice G, et al. Behavioral correlates of adherence to antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes. 2002;31(Suppl 3):S145–S148. doi: 10.1097/00126334-200212153-00012. [DOI] [PubMed] [Google Scholar]
- *57.Altice FL, Mostashari F, Friedland GH. Trust and the acceptance of and adherence to antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes. 2001;28(1):47–58. doi: 10.1097/00042560-200109010-00008. [DOI] [PubMed] [Google Scholar]
- *58.Ammassari A, Antinori A, Aloisi MS, Trotta MP, Murri R, Bartoli L, et al. Depressive symptoms, neurocognitive impairment, and adherence to highly active antiretroviral therapy among HIV-infected persons. Psychosomatics. 2004;45(5):394–402. doi: 10.1176/appi.psy.45.5.394. [DOI] [PubMed] [Google Scholar]
- *34.Antinori A, Cozzi-Lepri A, Ammassari A, Trotta MP, Nauwelaers D, Hoetelmans R. Relative prognostic value of self-reported adherence and plasma NNRTI/PI concentrations to predict virological rebound in patients initially responding to HAART. Antiviral Therapy. 2004;9(2):291–296. [PubMed] [Google Scholar]
- *2.Arnsten JH, Demas PA, Farzadegan H, Grant RW, Gourevitch MN, Chang CJ. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: Comparison of self-report and electronic monitoring. Clinical Infectious Diseases. 2001;33(8):1417–1423. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *8.Bangsberg DR, Hecht FM, Charlebois ED, Zolopa AR, Holodniy M, Sheiner L, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14(4):357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- *9.Bangsberg DR, Bronstone A, Hofmann R, et al. A computer-based assessment detects regimen misunderstandings and nonadherence for patients on HIV antiretroviral therapy. AIDS Care. 2002;14(1):3–15. doi: 10.1080/09540120220097892. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR, Charlebois ED, Grant RM, Holodniy M, Deeks SG, Perry S, et al. High levels of adherence do not prevent accumulation of HIV drug resistanc mutations. AIDS. 2003;17(13):1925–1932. doi: 10.1097/00002030-200309050-00011. [DOI] [PubMed] [Google Scholar]
- *66.Bangsberg DR, Hecht FM, Clague H, Charlebois ED, Ciccarone D, Chesney M, et al. Provider assessment of adherence to HIV antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes. 2001;26(5):435–442. doi: 10.1097/00126334-200104150-00005. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR, Moss AR, Deeks SG. Paradoxes of adherence and drug resistance to HIV antiretroviral therapy. Journal of Antimicrobial Chemotherapy. 2004;53(5):696–699. doi: 10.1093/jac/dkh162. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR, Charlebois ED, Grant RM, Holodniy M, Deeks SG, Perry S, et al. High levels of adherence do not prevent accumulation of HIV drug resistant mutations. AIDS. 2003;17:1925–1932. doi: 10.1097/00002030-200309050-00011. [DOI] [PubMed] [Google Scholar]
- *40.Barroso PF, Schechter M, Gupta P, Bressan C, Bomfim A, Harrison LH. Adherence to antiretroviral therapy and persistence of HIV RNA in semen. Journal of Acquired Immune Deficiency Syndromes. 2003;32(4):435–440. doi: 10.1097/00126334-200304010-00014. [DOI] [PubMed] [Google Scholar]
- Bartlett JA. Addressing the challenges of adherence. Journal of Acquired Immune Deficiency Syndromes. 2002;29(Suppl 1):S2–S10. doi: 10.1097/00126334-200202011-00002. [DOI] [PubMed] [Google Scholar]
- Bartlett JG, Gallant JE. Medical management of HIV infection. 2004 ed. Baltimore, MD: Johns Hopkins Medicine Health Publishing Business Group; 2004. [Google Scholar]
