INTRODUCTION
Eosinophils and mast cells coexist in tissues in some benign conditions, and also in bone marrow biopsies of patients affected by clonal diseases. Some clonal and nonclonal disorders in which mast cells are affected are also associated with peripheral eosinophilia. These disorders include such varied presentations as allergic and asthmatic disorders, eosinophilic esophagitis (EoE), and both clonal and probably nonclonally expanded lines within the category of malignancies.
Discussed are the complex interplay of eosinophils and mast cells in these disorders. Also what is known about these disorders is described, a schematic in thinking about these disorders in one rubric is offered, and treatment options, which are usually tailored specifically to the patient presentation and underlying disorder, if it can be elucidated, are discussed.
MAST CELL AND EOSINOPHIL BIOLOGY
Mast Cells
Mast cells derive from the pluripotent precursor cell (CD34+, CD117+(Kit)). They develop and mature with the influence of stem cell factor (SCF) via Kit, the transmembrane tyrosine kinase receptor for SCF.1 Many other cytokines, including interleukins (IL) IL-3, IL-4, IL-5, IL-6, IL-9, and IL-15, can potentiate the growth and maturation of mast cells.
Mast cells are a rich source of inflammatory mediators which include histamine, prostaglandin D2, cysteinyl leukotrienes (LTC4), platelet activating factor, IL-3, IL-5, IL-6, IL-16, and SCF. Mast cell products that likely provide some interaction with eosinophils include IL-5, which is a potent growth and survival factor, CCL5 (RANTES), which is a chemotactic molecule, chymase (eosinophil apoptosis suppressor), tumor necrosis factor (survival and chemotaxis), heparin (stabilize eotaxins), and Kit, which interacts with eosinophil-derived SCF to induce differentiation, proliferation, and activation of mast cells.2
Eosinophils
Eosinophils also derive from the pluripotent CD34+ cell line. Granulocyte-macrophage colony-stimulating factor, IL-3, and IL-5 are all growth factors for eosinophils. IL-5 is the major cytokine that influences eosinophilopoiesis, as well as eliciting their activation and chemotaxis.3 As discussed above, mast cells do produce both IL-3 and IL-5. Eosinophil mediators include many chemokines and cytokines. A notable feature of eosinophils as a source of cytokines is that they store these cytokines preformed within eosinophil granules and secretory vesicles. Some of these mediators that have potential for mast cell interactions include IL-3, IL-5, IL-6, IL-16, and LTC4. More recently IL-9 was found to be produced by eosinophils in the context of mast cell interactions in EoE.4 Platelet activating factor is a known chemoattractant for eosinophils. IL-16, prostaglandin D2, and LTC4 are produced by eosinophils and eosinophils express receptors for these agents, enhancing the interaction between mast cells and eosinophils.5
Therefore, in addition to interacting with each other, these 2 cells have the capacity to influence the tissue microenvironment, which self-promotes their own existence and attracts cells that help activate and stimulate them into the area (such as TH2 CD4+ T cells, macrophages).
The Mast Cell-Eosinophil Pair
One interesting concept regarding the interaction between them is the existence of the eosinophil and mast cell couplets or pairs, reported in papers from 2011 and 2013.6,7 The research was mainly done on tissues with allergic inflammation, and the authors found several colocalized pairs of mast cells and eosinophils in human nasal polyps, asthmatic bronchi, as well as in mouse atopic dermatitis tissues. In vitro, they found that the 2 cells form stable conjugates and there is clear membrane contact established between them. Eosinophils were more viable when mast cells were present, dependent on soluble mediators and on physical cell contact (interestingly more so in the presence of SCF-enriched media than in granulocyte-macrophage colony-stimulating factor–enriched media). Mast cells were not as clearly affected or made more viable by eosinophil coculture. They were found to influence each other in a paracrine/physical pathway, using human and murine cells in vitro.7 This concept is discussed later when discussing the role of anti-IL-5 in EoE.
