We are on the eve of a new era in hepatitis C virus (HCV) treatment. For the first two decades after the virus was discovered, only ribavirin and interferon alfa-related compounds were approved for HCV treatment, and nearly a decade has passed since the last substantive upgrade. During the same two-decade interval, 24 new compounds from 6 classes were approved for treatment of HIV. However, the long wait for new HCV treatments is about to end. Clinical trials are now underway for multiple compounds from each of at least five novel classes, and data have already been published or disclosed for medications scheduled for release in 2011 that suggest sustained response rates may go as high as 75%, even for the most difficult to treat genotype 1 infections.1, 2 Considering the medications that will follow, in the foreseeable future it may be possible to cure nearly all those who are treated.
In this issue of the Lancet, Gane et al report the outcome of a series of HCV-infected persons treated with two new drugs: RG7128 and danoprevir, compounds that directly inhibit the HCV polymerase and HCV protease, respectively.3 All individuals had genotype 1 HCV infection. Some had never been treated before, while others had failed interferon-based standard of care. Eighty-seven persons were enrolled into one of 7 ‘cohorts’, randomized to various doses or schedules of study drugs or placebo for 13 days, then transitioned to peginterferon and ribavirin. The main finding is that persons who received the higher doses of the two study drugs had an average plasma HCV RNA reduction at 13 days of 5 log10 IU, a value that was not far from the average starting viral load of 6.4 log IU/ml. In some individuals, plasma HCV RNA could no longer be detected after 13 days of two orally-administered compounds. Treatment was well tolerated, and there was no direct evidence resistant viruses were selected.
Although the study met its primary safety objectives, there are important limitations to early phase trials. The goal of HCV treatment is to eradicate infection, an end point that is achieved when HCV RNA cannot be detected in the blood at the end of treatment and 6 months later (referred to as a sustained virologic response or ‘cure’). Because persons in the present study rolled over to peginterferon and ribavirin after the 13 day trial, the study will never tell us about the ultimate efficacy of the combined use of the two direct-acting agents. This limitation could be important if, despite the potent viral suppression of the direct-acting compounds, the immunologic effects of interferon and ribavirin ultimately are needed to cure infection. Likewise, one of the primary safety concerns, the long term risk of viral resistance with a two-drug, direct-acting regimen, cannot be confidently assessed because medication use was directly observed in a clinical trial unit, and only limited resistance testing is presented. So, how does this small study contribute to the rapidly unfolding era of HCV treatment? The results are an important step toward developing an interferon-sparing cure for HCV infection, and achieving that goal may indirectly determine the ultimate global impact of the new medications.
Part of the benefit of interferon-sparing HCV treatment will be overcoming the limited efficacy and tolerability of interferon alfa. Although nearly everyone cured of HCV infection has received interferon alfa injections, some individuals cannot tolerate interferon alfa. In others, HCV infection is ‘resistant’ to the drug’s effect. Even in the carefully-selected patients screened for phase 3 registration trials, 10–14% stop medications because of adverse events, and more than half of those with genotype 1 HCV infection fail the current interferon alfa based standard of care.4, 5 These interferon “intolerant” or “resistant” persons are obvious beneficiaries of interferon-sparing HCV therapy. However, quantitatively, it is possible the much larger impact of efficacious, interferon-sparing HCV treatment will be to expand HCV testing and treatment initiation.
Only a small fraction of the estimated 170 million persons with chronic hepatitis C infection know they are infected; far fewer ever start treatment. In the United States, some have estimated only one-third of those with hepatitis C are aware of their infections, and only a fraction of those go on to care.6 For example, from 2002–2007, approximately 663,000 of ~4–5 million persons with chronic hepatitis C were treated.7 In Europe it is estimated that by 2006 chronic hepatitis C treatment was provided for only 308,000 persons who comprised no more than 16% of the HCV-infected persons in any single country.8 Thus, without changing the extent to which HCV infection is detected and treatment is started, even 100% efficacious treatment will not cure many of those infected worldwide. On the other hand, if interferon-sparing treatment is safer, more effective, and simpler to administer, it may markedly expand testing, treatment uptake, and treatment efficacy, similar to how development of highly active antiretroviral therapy led to expanded testing and treatment of HIV.