- Bova CA, Fennie KP, Knafl GJ, Dieckhaus KD, Watrous E, Williams AB. Use of electronic monitoring devices to measure antiretroviral adherence: Practical considerations. AIDS and behavior. 2005;9(1):103–110. doi: 10.1007/s10461-005-1685-0. [DOI] [PubMed] [Google Scholar]
- *41.Brigido LF, Rodrigues R, Casseb J, Oliveira D, Rossetti M, Menezes P, et al. Impact of adherence to antiretroviral therapy in HIV-1-infected patients at a university public service in Brazil. AIDS Patient Care and STDS. 2001;15(11):587–593. doi: 10.1089/108729101753287685. [DOI] [PubMed] [Google Scholar]
- Caplan RD, Harrison RV, Wellons RV, Frech JRP. Social support and patient adherence: Experimental and survey findings. Ann Arbor, MI: Survey Research Center, University of Michigan; 1980. [Google Scholar]
- *13.Carrieri P, Cailleton V, Le Moing V, Spire B, Dellamonica P, Bouvet E, et al. The dynamic of adherence to highly active antiretroviral therapy: Results from the French National APROCO cohort. Journal of Acquired Immune Deficiency Syndromes. 2001;28(3):232–239. doi: 10.1097/00042560-200111010-00005. [DOI] [PubMed] [Google Scholar]
- *14.Carrieri MP, Raffi F, Lewden C, Sobel A, Michelet C, Cailleton V, et al. Impact of early versus late adherence to highly active antiretroviral therapy on immuno-virological response: A 3-year follow-up study. Antiviral Therapy. 2003;8(6):585–594. doi: 10.1177/135965350300800606. [DOI] [PubMed] [Google Scholar]
- *48.Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychology. 2000;19(2):124–133. [PubMed] [Google Scholar]
- *42.Cederfjall C, Langius-Eklof A, Lidman K, Wredling R. Self-reported adherence to antiretroviral treatment and degree of sense of coherence in a group of HIV-infected patients. AIDS Patient Care and STDS. 2002;16(12):609–616. doi: 10.1089/108729102761882143. [DOI] [PubMed] [Google Scholar]
- *51.Cingolani A, Antinori A, Rizzo MG, Murri R, Ammas-sari A, Baldini F, et al. Usefulness of monitoring HIV drug resistance and adherence in individuals failing highly active antiretroviral therapy: A randomized study (ARGENTA) AIDS. 2002;16(3):369–379. doi: 10.1097/00002030-200202150-00008. [DOI] [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. Patient Care Committee and Adherence Working Group of the Outcomes Committeeofthe Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12(3):255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- *5.Cohn SE, Kammann E, Williams P, Currier JS, Chesney MA. Association of adherence to Mycobac-terium avium complex prophylaxis and antiretroviral therapy with clinical outcomes in acquired immunodeficiency syndrome. Clinical Infectious Diseases. 2002;34(8):1129–1136. doi: 10.1086/339542. [DOI] [PubMed] [Google Scholar]
- DiMatteo MR. Variations in patients’ adherence to medical recommendations: A quantitative review of 50 years of research. Medical Care. 2004;42(3):200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- *23.Dorz S, Lazzarini L, Cattelan A, Meneghetti F, Novara C, Concia E, et al. Evaluation of adherence to an-tiretroviral therapy in Italian HIV patients. AIDS Patient Care and STDs. 2003;17(1):33. doi: 10.1089/108729103321042890. [DOI] [PubMed] [Google Scholar]
- *52.Duong M, Piroth L, Peytavin G, Forte F, Kohli E, Grappin M, et al. Value of patient self-report and plasma human immunodeficiency virus protease inhibitor level as markers of adherence to antiretroviral therapy: Relationship to virologic response. Clinical Infectious Diseases. 2001;33(3):386–392. doi: 10.1086/321876. [DOI] [PubMed] [Google Scholar]