NONCLONAL DISORDERS IN WHICH BOTH CELLS ARE PRESENT AND LIKELY DRIVE DISEASE PATHOGENESIS
Asthma and Allergic Rhinitis
In allergic disorders, mast cells degranulate in response to immunoglobulin E–mediated allergic stimulation. Mediators released locally recruit eosinophils to cause further damage. Previous work has shown that both cells are present in asthmatic airways more often than in patients without allergic asthma.8 They are also present in the nasal fluid of patients with allergic rhinitis.9 Bronchoalveolar lavage (BAL) tryptase level, albeit elevated, however, did not correlate with worse lung function in asthmatic patients. In the same way, in a study done to evaluate tryptase/mast cell burden in eosinophilic pneumonia patients, while tryptase was elevated in BAL of these patients versus normal controls, the actual level of elevation between those patients did not correlate with lung function parameters.10
EoE
Both mast cells and eosinophils are present in biopsy specimens of patients with EoE. There is a great mast cell signature in EoE,11 and the presence of mast cells has been found to be useful in distinguishing between EoE and gastroesophageal reflux disease in biopsy specimens.12 The relative contribution of both cell types to disease pathogenesis is under investigation. Some patients with EoE respond to anti-IL-5 therapy. In those who respond, mast cells were found to be decreased along with eosinophils after therapy in biopsy specimens.4 Interestingly, these cells existed as couplets in EoE as with other disorders mentioned above within biopsy specimens. The mechanism under which this occurred was thought to be due to mutual cross-talk as others previously had shown, as well as through the role of IL-9-producing cells (70% of which were eosinophils per their evaluation). IL-9 produced by mostly eosinophils served as a potent mast cell growth factor.
Idiopathic Hypereosinophilic Syndrome
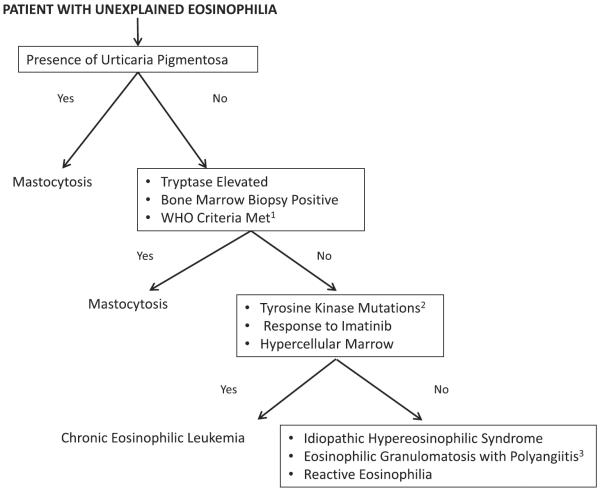
In the general workup of uncharacterized eosinophilia, sometimes one reaches the conclusion that a patient has idiopathic hypereosinophilic syndrome (HES) (no myeloproliferative criteria or lymphoproliferative criteria are met, no allergic cause is found, no vasculitis or features of it are elucidated such as in eosinophilic polyangiitis with granulomatous formerly known as Churg-Strauss syndrome) (Box 1, Fig. 1). In the paper with the largest database of published HES patients from 2009, 20% of patients without myeloproliferative variant HES or chronic eosinophilic leukemia (CEL) had elevated tryptase levels (66% of the patients total had tryptase levels checked during workup in this population).13 Idiopathic HES can be α-interferon-responsive,14 something that is also used to control systemic mastocytosis in more severe cases.15
Box 1 World Health Organization criteria for systemic mastocytosis.
One major and one minor criterion or 3 minor criteria must be met for diagnosis.
Major Criterion
Multifocal, dense aggregates of mast cells (15 or more) detected in sections of bone marrow and confirmed by tryptase immunohistochemistry or other special stains
Minor Criteria
In biopsy section, more than 25% of the mast cells in the infiltrate have atypical morphology, or, of all the mast cells in the aspirate smear, more than 25% are immature or atypical
Mast cells coexpress CD117 with CD2 and/or CD25
Detection of KIT point mutation at codon 816 in bone marrow, blood, or other extracutaneous organs
Serum total tryptase persistently >20 ng/mL (not a valid criteria in cases of systemic mastocytosis with associated clonal hematologic non-mast-cell lineage disease)
Adapted from Valent P, Horny HP, Escribano L, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res 2001;25:603–25.
Fig. 1.
Workup of uncharacterized eosinophilia. 1. World Health Organization criteria for systemic mastocytosis (see Box 1). 2. Tyrosine kinase mutations in chronic eosinophilic leukemia. Including but not limited to FIP1L1-PDGFRA fusion, fusion of PDGFRA with other partner genes, fusions involving PDGFRB. 3. Formerly known as Churg-Strauss syndrome.