We are indeed on the dawn of a new era in the treatment of HCV, and it is worth noting that this curable infection with no natural nonhuman reservoir can be eliminated from any setting in which robust testing and treatment can be sustained. What is unclear at this stage is whether HCV testing and treatment will penetrate to the prisons, drug treatment centers, and other venues where many HCV infected persons are found and unknowingly harbor the virus. On May 21, 2010 the World Health Assembly passed a resolution that called for the World Health Organization to develop a comprehensive approach to control of chronic hepatitis. This measure and the work published by Gane et al are exciting steps in the right direction. But, as with HIV testing and treatment, it will take both safe effective medications and a coordinated, well-resourced response to achieve major global impact.
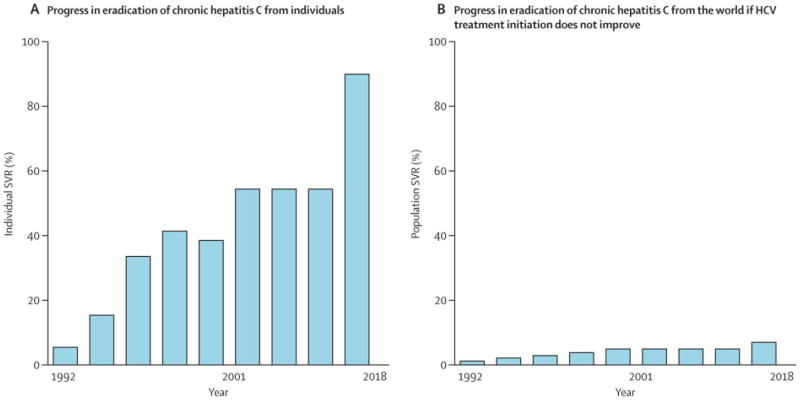
Figure 1.
Schematic representation of low global impact of improving HCV treatment efficacy without expanding HCV testing and treatment initiation.
A=overall sustained virological response rates (SVR) possible with major developments in HCV treatment and projections of 90% SVR with new drugs.
B=population impact calculated by multiplying efficacy figures from A by estimate of percent of individuals worldwide who have started HCV treatment.
2001 shown because pegylated interferon alfa and ribavirin were first approved then (peginterferon alfa 2a approved in 2002), the latest upgrade in HCV treatment
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McHutchison JG, Everson GT, Gordon SC, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360:1827–1838. doi: 10.1056/NEJMoa0806104. [DOI] [PubMed] [Google Scholar]
- 2.Kwo PY, Lawitz EJ, McCone J, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376:705–716. doi: 10.1016/S0140-6736(10)60934-8. [DOI] [PubMed] [Google Scholar]
- 3.Gane, et al. Lancet [Google Scholar]
- 4.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 5.McHutchison JG, Lawitz EJ, Shiffman ML, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580–593. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- 6.Falck-Ytter Y, Kale H, Mullen KD, et al. Surprisingly small effect of antiviral treatment in patients with hepatitis C. Ann Intern Med. 2002;136:288–292. doi: 10.7326/0003-4819-136-4-200202190-00008. [DOI] [PubMed] [Google Scholar]
- 7.Volk ML, Tocco R, Saini S, et al. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology. 2009 doi: 10.1002/hep.23220. [DOI] [PubMed] [Google Scholar]
- 8.Lettmeier B, Muhlberger N, Schwarzer R, et al. Market uptake of new antiviral drugs for the treatment of hepatitis C. J Hepatol. 2008;49:528–536. doi: 10.1016/j.jhep.2008.04.021. [DOI] [PubMed] [Google Scholar]