- *15.Duran S, Peytavin G, Carrieri P, Raffi F, Ecobichon JL, Pereira E, et al. The detection of non-adherence by self-administered questionnaires can be optimized by protease inhibitor plasma concentration determination. AIDS. 2003;17(7):1096–1099. doi: 10.1097/00002030-200305020-00025. [DOI] [PubMed] [Google Scholar]
- *53.Duran S, Saves M, Spire B, Cailleton V, Sobel A, Carrieri P, et al. Failure to maintain long-term adherence to highly active antiretroviral therapy: The role of lipodystrophy. AIDS. 2001;15(18):2441–2444. doi: 10.1097/00002030-200112070-00012. [DOI] [PubMed] [Google Scholar]
- *24.Duran S, Solas C, Spire B, Carrieri MP, Fuzibet JG, Costagliola D, et al. ‘Do HIV-infected injecting drug users over-report adherence to highly active antiretroviral therapy?’ A comparison between patients’ self-reports and serum protease inhibitor concentrations in the French Manif 2000 cohort study. AIDS. 2001;15(8):1075–1077. doi: 10.1097/00002030-200105250-00024. [DOI] [PubMed] [Google Scholar]
- *70.Eldred L, Wu A, Chaisson R, Moore R. Adherence to antiretroviral and pneumocystis prophylaxis in HIV disease. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1998;18(2):117–125. doi: 10.1097/00042560-199806010-00003. [DOI] [PubMed] [Google Scholar]
- *54.Fong OW, Ho CF, Fung LY, Lee FK, Tse WH, Yuen CY, et al. Determinants of adherence to highly active antiretroviral therapy (HAART) in Chinese HIV/AIDS patients. HIV Medicine. 2003;4(2):133–138. doi: 10.1046/j.1468-1293.2003.00147.x. [DOI] [PubMed] [Google Scholar]
- *61.Gao X, Nau D, Rosenbluth S, Scott V, Woodward C. The relationship of disease severity, health beliefs and medication adherence among HIV patients. AIDS Care. 2000;12(4):387–398. doi: 10.1080/09540120050123783. [DOI] [PubMed] [Google Scholar]
- *62.Garcia de Olalla P, Knobel H, Carmona A, Guelar A, Lopez-Colomes JL, Cayla JA. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. Journal of Acquired Immune Deficiency Syndromes. 2002;30:105–110. doi: 10.1097/00042560-200205010-00014. [DOI] [PubMed] [Google Scholar]
- Geletko SM, Segarra M, Mayer KH, Fiore TC, Bettencourt FA, Flanigan TP, et al. Electronic compliance assessment of antifungal prophylaxis for human immunodeficiency virus-infected women. Antimicrobial Agents and Chemotherapy. 1996;40(6):1338–1341. doi: 10.1128/aac.40.6.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *25.Gifford A, Bormann J, Shively M, Wright B, Richman D, Bozzette S. Predictors of self-reported adherence and plasma HIV concentrates in patients on multi drug an-tiretroviral regimens. Journal of Acquired Immune Deficiency Syndromes. 2000;23(5):386–395. doi: 10.1097/00126334-200004150-00005. [DOI] [PubMed] [Google Scholar]
- *43.Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clinical Trials. 2004;5(2):74–79. doi: 10.1310/JFXH-G3X2-EYM6-D6UG. [DOI] [PubMed] [Google Scholar]
- Godin G, Gagne C, Naccache H. Validation of a self-reported questionnaire assessing adherence to an-tiretroviral medication. AIDS Patient Care and STDs. 2003;17(7):325. doi: 10.1089/108729103322231268. [DOI] [PubMed] [Google Scholar]
- *72.Golin CE, Liu H, Hays RD, Miller LG, Beck CK, Ickovics J, et al. A prospective study of predictors of adherence to combination antiretroviral medication. Journal of General Internal Medicine. 2002;17(10):756–765. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *59.Gordillo V, del Amo J, Soriano V, Gonzalez-Lahoz J. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS. 1999;13(13):1763–1769. doi: 10.1097/00002030-199909100-00021. [DOI] [PubMed] [Google Scholar]
- Gordis L. Conceptual and methodologic problems in measuring patient compliance. In: Haynes DWTRB, Sackett DL, editors. Compliance in health care. Baltimore, MD: Johns Hopkins University; 1979. pp. 23–48. [Google Scholar]