CLONAL DISORDERS AFFECTING MAST CELLS AND EOSINOPHILS
Chronic Eosinophilic Leukemia
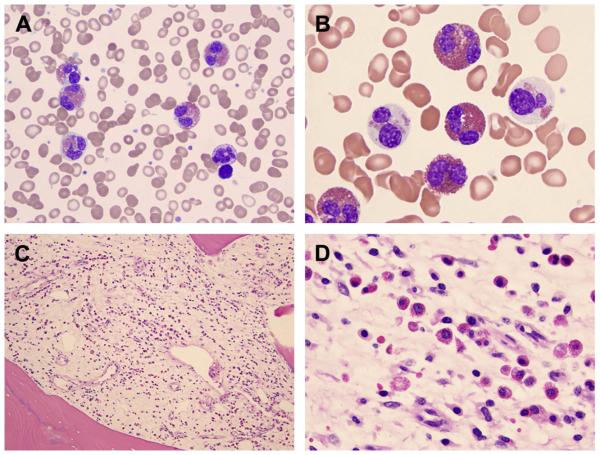
FIP1L1/PDFGRA-positive HES, described in 2003, also variably called myeloproliferative HES versus CEL, is the most significant entity in which an aberrant population of eosinophils is found with increased numbers of mast cells. These eosinophils are activated to cause end-organ damage, and there are elevated numbers of mast cells as well as serum tryptase levels. However, the mast cells do not carry the c-kit D816V mutation typically associated with mastocytosis nor do they cause any clinical symptoms of systemic mastocytosis.16 Eleven percent of HES patients have this mutation.13 The mutation is found in eosinophils, neutrophils, mast cells, T lymphocytes, B lymphocytes, and monocytes; hence, a pluripotent hematopoietic progenitor cell is affected. However, it seems to preferentially cause expansion of the eosinophil and mast cell populations.17 Dysplastic eosinophils and spindle-shaped mast cells are present, along with a hypercellular marrow, and usually elevated B12 and tryptase levels (Fig. 2). The mast cells also have CD25 positivity, yet they do not usually have the CD2 positivity often seen in systemic mastocytosis.18 Tryptase levels seem to at least partially normalize in response to imatinib treatment, like the eosinophilia does.19
Fig. 2.
Bone marrow biopsy and aspirate in a patient with chronic eosinophilic leukemia carrying the FIP1L1-PDGFRA rearrangement. Increased numbers of hypogranulated neoplastic eosinophils are observed in the aspirate (A, B) (Wright-Giemsa, original magnification ×100). The infiltrate predominantly consists of eosinophils in a background of fibrosis in the biopsy (C, D) (Hematoxylin and Eosin, original magnification ×400) as opposed to mastocytosis whereby the predominant infiltrate is that of mast cells. (Courtesy of German A. Pihan, MD, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA.)
CEL associated with F/P mutation can be confused with systemic mastocytosis due to the histopathologic and immunohistochemical features of mast cells shared among the 2 disorders. As mentioned above, mast cells in both of these disorders are spindle-shaped and express CD25+, which is an abnormal marker. However, they do not form compact aggregates, express CD2, or carry c-Kit D816V mutation in CEL. This confusion has contributed to the suggestion of imatinib as a therapy for mast cell disease in earlier reports, whereas an overwhelming majority of mastocytosis cases do not respond to imatinib due to the presence of D816V c-Kit mutation rendering resistance to this drug.20 Therefore, establishing the correct diagnosis is crucial for selecting the appropriate therapy. One paper suggests an algorithm based on the extent of eosinophilia and tryptase levels (see discussion below).16
Mastocytosis
Most patients with mastocytosis carry a D816V gain-of-function mutation in KIT, the gene encoding the c-kit receptor. There are limited data that SCF overexpression may play a role in some cases.21,22
Up to 28% of patients with systemic mastocytosis have peripheral eosinophilia, greater than 650 cells/mm3, although the frequency increases in advanced forms of mastocytosis.16,23 Eosinophilia is frequently noted in bone marrow biopsies and aspirates even in patients without significant peripheral eosinophilia (Fig. 3). Histopathology on these patients is usually positive for aberrant expression of CD2+ and CD25+. CKIT D816V mutation is positive in bone marrow or peripheral blood by sensitive PCR techniques24; no mutation for the FIP1L1-PDGFRA fusion is present. The CKIT mutation in mastocytosis is also interestingly multilineage yet preferentially affects mast cell expansion.25,26 The eosinophilia in mastocytosis is often not pathologic and does not necessitate treatment. The presentation is that of systemic mastocytosis, rather than that of clonal eosinophilia with end-organ damage.