- *23.Goujard C, Bernard N, Sohier N, Peyramond D, Lanc¸on F, Chwalow J, et al. Impact of a patient education program on adherence to HIV medication: A randomized clinical trial. Journal of Acquired Immune Deficiency Syndromes. 2003;34(2):191–194. doi: 10.1097/00126334-200310010-00009. [DOI] [PubMed] [Google Scholar]
- Grossberg R, Zhang Y, Gross R. A time-to-prescription-refill measure of antiretroviral adherence predicted changes in viral load in HIV. Journal of Clinical Epidemiology. 2004;57(10):1107–1110. doi: 10.1016/j.jclinepi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- *37.Haubrich RH, Little SJ, Currier JS, Forthal DN, Kemper CA, Beall GN, et al. The value of patient-reported adherence to antiretroviral therapy in predicting virologic and immunologic response. AIDS. 1999;13:1099–1107. doi: 10.1097/00002030-199906180-00014. [DOI] [PubMed] [Google Scholar]
- *39.Ho CF, Fong OW, Wong KH. Patient self-report as a marker of adherence to antiretroviral therapy. Clinical Infectious Diseases. 2002;34(11):1534–1535. doi: 10.1086/340267. [DOI] [PubMed] [Google Scholar]
- Hogg R, Heath K, Bangsberg DR, Yip B, Press N, O’shaughnessy MV, et al. Intermittent use of triple combination therapy is predictive of mortality at baseline and after one year of follow-up. AIDS. 2002;16:1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- *74.Horne R, Buick D, Fisher M, Leake H, Cooper V, Weinman J. Doubts about necessity and concerns about adverse effects: Identifying the types of beliefs that are associated with non-adherence to HAART. International Journal of STD AIDS. 2004;15(1):38–44. doi: 10.1258/095646204322637245. [DOI] [PubMed] [Google Scholar]
- *75.Hugen PW, Langebeek N, Burger DM, Zomer B, van Leusen R, Schuurman R, et al. Assessment of adherence to HIV protease inhibitors: Comparison and combination of various methods, including MEMS (electronic monitoring), patient and nurse report, and therapeutic drug monitoring. Journal of Acquired Immune Deficiency Syndromes. 2002;30(3):324–334. doi: 10.1097/00126334-200207010-00009. [DOI] [PubMed] [Google Scholar]
- Ickovics J. Measures of adhference; Washington, DC. Paper presented at the Adherence to New HIV Therapies: A Research Conference.1997. [Google Scholar]
- *16.Ickovics JR, Cameron A, Zackin R, Bassett R, Chesney M, Johnson VA, et al. Consequences and determinants of adherence to antiretroviral medication: Results from Adult AIDS Clinical Trials Group protocol 370. Antiviral Therapy. 2002;7(3):185–193. doi: 10.1177/135965350200700308. [DOI] [PubMed] [Google Scholar]
- *26.Ingersoll K. The impact of psychiatric symptoms, drug use, and medication regimen on non-adherence to HIV treatment. AIDS Care. 2004;16(2):199–211. doi: 10.1080/09540120410001641048. [DOI] [PubMed] [Google Scholar]
- *63.Kimmerling M, Wagner G, Ghosh-Dastidar B. Factors associated with accurate self-reported adherence to HIV antiretrovirals. International Journal of STD AIDS. 2003;14(4):281–284. doi: 10.1258/095646203321264917. [DOI] [PubMed] [Google Scholar]
- *17.Kleeberger CA, Phair JP, Strathdee SA, Detels R, Kingsley L, Jacobson LP. Determinants of heterogeneous adherence to HIV-antiretroviral therapies in the Multicenter AIDS Cohort Study. Journal of Acquired Immune Deficiency Syndromes. 2001;26(1):82–92. doi: 10.1097/00126334-200101010-00012. [DOI] [PubMed] [Google Scholar]