Fig. 3.
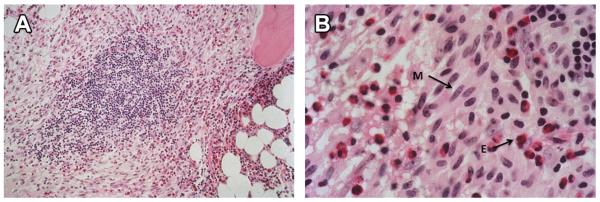
(A) Bone marrow biopsy in systemic mastocytosis, intermediate magnification (Hematoxylin and Eosin, 100×) featuring atypical, spindle-shaped mast cells and intermingled eosinophils surrounding a lymphoid aggregate. (B) Bone marrow biopsy in systemic mastocytosis, high magnification (Hematoxylin and Eosin, 400×) showing atypical, spindle-shaped mast cells (M) with numerous intermingled eosinophils (E). (Courtesy of Charles W. Ross, MD, University of Michigan, Ann Arbor, MI.)
In a paper from 2007 comparing the 2 entities (D816V-positive systemic mastocytosis vs FIP1L1/PDGFRA-positive CEL), distinguishing features for CEL included the degree of eosinophilia in relation to tryptase level, the absence of dense mast cell aggregates, the degree of B12 elevation, pulmonary symptoms, and cardiac symptoms.16 Mastocytosis rather than CEL was more likely in those with elevated tryptase to absolute eosinophil count ratio, dense mast cell aggregates on bone marrow biopsy, gastrointestinal symptoms, urticaria pigmentosa, female sex, and thrombocytosis. The 2 disorders can occasionally coexist, although it is very unusual to exhibit both D816V c-Kit mutation and PDGFRA fusion concurrently.27
FUTURE DIRECTIONS
Therapies
Although some therapies are known to target both mast cell disease and eosinophilia (systemic steroids, α-interferon), others are potentially used in one versus the other disorder13,28,29; hence, distinguishing between entities is of vital importance with careful evaluation. Imatinib is useful in FIP1L1-PDGFRA fusion disease but has no effect and theoretically can worsen c-Kit D816V-positive mastocytosis, by selectively inhibiting the cells carrying the wild-type c-Kit.19,30,31 Conversely, anti-IL5 therapy, although promising in HES,32 EoE,4 eosinophilic asthma33 (diseases which all have a mast cell signature), remains to be studied in the treatment of mastocytosis, which has associated eosinophilia. Omalizumab (anti-immunoglobulin E therapy), although useful in allergic asthma,34,35 has not been carefully studied in mastocytosis, although case reports have shown it to be useful in recurrent anaphylaxis associated with mastocytosis.36 Finally, up and coming therapies, such as midostaurin (PKC412), one of the tyrosine kinase inhibitors currently under study, have reportedly been shown to help with both systemic mastocytosis and associated eosinophilia.2
Bone marrow transplantation is a last-resort therapy in the more aggressive and malignant forms of both mastocytosis and eosinophilic disorders. Further papers on experience with bone marrow transplant in these disorders (often written as case studies) will shed some light on both the cure rate and the complications related to treating these disorders.
KEY POINTS.
The interplay between mast cells and eosinophils is complicated and those interactions are currently being studied.
Certain clonal and nonclonal entities exist in which these 2 cell types are increased in tissues and other sites, and in which they play a role in pathogenesis.
The specific type of clonal disorder is important to diagnose correctly, because treatment needs to be carefully tailored to the specific entity.
Footnotes
The authors have nothing to disclose.
REFERENCES
- 1.Metcalfe DD. Mastocytosis. In: Adkinson NF Jr, editor. Middleton's allergy principles and practice. 8th edition Vol. 2. Saunders Elsevier; Philadelphia: 2014. pp. 1224–36. [Google Scholar]
- 2.Gotlib J, Akin C. Mast cells and eosinophils in mastocytosis, chronic eosinophilic leukemia, and non-clonal disorders. Semin Hematol. 2012;49(2):128–37. doi: 10.1053/j.seminhematol.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Klion AD, Weller PF. Eosinophilia and eosinophil related disorders. In: Adkinson NF Jr, editor. Middleton's allergy principles and practice. 8th edition Vol. 2. Saunders Elsevier; Philadelphia: 2014. pp. 1205–23. [Google Scholar]
- 4.Otani IM, Anilkumar AA, Newbury RO, et al. Anti-IL-5 therapy reduces mast cell and IL-9 cell numbers in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2013;131(6):1576–82. doi: 10.1016/j.jaci.2013.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovalszki A, Sheikh J, Weller PF. Eosinophils and eosinophilia. In: Rich RR, editor. Clinical immunology principles and practice. 4th edition Vol. 1. Elsevier Saunders; Philadelphia: 2013. pp. 298–309. [Google Scholar]
- 6.Elishmereni M, Alenius HT, Bradding P, et al. Physical interactions between mast cells and eosinophils: a novel mechanism enhancing eosinophil survival in vitro. Allergy. 2011;66(3):376–85. doi: 10.1111/j.1398-9995.2010.02494.x. [DOI] [PubMed] [Google Scholar]
- 7.Elishmereni M, Bachelet I, Nissim Ben-Efraim AH, et al. Interacting mast cells and eosinophils acquire an enhanced activation state in vitro. Allergy. 2013;68(2):171–9. doi: 10.1111/all.12059. [DOI] [PubMed] [Google Scholar]
- 8.Jarjour NN, Calhoun WJ, Schwartz LB, et al. Elevated bronchoalveolar lavage fluid histamine levels in allergic asthmatics are associated with increased airway obstruction. Am Rev Respir Dis. 1991;144(1):83–7. doi: 10.1164/ajrccm/144.1.83. [DOI] [PubMed] [Google Scholar]
- 9.Rasp G, Hochstrasser K. Tryptase in nasal fluid is a useful marker of allergic rhinitis. Allergy. 1993;48(2):72–4. doi: 10.1111/j.1398-9995.1993.tb00688.x. [DOI] [PubMed] [Google Scholar]
- 10.Bargagli E, Bigliazzi C, Leonini A, et al. Tryptase concentrations in bronchoalveolar lavage from patients with chronic eosinophilic pneumonia. Clin Sci. 2005;108(3):273–6. doi: 10.1042/CS20040178. [DOI] [PubMed] [Google Scholar]
- 11.Abonia JP, Blanchard C, Butz BB, et al. Involvement of mast cells in eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126(1):140–9. doi: 10.1016/j.jaci.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirsch R, Bokhary R, Marcon MA, et al. Activated mucosal mast cells differentiate eosinophilic (allergic) esophagitis from gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2007;44(1):20–6. doi: 10.1097/MPG.0b013e31802c0d06. [DOI] [PubMed] [Google Scholar]
- 13.Ogbogu PU, Bochner BS, Butterfield JH, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009;124(6):1319–25.e3. doi: 10.1016/j.jaci.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butterfield JH, Weiler CR. Use of pegylated interferon in hypereosinophilic syndrome. Leuk Res. 2012;36(2):192–7. doi: 10.1016/j.leukres.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Cardet JC, Akin C, Lee MJ. Mastocytosis: update on pharmacotherapy and future directions. Expert Opin Pharmacother. 2013;14(15):2033–45. doi: 10.1517/14656566.2013.824424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maric I, Robyn J, Metcalfe DD, et al. KIT D816V-associated systemic mastocytosis with eosinophilia and FIP1L1/PDGFRA-associated chronic eosinophilic leukemia are distinct entities. J Allergy Clin Immunol. 2007;120(3):680–7. doi: 10.1016/j.jaci.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 17.Robyn J, Lemery S, McCoy JP, et al. Multilineage involvement of the fusion gene in patients with FIP1L1/PDGFRA-positive hypereosinophilic syndrome. Br J Haematol. 2006;132(3):286–92. doi: 10.1111/j.1365-2141.2005.05863.x. [DOI] [PubMed] [Google Scholar]
- 18.Klion AD, Noel P, Akin C, et al. Elevated serum tryptase levels identify a subset of patients with a myeloproliferative variant of idiopathic hypereosinophilic syndrome associated with tissue fibrosis, poor prognosis, and imatinib responsiveness. Blood. 2003;101(12):4660–6. doi: 10.1182/blood-2003-01-0006. [DOI] [PubMed] [Google Scholar]