- Kitahata MM, Reed SD, Dillingham PW, Van Rompaey SE, Young AA, Harrington RD, et al. Pharmacy-based assessment of adherence to HAART predicts virologic and immunologic treatment response and clinical progression to AIDS and death. International Journal of STD AIDS. 2004;15(12):803–810. doi: 10.1258/0956462042563666. [DOI] [PubMed] [Google Scholar]
- *55.Knobel H, Alonso J, Casado J, Collazos J, Gonzalez J, Ruiz I, et al. Validation of a simplified medication adherence questionnaire in a large cohort of HIV-infected patients: The GEEMA Study. AIDS. 2002;16:605–613. doi: 10.1097/00002030-200203080-00012. [DOI] [PubMed] [Google Scholar]
- *44.Knobel H, Guelar A, Carmona A, Espona M, Gonzalez A, Lopez-Colomes JL, et al. Virologic outcome and predictors of virologic failure of highly active antiretroviral therapy containing protease inhibitors. AIDS Patient Care and STDs. 2001;15:193–199. doi: 10.1089/10872910151133729. [DOI] [PubMed] [Google Scholar]
- *56.Knobel H, Vallecillo G, Guelar A, Pedrol E, Soler A, Carmona A, et al. Simplified therapy with zidovudine, lamivudine, and abacavir for very nonadherent, treatment-failing patients. HIV Clinical Trials. 2004;5(2):65–73. doi: 10.1310/CW63-E5E4-M51K-91DR. [DOI] [PubMed] [Google Scholar]
- Konkle-Parker DJ. What is the best way to measure medication adherence? HIV Clinical Trials. 2000;12(3):11–12. [PubMed] [Google Scholar]
- Laniece I, Ciss M, Desclaux A, Diop K, Mbodj F, Ndiaye B, et al. Adherence to HAART and its principal determinants in a cohort of Senegalese adults. AIDS. 2003;17(Suppl 3):S103–S108. doi: 10.1097/00002030-200317003-00014. [DOI] [PubMed] [Google Scholar]
- Le Moing V, Chene G, Carrieri MP, Alioum A, Brun-Vezinet F, Piroth L, et al. Predictors of virological rebound in HIV-1-infected patients initiating a protease inhibitor-containing regimen. AIDS. 2002;16(1):21–29. doi: 10.1097/00002030-200201040-00004. [DOI] [PubMed] [Google Scholar]
- Le Moing V, Chene G, Carrieri MP, Besnier JM, Masquelier B, Salamon R, et al. Clinical, biologic, and behavioral predictors of early immunologic and virologic response in HIV-infected patients initiating protease inhibitors. Journal of Acquired Immune Deficiency Syndromes. 2001;27(4):372–376. doi: 10.1097/00126334-200108010-00007. [DOI] [PubMed] [Google Scholar]
- *27.Liu H, Golin CE, Miller LG, Hays RD, Beck CK, Sanandaji S, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Annals of Internal Medicine. 2001;134(10):968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- *60.Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. Journal of Acquired Immune Deficiency Syndromes. 2001;27(3):251–259. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- *76.Lopez-Suarez A, Fernandez-Gutierrez del Almo C, Perez-Guzman E, Giron-Gonzalez JA. Adherence to the antiretroviral treatment in asymptomatic HIV-infected patients. AIDS. 1998;12(6):685–686. [PubMed] [Google Scholar]
- *49.Maggiolo F, Ripamonti D, Arici C, Gregis G, Quinzan G, Camacho GA, et al. Simpler regimens may enhance adherence to antiretrovirals in HIV-infected patients. HIV Clinical Trials. 2002;3(5):371–378. doi: 10.1310/98b3-pwg8-pmyw-w5bp. [DOI] [PubMed] [Google Scholar]
- *28.Mannheimer S, Friedland G, Matts J, Child C, Chesney M. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clinical Infectious Diseases. 2002;34(8):1115–1121. doi: 10.1086/339074. [DOI] [PubMed] [Google Scholar]
- Martin J, Sabugal GM, Rubio R, Sainz-Maza M, Blanco JM, Alonso JL, et al. Outcomes of a health education intervention in a sample of patients infected by HIV, most of them injection drug users: Possibilities and limitations. AIDS Care. 2001;13(4):467–473. doi: 10.1080/09540120120057996. [DOI] [PubMed] [Google Scholar]
- Martin-Fernandez J, Escobar-Rodriguez I, Campo-Angora M, Rubio-Garcia R. Evaluation of adherence to highly active antiretroviral therapy. Archives of Internal Medicine. 2001;161(22):2739–2740. doi: 10.1001/archinte.161.22.2739. [DOI] [PubMed] [Google Scholar]
- *67.Martin J, Sabugal GM, Rubio R, Sainz-Maza M, Blanco JM, Alonso JL, et al. Outcomes of a health education intervention in a sample of patients infected by HIV, most of them injection drug users: Possibilities and limitations. AIDS Care. 2001;13(4):467–473. doi: 10.1080/09540120120057996. [DOI] [PubMed] [Google Scholar]
- *46.Mathews WC, Mar-Tang M, Ballard C, Colwell B, Abulhosn K, Noonan C, et al. Prevalence, predictors, and outcomes of early adherence after starting or changing antiretroviral therapy. AIDS Patient Care and STDS. 2002;16(4):157–172. doi: 10.1089/10872910252930867. [DOI] [PubMed] [Google Scholar]
- *65.Melbourne KM, Geletko SM, Brown SL, Willey-Lessne C, Chase S, Fisher A. Medication adherence in patients with HIV infection: A comparison of two measurement methods. The AIDS Reader. 1999;9(5):329–338. [PubMed] [Google Scholar]
- Miller LG, Hays RD. Adherence to combination antiretroviral therapy: Synthesis of the literature and clinical implications. The AIDS Reader. 2000;10(3):177–185. [PubMed] [Google Scholar]
- Miller LG, Hays RD. Measuring adherence to antiretroviral medications in clinical trials. HIV Clinical Trials. 2000;1(1):36–46. doi: 10.1310/enxw-95pb-5ngw-1f40. [DOI] [PubMed] [Google Scholar]
- *29.Moatti JP, Carrieri MP, Spire B, Gastaut JA, Cassuto JP, Moreau J. Adherence to HAART in French HIV-infected injecting drug users: The contribution of buprenorphine drug maintenance treatment. The Manif 2000 Study Group. AIDS. 2000;14(2):151–155. doi: 10.1097/00002030-200001280-00010. [DOI] [PubMed] [Google Scholar]
- *29.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Medical Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- *4.Murri R, Ammassari A, De Luca A, Cingolani A, Marconi P, Wu AW, et al. Self-reported nonadherence with antiretroviral drugs predicts persistent condition. HIV Clinical Trials. 2001;2(4):323–329. doi: 10.1310/KDM0-RU5W-NVTW-N9MC. [DOI] [PubMed] [Google Scholar]
- *30.Nieuwkerk P, Gisolf E, Sprangers M, Danner S. Adherence over 48 weeks in an antiretroviral clinical trial: Variable within patients, affected by toxicities and independently predictive of virological response. Antiviral Therapy. 2001;6(2):97–103. [PubMed] [Google Scholar]
- Nieuwkerk PT, Oort FJ. Self-reported adherence to antiretroviral therapy for HIV-1 infection and virologic treatment response: A meta-analysis. Journal of Acquired Immune Deficiency Syndromes. 2005;38(4):445–448. doi: 10.1097/01.qai.0000147522.34369.12. [DOI] [PubMed] [Google Scholar]
- *35.Nieuwkerk PT, Sprangers MA, Burger DM, Hoetelmans RM, Hugen PW, Danner SA, et al. Limited patient adherence to highly active antiretroviral therapy for HIV-1 infection in an observational cohort study. Archives of Internal Medicine. 2001;161(16):1962–1968. doi: 10.1001/archinte.161.16.1962. [DOI] [PubMed] [Google Scholar]
- *10.Oyugi JH, Byakika-Tusiime J, Charlebois ED, Kityo C, Mugerwa R, Mugyenyi P, et al. Multiple validated measures of adherence indicate high levels of adherence to generic HIV antiretroviral therapy in a resource-limited setting. Journal of Acquired Immune Deficiency Syndromes. 2004;36(5):1100–1102. doi: 10.1097/00126334-200408150-00014. [DOI] [PubMed] [Google Scholar]