- 19.Klion AD, Robyn J, Akin C, et al. Molecular remission and reversal of myelofibrosis in response to imatinib mesylate treatment in patients with the myeloproliferative variant of hypereosinophilic syndrome. Blood. 2004;103(2):473–8. doi: 10.1182/blood-2003-08-2798. [DOI] [PubMed] [Google Scholar]
- 20.Akin C, Metcalfe DD. The biology of Kit in disease and the application of pharmacogenetics. J Allergy Clin Immunol. 2004;114(1):13–9. doi: 10.1016/j.jaci.2004.04.046. quiz: 20. [DOI] [PubMed] [Google Scholar]
- 21.Valent P, Akin C, Sperr WR, et al. Mastocytosis: pathology, genetics, and current options for therapy. Leuk Lymphoma. 2005;46(1):35–48. doi: 10.1080/10428190400010775. [DOI] [PubMed] [Google Scholar]
- 22.Valent P, Horny HP, Escribano L, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25(7):603–25. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 23.Pardanani A, Lim KH, Lasho TL, et al. Prognostically relevant breakdown of 123 patients with systemic mastocytosis associated with other myeloid malignancies. Blood. 2009;114(18):3769–72. doi: 10.1182/blood-2009-05-220145. [DOI] [PubMed] [Google Scholar]
- 24.Kristensen T, Vestergaard H, Moller MB. Improved detection of the KIT D816V mutation in patients with systemic mastocytosis using a quantitative and highly sensitive real-time qPCR assay. J Mol Diagn. 2011;13(2):180–8. doi: 10.1016/j.jmoldx.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akin C, Kirshenbaum AS, Semere T, et al. Analysis of the surface expression of c-kit and occurrence of the c-kit Asp816Val activating mutation in T cells, B cells, and myelomonocytic cells in patients with mastocytosis. Exp Hematol. 2000;28(2):140–7. doi: 10.1016/s0301-472x(99)00145-9. [DOI] [PubMed] [Google Scholar]
- 26.Yavuz AS, Lipsky PE, Yavuz S, et al. Evidence for the involvement of a hematopoietic progenitor cell in systemic mastocytosis from single-cell analysis of mutations in the c-kit gene. Blood. 2002;100(2):661–5. doi: 10.1182/blood-2002-01-0203. [DOI] [PubMed] [Google Scholar]
- 27.Florian S, Esterbauer H, Binder T, et al. Systemic mastocytosis (SM) associated with chronic eosinophilic leukemia (SM-CEL): detection of FIP1L1/PDGFRalpha, classification by WHO criteria, and response to therapy with imatinib. Leuk Res. 2006;30(9):1201–5. doi: 10.1016/j.leukres.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Valent P, Sperr WR, Akin C. How I treat patients with advanced systemic mastocytosis. Blood. 2010;116(26):5812–7. doi: 10.1182/blood-2010-08-292144. [DOI] [PubMed] [Google Scholar]
- 29.Kluin-Nelemans HC, Jansen JH, Breukelman H, et al. Response to interferon alfa-2b in a patient with systemic mastocytosis. N Engl J Med. 1992;326(9):619–23. doi: 10.1056/NEJM199202273260907. [DOI] [PubMed] [Google Scholar]
- 30.Cools J, DeAngelo DJ, Gotlib J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348(13):1201–14. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 31.Akin C, Brockow K, D'Ambrosio C, et al. Effects of tyrosine kinase inhibitor STI571 on human mast cells bearing wild-type or mutated c-kit. Exp Hematol. 2003;31(8):686–92. doi: 10.1016/s0301-472x(03)00112-7. [DOI] [PubMed] [Google Scholar]
- 32.Rothenberg ME, Klion AD, Roufosse FE, et al. Treatment of patients with the hyper-eosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358(12):1215–28. doi: 10.1056/NEJMoa070812. [DOI] [PubMed] [Google Scholar]
- 33.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–9. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 34.Corren J, Casale T, Deniz Y, et al. Omalizumab, a recombinant humanized anti-IgE antibody, reduces asthma-related emergency room visits and hospitalizations in patients with allergic asthma. J Allergy Clin Immunol. 2003;111(1):87–90. doi: 10.1067/mai.2003.49. [DOI] [PubMed] [Google Scholar]
- 35.Chiang DT, Clark J, Casale TB. Omalizumab in asthma: approval and postapproval experience. Clin Rev Allergy Immunol. 2005;29(1):3–16. doi: 10.1385/CRIAI:29:1:003. [DOI] [PubMed] [Google Scholar]
- 36.Carter MC, Robyn JA, Bressler PB, et al. Omalizumab for the treatment of unprovoked anaphylaxis in patients with systemic mastocytosis. J Allergy Clin Immunol. 2007;119(6):1550–1. doi: 10.1016/j.jaci.2007.03.032. [DOI] [PubMed] [Google Scholar]