- *47.Palepu A, Horton NJ, Tibbetts N, Meli S, Samet JH. Uptake and adherence to highly active antiretroviral therapy among HIV-infected people with alcohol and other substance use problems: The impact of substance abuse treatment. Addiction. 2004;99(3):361–368. doi: 10.1111/j.1360-0443.2003.00670.x. [DOI] [PubMed] [Google Scholar]
- Paterson D, Potoski B, Capitano B. Measurement of adherence to antiretroviral medications. Journal of Acquired Immune Deficiency Syndromes. 2002;31:S103–S106. doi: 10.1097/00126334-200212153-00003. [DOI] [PubMed] [Google Scholar]
- Paterson D, Swindells S, Mohr J, Brester M, Vergis E, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Pinheiro CA, de-Carvalho-Leite JC, Drachler ML, Silveira VL. Factors associated with adherence to antiretroviral therapy in HIV/AIDS patients: A cross-sectional study in Southern Brazil. Brazilian Journal of Medical and Biological Research. 2002;35(10):1173–1181. doi: 10.1590/s0100-879x2002001000010. [DOI] [PubMed] [Google Scholar]
- *31.Pradier C, Carrieri P, Bentz L, Spire B, Dellamonica P, Moreau J, et al. Impact of short-term adherence on virological and immunological success of HAART: A case study among French HIV-infected IDUs. International Journal of STD AIDS. 2001;12(5):324–328. doi: 10.1258/0956462011923165. [DOI] [PubMed] [Google Scholar]
- *38.Raboud JM, Harris M, Rae S, Montaner JS. Impact of adherence on duration of virological suppression among patients receiving combination antiretroviral therapy. HIV Medicine. 2002;3(2):118–124. doi: 10.1046/j.1468-1293.2002.00109.x. [DOI] [PubMed] [Google Scholar]
- Rudd P. In search of the gold standard for compliance measurement. Archives of Internal Medicine. 1979;139(6):627–628. [PubMed] [Google Scholar]
- Rudd P, Ahmed S, Zachary V, Barton C, Bonduelle D. Improved compliance measures: Applications in an ambulatory hypertensive drug trial. Clinical Pharmacology and Therapeutics. 1990;48(6):676–685. doi: 10.1038/clpt.1990.211. [DOI] [PubMed] [Google Scholar]
- Samet JH, Sullivan LM, Traphagen ET, Ickovics JR. Measuring adherence among HIV-infected persons: Is MEMS consummate technology? AIDS and behavior. 2001;5(1):21–30. [Google Scholar]
- Schroder KE, Carey MP, Vanable PA. Methodological challenges in research on sexual risk behavior: I. Item content, scaling, and data analytical options. Annals of Behavioral Medicine. 2003;26(2):76–103. doi: 10.1207/s15324796abm2602_02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *36.Schuman P, Ohmit E, Cohen M, Sacks H, et al. Prescription of and adherence to antiretroviral therapy among women with AIDS. AIDS and behavior. 2001;5(4):371–000. [Google Scholar]
- *7.Silveira MP, Draschler Mde L, Leite JC, Pinheiro CA, da Silveira VL. Predictors of undetectable plasma viral load in HIV-positive adults receiving antiretroviral therapy in southern Brazil. The Brazilian Journal of Infectious Diseases. 2002;6(4):164–171. doi: 10.1590/s1413-86702002000400003. [DOI] [PubMed] [Google Scholar]
- *20.Spire B, Duran S, Souville M, Leport C, Raffi F, Moatti JP. Adherence to highly active antiretroviral therapies (HAART) in HIV-infected patients: From a predictive to a dynamic approach. Social Science and Medicine. 2002;54(10):1481–1496. doi: 10.1016/s0277-9536(01)00125-3. [DOI] [PubMed] [Google Scholar]
- Straka RJ, Fish JT, Benson SR, Suh JT. Patient self-reporting of compliance does not correspond with electronic monitoring: An evaluation using isosorbide dinitrate as a model drug. Pharmacotherapy. 1997;17(1):126–132. [PubMed] [Google Scholar]
- *32.Trotta MP, Ammassari A, Cozzi-Lepri A, Zaccarelli M, Castelli F, Narciso P, et al. Adherence to highly active antiretroviral therapy is better in patients receiving non-nucleoside reverse transcriptase inhibitor-containing regimens than in those receiving protease inhibitor-containing regimens. AIDS. 2003;17(7):1099–1102. doi: 10.1097/00002030-200305020-00026. [DOI] [PubMed] [Google Scholar]
- Turner B. Adherence to antiretroviral therapy by human immunodeficiency virus-infected patients. The Journal of Infectious Disease. 2002;185(Suppl 2):S143–S151. doi: 10.1086/340197. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Hecht FM. Improving on a coin toss to predict patient adherence to medications. Annals of Internal Medicine. 2001;134(10):1004–1006. doi: 10.7326/0003-4819-134-10-200105150-00015. [DOI] [PubMed] [Google Scholar]
- *69.Vincke J, Bolton R. Therapy adherence and highly active antiretroviral therapy: Comparison of three sources of information. AIDS Patient Care and STDS. 2002;16(10):487–495. doi: 10.1089/10872910260351267. [DOI] [PubMed] [Google Scholar]
- *64.Wagner GJ. Predictors of antiretroviral adherence as measured by self-report, electronic monitoring, and medication diaries. AIDS Patient Care and STDS. 2002;16(12):599–608. doi: 10.1089/108729102761882134. [DOI] [PubMed] [Google Scholar]
- *11.Wagner GJ, Kanouse DE, Koegel P, Sullivan G. Adherence to HIV antiretrovirals among persons with serious mental illness. AIDS Patient Care and STDs. 2003;17(4):179. doi: 10.1089/108729103321619782. [DOI] [PubMed] [Google Scholar]
- Wagner G, Miller LG. Is the influence of social desirability on patients’ self-reported adherence overrated? Journal of Acquired Immune Deficiency Syndromes. 2004;35(2):203–204. doi: 10.1097/00126334-200402010-00016. [DOI] [PubMed] [Google Scholar]
- *21.Wagner JH, Justice AC, Chesney M, Sinclair G, Weissman S, Rodriguez-Barradas M. Patient-and provider-reported adherence: Toward a clinically useful approach to measuring antiretroviral adherence. Journal of Clinical Epidemiology. 2001;54(Suppl 1):S91–S98. doi: 10.1016/s0895-4356(01)00450-4. [DOI] [PubMed] [Google Scholar]
- *68.Walsh JC, Horne R, Dalton M, Burgess AP, Gazzard BG. Reasons for non-adherence to antiretroviral therapy: Patients’ perspectives provide evidence of multiple causes. AIDS Care. 2001;13(6):709–720. doi: 10.1080/09540120120076878. [DOI] [PubMed] [Google Scholar]
- *12.Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002;16(2):269–277. doi: 10.1097/00002030-200201250-00017. [DOI] [PubMed] [Google Scholar]
- Waterhouse DM, Calzone KA, Mele C, Brenner DE. Adherence to oral tamoxifen: A comparison of patient self-report, pill counts, and microelectronic monitoring. Journal of Clinical Oncology. 1993;11(6):1189–1197. doi: 10.1200/JCO.1993.11.6.1189. [DOI] [PubMed] [Google Scholar]
- *70.Weiser S, Wolfe W, Bangsberg D, Thior I, Gilbert P, Makhema J, et al. Barriers to antiretroviral adherence for patients living with HIV infection and AIDS in Botswana. Journal of Acquired Immune Deficiency Syndromes. 2003;34(3):281–288. doi: 10.1097/00126334-200311010-00004. [DOI] [PubMed] [Google Scholar]
- *33.Wutoh AK, Brown CM, Kumoji EK, Daftary MS, Jones T, Barnes NA, et al. Antiretroviral adherence and use of alternative therapies among older HIV-infected adults. Journal of the National Medical Association. 2001;93(7-8):243–250. [PMC free article] [PubMed] [Google Scholar]
- Wutoh A, Elekwachi O, Clarke-Tasker V, Daftary M, Powell N, Campusano G. Assessment and predictors of antiretroviral adherence in older HIV-infected patients. Journal of Acquired Immune Deficiency Syndromes. 2003;33(2):S106–S114. doi: 10.1097/00126334-200306012-00007. [DOI] [PubMed] [Google Scholar]